Valorizing Biomass Waste: Hydrothermal Carbonization and Chemical Activation for Activated Carbon Production
Abstract
1. Introduction
2. Methodology
2.1. Biomass Utilized
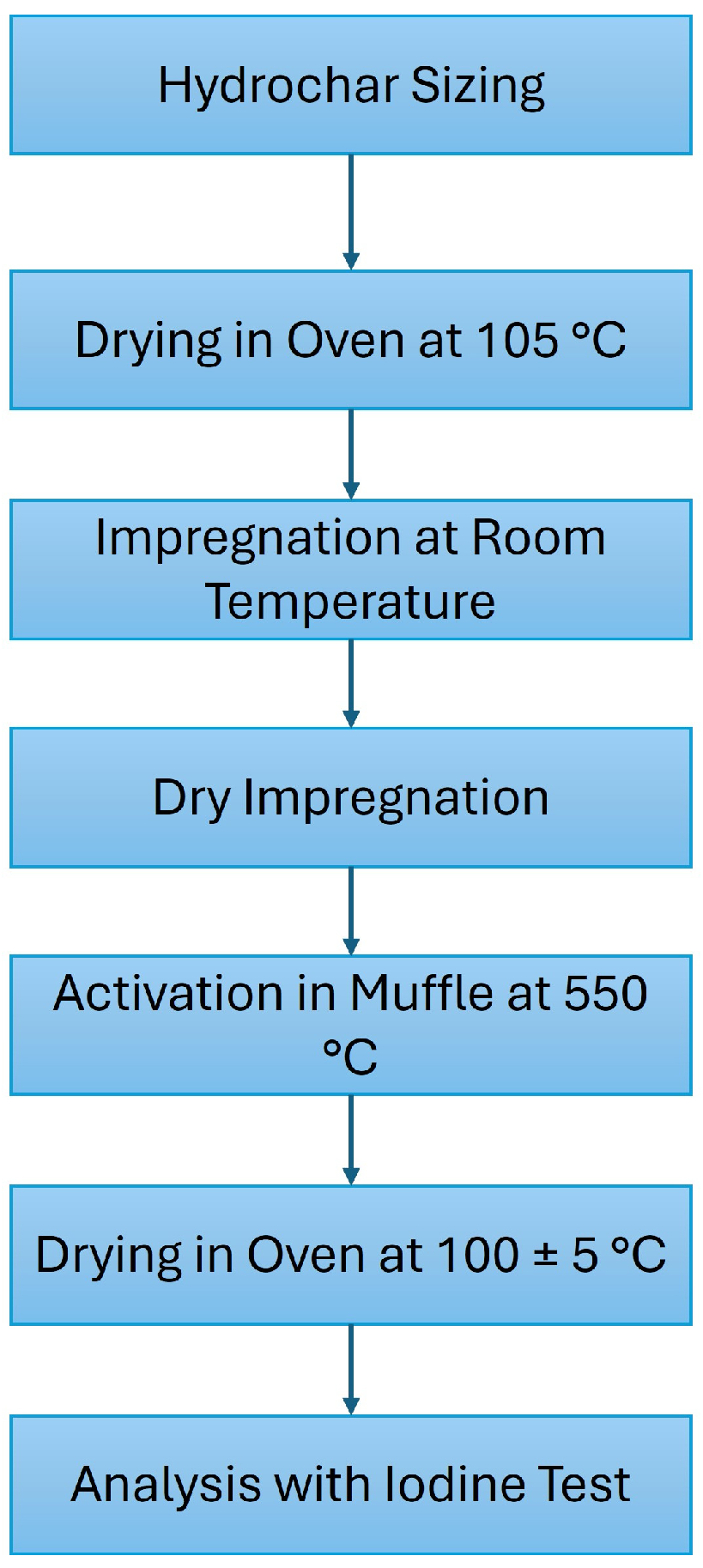
2.2. Experimental Procedure
2.3. Experimental Design
3. Results
3.1. Effect of Hydrothermal Carbonization
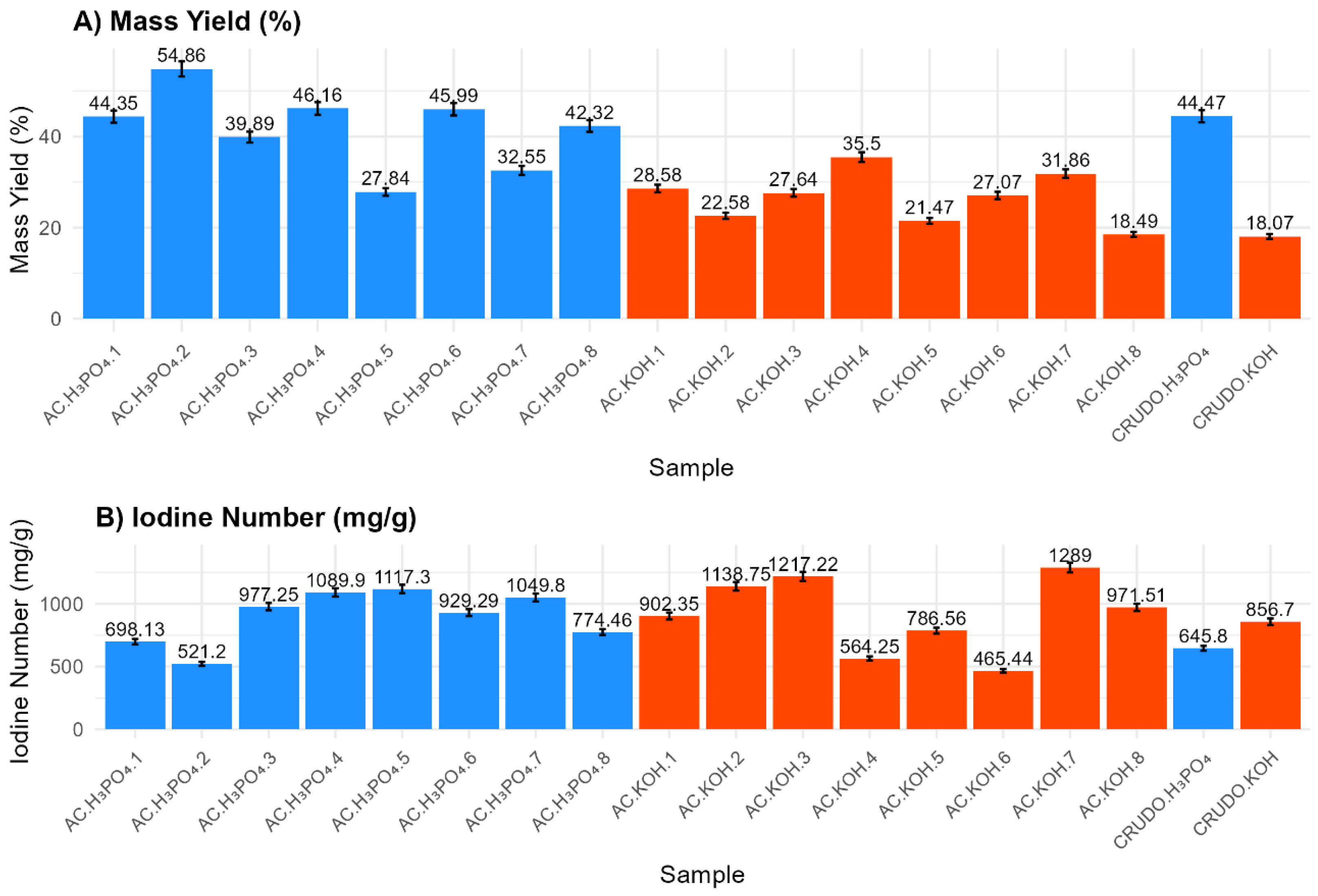
3.2. Effect of Activating Agent and Agent-to-Precursor Ratio
3.3. Effect of Activation Time and Operational Conditions
3.4. ANOVA and Contour Analysis for Mass Yield and Iodine Number
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jain, A.; Sarsaiya, S.; Kumar Awasthi, M.; Singh, R.; Rajput, R.; Mishra, U.C.; Chen, J.; Shi, J. Bioenergy and bio-products from bio-waste and its associated modern circular economy: Current research trends, challenges, and future outlooks. Fuel 2022, 307, 121859. [Google Scholar] [CrossRef]
- Chen, Y.-H.R.; Lee, W.-C.; Liu, B.-C.; Yang, P.-C.; Ho, C.-C.; Hwang, J.-S.; Huang, T.-H.; Lin, H.-H.; Lo, W.-C. Quantifying the potential effects of air pollution reduction on population health and health expenditure in Taiwan. Environ. Pollut. 2023, 336, 122405. [Google Scholar] [CrossRef]
- Balogun, A.O.; Weigel, M.M.; Estévez, E.; Armijos, R.X. Chronic Occupational Exposure to Traffic Pollution Is Associated with Increased Carotid Intima-Media Thickness in Healthy Urban Traffic Control Police. Int. J. Environ. Res. Public Health 2023, 20, 6701. [Google Scholar] [CrossRef]
