The Efficacy of Hyaluronic Acid Binding (HAB) in the Treatment of Male Infertility: A Systematic Review of the Literature
Abstract
1. Introduction
- Provides prognostic information regarding the likelihood of success of treatment via HAB scores? (Study question 1)
- Improves the incidence of miscarriage/pregnancy loss following insemination with HAB selected sperm compared with conventional insemination techniques? (Study question 2)
- Improves clinical outcomes (e.g., live birth rates) compared with conventional IVF/ICSI in the unexplained infertility population? (Study question 3)
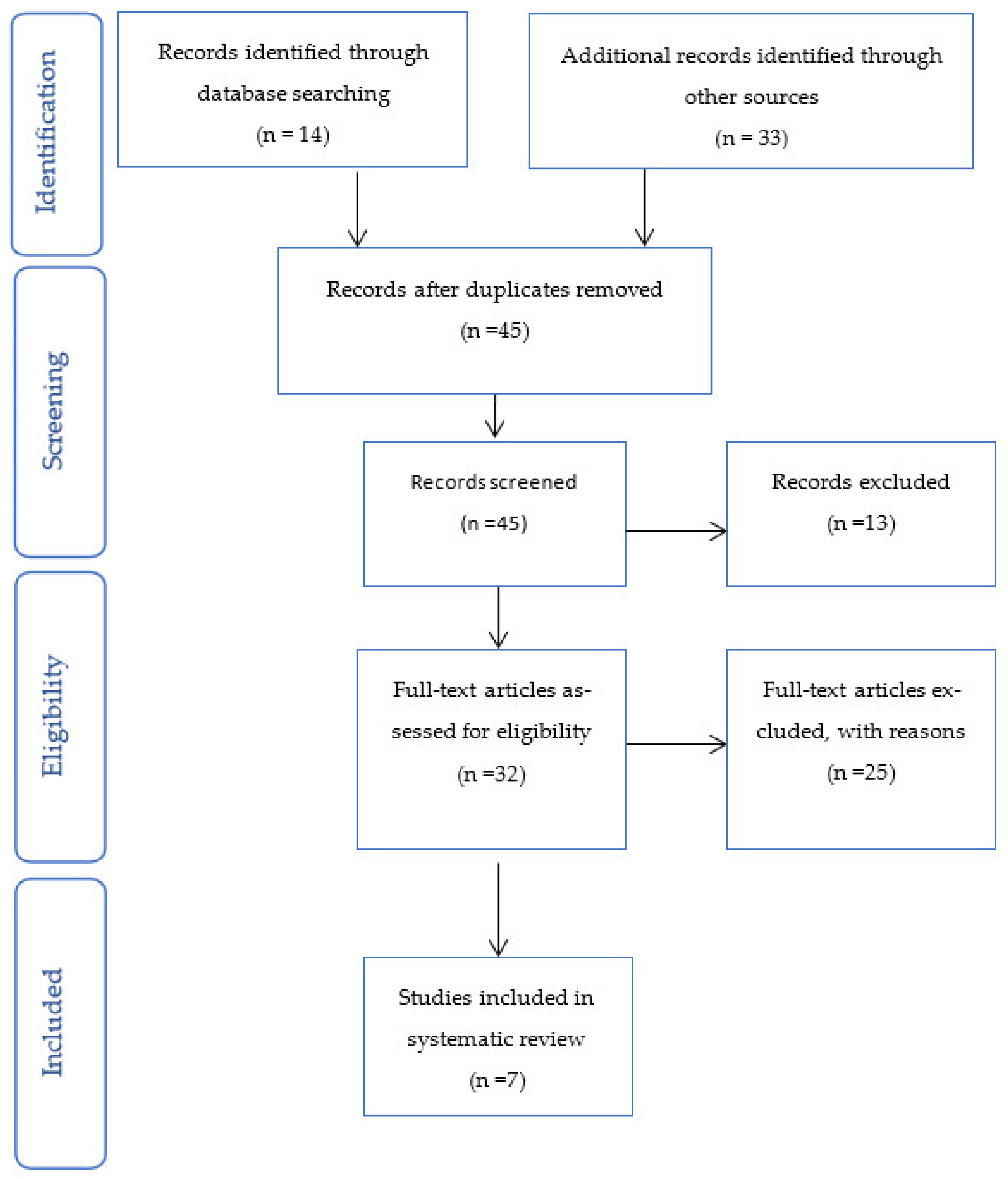
2. Materials and Methods
- Cochrane Central Register of Controlled Trials (CENTRAL)
- US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
- Hand-searching through reference lists and citing articles of selected studies. This review was not registered.
2.1. Search Terms for Each of the Research Questions
- Is sperm HAB score prior to insemination predictive of clinical outcomes?
- Does sperm selection by HAB reduce the rate of pregnancy loss in IVF or ICSI cycles?
- Does sperm selection by HAB improve clinical outcomes for all infertility patients?
2.2. Types of Studies
2.3. Types of Participants
- Advanced maternal age (>43 years)
