Valorization of Forest Biomass Through Biochar for Static Floating Applications in Agricultural Uses
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Materials
2.2. Biochar Synthesis and Characterization
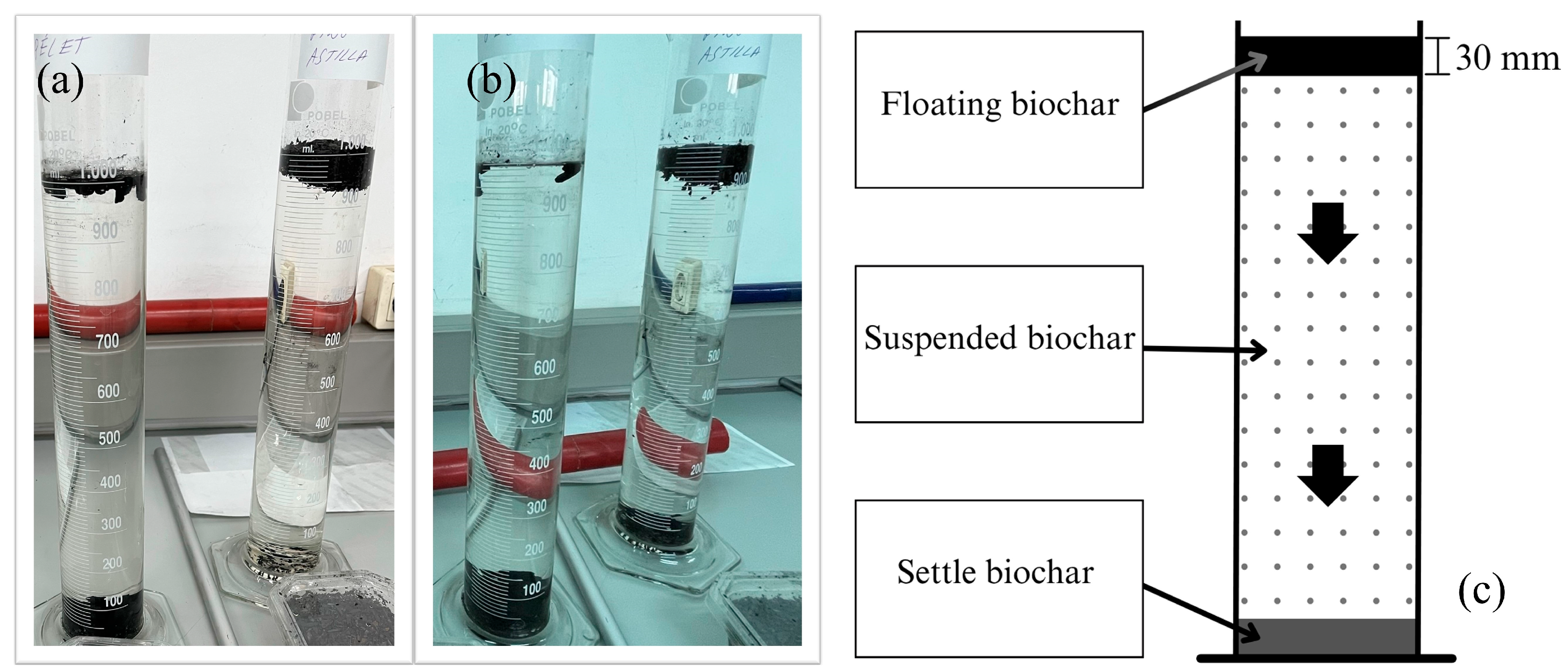
2.3. Floatability Analysis
3. Results and Discussion
3.1. Results and Discussion of the Biomass Materials
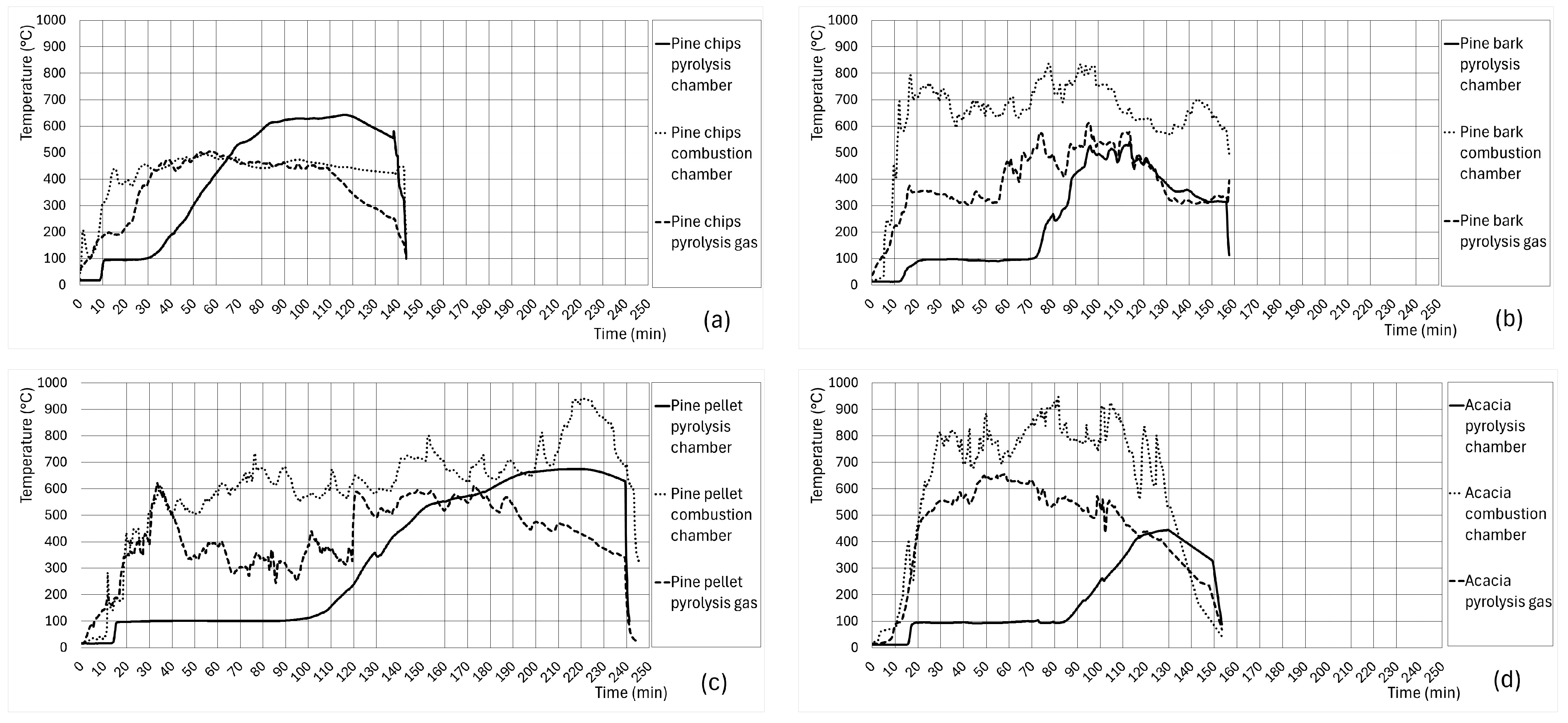
3.2. Results and Discussion of Biochar Synthesis and Characterization
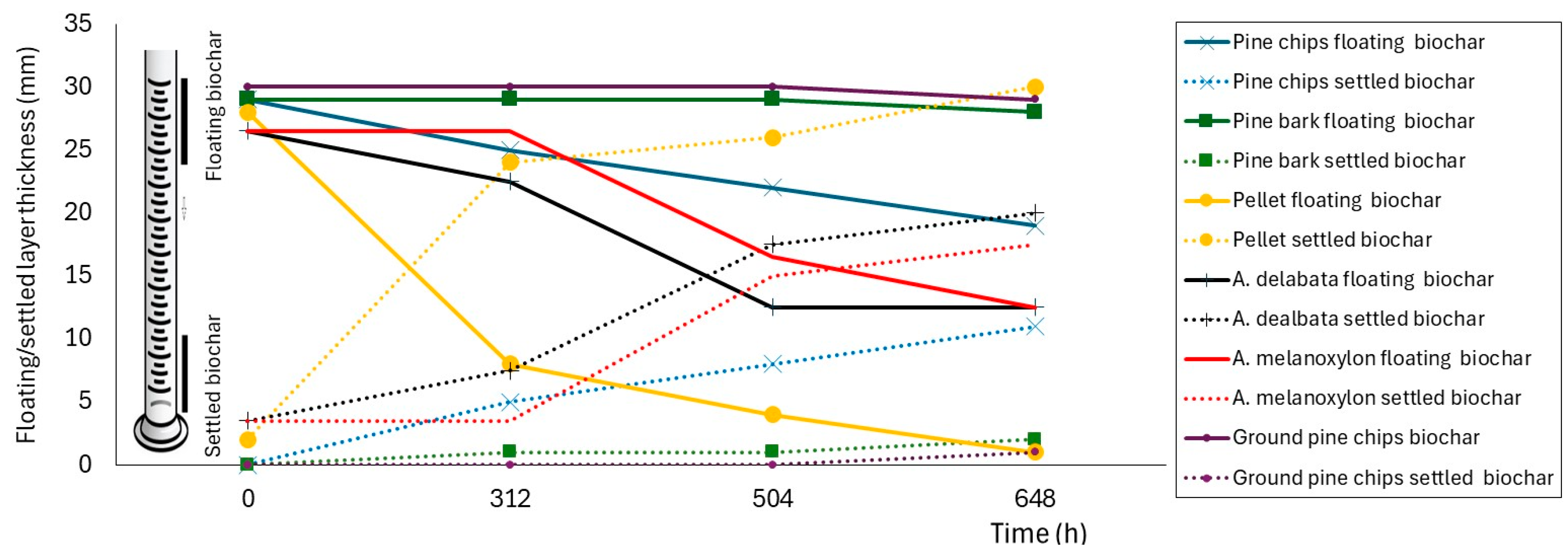
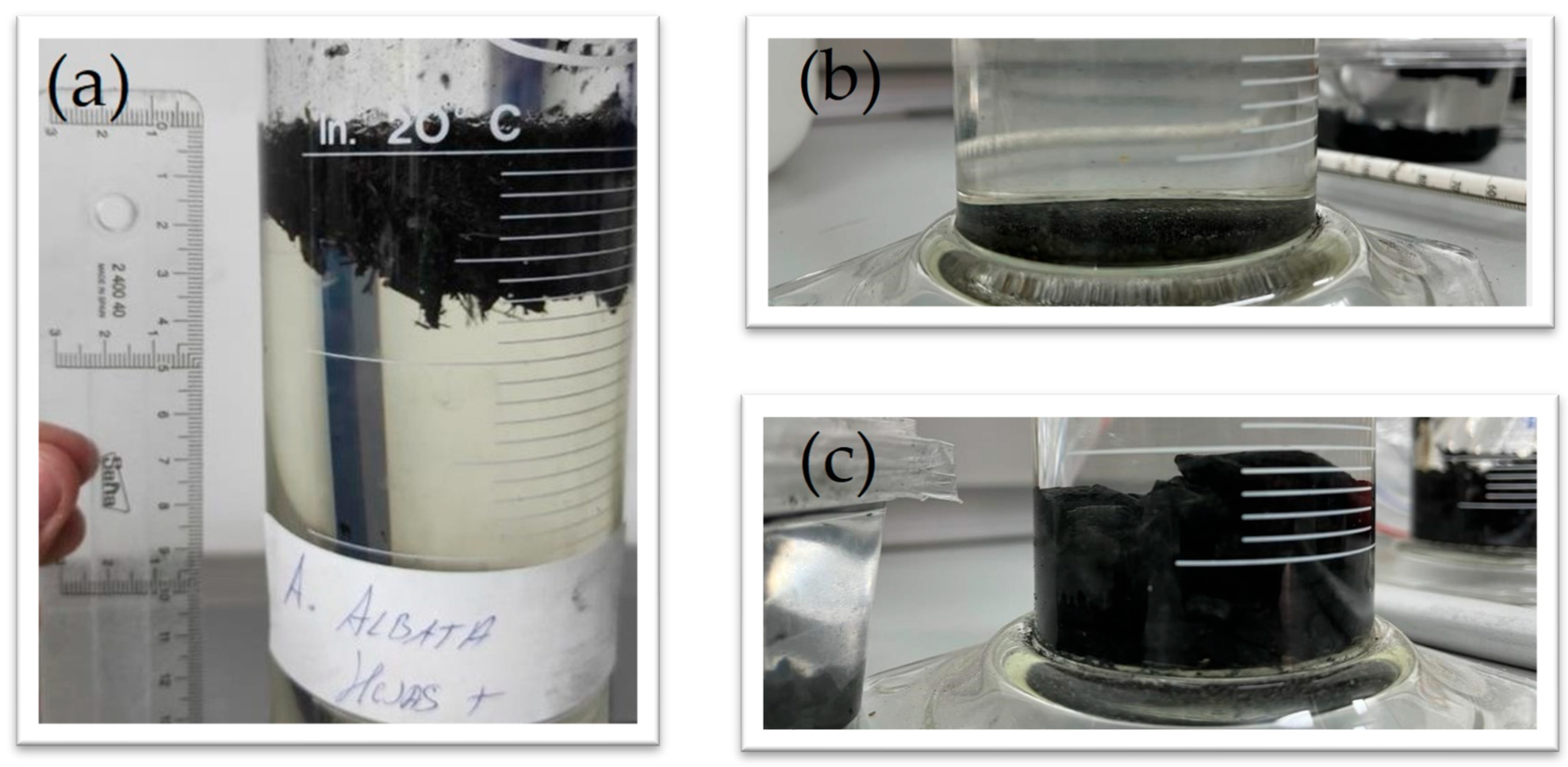
3.3. Results and Discussion of Floatability Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahng, M.-K.; Mukarakate, C.; Robichaud, D.J.; Nimlos, M.R. Current technologies for analysis of biomass thermochemical processing: A review. Anal. Chim. Acta 2009, 651, 117–138. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Fang, J.; Ma, Z.; Li, C.; Yan, M.; Qiao, N.; Liu, Y.; Bian, M. Reduction and Reuse of Forestry and Agricultural Bio-Waste through Innovative Green Utilization Approaches: A Review. Forests 2024, 15, 1372. [Google Scholar] [CrossRef]