- González-Arias, J.; Baena-Moreno, F.M.; Sánchez, M.E.; Cara-Jiménez, J. Optimizing hydrothermal carbonization of olive tree pruning: A techno-economic analysis based on experimental results. Sci. Total Environ. 2021, 784, 147169. [Google Scholar] [CrossRef]
- Yuan, H.; Li, C.; Shan, R.; Zhang, J.; Wu, Y.; Chen, Y. Recent developments on the zeolites catalyzed polyolefin plastics pyrolysis. Fuel Process. Technol. 2022, 238, 107531. [Google Scholar] [CrossRef]
- Vallejo, F.; Díaz-Robles, L.A.; Vega, R.E.; Cubillos, F. A novel approach for prediction of mass yield and higher calorific value of hydrothermal carbonization by a robust multilinear model and regression trees. J. Energy Inst. 2020, 93, 1755–1762. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Bai, S.X.; Zhou, L.M.; Chang, Z.B.; Zhang, C.; Chu, M. Synthesis of Na-X zeolite from Longkou oil shale ash by alkaline fusion hydrothermal method. Carbon Resour. Convers. 2018, 1, 245–250. [Google Scholar] [CrossRef]
- Dhull, S.B.; Rose, P.K.; Rani, J.; Goksen, G.; Bains, A. Food waste to hydrochar: A potential approach towards the Sustainable Development Goals, carbon neutrality, and circular economy. Chem. Eng. J. 2024, 490, 151609. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Liang, K.; Chen, Y.; Wang, S.; Wang, D.; Wang, W.; Jia, S.; Mitsuzakic, N.; Chen, Z. Peanut shell waste derived porous carbon for high-performance supercapacitors. J. Energy Storage 2023, 70, 107947. [Google Scholar] [CrossRef]
- Sandeep, A.; T, P.; Ravindra, A.V. Activated carbon derived from corncob via hydrothermal carbonization as a promising electrode for supercapacitors. Mater. Res. Bull. 2024, 179, 112991. [Google Scholar] [CrossRef]