- Use of donated gametes
- Use of surgically retrieved sperm
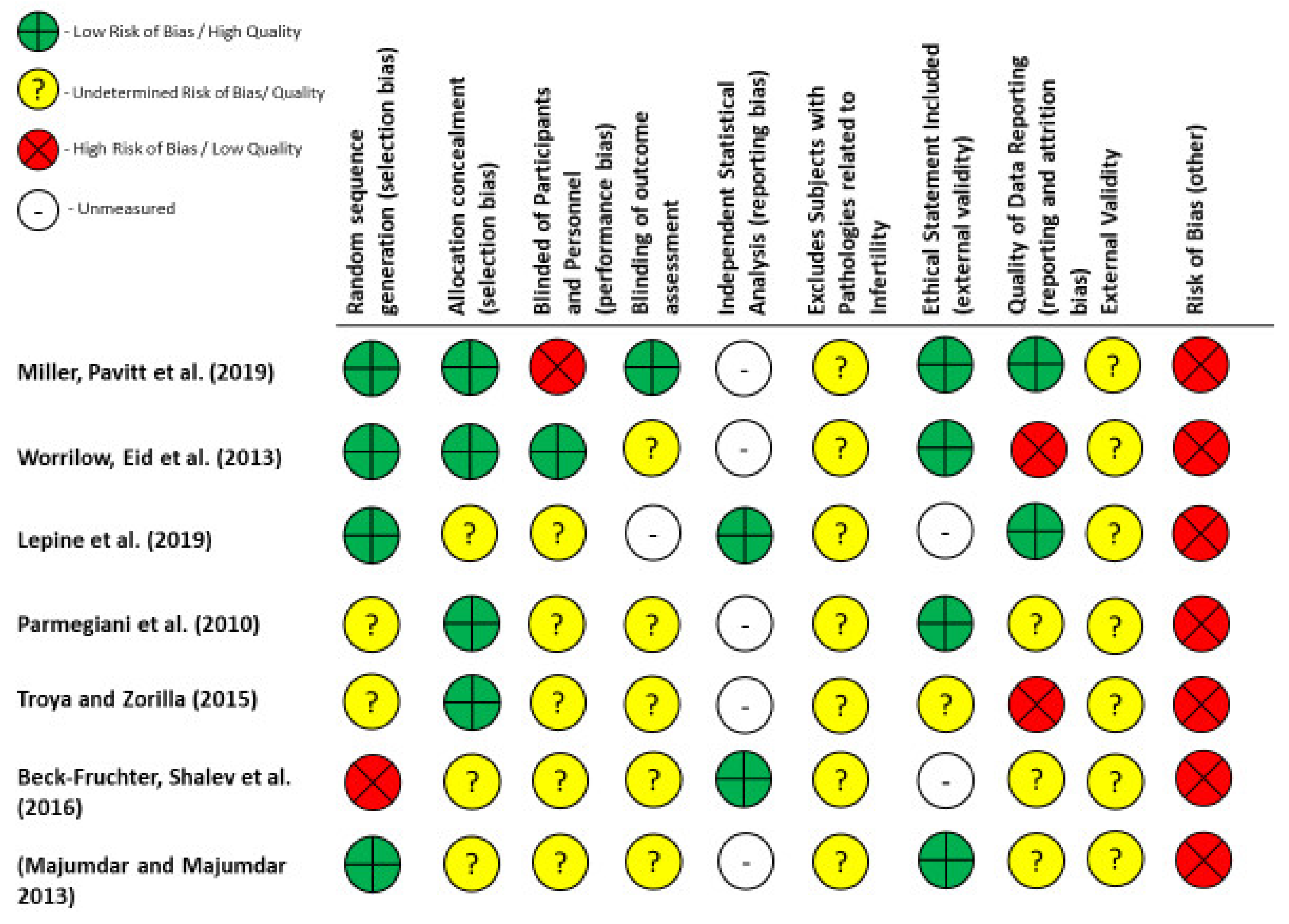
2.4. Quality and Risk of Bias Assessments
3. Results
3.1. Can Assessment of Sperm Function by HA-Binding Predict Clinical Outcomes?
3.1.1. Fertilisation Rate (FR)
3.1.2. Clinical Pregnancy Rates (CPR)
3.1.3. Live Birth Rates (LBR)
3.2. Is the Incidence of Miscarriage/Pregnancy Loss Reduced following Insemination with HAB Selected Sperm Compared with Conventional Insemination Techniques?
3.3. Can HAB-Sperm Selection Improve Clinical Outcomes Compared with Conventional IVF/ICSI in the Unexplained Infertility Population?
3.3.1. Fertilisation Rates (FR)
3.3.2. Clinical Pregnancy Rates (CPR)
3.3.3. Live Birth Rates (LBR)
4. Discussion
4.1. Confounding Variables
4.2. HAB Score and Clinical Outcomes
4.3. HAB-Sperm Selection and Pregnancy Loss
4.4. HAB-Sperm Selection and Clinical Outcomes
4.5. Limitations of Included Studies
4.6. HAB Score as a Screening Tool
4.7. Future Approaches by the Clinic and the HFEA
4.8. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V.; et al. ART in Europe, 2014: Results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. 2018, 33, 1586–1601. [Google Scholar] [CrossRef] [PubMed]
- Colaco, S.; Sakkas, D. Paternal factors contributing to embryo quality. J. Assist. Reprod. Genet. 2018, 35, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.; Kumar, M.; Kalmbach, K. Oocyte competency is the key to embryo potential. Fertil. Steril. 2015, 103, 317–322. [Google Scholar] [CrossRef]
- Qiu, J.; Hales, B.F.; Robaire, B. Damage to Rat Spermatozoal DNA after Chronic Cyclophosphamide Exposure1. Biol. Reprod. 1995, 53, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hales, B.F.; Robaire, B. Effects of Chronic Low-Dose Cyclophosphamide Exposure on the Nuclei of Rat Spermatozoa1. Biol. Reprod. 1995, 52, 33–40. [Google Scholar] [CrossRef]
- Sakkas, D.; Manicardi, G.C.; Bizzaro, D.; Bianchi, P.G. Possible consequences of performing intracytoplasmic sperm injection (ICSI) with sperm possessing nuclear DNA damage. Hum. Fertil. 2000, 3, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Borges, E., Jr.; Dutta, S.; Krajewska-Kułak, E. Decline in sperm count in European men during the past 50 years. Hum. Exp. Toxicol. 2017, 37, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y. The cycle of the seminiferous epithelium in man. Am. J. Anat. 1963, 112, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Huszar, G.; Vigue, L. Spermatogenesis-related change in the synthesis of the creatine kinase B-type and M-type isoforms in human spermatozoa. Mol. Reprod. Dev. 1990, 25, 258–262. [Google Scholar] [CrossRef]
- Huszar, G.; Vigue, L. Correlation between the rate of lipid peroxidation and cellular maturity as measured by creatine kinase activity in human spermatozoa. J. Androl. 1994, 15, 71–77. [Google Scholar]
- Aitken, J.; Krausz, C.; Buckingham, D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol. Reprod. Dev. 1994, 39, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Effectiveness and treatment for unexplained infertility. Fertil. Steril. 2006, 86, S111–S114. [Google Scholar] [CrossRef] [PubMed]
- Prinosilova, P.; Kruger, T.; Sati, L.; Ozkavukcu, S.; Vigue, L.; Kovanci, E.; Huszar, G. Selectivity of hyaluronic acid binding for spermatozoa with normal Tygerberg strict morphology. Reprod. Biomed. Online 2009, 18, 177–183. [Google Scholar] [CrossRef]
- Huszar, G.; Ozkavukcu, S.; Jakab, A.; Celik-Ozenci, C.; Sati, G.L.; Cayli, S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: Selection of sperm for intracytoplasmic sperm injection. Curr. Opin. Obstet. Gynecol. 2006, 18, 260–267. [Google Scholar] [CrossRef]
- Gasca, S.; Pellestor, F.; Assou, S.; Loup, V.; Anahory, T.; Dechaud, H.; De Vos, J.; Hamamah, S. Identifying new human oocyte marker genes: A microarray approach. Reprod. Biomed. Online 2007, 14, 175–183. [Google Scholar] [CrossRef]
- Cayli, S.; Jakab, A.; Ovari, L.; Delpiano, E.; Celik-Ozenci, C.; Sakkas, D.; Ward, D.; Huszar, G. Biochemical markers of sperm function: Male fertility and sperm selection for ICSI. Reprod. Biomed. Online 2003, 7, 462–468. [Google Scholar] [CrossRef]