- Maaoui, A.; Trabelsi, A.B.H.; Chagtmi, R.; Lopez, G.; Cortazar, M.; Olazar, M. Assessment of pine wood biomass wastes valorization by pyrolysis with focus on fast pyrolysis biochar production. J. Energy Inst. 2023, 108, 101242. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Progress in Thermochemical Biomass Conversion; Blackwell Science: Oxford, UK, 2001; ISBN 0-632-05533-2. [Google Scholar]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Xiao, R.; Awasthi, M.K.; Li, R.; Park, J.; Pensky, S.M.; Wang, Q.; Wang, J.J.; Zhang, Z. Recent developments in biochar utilization as an additive in organic solid waste composting: A review. Bioresour. Technol. 2017, 246, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Meiirkhanuly, Z.; Koziel, J.A.; Białowiec, A.; Banik, C.; Brown, R.C. The-Proof-of-Concept of Biochar Floating Cover Influence on Water pH. Water 2019, 11, 1802. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Díaz, M.A.D.; Frómeta, A.E.N.; Muñoz, C.L.S. Improved sorbent for the removal of hydrocarbons spilled in water. Front. Sustain. 2022, 3, 1–11. [Google Scholar] [CrossRef]
- Sileshi, G.W.; Barrios, E.; Lehmann, J.; Tubiello, F.N. An organic matter database (OMD): Consolidating global residue data from agriculture, fisheries, forestry and related industries. Earth Syst. Sci. Data. 2025, 17, 369–391. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.K.; Goyal, N.; Bobde, P.; Kwon, E.E.; Lin, K.-Y.A.; Chen, W.-H. A critical review on biochar production from pine wastes, upgradation techniques, environmental sustainability, and challenges. Bioresour. Technol. 2023, 387, 129632. [Google Scholar] [CrossRef]
- González-Prieto, Ó.; Ortiz Torres, L. Use of Invasive Acacia Biomass to Produce Biochar and Solid Biofuels. App. Sci. 2025, 15, 5755. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Bialowiec, A.; O’Brien, S.C. The potential role of biochar in mitigating gaseous emissions from livestock waste—A mini-review. J. Environ. Manag. 2024, 370, 122692. [Google Scholar] [CrossRef]
- Lee, J.; Wardhani, R.; Shin, J.; Lee, S.; Lee, Y.; Ahn, H. Effectiveness of Floating Covers in Mitigating Ammonia and Hydrogen Sulfide Emissions from Lab-Scale Swine Slurry Pits. Sustainability 2025, 17, 374. [Google Scholar] [CrossRef]