- Sandeep, A.; Ravindra, A.V. Peanut shell-derived porous carbons activated with iron and zinc chlorides as electrode materials with improved electrochemical performance for supercapacitors. Emergent Mater. 2024, 8, 235–250. [Google Scholar] [CrossRef]
- Sandeep, A.; Ravindra, A.V. Highly efficient peanut shell activated carbon via hydrothermal carbonization and chemical activation for energy storage applications. Diam. Relat. Mater. 2024, 146, 111158. [Google Scholar] [CrossRef]
- Marrakchi, F.; Sohail Toor, S.; Haaning Nielsen, A.; Helmer Pedersen, T.; Aistrup Rosendahl, L. Bio-crude oils production from wheat stem under subcritical water conditions and batch adsorption of post-hydrothermal liquefaction aqueous phase onto activated hydrochars. Chem. Eng. J. 2023, 452, 139293. [Google Scholar] [CrossRef]
- Vallejo, F.; Yánez-Sevilla, D.; Díaz-Robles, L.A.; Cubillos, F.; Espinoza-Pérez, A.; Espinoza-Pérez, L.; Pino-Cortés, E.; Cereceda-Balic, F. Insights into hydrothermal treatment of biomass blends: Assessing energy yield and ash content for biofuel enhancement. PLoS ONE 2024, 19, e0304054. [Google Scholar] [CrossRef]
- Vallejo, F.; Díaz-Robles, L.; Carné-Seco, V.; Pino-Cortés, E.; Espinoza-Pérez, A.; Espinoza-Pérez, L. Hybrid porous media gasification of urban solid waste pre-treated by hydrothermal carbonization. PLoS ONE 2023, 18, e0291838. [Google Scholar] [CrossRef]
- ISO 18122; Solid Biofuels—Determination of Ash Content. ISO: Geneva, Switzerland, 2016.