- Tarozzi, N.; Nadalini, M.; Bizzaro, D.; Serrao, L.; Fava, L.; Scaravelli, G.; Borini, A. Sperm–hyaluronan-binding assay: Clinical value in conventional IVF under Italian law. Reprod. Biomed. Online 2009, 19 (Suppl 3), 35–43. [Google Scholar] [CrossRef]
- Ye, H.; Huang, G.-N.; Gao, Y.; Liu, D.Y. Relationship between human sperm-hyaluronan binding assay and fertilization rate in conventional in vitro fertilization. Hum. Reprod. 2006, 21, 1545–1550. [Google Scholar] [CrossRef][Green Version]
- Nijs, M.; Creemers, E.; Cox, A.; Janssen, M.; Vanheusden, E.; Van Der Elst, J.; Ombelet, W. Relationship between hyaluronic acid binding assay and outcome in ART: A pilot study. Andrologia 2010, 42, 291–296. [Google Scholar] [CrossRef]
- Balaban, B.; Lundin, K.; Morrell, J.M.; Tjellstrom, H.; Urman, B.; Holmes, P.V. An alternative to PVP for slowing sperm prior to ICSI. Hum. Reprod. 2003, 18, 1887–1889. [Google Scholar] [CrossRef]
- Barak, Y.; Menezo, Y.; Veiga, A.; Elder, K. A physiological replacement for polyvinylpyrrolidone (PVP) in assisted reproductive technology. Hum. Fertil. 2001, 4, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bergh, M.J.; Fahy-Deshe, M.; Hohl, M.K. Pronuclear zygote score following intracytoplasmic injection of hyaluronan-bound spermatozoa: A prospective randomized study. Reprod Biomed Online 2009, 19, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Kuo-Kuang Lee, R.; Li, S.-H.; Lu, C.-H.; Sun, F.-J.; Hwu, Y.-M. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil. Steril. 2008, 90, 352–359. [Google Scholar] [CrossRef]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 998–1005.e8. [Google Scholar] [CrossRef]
- Worrilow, K.C.; Eid, S.; Woodhouse, D.; Perloe, M.; Smith, S.; Witmyer, J.; Ivani, K.; Khoury, C.; Ball, G.D.; Elliot, T.; et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): Significant improvement in clinical outcomes—Multicenter, double-blinded and randomized controlled trial. Hum. Reprod. 2013, 28, 306–314. [Google Scholar] [CrossRef]
- Parmegiani, L.; Cognigni, G.E.; Bernardi, S.; Troilo, E.; Taraborrelli, S.; Arnone, A.; Maccarini, A.M.; Filicori, M. Comparison of two ready-to-use systems designed for sperm–hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs. Sperm Slow: A prospective, randomized trial. Fertil. Steril. 2012, 98, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Parmegiani, L.; Cognigni, G.E.; Bernardi, S.; Troilo, E.; Ciampaglia, W.; Filicori, M. “Physiologic ICSI”: Hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil. Steril. 2010, 93, 598–604. [Google Scholar] [CrossRef]
- Parmegiani, L.; Cognigni, G.E.; Ciampaglia, W.; Pocognoli, P.; Marchi, F.; Filicori, M. Efficiency of hyaluronic acid (HA) sperm selection. J. Assist. Reprod. Genet. 2010, 27, 13–16. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.H.; Razavi, S.; Vahdati, A.A.; Fathi, F.; Tavalaee, M. Evaluation of sperm selection procedure based on hyaluronic acid binding ability on ICSI outcome. J. Assist. Reprod. Genet. 2008, 25, 197–203. [Google Scholar] [CrossRef]
- Miller, D.; Pavitt, S.; Sharma, V.; Forbes, G.; Hooper, R.; Bhattacharya, S.; Kirkman-Brown, J.; Coomarasamy, A.; Lewis, S.; Cutting, R.; et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): A parallel, two-group, randomised trial. Lancet 2019, 393, 416–422. [Google Scholar] [CrossRef]
- Titus, S.; Stobezki, R.; Oktay, K. Impaired DNA Repair as a Mechanism for Oocyte Aging: Is It Epigenetically Determined? Semin. Reprod. Med. 2015, 33, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Alegre, L.; Garrido, N.; Munoz, M.; De Los Santos, M.; Remohi Gimenez, J.; Meseguer, M. Sperm selection with hyaluronic acid (PICSI) improves LBR in IVF treatments. Fertil. Steril. 2017, 108, e130. [Google Scholar] [CrossRef][Green Version]
- Lepine, S.; McDowell, S.; Searle, L.M.; Kroon, B.; Glujovsky, D.; Yazdani, A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst. Rev. 2019, 7, CD010461. [Google Scholar] [CrossRef]
- McDowell, S.; Kroon, B.; Ford, E.; Hook, Y.; Glujovsky, D.; Yazdani, A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst. Rev. 2014, 10, Cd010461. [Google Scholar] [CrossRef]