- di Perta, E.S.; Giudicianni, P.; Mautone, A.; Caro, S.; Cervelli, E.; Ragucci, R.; Pindozzi, S. Is the biochar an effective floating cover for manure storage to reduce ammonia emissions, adsorbing nitrogen at the same time ƒ. In Proceedings of the IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento, Italy, 4–6 November 2020; pp. 44–48. [Google Scholar] [CrossRef]
- Viaene, J.; Peiren, N.; Vandamme, D.; Lataf, A.; Cuypers, A.; Jozefczak, M.; Vandecasteele, B. Biochar amendment to cattle slurry reduces NH3 emissions during storage without risk of higher NH3 emissions after soil application of the solid fraction. Waste Manag. 2023, 167, 39–45. [Google Scholar] [CrossRef] [PubMed]
- González-Prieto, Ó.; Ortiz Torres, L.; Vazquez Torres, A. Comparison of Waste Biomass from Pine, Eucalyptus, and Acacia and the Biochar Elaborated Using Pyrolysis in a Simple Double Chamber Biomass Reactor. Appl. Sci. 2024, 14, 1851. [Google Scholar] [CrossRef]
- EBC. European Biochar Certificate—Guidelines for Sustainable Production of Biochar, Version 10.4 E (updated on 20th December 2024); European Biochar Foundation (EBC): Arbaz, Switzerland, 2024; Available online: http://european-biochar.org (accessed on 12 February 2025).