- Vallejo, F.; Diaz-Robles, L.A.; Poblete, J.; Cubillos, F. Experimental study and validation of a kinetic scheme for Hydrothermal Carbonization reactions. Biofuels 2020, 13, 461–466. [Google Scholar] [CrossRef]
- Vallejo, F.; Díaz-Robles, L.A.; Cubillos, F.; Vega, R.; Gómez, J.; Pino-Cortés, E.; Bascuñan, B.; Carcamo, P.; Parra, F.; Urzua, A.; et al. Performance evaluation of biomass blends with additives treated by hydrothermal carbonization. Chem. Eng. Trans. 2019, 76, 1459–1464. [Google Scholar] [CrossRef]
- Chen, B.; Guan, H.; Zhang, Y.; Liu, S.; Zhao, B.; Zhong, C.; Zhang, H.; Ding, W.; Song, A.; Zhu, D.; et al. Performance and mechanism of Pb2+ and Cd2+ ions’ adsorption via modified antibiotic residue-based hydrochar. Heliyon 2023, 9, e14930. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Zhao, Y.; Yuan, H.; Chen, Y. Ultra-highly porous carbon from Wasted soybean residue with tailored porosity and doped structure as renewable multi-purpose absorbent for efficient CO2, toluene and water vapor capture. J. Clean. Prod. 2022, 337, 130283. [Google Scholar] [CrossRef]
- Chatir, E.M.; El Hadrami, A.; Ojala, S.; Brahmi, R. Production of activated carbon with tunable porosity and surface chemistry via chemical activation of hydrochar with phosphoric acid under oxidizing atmosphere. Surf. Interfaces 2022, 30, 101849. [Google Scholar] [CrossRef]
- Villamil, J.A.; Diaz, E.; de la Rubia, M.A.; Mohedano, A.F. Potential Use of Waste Activated Sludge Hydrothermally Treated as a Renewable Fuel or Activated Carbon Precursor. Molecules 2020, 25, 3534. [Google Scholar] [CrossRef] [PubMed]
- Purnomo, C.W.; Castello, D.; Fiori, L. Granular Activated Carbon from Grape Seeds Hydrothermal Char. Appl. Sci. 2018, 8, 331. [Google Scholar] [CrossRef]
- ASTM-D4607; Test Method for Determination of Iodine Number of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Zhu, F.; Wang, Z.; Huang, J.; Hu, W.; Xie, D.; Qiao, Y. Efficient adsorption of ammonia on activated carbon from hydrochar of pomelo peel at room temperature: Role of chemical components in feedstock. J. Clean. Prod. 2023, 406, 137076. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Env. Chem Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Long, Y.; An, X.; Zhang, H.; Yang, J.; Liu, L.; Tian, Z.; Yang, G.; Cheng, Z.; Cao, H.; Liu, H.; et al. Highly graphitized lignin-derived porous carbon with hierarchical N/O co-doping “core-shell” superstructure supported by metal-organic frameworks for advanced supercapacitor performance. Chem. Eng. J. 2023, 451, 138877. [Google Scholar] [CrossRef]
- Sánchez, D.A.; Díaz-Robles, L.A.; Cubillos, F.; Gómez, J.; Reyes, A.; Vallejo, F.; Pino-Cortés, E. Drying process study of hydrothermal carbonized biomass. Dry. Technol. 2020, 40, 273–283. [Google Scholar] [CrossRef]