- Miller, D. Hyaluronan-selected sperm should not be considered an add-on—Author’s reply. Lancet 2019, 394, 1320. [Google Scholar] [CrossRef]
- Parmegiani, L. Hyaluronan-selected sperm should not be considered an add-on. Lancet 2019, 394, 1319–1320. [Google Scholar] [CrossRef]
- Treatment add-ons with limited evidence, HFEA. 2022. Available online: https://www.hfea.gov.uk/treatments/treatment-add-ons/ (accessed on 4 July 2022).
- Macklon, N.S.; Ahuja, K.K.; Fauser, B. Building an evidence base for IVF ‘add-ons’. Reprod. Biomed. Online 2019, 38, 853–856. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Reprint—Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen, A.D.; Franken, D.R.; Bosman, E.; Rodrigues, F.A.; Van Rensburg, J.H.; Van Schouwenburg, J.A.; Lombaard, C. Relationship between human spermatozoa-hyaluronan-binding assay, conventional semen parameters and fertilisation rates in intracytoplasmic spermatozoa injection. Andrologia 2015, 47, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, P.; Kovats, T.; Sajgo, A.; Szollosi, J.; Matyas, S.; Kaali, S.G. The role of hyaluronic acid binding assay in choosing the fertilization method for patients undergoing IVF for unexplained infertility. J. Assist. Reprod. Genet. 2011, 28, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Mokánszki, A.; Tóthné, E.V.; Bodnár, B.; Tándor, Z.; Molnár, Z.; Jakab, A.; Ujfalusi, A.; Oláh, E. Is sperm hyaluronic acid binding ability predictive for clinical success of intracytoplasmic sperm injection: PICSI vs. ICSI? Syst. Biol. Reprod. Med. 2014, 60, 348–354. [Google Scholar] [CrossRef]
- Pregl Breznik, B.; Kovačič, B.; Vlaisavljević, V. Are sperm DNA fragmentation, hyperactivation, and hyaluronan-binding ability predictive for fertilization and embryo development in in vitro fertilization and intracytoplasmic sperm injection? Fertil. Steril. 2013, 99, 1233–1241. [Google Scholar] [CrossRef]
- Said, T.M.; Land, J.A. Effects of advanced selection methods on sperm quality and ART outcome: A systematic review. Hum. Reprod. Update 2011, 17, 719–733. [Google Scholar] [CrossRef]
- Troya, J.; Zorrilla, I. Annexin V-MACS in infertile couples as method for separation of sperm without DNA fragmentation. JBRA Assist. Reprod. 2015, 19, 66–69. [Google Scholar] [CrossRef]
- Majumdar, G.; Majumdar, A. A prospective randomized study to evaluate the effect of hyaluronic acid sperm selection on the intracytoplasmic sperm injection outcome of patients with unexplained infertility having normal semen parameters. J. Assist. Reprod. Genet. 2013, 30, 1471–1475. [Google Scholar] [CrossRef]
- Beck-Fruchter, R.; Shalev, E.; Weiss, A. Clinical benefit using sperm hyaluronic acid binding technique in ICSI cycles: A systematic review and meta-analysis. Reprod. Biomed. Online 2016, 32, 286–298. [Google Scholar] [CrossRef]
- Worrilow, K.; Eid, S.; Woodhouse, D.; Matthews, J.; Khoury, C.; Witmyer, J. Prospective, multi-center, double-blind, randomized clinical trial evaluating the use of hyaluronan bound sperm (HBS) in ICSI: Statistically significant improvement in clinical outcomes. Fertil. Steril. 2011, 96, S58. [Google Scholar] [CrossRef]
- Worrilow, K.; Huynh, H.; Bower, J.; Anderson, A.; Schillings, W.; Crain, J. PICSI™ vs. ICSI: Statistically significant improvement in clinical outcomes in 240 in vitro fertilization (IVF) patients. Fertil. Steril. 2007, 88, S37. [Google Scholar] [CrossRef]
- Lauga, E.; Powers, T.R. The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 2009, 72. [Google Scholar] [CrossRef]
- Zalata, A.; Hafez, T.; Comhaire, F. Evaluation of the role of reactive oxygen species in male infertility. Hum. Reprod. 1995, 10, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Rappa, K.L.; Rodriguez, H.F.; Hakkarainen, G.C.; Anchan, R.M.; Mutter, G.L.; Asghar, W. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol. Adv. 2016, 34, 578–587. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- Pasqualotto, F.F.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J.; Agarwal, A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil. Steril. 2000, 73, 459–464. [Google Scholar] [CrossRef]