- UNE-EN ISO 17828; Solid Biofuels—Determination of Bulk Density (ISO 17828:2015). AENOR, Spanish Association for Standardization and Certification: Madrid, Spain, 2016.
- UNE-EN ISO 18134-3; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 3: Moisture in General Analysis Sample (ISO 18134-3:2023). AENOR, Spanish Association for Standardization and Certification: Madrid, Spain, 2024.
- UNE-EN ISO 18122; Solid Biofuels—Determination of Ash Content (ISO 18122:2022). AENOR, Spanish Association for Standardization and Certification: Madrid, Spain, 2023.
- UNE-EN ISO 18123; Solid Biofuels—Determination of the Content of Volatile Matter (ISO 18123:2023). AENOR, Spanish Association for Standardization and Certification: Madrid, Spain, 2024.
- UNE-EN ISO 10390; Soil, Treated Biowaste and Sludge—Determination of pH (ISO 10390:2021). AENOR, Spanish Association for Standardization and Certification: Madrid, Spain, 2022.
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Filipe dos Santos Viana, H.; Martins Rodrigues, A.; Godina, R.; Carlos de Oliveira Matias, J.; Jorge Ribeiro Nunes, L. Evaluation of the Physical, Chemical and Thermal Properties of Portuguese Maritime Pine Biomass. Sustainability 2018, 10, 2877. [Google Scholar] [CrossRef]
- Lerma-Arce, V.; Oliver-Villanueva, J.-V.; Segura-Orenga, G. Influence of raw material composition of Mediterranean pinewood on pellet quality. Biomass Bioenergy 2017, 99, 90–96. [Google Scholar] [CrossRef][Green Version]
- Santos, L.B.; Striebeck, M.V.; Crespi, M.S.; Ribeiro, C.A.; De Julio, M. Characterization of biochar of pine pellet. J. Therm. Anal. Calorim. 2015, 122, 21–32. [Google Scholar] [CrossRef]
- Ilek, A.; Kucza, J.; Morkisz, K. Hygroscopicity of the bark of selected forest tree species. IForest 2017, 10, 220–226. [Google Scholar] [CrossRef]
- Iglesias Canabal, A.; Proupín Castiñeiras, J.; Rodríguez Añón, J.A.; Eimil Fraga, C.; Rodríguez Soalleiro, R. Elemental composition of raw and torrefied pellets made from pine and pine-eucalyptus blends. Biomass Bioenergy 2023, 177, 106951. [Google Scholar] [CrossRef]
- Fraga, L.G.; Silva, J.; Teixeira, J.C.; Ferreira, M.E.C.; Teixeira, S.F.; Vilarinho, C.; Gonçalves, M.M. Study of mass loss and elemental analysis of pine wood pellets in a small-scale reactor. Energies 2022, 15, 5253. [Google Scholar] [CrossRef]
- Santana, D.A.R.; Scatolino, M.V.; Lima, M.D.R.; de Oliveira Barros Junior, U.; Garcia, D.P.; Andrade, C.R.; de Cássia Oliveira Carneiro, A.; Trugilho, P.F.; de Paula Protásio, T. Pelletizing of lignocellulosic wastes as an environmentally friendly solution for the energy supply: Insights on the properties of pellets from Brazilian biomasses. Env. Sci. Pollut. Res. 2021, 28, 11598–11617. [Google Scholar] [CrossRef] [PubMed]
