Biogas and Hydrogen Production from Waste Biomass via Dark Fermentation Evaluating VFAs, COD, and HRT for Process Optimization
Abstract
1. Introduction
2. Materials and Methods
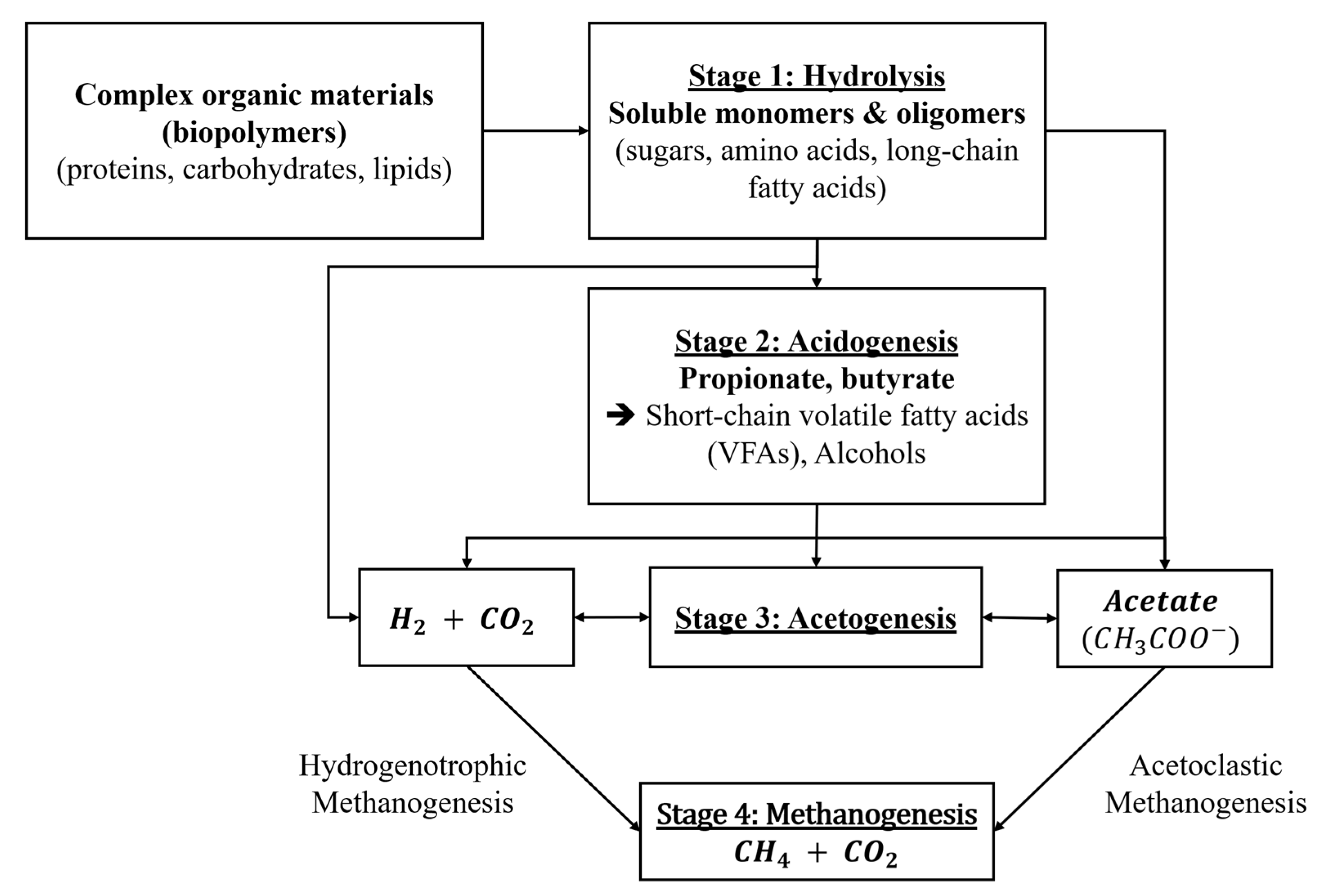
2.1. Anaerobic Digestion, Dark Fermentation, and VFA Production
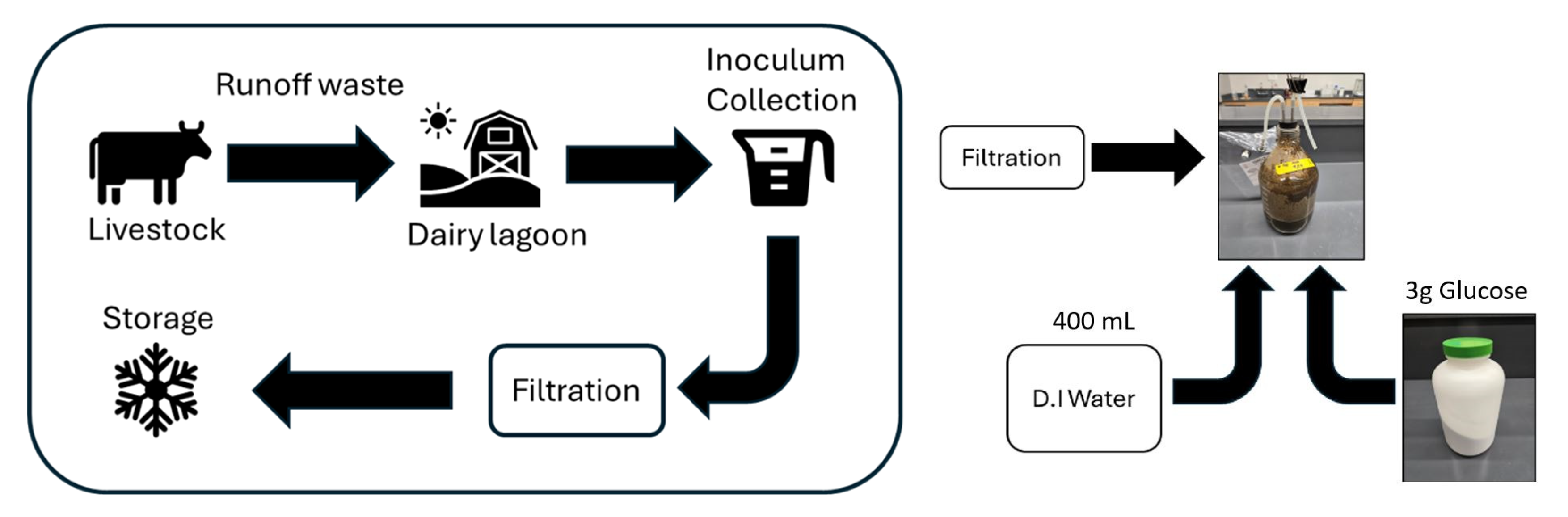
2.2. Preparation and Activation of Anaerobic Inoculum and Substrates
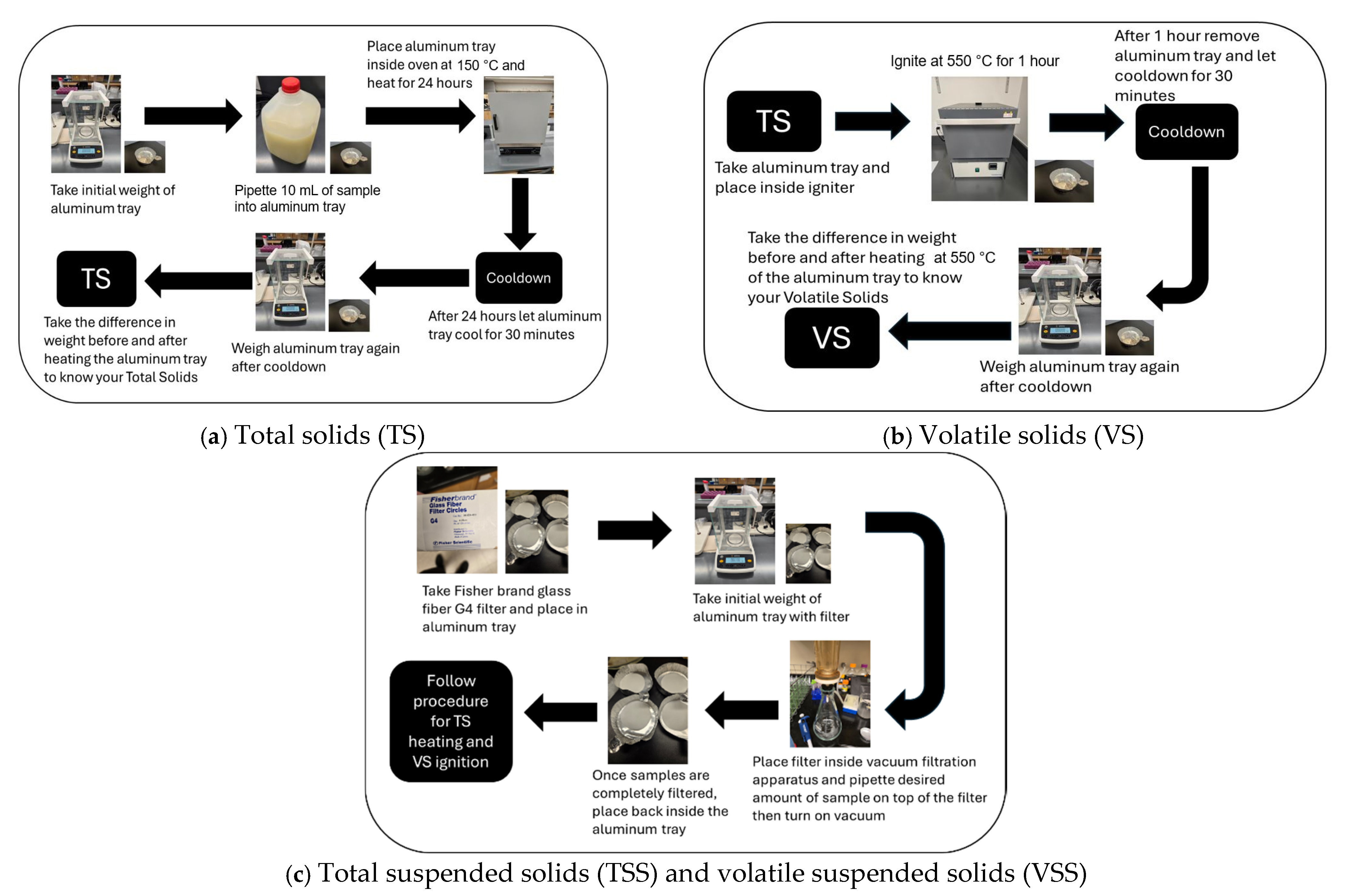
- Sample aliquots of 25–50 g are dried at 103 °C to 105 °C to drive off water in the sample;
- The residue from step 1 is cooled, weighed, and dried again at 550 °C to drive off volatile solids in the sample;
- The total, fixed, and volatile solids are determined by comparing the mass of the sample before and after each drying step.