- Pelliccione, F.; Micillo, A.; Cordeschi, G.; D’Angeli, A.; Necozione, S.; Gandini, L.; Lenzi, A.; Francavilla, F.; Francavilla, S. Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil. Steril. 2011, 95, 641–646. [Google Scholar] [CrossRef]
- Mundy, A.J.; Ryder, T.A.; Edmonds, D.K. Asthenozoospermia and the human sperm mid-piece. Hum. Reprod. 1995, 10, 116–119. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenco, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef]
- Simon, L.; Murphy, K.; Shamsi, M.B.; Liu, L.; Emery, B.; Aston, K.I.; Hotaling, J.; Carrell, D.T. Paternal influence of sperm DNA integrity on early embryonic development. Hum. Reprod. 2014, 29, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Brunborg, G.; Stevenson, M.; Lutton, D.; McManus, J.; Lewis, S.E. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum. Reprod. 2010, 25, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Benchaib, M.; Lornage, J.; Mazoyer, C.; Lejeune, H.; Salle, B.; Francois Guerin, J. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil. Steril. 2007, 87, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Laverge, H.; De Sutter, P.; Van der Elst, J.; Dhont, M. A prospective, randomized study comparing day 2 and day 3 embryo transfer in human IVF. Hum. Reprod. 2001, 16, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Lane, M.; Calderon, I.; Leeton, J. Environment of the preimplantation human embryo in vivo: Metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil. Steril. 1996, 65, 349–353. [Google Scholar] [CrossRef]
- Carvalho, B.R.; Barbosa, M.; Bonesi, H.; Gomes, D.B.S.; Cabral, I.O.; Barbosa, A.C.P.; Silva, A.A.; Iglesias, J.R.; Nakagawa, H.M. Embryo stage of development is not decisive for reproductive outcomes in frozen-thawed embryo transfer cycles. JBRA Assist. Reprod. 2017, 21, 23–26. [Google Scholar] [CrossRef]
- Barnhart, K.; Dunsmoor-Su, R.; Coutifaris, C. Effect of endometriosis on in vitro fertilization. Fertil. Steril. 2002, 77, 1148–1155. [Google Scholar] [CrossRef]
- Vandromme, J.; Chasse, E.; Lejeune, B.; Van Rysselberge, M.; Delvigne, A.; Leroy, F. Infertility: Hydrosalpinges in in-vitro fertilization: An unfavourable prognostic feature. Hum. Reprod. 1995, 10, 576–579. [Google Scholar] [CrossRef]
- Chu, J.; Harb, H.M.; Gallos, I.D.; Dhillon, R.; Al-Rshoud, F.M.; Robinson, L.; Coomarasamy, A. Salpingostomy in the treatment of hydrosalpinx: A systematic review and meta-analysis. Hum. Reprod. 2015, 30, 1882–1895. [Google Scholar] [CrossRef]
- Somigliana, E.; De Benedictis, S.; Vercellini, P.; Nicolosi, A.E.; Benaglia, L.; Scarduelli, C.; Ragni, G.; Fedele, L. Fibroids not encroaching the endometrial cavity and IVF success rate: A prospective study. Hum. Reprod. 2011, 26, 834–839. [Google Scholar] [CrossRef]
- Heytens, E.; Parrington, J.; Coward, K.; Young, C.; Lambrecht, S.; Yoon, S.-Y.; Fissore, R.; Hamer, R.; Deane, C.; Ruas, M.; et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLC) in spermatozoa from infertile men. Hum. Reprod. 2009, 24, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, D.E.; Feng, H.L. Sperm DNA Integrity From Sperm to Egg. J. Androl. 2007, 28, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.; Kim, T.H.; Jeong, J.; Lee, W.S.; Lyu, S.W. Effect of sperm selection using hyaluronan on fertilization and quality of cleavage-stage embryos in intracytoplasmic sperm injection (ICSI) cycles of couples with severe teratozoospermia. Gynecol. Endocrinol. 2020, 36, 456–459. [Google Scholar] [CrossRef]
- Kirkman-Brown, J.; Pavitt, S.; Khalaf, Y.; Lewis, S.; Hooper, R.; Bhattacharya, S.; Coomarasamy, A.; Sharma, V.; Brison, D.; Forbes, G.; et al. Efficacy and Mechanism Evaluation. In Sperm Selection for Assisted Reproduction by Prior Hyaluronan Binding: The HABSelect RCT; NIHR Journals Library: Southampton, UK, 2019. [Google Scholar]
- Yagci, A.; Murk, W.; Stronk, J.; Huszar, G. Spermatozoa Bound to Solid State Hyaluronic Acid Show Chromatin Structure With High DNA Chain Integrity: An Acridine Orange Fluorescence Study. J. Androl. 2010, 31, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V.; Kumar, M.; Deka, D.; Malhotra, N.; Singh, N.; Dadhwal, V.; Dada, R. Paternal factors and embryonic development: Role in recurrent pregnancy loss. Andrologia 2019, 51, e13171. [Google Scholar] [CrossRef] [PubMed]