- Puccini, M.; Stefanelli, E.; Hiltz, M.; Seggiani, M. Activated Carbon from Hydrochar Produced by Hydrothermal Carbonization of Wastes. Chem. Eng. Trans. 2017, 57, 169–174. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, T.; Zhang, G.; Liu, Q.; Kong, G.; Wang, K.; Jiang, Y.; Zhang, X.; Han, L. Sustainable hydrothermal carbon for advanced electrochemical energy storage. J. Mater. Chem. A 2024, 12, 4996–5039. [Google Scholar] [CrossRef]
- Genli, N.; Kutluay, S.; Baytar, O.; Şahin, Ö. Preparation and characterization of activated carbon from hydrochar by hydrothermal carbonization of chickpea stem: An application in methylene blue removal by RSM optimization. Int. J. Phytorem. 2021, 24, 88–100. [Google Scholar] [CrossRef]
- Wilk, M.; Czerwińska, K.; Śliz, M.; Imbierowicz, M. Hydrothermal carbonization of sewage sludge: Hydrochar properties and processing water treatment by distillation and wet oxidation. Energy Rep. 2023, 9, 39–58. [Google Scholar] [CrossRef]
- Suresh, A.; Alagusundaram, A.; Kumar, P.S.; Vo, D.V.N.; Christopher, F.C.; Balaji, B.; Viswanathan, V.; Sankar, S. Microwave pyrolysis of coal, biomass and plastic waste: A review. Environ. Chem. Lett. 2021, 19, 3609–3629. [Google Scholar] [CrossRef]
- Duan, D.; Wang, Y.; Dai, L.; Ruan, R.; Zhao, Y.; Fan, L.; Tayier, M.; Liu, Y. Ex-situ catalytic co-pyrolysis of lignin and polypropylene to upgrade bio-oil quality by microwave heating. Bioresour. Technol. 2017, 241, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Diaz-Robles, L.A.; Gonzalez, P.; Poblete, J. Energy efficiency evaluation of a continuos treatment of agroforestry waste biomass by hydrothermal carbonization. Maderas Cienc. y Tecnol. 2021, 23, 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Ru, B.; Dai, G.; Shi, Z.; Zhou, J.; Luo, Z.; Ni, M.; Cen, K. Mechanism study on the pyrolysis of a synthetic β-O-4 dimer as lignin model compound. Proc. Combust. Inst. 2017, 36, 2225–2233. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, H.T.; Yang, L.; Zhang, W.; Hu, Y.; Guo, Y.; Yang, L.; Leng, S.; Chen, J.; Chen, J.; et al. Co-hydrothermal carbonization of cellulose, hemicellulose, and protein with aqueous phase recirculation: Insight into the reaction mechanisms on hydrochar formation. Energy 2022, 251, 123965. [Google Scholar] [CrossRef]
- Waribam, P.; Ngo, S.D.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Chanlek, N.; Wei, L.; Zhang, H.; Guan, G.; Samart, C. Waste biomass valorization through production of xylose-based porous carbon microspheres for supercapacitor applications. Waste Manag. 2020, 105, 492–500. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Lyczko, N.; Minh, D.P. The catalytic effect of inherent and adsorbed metals on the fast/flash pyrolysis of biomass: A review. Energy 2019, 170, 326–337. [Google Scholar] [CrossRef]