2.3. Batch Experimental Design for Dark Fermentation Processes: DF 1 and DF 2
3. Results and Discussion
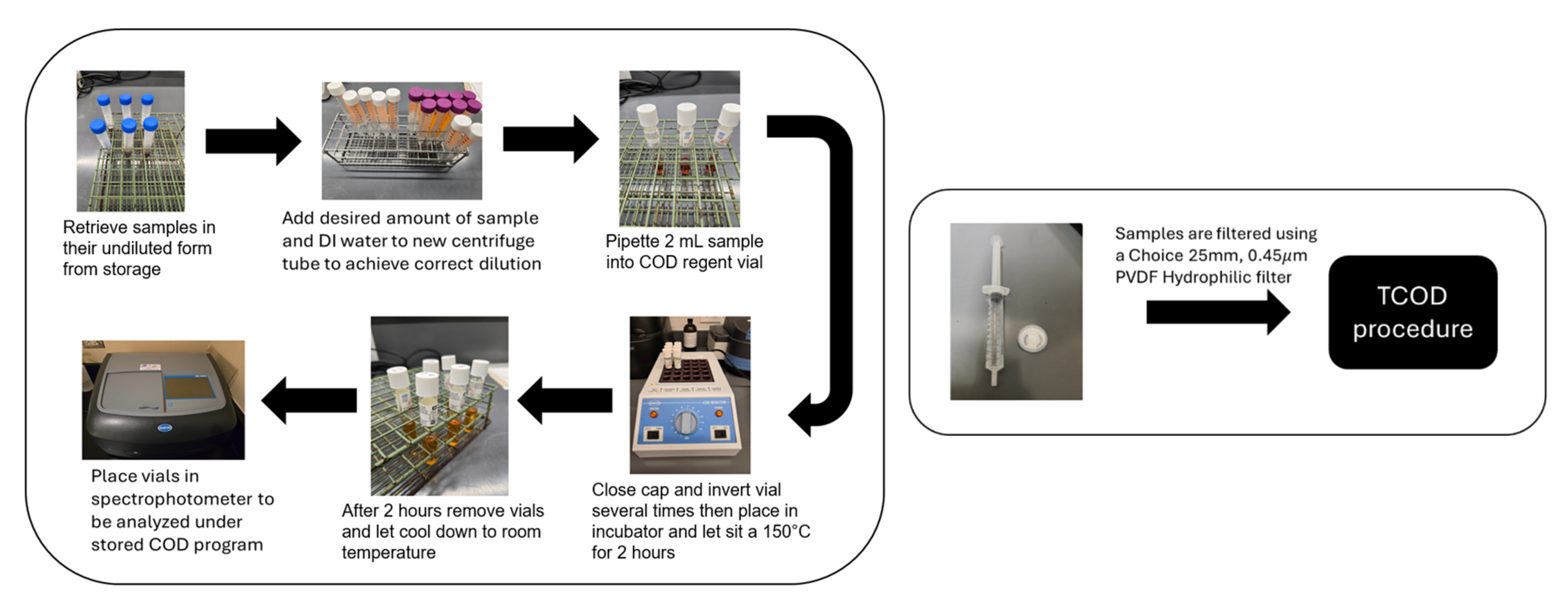
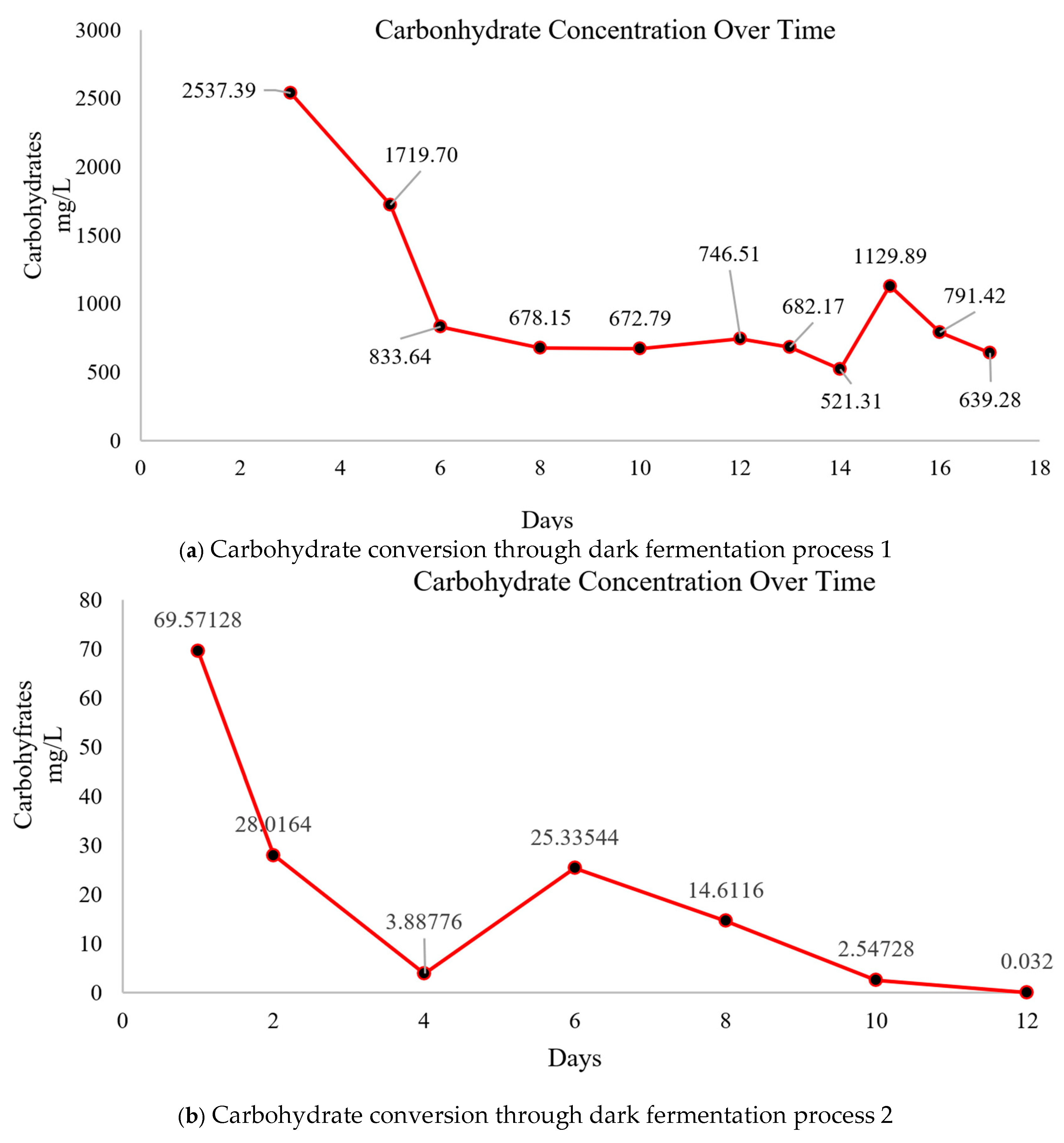
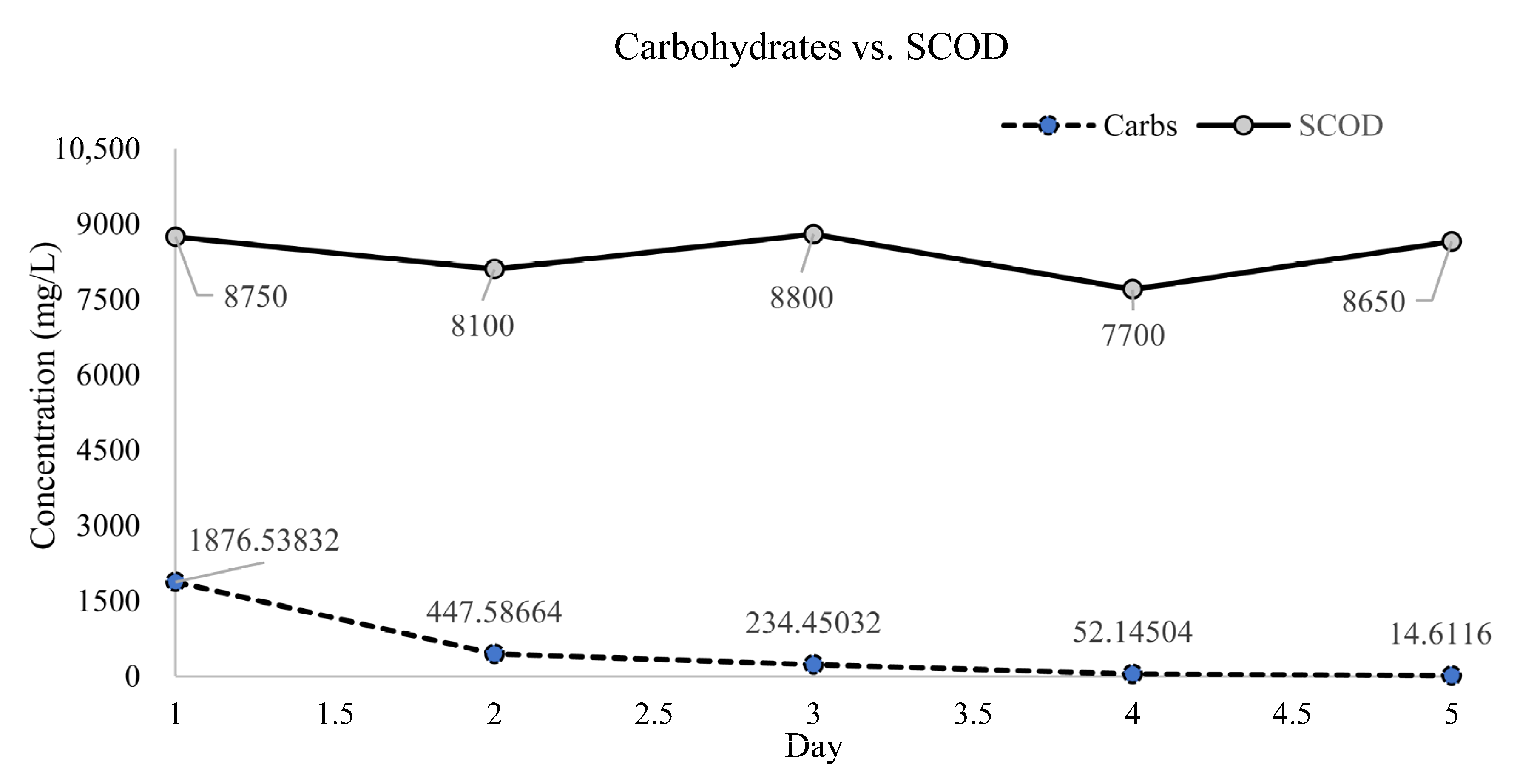