- McQueen, D.B.; Zhang, J.; Robins, J.C. Sperm DNA fragmentation and recurrent pregnancy loss: A systematic review and meta-analysis. Fertil. Steril. 2019, 112, 54–60.e3. [Google Scholar] [CrossRef]
- Tan, J.; Taskin, O.; Albert, A.; Bedaiwy, M.A. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 38, 951–960. [Google Scholar] [CrossRef]
- Kirshenbaum, M.; Orvieto, R. Should We Offer In Vitro Fertilization to Couples with Unexplained Recurrent Pregnancy Loss? J. Clin. Med. 2019, 8, 2001. [Google Scholar] [CrossRef]
- Sustar, K.; Rozen, G.; Agresta, F.; Polyakov, A. Use of intracytoplasmic sperm injection (ICSI) in normospermic men may result in lower clinical pregnancy and live birth rates. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 706–711. [Google Scholar] [CrossRef]
- Erberelli, R.F.; Salgado, R.M.; Pereira, D.H.; Wolff, P. Hyaluronan-binding system for sperm selection enhances pregnancy rates in ICSI cycles associated with male factor infertility. JBRA Assist. Reprod. 2017, 21, 2–6. [Google Scholar] [CrossRef]
- Bradley, C.K.; McArthur, S.J.; Gee, A.J.; Weiss, K.A.; Schmidt, U.; Toogood, L. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: A retrospective analysis. Andrology 2016, 4, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Casciano, I.; De Leo, C.; Massarotti, C.; Anserini, P.; Remorgida, V.; Scaruffi, P. A successful healthy childbirth and an ongoing evolutive pregnancy in a case of partial globozoospermia by hyaluronic acid sperm selection. Andrologia 2019, 51, e13178. [Google Scholar] [CrossRef] [PubMed]
- Cairo Consensus Workshop, G. The current status and future of andrology: A consensus report from the Cairo workshop group. Andrology 2020, 8, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 30, 120–127. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management—Meta-analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef]
- Cissen, M.; Wely, M.V.; Scholten, I.; Mansell, S.; Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef]
- Simon, L.; Zini, A.; Dyachenko, A.; Ciampi, A.; Carrell, D.T. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 2017, 19, 80–90. [Google Scholar] [CrossRef]
- Marchlewska, K.; Filipiak, E.; Walczak-Jedrzejowska, R.; Oszukowska, E.; Sobkiewicz, S.; Wojt, M.; Chmiel, J.; Kula, K.; Slowikowska-Hilczer, J. Sperm DNA Fragmentation Index and Hyaluronan Binding Ability in Men from Infertile Couples and Men with Testicular Germ Cell Tumor. Biomed Res. Int. 2016, 2016, 7893961. [Google Scholar] [CrossRef]
- Spencer, E.A.; Mahtani, K.R.; Goldacre, B.; Heneghan, C. Claims for fertility interventions: A systematic assessment of statements on UK fertility centre websites. BMJ Open 2016, 6, e013940. [Google Scholar] [CrossRef]
- Harper, J.; Jackson, E.; Sermon, K.; Aitken, R.J.; Harbottle, S.; Mocanu, E.; Hardarson, T.; Mathur, R.; Viville, S.; Vail, A.; et al. Adjuncts in the IVF laboratory: Where is the evidence for ‘add-on’ interventions? Hum. Reprod. 2017, 32, 485–491. [Google Scholar] [CrossRef]
- Humaidan, P.; Haahr, T. Bureaucratic overheating is a parasite hampering modern clinical research—A viewpoint from the ‘belly of the beast’. Reprod. Biomed. Online 2019, 38, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.; Chambers, G.M.; de Mouzon, J.; Nygren, K.G.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Adamson, G.D. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum. Reprod. 2016, 31, 1588–1609. [Google Scholar] [CrossRef] [PubMed]
- Evers, J.L. Santa Claus in the fertility clinic. Hum. Reprod. 2016, 31, 1381–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, A.Y.; Bowman, M.; Hammarberg, K.; Farquhar, C.; Johnson, L.; Safi, N.; A Sullivan, E. ICSI does not increase the cumulative live birth rate in non-male factor infertility. Hum. Reprod. 2018, 33, 1322–1330. [Google Scholar] [CrossRef]
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; Van Der Poel, S.; St John, B.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance—Challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef]