- Gallant, R.; Farooque, A.A.; He, S.; Kang, K.; Hu, Y. A mini-review: Biowaste-derived fuel pellet by hydrothermal carbonization followed by pelletizing. Sustainability 2022, 14, 12530. [Google Scholar] [CrossRef]
- Carrasco, S.; Silva, J.; Pino-Cortés, E.; Gómez, J.; Vallejo, F.; Díaz-Robles, L.; Campos, V.; Cubillos, F.; Pelz, S.; Paczkowski, S.; et al. Experimental study on hydrothermal carbonization of lignocellulosic biomass with magnesium chloride for solid fuel production. Processes 2020, 8, 444. [Google Scholar] [CrossRef]
- Banifateme, M.; Behbahaninia, A.; Pignatta, G. Estimating the chemical composition of municipal solid waste using the inverse method. J. Clean. Prod. 2023, 393, 136156. [Google Scholar] [CrossRef]
- Chai, H.; Ma, J.; Ma, H.; Cheng, H.; Weng, Z.; Kong, Z.; Shao, Z.; Yuan, Y.; Xu, Y.; Ni, Q.; et al. Enhanced nutrient removal of agricultural waste-pyrite bioretention system for stormwater pollution treatment. J. Clean. Prod. 2023, 395, 136457. [Google Scholar] [CrossRef]
- Vo, T.A.; Tran, Q.K.; Ly, H.V.; Kwon, B.; Hwang, H.T.; Kim, J.; Kim, S.-S. Co-pyrolysis of lignocellulosic biomass and plastics: A comprehensive study on pyrolysis kinetics and characteristics. J. Anal. Appl. Pyrolysis 2022, 163, 105464. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Sun, R.; Yao, L.; Zuo, H.; Ruan, F.; Feng, Q.; Wang, J. Preparation of hierarchical micro-meso porous carbon and carbon nanofiber from polyacrylonitrile/polysulfone polymer via one-step carbonization for supercapacitor electrodes. Electrochim. Acta 2023, 441, 141827. [Google Scholar] [CrossRef]
- Chen, T.; Luo, L.; Luo, L.; Deng, J.; Wu, X.; Fan, M.; Du, G.; Zhao, W. High energy density supercapacitors with hierarchical nitrogen-doped porous carbon as active material obtained from bio-waste. Renew. Energy 2021, 175, 760–769. [Google Scholar] [CrossRef]
- Ni, J.; Qian, L.; Wang, Y.; Zhang, B.; Gu, H.; Hu, Y.; Wang, Q. A review on fast hydrothermal liquefaction of biomass. Fuel 2022, 327, 125315. [Google Scholar] [CrossRef]
- Falco, C.; Marco-Lozar, J.P.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D.; Titirici, M.M.; Lozano-Castelló, D. Tailoring the porosity of chemically activated hydrothermal carbons: Influence of the precursor and hydrothermal carbonization temperature. Carbon 2013, 62, 346–355. [Google Scholar] [CrossRef]
- Liu, S.; Liang, Y.; Zhou, W.; Hu, W.; Dong, H.; Zheng, M.; Hu, H.; Lei, B.; Xiao, Y.; Liu, Y. Large-scale synthesis of porous carbon via one-step CuCl2 activation of rape pollen for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 12046–12055. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Villamil, J.A.; Qaramaleki, S.V.; Coronella, C.J.; Mohedano, A.F.; la Rubia, M.A.d. Energy valorization of cow manure by hydrothermal carbonization and anaerobic digestion. Renew. Energy 2020, 160, 623–632. [Google Scholar] [CrossRef]