- Basu, P. Chapter 4—Torrefaction. In Biomass Gasification, Pyrolysis and Torrefaction, 3rd ed.; Basu, P., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 93–154. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Glob. Change Biol. Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Meiirkhanuly, Z.; Koziel, J.A.; Bialowiec, A.; Banik, C.; Brown, R.C. The Proof-of-the Concept of Biochar Floating Cover Influence on Swine Manure pH: Implications for Mitigation of Gaseous Emissions From Area Sources. Front. Chem. 2020, 8, 656. [Google Scholar] [CrossRef]



| Biomass | Moisture Content (%) | Bulk Density (kg/m3) | Volatile * (%) | Ash Content * (%) | Fixed Carbon (%) | pH |
|---|---|---|---|---|---|---|
| Pine chips | 11.2 ± [0.08] | 298.5 ± [2.69] | 85.0 ± [0.04] | 0.47 ± [0.10] | 14.5 ± [0.10] | 4.1 ± [0.02] |
| Pine bark | 17.8 ± [0.49] | 193.7 ± [2.40] | 71.4 ± [1.30] | 0.92 ± [0.30] | 27.9 ± [1.22] | 3.3 ± [0.02] |
| Pellet | 8.4 ± [1.66] | 647.6 ± [14.85] | 77.4 ± [0.64] | 0.69 ± [0.01] | 22.0 ± [0.64] | 4.5 ± [0.30] |
| A. dealbata | 13.6 ± [0.53] | 212.8 ± [36.53] | 82.3 ± [2.14] | 1.65 ± [0.82] | 16.1 ± [1.34] | 4.7 ± [0.20] |
| A. melanoxylon | 17.7 ± [2.93] | 230.6 ± [10.00] | 81.3 ± [2.75] | 2.50 ± [1.05] | 16.3 ± [1.74] | 4.9 ± [0.13] |
| Biochar | Mass Yield (%) | Losses (%) | Mean Temperature of Pyrolysis (°C) | Pyrolysis Time (>300 °C) (min) |
|---|---|---|---|---|
| Pine chips | 54 | 46 | 499.4 ± [160.3] | 100 |
| Pine bark | 75 | 25 | 386.5 ± [97.4] | 70 |
| Pine pellet | 70 | 30 | 504.5 ± [181.3] | 120 |
| A. dealbata | 52 | 48 | 401.4 ± [44.93] | 60 |
| A. melanoxylon | 46 | 54 | 460.0 ± [79.70] | 60 |
| Biochar | Bulk Density (kg/m3) | Volatile * (%) | Ash Content * (%) | Fixed Carbon (%) | pH |
|---|---|---|---|---|---|
| Pine chips | 215.1 ± [9.63] | 35.3 ± [3.03] | 1.28 ± [0.42] | 63.4 ± [3.24] | 5.3 ± [0.09] |
| Pine bark | 139.4 ± [4.58] | 29.3 ± [0.05] | 1.19 ± [0.07] | 69.5 ± [0.03] | 7.6 ± [0.34] |
| Pellet | 384.9 ± [20.00] | 30.1 ± [17.76] | 2.52 ± [0.28] | 67.4 ± [17.49] | 6.5 ± [2.05] |
| A. dealbata | 177.2 ± [18.33] | 39.9 ± [8.58] | 3.70 ± [0.54] | 56.4 ± [9.13] | 6.9 ± [1.28] |
| A. melanoxylon | 155.4 ± [5.39] | 29.1 ± [6.26] | 12.28 ± [6.03] | 58.6 ± [0.47] | 8.8 ± [0.76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Prieto, Ó.; Ortiz Torres, L.; Costas Costas, M.E. Valorization of Forest Biomass Through Biochar for Static Floating Applications in Agricultural Uses. Biomass 2025, 5, 44. https://doi.org/10.3390/biomass5030044
González-Prieto Ó, Ortiz Torres L, Costas Costas ME. Valorization of Forest Biomass Through Biochar for Static Floating Applications in Agricultural Uses. Biomass. 2025; 5(3):44. https://doi.org/10.3390/biomass5030044
Chicago/Turabian StyleGonzález-Prieto, Óscar, Luis Ortiz Torres, and María Esther Costas Costas. 2025. "Valorization of Forest Biomass Through Biochar for Static Floating Applications in Agricultural Uses" Biomass 5, no. 3: 44. https://doi.org/10.3390/biomass5030044
APA StyleGonzález-Prieto, Ó., Ortiz Torres, L., & Costas Costas, M. E. (2025). Valorization of Forest Biomass Through Biochar for Static Floating Applications in Agricultural Uses. Biomass, 5(3), 44. https://doi.org/10.3390/biomass5030044