3.1. Carbohydrates, TCOD, and SCOD
3.2. Total Solids (TS), Volatile Solids (VS), Total Suspended Solids (TSS), and Volatile Suspended Solids (VSS)
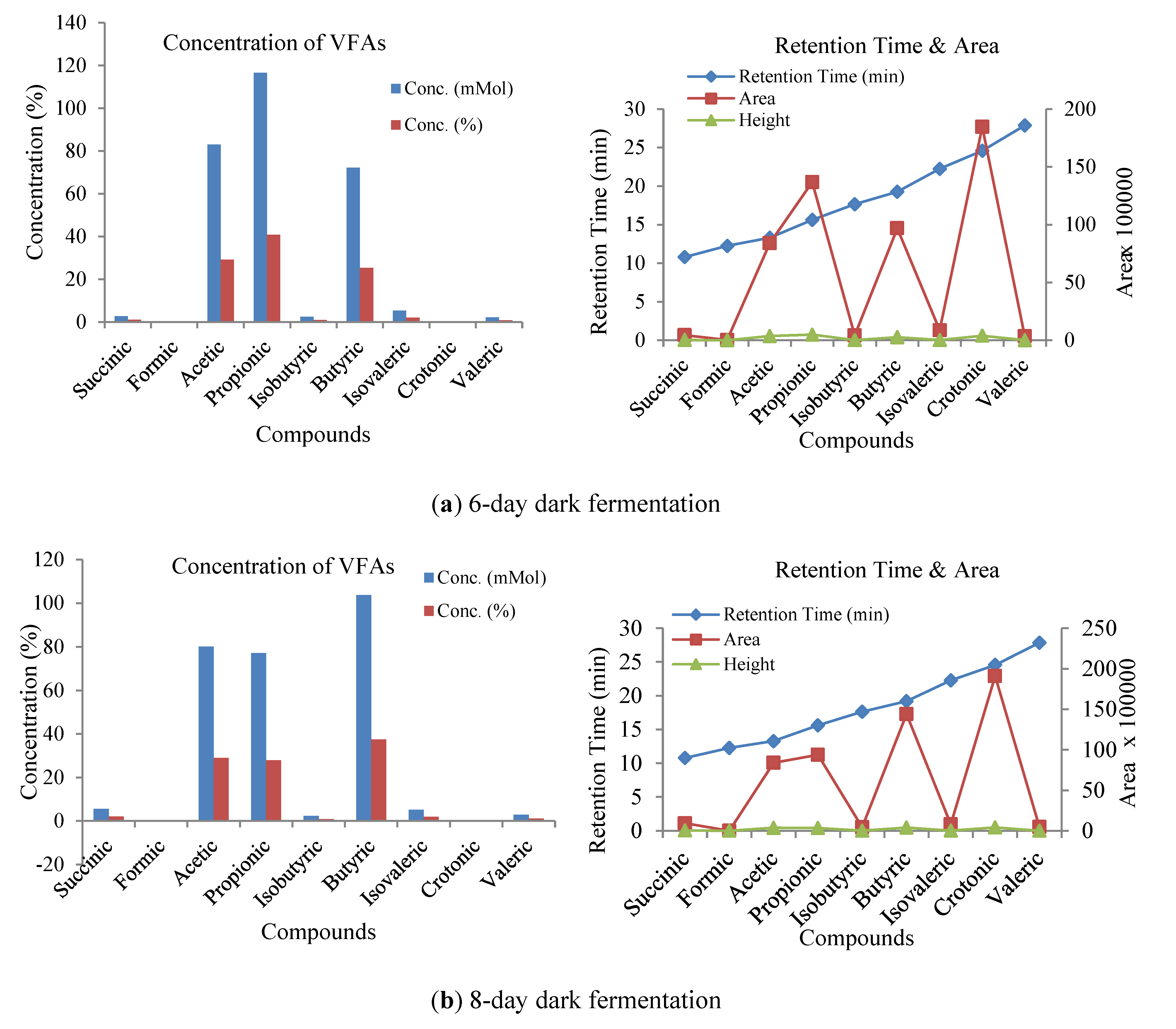
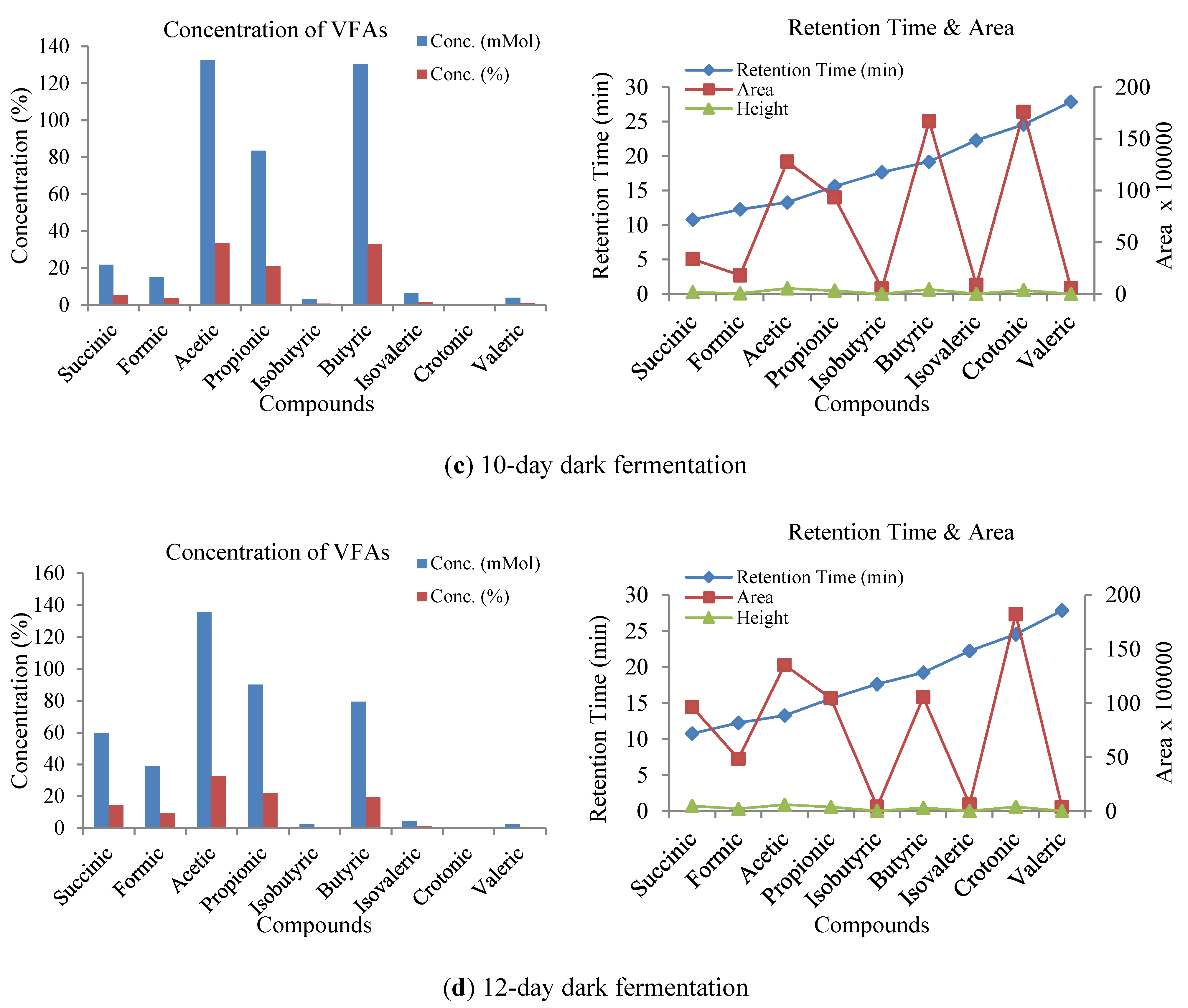