- West, R.; Coomarasamy, A.; Frew, L.; Hutton, R.; Kirkman-Brown, J.; Lawlor, M.; Lewis, S.; Partanen, R.; Payne-Dwyer, A.; Román-Montañana, C.; et al. Sperm selection with hyaluronic acid improved live birth outcomes among older couples and was connected to sperm DNA quality, potentially affecting all treatment outcomes. Hum. Reprod. 2022, 37, 1106–1125. [Google Scholar] [CrossRef]
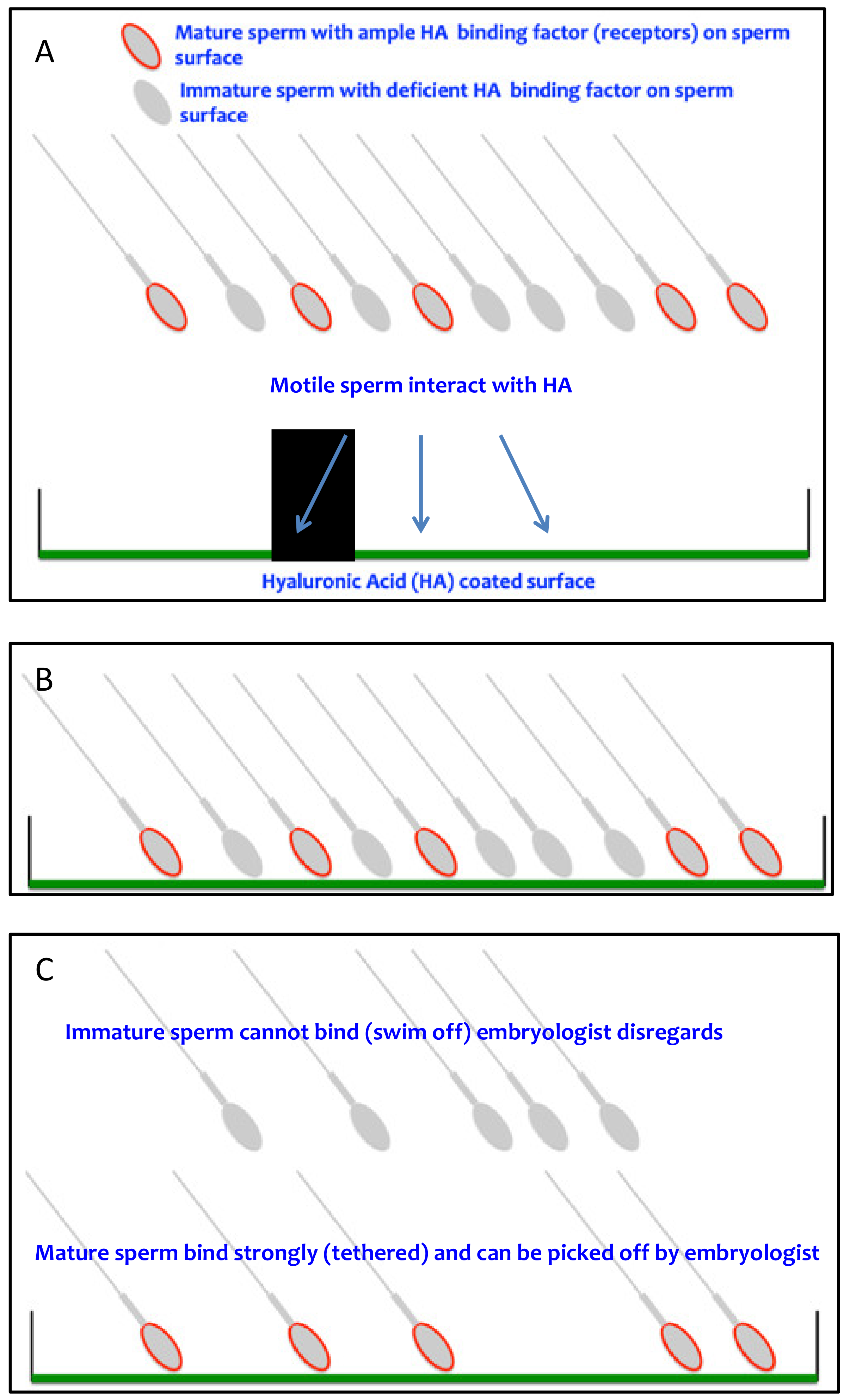
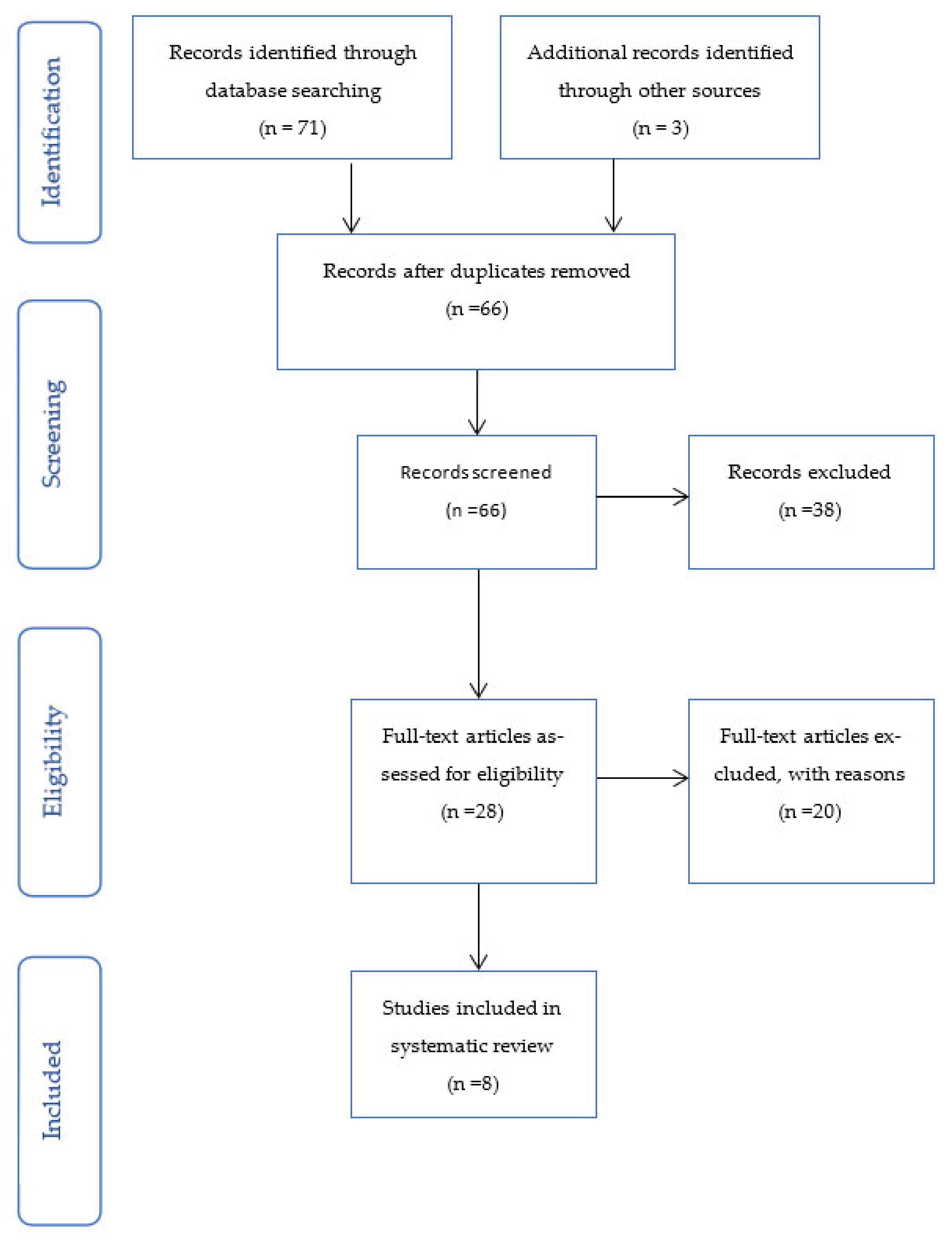
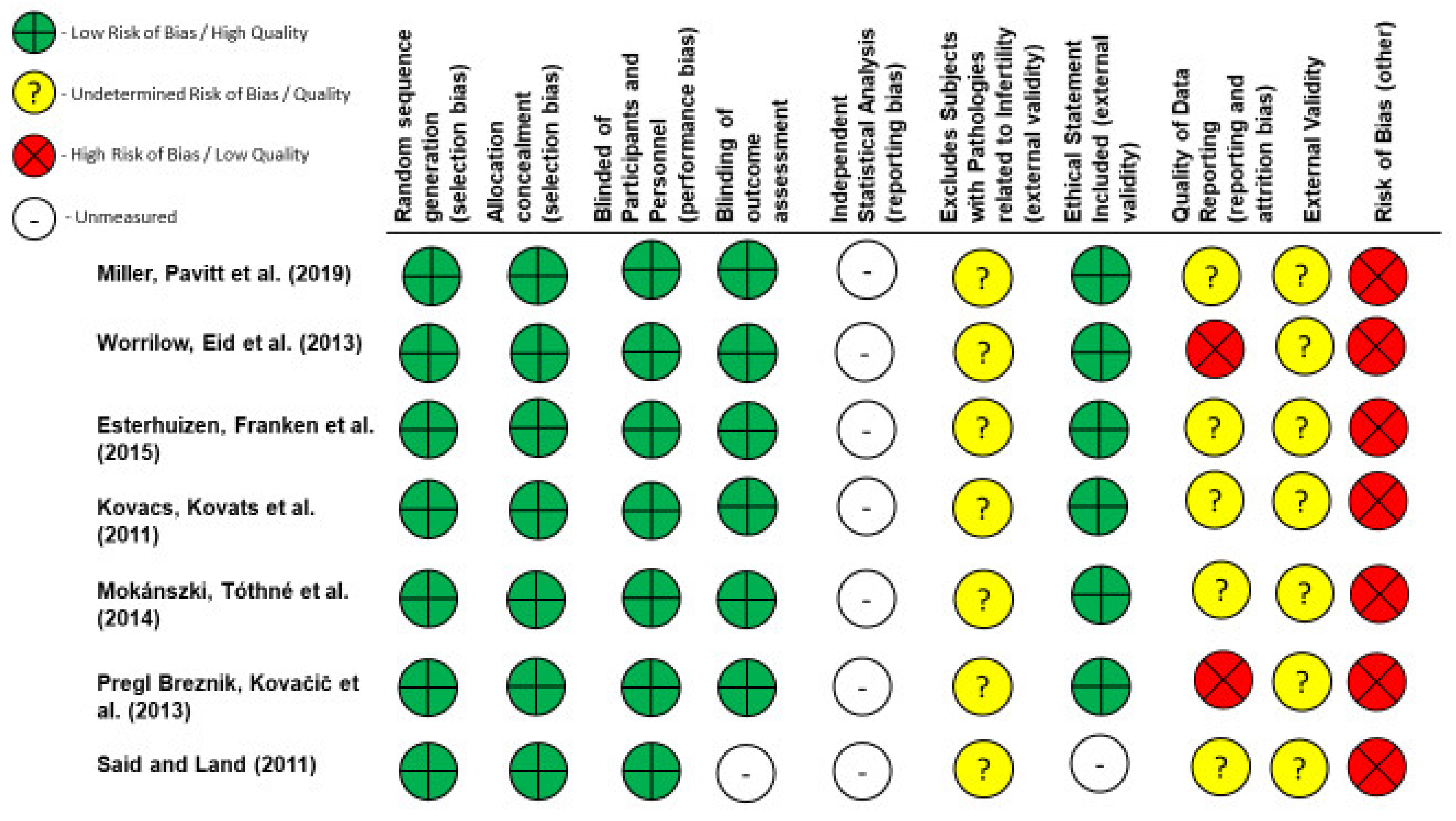

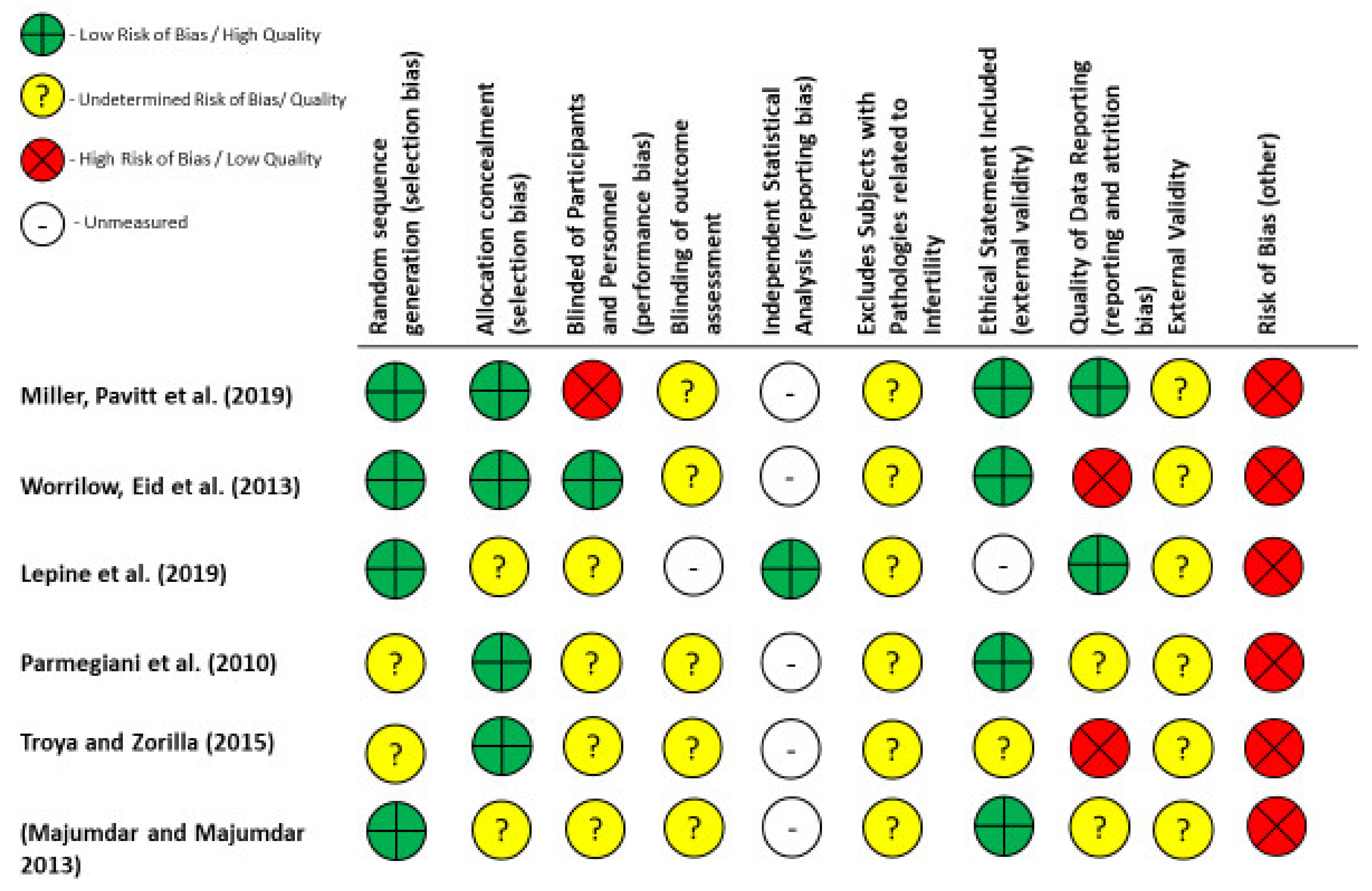
| Author | Intervention | Study Type | Indication | Reported Outcomes | Results |
|---|---|---|---|---|---|
| Esterhuizen, Franken et al. (2015) [44] | ICSI | Prospective, Controlled | Mild–Moderate endometriosis | HAB score and FR, BPR, and CPR | R = 0.60 (p = 0.0001 *), R = 0.24 (p = 0.02 *), R = 0.14 (p = 0.14) |
| Kovacs, Kovats et al. (2011) [45] | IVF/ICSI | Prospective, Blinded Randomised Controlled | Unexplained infertility Female age <40 Normozoospermic patients | HAB score and FR | No significant correlation (data not presented) |
| Miller et al. (2019) [31] | PICSI | Prospective, blinded, randomised, controlled | Unexplained infertility Female age 18–38 Patients able to produce fresh ejaculate on the day of OR | HAB score, FR, CPR, PLR, LBR | No significant correlations reported (data not presented) |
| Mokánszki, Tóthné et al. (2014) [46] | PICSI | Prospective Controlled Non-randomised (treatment allocation based on HAB score) | Female age <40 years Sperm concentration >10,000/mL on the day of OR | FR | 64.5% vs. 56.5% (p > 0.05) HAB score >60%:73.36% vs. 60.1% (p < 0.05 *) HAB score ≤60%:55.7% vs. 52.8% (p > 0.05) |
| IR | 21.7% vs. 17.12% (p > 0.05) HAB score >60%:20.8% vs. 21.47% (p > 0.05) HAB score ≤60%:22.6% vs. 12.76% (p < 0.05 *) | ||||
| CPR | 40.46% vs. 29.22% (p < 0.05 *) HAB score >60%:41.67% vs. 31.85% (p < 0.05 *) HAB score ≤60%:39.3% vs. 26.6% (p < 0.05 *) | ||||
| PLR | 2% vs. 5.14% (p < 0.05 *) HAB score >60%:2.2% vs. 8.37% (p < 0.05 *) HAB score ≤60%:1.99% vs. 1.9% (p > 0.05) | ||||
| LBR | 0.45% vs. 0.42% (p > 0.05) HAB score >60%:0.42% vs. 0.58% (p > 0.05) HAB score ≤60%:0.49% vs. 0.27% (p < 0.05 *) | ||||
| Pregl Breznik, Kovačič et al. (2013) [47] | IVF/ICSI | Prospective | Mild male factor Unexplained infertility Female factor infertility | HAB score and FR | FR <50%, HAB 85.1% vs. FR >50%, HAB 93% (p = 0.019 *) |
| Said and Land (2011) [48] | Systematic Review | HAB score, FR, CPR, | No significant correlations reported | ||
| Worrilow, Eid et al. (2013) [26] | PICSI | Prospective, Double blinded, randomised, controlled | Female age <40 HAB score >2% Sperm concentration >10,000/mL | IR | 33.5% vs. 32.2% (p > 0.05) HAB score >65%:37.9% vs. 34.8% (p > 0.05) HAB score ≤65%:37.4% vs. 30.7% (p > 0.05) 47.3% vs. 47.8% (p > 0.05) |
| CPR | HAB score >65%:46.2% vs. 51.1% (p > 0.05) HAB score ≤65%:50.8% vs. 37.9% (p > 0.05) | ||||
| PLR (HAB score unwashed) | 4.3% vs. 10% (p > 0.05) | ||||
| PLR (HAB score washed) | HAB score >65%:5.3% vs. 3.5% (p > 0.05) HAB score ≤65%–3.3% vs. 15.1% (p = 0.021 *) 4.3% vs. 10% (p > 0.05) HAB score ≤65%:0% vs. 18.5% (p = 0.016 *) |
| Author | Intervention | Control | Study Type | Indication | Reported Outcomes | Results |
|---|---|---|---|---|---|---|
| Lepine, McDowell et al. (2019) [34] | PICSI | ICSI | Systematic Review | Unexplained Infertility | Miscarriage per woman randomly assigned | 43 of 1000 vs. 70 of 1000 (RR = 0.61) |
| Miscarriage per Clinical Pregnancy | 122 per 1000 vs. 197 per 1000 (RR = 0.62) | |||||
| Miller et al. (2019) [31] | PICSI | ICSI | Prospective, blinded, randomised, controlled | Unexplained infertility Female age 18-38 Patients able to produce fresh ejaculate on the day of OR | Miscarriage per Clinical Pregnancy | 4.3% vs. 7% (OR = 0.61, p = 0.003) |
| Parmegiani, Cognigni et al. (2010) [28] | SpermSlow | ICSI | Prospective, randomised, controlled | No female age range reported Motility >5%, Sperm concentration >1 million/mL | Miscarriage per Clinical Pregnancy | 18.2% vs. 19.3% (p > 0.05) |