- Qaramaleki, S.V.; Cardenas, J.; Jackson, M.A.; Compton, D.L.; Szogi, A.A.; Ro, K.S.; Coronella, C.J. Characterization of Products from Catalytic Hydrothermal Carbonization of Animal Manure. Agronomy 2023, 13, 2219. [Google Scholar] [CrossRef]
- Spencer, W.; Ibana, D.; Singh, P.; Nikoloski, A.N. Effect of Surface Area, Particle Size and Acid Washing on the Quality of Activated Carbon Derived from Lower Rank Coal by KOH Activation. Sustainability 2024, 16, 5876. [Google Scholar] [CrossRef]

| Precursor Agent | Agent/Precursor Ratio (g/g) | Dry Impregnation (h) | Activation Time (h) | |||
|---|---|---|---|---|---|---|
| − | + | − | + | − | + | |
| KOH | 2:1 | 4:1 | 0 | 1 | 1 | 2 |
| H3PO4 | 1.3:1 | 2:1 | 0 | 1 | 1 | 2 |
| Sample Name | Activating Agent | Agent/Precursor Ratio | Dry Impregnation (h) | Activation Time (h) |
|---|---|---|---|---|
| AC.KOH.1 | KOH | 2:1 (−) | 0 (−) | 1 (−) |
| AC.KOH.2 | KOH | 4:1 (+) | 0 (−) | 1 (−) |
| AC.KOH.3 | KOH | 2:1 (−) | 1 (+) | 1 (−) |
| AC.KOH.4 | KOH | 4:1 (+) | 1 (+) | 1 (−) |
| AC.KOH.5 | KOH | 2:1 (−) | 0 (−) | 2 (+) |
| AC.KOH.6 | KOH | 4:1 (+) | 0 (−) | 2 (+) |
| AC.KOH.7 | KOH | 2:1 (−) | 1 (+) | 2 (+) |
| AC.KOH.8 | KOH | 4:1 (+) | 1 (+) | 2 (+) |
| AC.H3PO4.1 | H3PO4 | 1.3:1 (−) | 0 (−) | 1 (−) |
| AC.H3PO4.2 | H3PO4 | 2:1 (+) | 0 (−) | 1 (−) |
| AC.H3PO4.3 | H3PO4 | 1.3:1 (−) | 1 (+) | 1 (−) |
| AC.H3PO4.4 | H3PO4 | 2:1 (+) | 1 (+) | 1 (−) |
| AC.H3PO4.5 | H3PO4 | 1.3:1 (−) | 0 (−) | 2 (+) |
| AC.H3PO4.6 | H3PO4 | 2:1 (+) | 0 (−) | 2 (+) |
| AC.H3PO4.7 | H3PO4 | 1.3:1 (−) | 1 (+) | 2 (+) |
| AC.H3PO4.8 | H3PO4 | 2:1 (+) | 1 (+) | 2 (+) |
| CRUDO.KOH | KOH | 4:1 (+) | 1 (+) | 1 (−) |
| CRUDO.H3PO4 | H3PO4 | 2:1 (+) | 0 (−) | 1 (−) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejo, F.; Yánez, D.; Díaz-Robles, L.; Oyaneder, M.; Alejandro-Martín, S.; Zalakeviciute, R.; Romero, T. Valorizing Biomass Waste: Hydrothermal Carbonization and Chemical Activation for Activated Carbon Production. Biomass 2025, 5, 45. https://doi.org/10.3390/biomass5030045
Vallejo F, Yánez D, Díaz-Robles L, Oyaneder M, Alejandro-Martín S, Zalakeviciute R, Romero T. Valorizing Biomass Waste: Hydrothermal Carbonization and Chemical Activation for Activated Carbon Production. Biomass. 2025; 5(3):45. https://doi.org/10.3390/biomass5030045
Chicago/Turabian StyleVallejo, Fidel, Diana Yánez, Luis Díaz-Robles, Marcelo Oyaneder, Serguei Alejandro-Martín, Rasa Zalakeviciute, and Tamara Romero. 2025. "Valorizing Biomass Waste: Hydrothermal Carbonization and Chemical Activation for Activated Carbon Production" Biomass 5, no. 3: 45. https://doi.org/10.3390/biomass5030045
APA StyleVallejo, F., Yánez, D., Díaz-Robles, L., Oyaneder, M., Alejandro-Martín, S., Zalakeviciute, R., & Romero, T. (2025). Valorizing Biomass Waste: Hydrothermal Carbonization and Chemical Activation for Activated Carbon Production. Biomass, 5(3), 45. https://doi.org/10.3390/biomass5030045