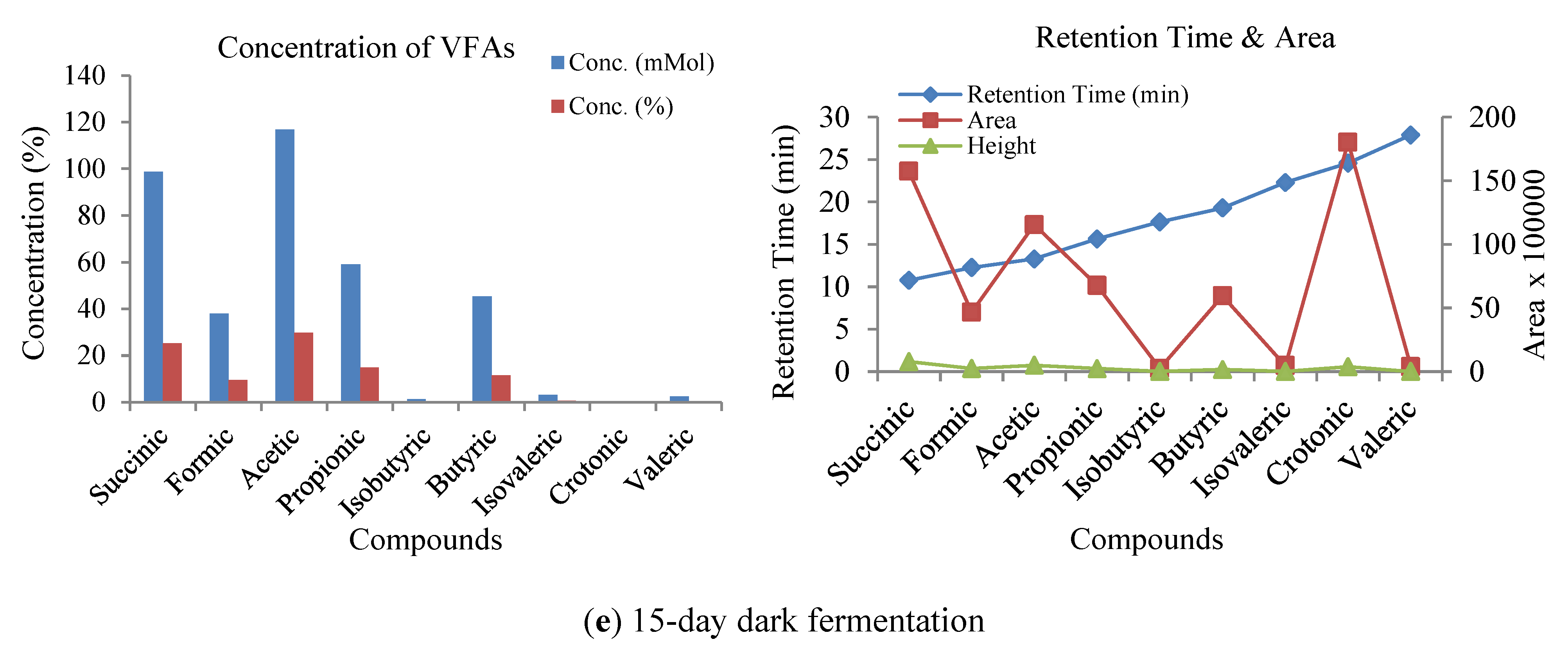
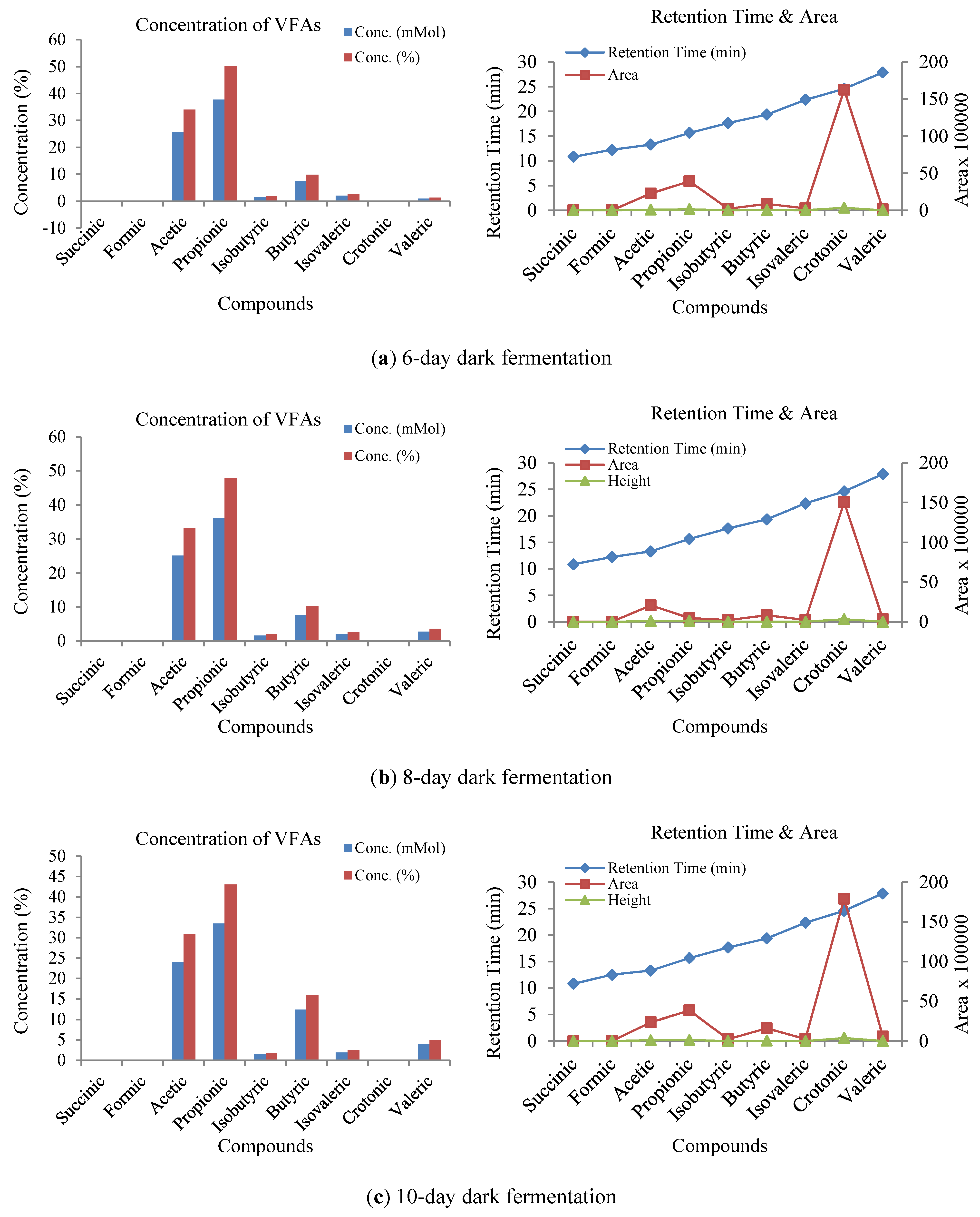
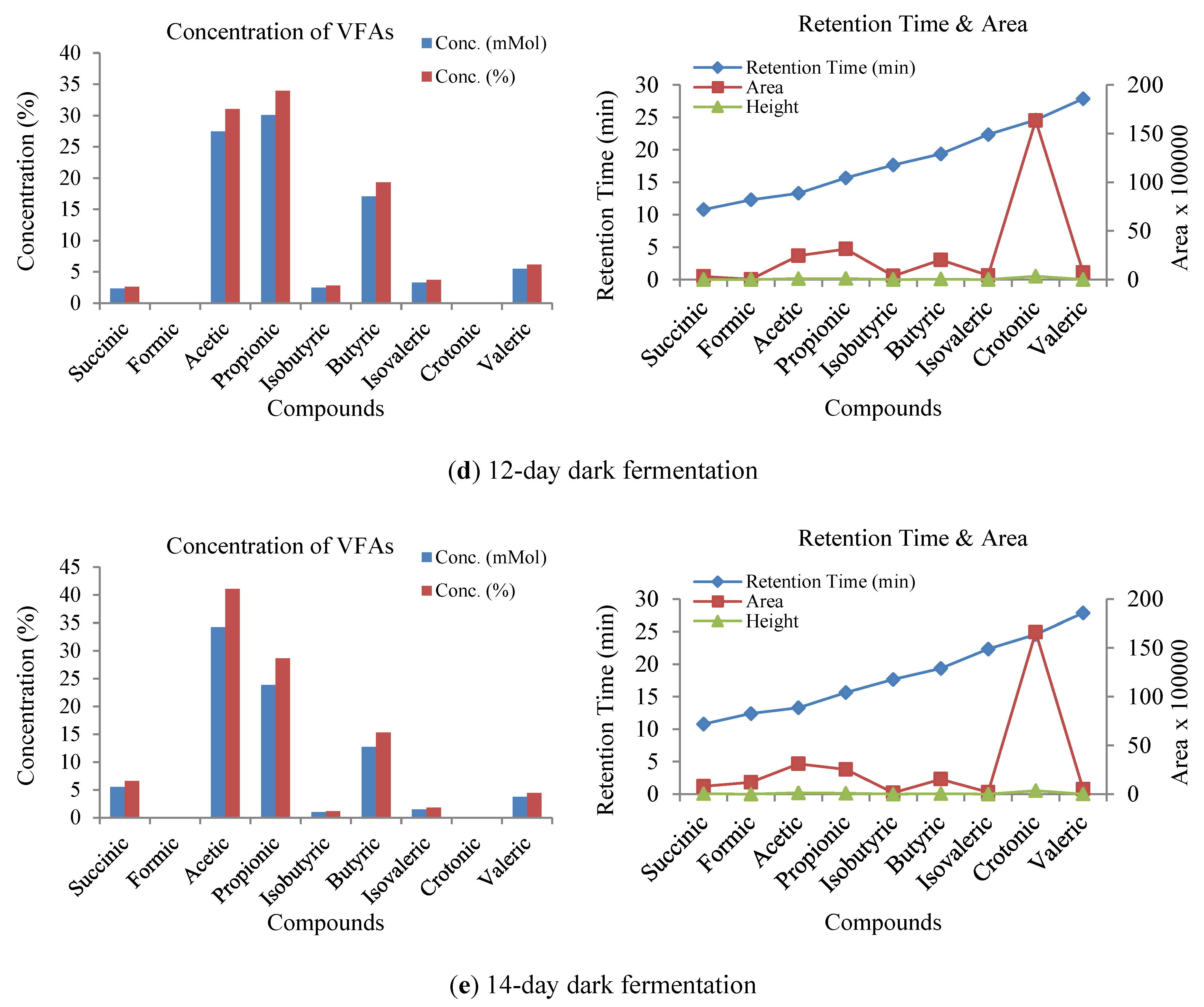
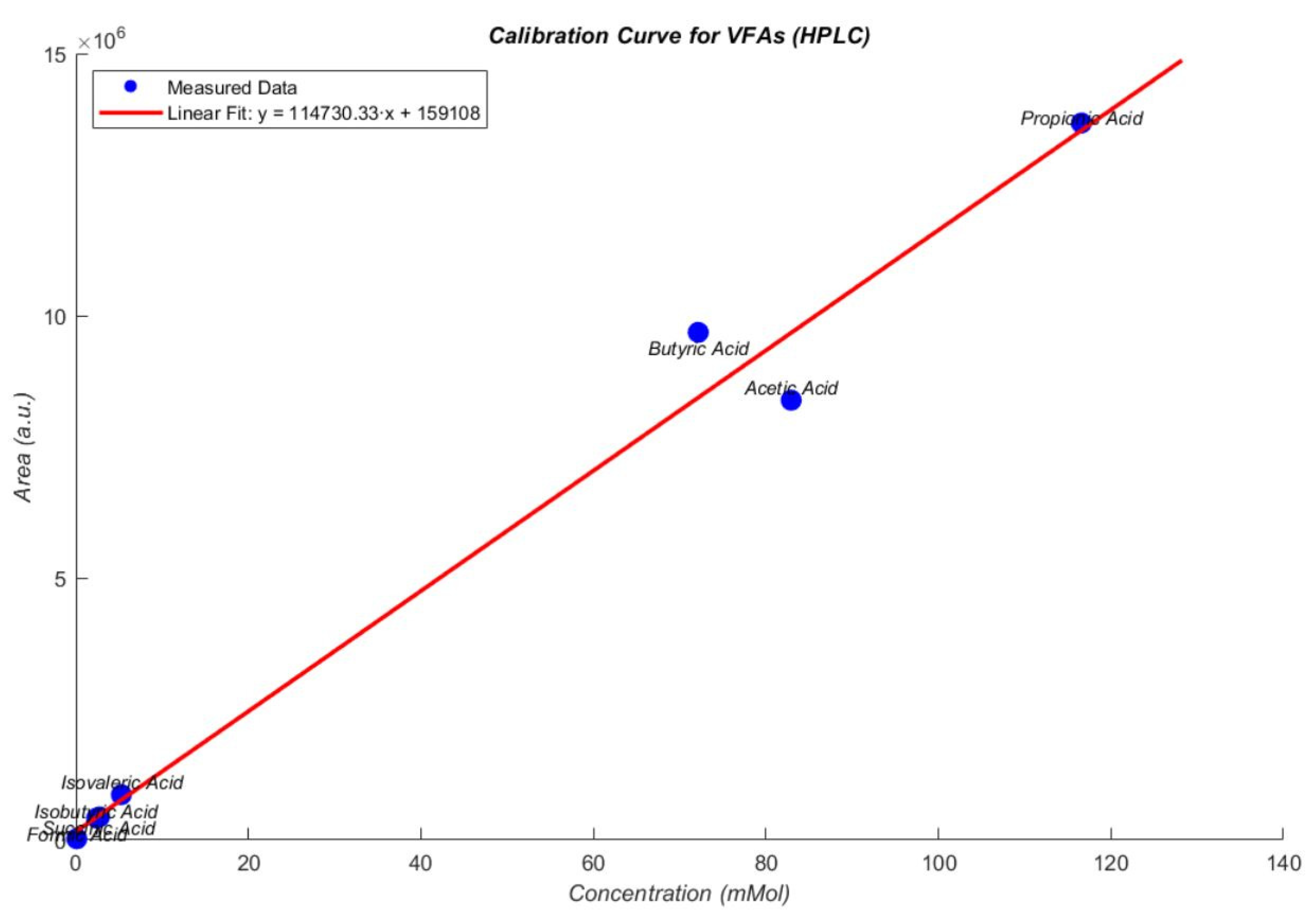
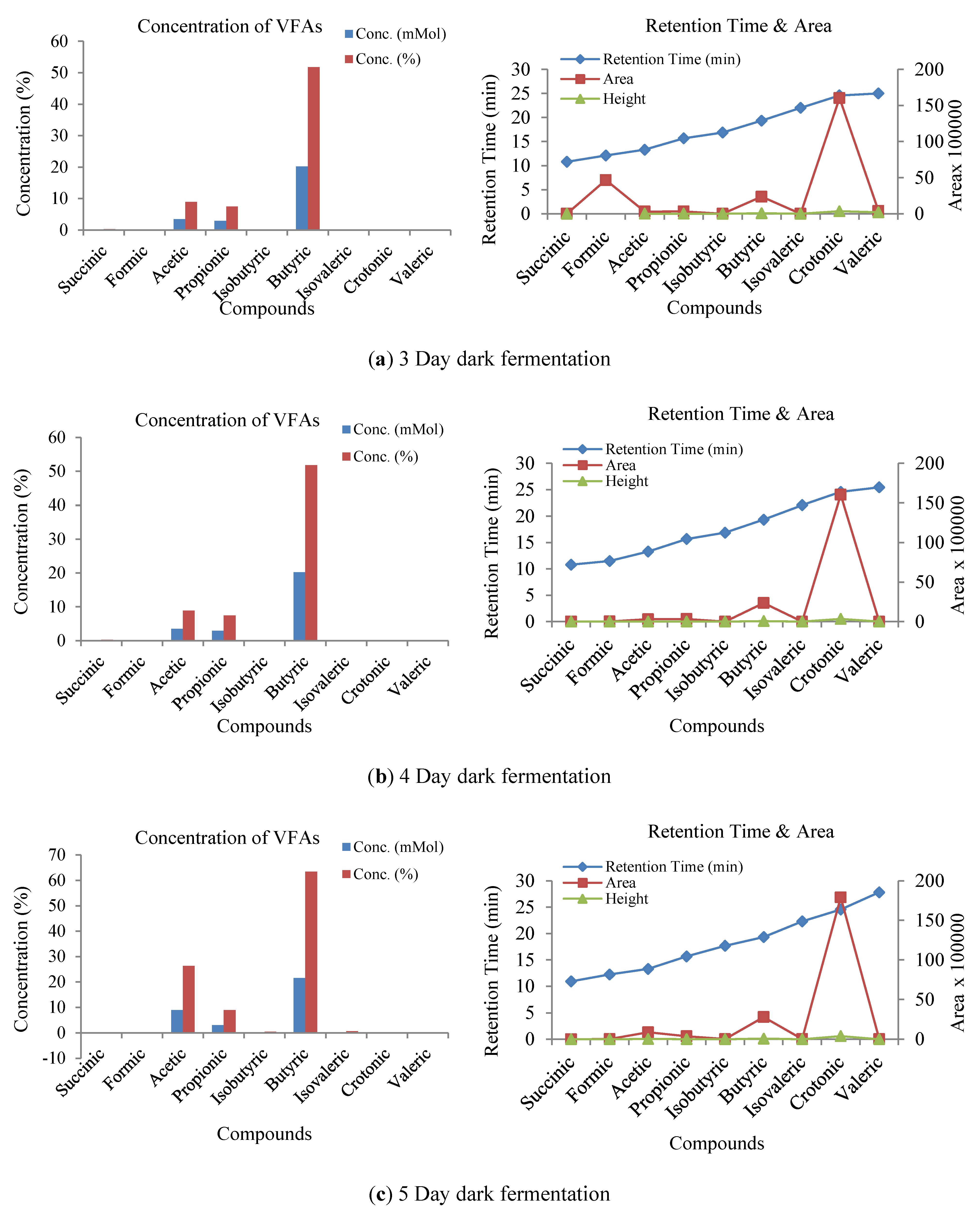