| Troya and Zorrilla (2015) [49] | PICSI | MACS and ICSI | Prospective, Randomised, Controlled | Unexplained infertility Normozoospermic Female age >35 | Miscarriage per Clinical Pregnancy | 5.3% vs. 5.5% and 13.3% (p > 0.05) |
| Worrilow, Eid et al. (2013) [26] | PICSI | ICSI | Prospective, Double blinded, randomised, controlled | Female age <40 HAB score >2% Sperm concentration >10,000/mL | Miscarriage per Clinical Pregnancy | 4.3% vs. 10 % (p > 0.05) |
| Miscarriage per Clinical Pregnancy, Final HAB score >65% | 5.9% vs. 7.8% (p > 0.05) | |||||
| Miscarriage per Clinical Pregnancy, Final HAB score ≤65% | 0% vs. 18.5% (p = 0.016 *) | |||||
| Majumdar and Majumdar (2013) [50] | PICSI | ICSI | Prospective, randomised, controlled | Unexplained infertility, female patients <43, >3 oocytes collected | Miscarriage per clinical pregnancy | 12% vs. 25% (p > 0.05) |
| Author | Intervention | Control | Study Type | Indication | Reported Outcomes | Results |
|---|---|---|---|---|---|---|
| Beck-Fruchter, Shalev et al. (2016) [51] | HA-ICSI | Systematic Review | Unexplained Infertility | FR, CPR | No significant difference | |
| Cleavage rate | RR = 0.94 in favour of Control (p = 0.0001) | |||||
| EQR | 35–36% vs. 22–24% (p < 0.05) RR = 1.53 in favour of HA-ICSI (p < 0.0001) | |||||
| IR | RR 1.34 in favour of HA-ICSI (p = 0.24) | |||||
| PLR | No significant difference (data not presented) | |||||
| LBR | No significant difference (data not presented) | |||||
| Miller et al. (2019) [31] | PICSI | Prospective, blinded, randomised, controlled | Unexplained infertility Female age 18–38 Patients able to produce fresh ejaculate on the day of OR | FR, CPR, LBR PLR | No significant difference (data not presented) 4.3% vs. 7.0% (p = 0.003) | |
| Worrilow, Eid et al. (2013) [26] | PICSI | Prospective, Double blinded, randomised, controlled | Female age <40 HABScore >2% Sperm concentration >10,000/mL on the day of OR | IR | 33.5% vs. 32.2% (p > 0.05) | |
| CPR | 47.3% vs. 47.8% | |||||
| PLR | 4.3% vs. 10% (p > 0.05) | |||||
| Lepine, McDowell et al. (2019) [34] | PICSI | Systematic Review | LBR per patient | RR = 1.09 in favour of HA-ICSI (p > 0.05) RR = 0.62 in favour of HA-ICSI (p > 0.05) | ||
| PLR per Clinical Pregnancy CPR per patient | No significant difference (data not presented) | |||||
| Parmegiani, Cognigni et al. (2010) [28] | SpermSlow | Prospective, randomised, controlled | No female age range reported Motility >5%, Sperm concentration >1 million/mL on the day of OR | FR, CPR, PLR EDR | No significant differences (data not presented) 95.0% vs. 84.0% (p = 0.001) | |
| Troya and Zorrilla (2015) [49] | PICSI | ICSI and MACS | Prospective, randomised, controlled | Endometriosis Excluded Normozoospermic patients undergoing ICSI | FR | 70.15% vs. 78.97% and 80.28% (p = 0.036) |
| CLR | 40.4% vs. 27.3% and 58.1% (p = 0.019) | |||||
| PLR per Clinical Pregnancy | 5.3% vs. 13.3% and 5.5% (p > 0.05) | |||||
| Majumdar and Majumdar (2013) [50] | PICSI | ICSI | Prospective, randomised, controlled | Female patient <43 years Unexplained infertility | Pregnancy FR, CPR, PLR, LBR | No significant difference reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ní Dhuifin, R.; Griffin, D.K.; Moodley, T. The Efficacy of Hyaluronic Acid Binding (HAB) in the Treatment of Male Infertility: A Systematic Review of the Literature. DNA 2022, 2, 149-171. https://doi.org/10.3390/dna2030011
Ní Dhuifin R, Griffin DK, Moodley T. The Efficacy of Hyaluronic Acid Binding (HAB) in the Treatment of Male Infertility: A Systematic Review of the Literature. DNA. 2022; 2(3):149-171. https://doi.org/10.3390/dna2030011
Chicago/Turabian StyleNí Dhuifin, Róisín, Darren K. Griffin, and Therishnee Moodley. 2022. "The Efficacy of Hyaluronic Acid Binding (HAB) in the Treatment of Male Infertility: A Systematic Review of the Literature" DNA 2, no. 3: 149-171. https://doi.org/10.3390/dna2030011
APA StyleNí Dhuifin, R., Griffin, D. K., & Moodley, T. (2022). The Efficacy of Hyaluronic Acid Binding (HAB) in the Treatment of Male Infertility: A Systematic Review of the Literature. DNA, 2(3), 149-171. https://doi.org/10.3390/dna2030011