3.3. Influence of Volatile Fatty Acids on Biogas and Hydrogen Production
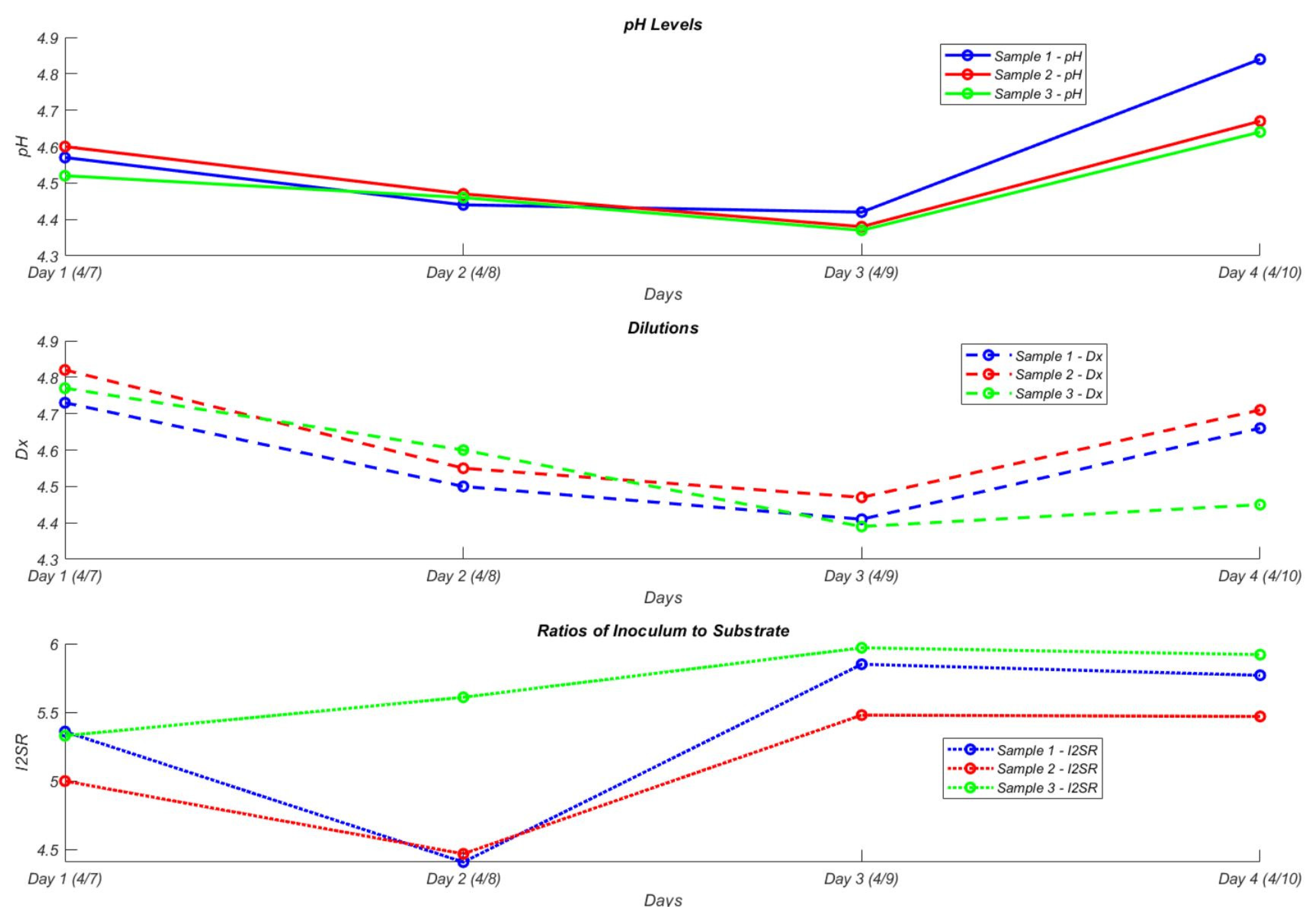
3.4. Sensitivity Analysis of VFA and pH Responses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salhotra, P.; Carver, J.L. Texas’ Water Infrastructure is Broken, Jeopardizing Quality and Supply for a Growing State. The Texas Tribune, 3 May 2023. Available online: https://www.texastribune.org/2023/05/03/texas-water-infrastructure-broken/ (accessed on 5 November 2024).
- Plainview Herald. Waco Sues Dairies to Get Relief from Pollution. Plainview Herald, 30 April 2004. Available online: https://www.myplainview.com/news/article/Waco-sues-dairies-to-get-relief-from-pollution-8958742.php (accessed on 5 November 2024).
- Ewusi-Mensah, N.; Logah, V.; Akrasi, E.J. Impact of Different Systems of Manure Management on the Quality of Cow Dung. Commun. Soil Sci. Plant Anal. 2014, 46, 137–147. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock Manure and the Impacts on Soil Health: A Review, MDPI soil systems. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Dadrasnia, A.; Muñoz, I.d.B.; Yáñez, E.H.; Lamkaddam, I.U.; Mora, M.; Ponsá, S.; Ahmed, M.; Argelaguet, L.L.; Williams, P.M.; Oatley-Radcliffe, D.L. Sustainable nutrient recovery from animal manure: A review of current best practice technology and the potential for freeze concentration. J. Clean. Prod. 2021, 315, 128106. [Google Scholar] [CrossRef]
- Meena, P.K.; Patane, P.M. Biohydrogen: Advancing a sustainable transition from fossil fuels to renewable energy. Int. J. Hydrogen Energy 2025, 100, 955–970. [Google Scholar] [CrossRef]
- Qureshi, F.; Kamyab, H.; Rajendran, S.; Vo, D.-V.N.; Rajamohan, N.; Yusuf, M. Unveiling the potentials of biohydrogen as an alternative energy source: Strategies, challenges and future perspectives. Mater. Today Sustain. 2025, 31, 101133. [Google Scholar] [CrossRef]
- Kanwal, F.; Torriero, A.A.J. Biohydrogen—A Green Fuel for Sustainable Energy Solutions. Energies 2022, 15, 7783. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, S.; Farrok, O.; Khan, T.M.Y. Biohydrogen Production From Biomass Sources: Metabolic Pathways and Economic Analysis. Front. Energy Res. 2021, 9, 753878. [Google Scholar] [CrossRef]
- Bertasini, D.; Battista, F.; Mancini, R.; Frison, N.; Bolzonella, D. Hydrogen and methane production through two stage anaerobic digestion of straw residues. Environ. Res. 2024, 247, 118101. [Google Scholar] [CrossRef]
- Mukherjee, T.; Senevirathne, N.; Kaparaju, P. Dark fermentative hydrogen production from sugar syrup at different temperatures and inoculum-to-substrate ratios. Int. J. Hydrogen Energy 2025, 102, 37–49. [Google Scholar] [CrossRef]
- Alvarez-Guzmán, C.L.; Rodríguez-Hipólito, F.; Chávez-Reyes, Y.; Valdez-Vazquez, I. Lignocellulosic biomass mixtures improve hydrogen production by promoting microbial complementation in a consolidated bioprocess. J. Clean. Prod. 2025, 489, 144691. [Google Scholar] [CrossRef]
- Sun, W.; Vasu, S.; Blais, M.S. Chapter 2—Fundamentals. In Machinery and Energy Systems for the Hydrogen Economy; Elsevier: Amsterdam, The Netherlands, 2022; Volume 9, pp. 11–30. [Google Scholar] [CrossRef]
- Yang, M.; Hunger, R.; Berrettoni, S.; Sprecher, B.; Wang, B. A review of hydrogen storage and transport technologies. Clean Energy 2023, 7, 190–216. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Medeiros, F.; Lopes, F.; Vasconcelos, B. Prospects and Technical Challenges in Hydrogen Production through Dry Reforming of Methane. Catalysts 2022, 12, 363. [Google Scholar] [CrossRef]
- Shahid, M.I.; Farhan, M.; Rao, A.; Hussain, M.S.; Salam, H.A.; Chen, T.; Zhang, Z.; Li, X.; Ma, F. Hydrogen production by waste heat recovery of hydrogen-enriched compressed natural gas via steam methane reforming process. Int. J. Hydrogen Energy 2025, 117, 374–392. [Google Scholar] [CrossRef]
- Karibe, H.; Sair, S.; Faik, A.; Ousaleh, H.A. Electrified steam methane reforming: A review of heating technologies, challenges, and prospects. Int. J. Hydrogen Energy 2025, 133, 200–213. [Google Scholar] [CrossRef]
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef]
- Mokhtarani, B.; Zanganeh, J.; Moghtaderi, B. A Review on Biohydrogen Production Through Dark Fermentation, Process Parameters and Simulation. Energies 2025, 18, 1092. [Google Scholar] [CrossRef]
- Sun, S.; Wang, X.; Cheng, S.; Lei, Y.; Sun, W.; Wang, K.; Li, Z. A review of volatile fatty acids production from organic wastes: Intensification techniques and separation methods. J. Environ. Manag. 2024, 360, 121062. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, P.; Zhang, R.; Chang, J.; Chen, L.; Zhang, G.; Wang, A. Bioconversion of volatile fatty acids from organic wastes to produce high-value products by photosynthetic bacteria: A review. Environ. Res. 2024, 242, 117796. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.-H. Dark fermentation: Production and utilization of volatile fatty acid from different wastes- A review. Chemosphere 2022, 288 Pt 1, 132444. [Google Scholar] [CrossRef]
- Chaitanya, N.K.; Chatterjee, P. Medium chain fatty acid production from CO2 in integrated dark fermentation-microbial electrosynthesis reactor. Bioresour. Technol. 2025, 426, 132371. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Hidayatullah, M.A.; Nurullah, A.; Suhendi, E.; Kustiningsih, I.; Susanti, D.Y.; Darsono, N.; Primeia, S.; Khaerudini, D.S. Enhanced biohydrogen production from palm oil mill effluent using single-stage process of dark fermentation and microbial electrolysis cell at various initial pHs. Renew. Energy 2025, 249, 123161. [Google Scholar] [CrossRef]
- Cardeña, R.; Buitrón, G.; Valdez-Vazquez, I.; Contreras, M. Biohydrogen production from cheese whey in UASB and packed bed reactors: Impact of microbial interactions on productivity. Int. J. Hydrogen Energy 2025, 141, 1061–1069. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Continuous biohydrogen and volatile fatty acids production from cheese whey in a tubular biofilm reactor: Substrate flow rate variations and microbial dynamics. Int. J. Hydrogen Energy 2024, 59, 1305–1316. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Rai, P.; Duke, M. Cheese whey to biohydrogen and useful organic acids: A non-pathogenic microbial treatment by L. acidophilus. Sci. Rep. 2019, 9, 8320. [Google Scholar] [CrossRef] [PubMed]
- Astals, S.; Esteban-Gutiérrez, M.; Fernández-Arévalo, T.; Aymerich, E.; García-Heras, J.; Mata-Alvarez, J. Anaerobic digestion of seven different sewage sludges: A biodegradability and modelling study. Water Res. 2013, 47, 6033–6043. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Bi, C.; Ma, C.; Shi, J.; Meng, Q.; Li, J.; Zhang, S.; Li, J.; Shan, A. Lactic acid bacteria mechanism of protein degradation in anaerobic co-fermentation of cabbage waste with wheat bran. Chem. Eng. J. 2025, 507, 160738. [Google Scholar] [CrossRef]
- Kontodimos, I.; Evaggelou, C.; Margaritis, N.; Grammelis, P.; Goula, M. Dark Fermentation and Anaerobic Digestion for H2 and CH4 Production, from Food Waste Leachates. Methane 2025, 4, 11. [Google Scholar] [CrossRef]
- Hussien, M.; Mohamed, H.O.; Jadhav, D.A.; Bahaa, A.; Jo, S.-M.; Jang, J.-H.; Kim, J.-H.; Kwon, J.-Y.; Sayed, E.T.; Abdelkareem, M.A.; et al. Synergistic integration of dark fermentation and microbial electrolysis cells for hydrogen production and sustainable swine manure treatment. Int. J. Hydrogen Energy 2025, 115, 299–309. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, G.; Zhang, X.; Lu, X.; Li, S.; Wang, X.; Yang, W.; Yuan, Y.; Jiao, P. The gas production, ruminal fermentation parameters, and microbiota in response to Clostridium butyricum supplementation on in vitro varying with media pH levels. Front. Microbiol. 2022, 13, 960623. [Google Scholar] [CrossRef]
- Liu, S.; Bischoff, K.M.; Leathers, T.D.; Qureshi, N.; Rich, J.O.; Hughes, S.R. Butyric acid from anaerobic fermentation of lignocellulosic biomass hydrolysates by Clostridium tyrobutyricum strain RPT-4213. Bioresour. Technol. 2013, 143, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.; Schröder, C.; Schönheit, P. Glucose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic eubacterium Thermotoga maritima: Involvement of the Embden-Meyerhof pathway. Arch. Microbiol. 1994, 161, 460–470. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Lynd, L.H.; Zeikus, J.G. Metabolism of H2-CO2, methanol, and glucose by Butyribacterium methylotrophicum. J. Bacteriol. 1983, 153, 1415–1423. [Google Scholar] [CrossRef]
- Yonehara, T.; Miyata, R. Fermentative production of pyruvate from glucose by Torulopsis glabrata. J. Ferment. Bioeng. 1994, 78, 155–159. [Google Scholar] [CrossRef]
- Sirisangsawang, R.; Samaikaew, P.; Chotiviriyavanich, B.; Kitchaiya, P. Conversion of Sodium Lactate to Lactic acid and Sodium Hydroxide with Cation Exchange Membrane Electrolytic Cell. In IOP Conference Series Materials Science and Engineering, Proceedings of the 2020 3rd International Conference on Chemistry and Energy Research, Shenzhen, China, 23–25 October 2020; IOP Publishing: Bristol, UK, 2019; Volume 639, p. 12052. [Google Scholar] [CrossRef]
- Ai, B.; Li, J.; Chi, X.; Meng, J.; Liu, C.; Shi, E. Butyric Acid Fermentation of Sodium Hydroxide Pretreated Rice Straw with Undefined Mixed Culture. J. Microbiol. Biotechnol. 2014, 24, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, R. EPA Microbiological Alternate Test Procedure (ATP) Protocol for Drinking Water, Ambient Water, Wastewater, and Sewage Sludge Monitoring Methods, EPA-Engineering and Analysis Division (4303T), September 2010, EPA-821-B-10-001. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/micro_atp_protocol_sept-2010.pdf (accessed on 21 January 2025).
- Zhang, P.; Chen, Y.; Zhou, Q. Waste activated sludge hydrolysis and short-chain fatty acids accumulation under mesophilic and thermophilic conditions: Effect of pH. Water Res. 2009, 43, 3735–3742. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Awasthi, M.K.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Hidalgo, D.; Garrote, L.; Infante, F.; Martín-Marroquín, J.M.; Pérez-Zapatero, E.; Corona, F. Targeted Acidogenic Fermentation of Waste Streams for the Selective Production of Volatile Fatty Acids as Bioplastic Precursors. Appl. Sci. 2025, 15, 5923. [Google Scholar] [CrossRef]
- Jalil, A.; Yu, Z. Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis. Sustainability 2024, 16, 10755. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; Wiley: Weinheim, Germany, October 2010. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Zonfa, T.; Gioannis, G.D.; Muntoni, A. Factor-based assessment of continuous bio-H2 production from cheese whey. Chemosphere 2022, 8 Pt 1, 136174. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Paez, K.; Buitron, G.; Vital-Jacome, M. Predicting metabolic pathways and microbial interactions in dark fermentation systems treating real cheese whey effluents. Bioresour. Technol. 2024, 413, 131536. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Páez, K.M.; Vargas, A.; Buitrón, G. Feedback Control-Based Strategy Applied for Biohydrogen Production from Acid Cheese Whey. Waste Biomass Valorization 2022, 14, 447–460. [Google Scholar] [CrossRef]
- Colombo, B.; Calvo, M.V.; Sciarria, T.P.; Scaglia, B.; Kizito, S.S.; Adani, F. Biohydrogen and polyhydroxyalkanoates (PHA) as products of a two-steps bioprocess from deproteinized dairy wastes. Waste Manag. 2019, 95, 22–31. [Google Scholar] [CrossRef]
- Zonfa, T.; Kamperidis, T.; Falzarano, M.; Lyberatos, G.; Polettini, A.; Pomi, R.; Rossi, A.; Tremouli, A. Two-Stage Process for Energy Valorization of Cheese Whey through Bio-Electrochemical Hydrogen Production Coupled with Microbial Fuel Cell. Fermentation 2023, 9, 306. [Google Scholar] [CrossRef]

| Cheese Whey | TCOD (g/L) | SCOD (g/L) | Carbohydrates (g/L) |
|---|---|---|---|
| 1× | 87.5 | 78.5 | 78.4 |
| 5× | 19.2 | 17.8 | 14.75 |
| Concentration (mg/L) | 0 | 100 | 200 |
| DW (mL) | 1 | 0.5 | 0 |
| Standard solution (mL) | 0 | 0.5 | 1 |
| Reagent A (mL) | 1 | 1 | 1 |
| Reagent B (mL) | 5 | 5 | 5 |
| Cheese Whey (Dilution) | TCOD (g/L) | SCOD (g/L) | Carbohydrates (g/L) |
|---|---|---|---|
| 1× | 87.5 | 78.5 | 78.4 |
| 5× | 19.2 | 17.8 | 14.746 |
| (TS) | (VS) | TSS | VSS | ||
|---|---|---|---|---|---|
| Dilution | CW (1×) | 0.6135 | 0.0551 | 0.00741 | 0.00731 |
| CW (5×) | 0.1080 | 0.0889 | 0.00148 | 0.00146 | |
| CW (10×) | 0.0540 | 0.0453 | 0.00016 | 0.00015 | |
| Inoculum | 0.5538 | 0.2891 | 0.00074 | 0.00093 | |
| Samples | pH | Dilution (D×) | Ratio of Inoculum to Substrate (I/S) |
|---|---|---|---|
| 1 | 6.5 | 5 | 20 to 80 |
| 2 | 6.5 | 5 | 30 to 70 |
| 3 | 6.5 | 5 | 50 to 50 |
| 4 | 6.5 | 1 | 30 to 70 |
| 5 | 6.5 | 5 | 30 to 70 |
| 6 | 6.5 | 10 | 30 to 70 |
| 7 | 9 | 5 | 30 to 70 |
| 8 | 7 | 5 | 30 to 70 |
| 9 | 5 | 5 | 30 to 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-G.; Dulany, Z. Biogas and Hydrogen Production from Waste Biomass via Dark Fermentation Evaluating VFAs, COD, and HRT for Process Optimization. Biomass 2025, 5, 57. https://doi.org/10.3390/biomass5030057
Lee H-G, Dulany Z. Biogas and Hydrogen Production from Waste Biomass via Dark Fermentation Evaluating VFAs, COD, and HRT for Process Optimization. Biomass. 2025; 5(3):57. https://doi.org/10.3390/biomass5030057
Chicago/Turabian StyleLee, Hoe-Gil, and Zachary Dulany. 2025. "Biogas and Hydrogen Production from Waste Biomass via Dark Fermentation Evaluating VFAs, COD, and HRT for Process Optimization" Biomass 5, no. 3: 57. https://doi.org/10.3390/biomass5030057
APA StyleLee, H.-G., & Dulany, Z. (2025). Biogas and Hydrogen Production from Waste Biomass via Dark Fermentation Evaluating VFAs, COD, and HRT for Process Optimization. Biomass, 5(3), 57. https://doi.org/10.3390/biomass5030057






