1. Introduction
Sri Lanka’s major sugar factories, Pelwatta, Sevanagala, Galoya, and Ethimale, have long been central to the country’s sugar industry, reducing dependence on imported sugar [
1]. Pelwatta, Sevanagala, and Ethimale, located in the Monaragala district, employ modern production methods and foster local economic development by purchasing sugarcane from nearby farmers. Galoya, situated in the Ampara district, is Sri Lanka’s first large-scale sugar factory. Pelwatta and Galoya are the largest facilities, primarily producing sugar, with electricity and ethanol as valuable by-products.
The sugarcane varieties used in Sri Lanka’s production include CO775, SL121, SL330S, and SL925588, with CO775 yielding the highest sugar content. However, the industry faces several challenges, such as inefficiency, outdated machinery, and fluctuating yields resulting from inconsistent rainfall and poor soil management. Despite these obstacles, the factories have made significant improvements in management and technological practices, enabling continued operations and growth.
The sugar industry generates a variety of waste products, including bagasse from mills, spent wash from distilleries, boiler ash from boilers [
2], press mud from processing houses, and cane tops from cultivation. Managing these diverse by-products presents significant challenges, as they are difficult to dispose of properly, often resulting in environmental pollution and high treatment costs [
3]. Addressing these issues requires advanced technology and infrastructure to implement efficient waste management strategies, such as bioenergy generation and composting. Notably, these waste materials hold considerable potential for the production of bio-based energy and electricity [
4,
5].
Proper waste management plays a vital role in the sustainable development of a country by protecting the environment, conserving natural resources, and enhancing public health [
6,
7]. In contrast, improper waste disposal can lead to serious environmental consequences, such as air, water, and soil pollution, which in turn harm ecosystems and human well-being. Environmentally friendly approaches, including recycling, composting, and waste-to-energy technologies, are essential for effective waste collection, recycling, and disposal. Such methods are crucial not only to reduce landfill accumulation, but also to limit the overuse of natural resources and mitigate greenhouse gas emissions.
Biochar is a carbon-rich material produced through pyrolysis of organic biomass, such as agricultural waste or wood, under low-oxygen conditions. The pyrolysis process can be classified into three main types: slow pyrolysis, fast pyrolysis, and flash pyrolysis [
8]. Biochar has a wide range of applications, most notably in agriculture, where it improves soil fertility, structure, and water retention. It provides essential nutrients for plant growth and, when combined with compost, stabilizes nutrients, reduces leaching, and prolongs nutrient availability [
9]. Additionally, biochar enhances soil fertility and crop yields while decreasing the need for chemical fertilizers. It also contributes to greenhouse gas reduction by increasing soil organic carbon and promoting carbon sequestration—key components of sustainable agriculture and healthy soil ecosystems [
10].
Organic compost is a natural fertilizer derived from decomposed plant and animal matter through microbial activity in the presence of oxygen, heat, and moisture. This process enhances soil structure, water retention, and fertility [
11]. Widely used in agriculture and gardening, organic compost supports soil health and plant growth, offering an eco-friendly alternative that aligns with sustainable development goals [
12]. Compost made from natural sources supplies essential nutrients such as nitrogen, phosphorus, and potassium, while also improving soil structure and its ability to retain moisture and nutrients—benefits not offered by synthetic fertilizers.
Biochar improves the nutrient retention of compost, promotes microbial activity, and enhances the carbon content of the final product [
13]. These effects contribute to improved soil quality, reduced reliance on chemical fertilizers, and increased crop resilience under environmental stresses, such as water scarcity [
14]. Furthermore, biochar helps stabilize soil pH, thereby fostering more favorable growing conditions [
15].
Sugarcane factories produce substantial amounts of waste daily, and improper disposal can result in severe ecological damage. However, through effective composting, this waste can be transformed into valuable organic matter, improving waste management while preventing environmental pollution [
16]. Composting sugar industry by-products—such as bagasse, spent wash, boiler ash, and cane tops—offers a promising solution for both sustainable agriculture and environmental management [
17].
Converting organic waste into compost represents a viable strategy for the sugar industry. It can generate additional revenue while reducing dependence on chemical fertilizers. Currently, some compost is produced by combining filter mud with other waste materials in sugar factories. However, a significant amount of bagasse is discarded, especially in facilities like Pelwatta and Galoya, where no effective solution for its reuse has been implemented. There is strong potential to incorporate bagasse into compost production. Moreover, integrating boiler ash and spent wash with bagasse could yield an even richer compost [
18]. The addition of nutrient-dense cane tops—often left unused in fields—can further improve compost quality. Producing biochar from bagasse and blending it with compost can result in a highly effective, nutrient-rich organic fertilizer.
Bagasse, being fibrous, improves soil aeration, structure, and water retention when composted, thereby minimizing the risk of groundwater contamination through adsorption of substances such as heavy metals, pesticides, and organic contaminants. [
19]. Boiler ash contributes micronutrients that enhance soil fertility. While spent wash is a potentially harmful liquid waste from distilleries, it contains many beneficial micronutrients. Sugarcane tops, though slow to decompose, encourage microbial activity and contribute to organic matter when composted.
Organic fertilizer manufacturing using waste from the sugar industry offers a sustainable waste management approach that significantly reduces environmental pollution and decreases reliance on chemical fertilizers. By converting materials like bagasse, spent wash, boiler ash, and cane tops into nutrient-rich compost, this process improves soil fertility through increased organic content, microbial activity, and nutrient availability. Continued application enhances soil structure, water retention, and long-term agricultural sustainability. Despite the vast volumes of organic waste produced by Sri Lanka’s sugar industry, much of it remains underutilized or improperly disposed of, leading to environmental and soil degradation. Meanwhile, Sri Lanka faces fertilizer shortages and declining soil health, highlighting the urgent need for cost-effective, organic alternatives. This study aims to evaluate the potential of biochar-enriched compost derived from sugar industry by-products as a solution to these challenges. By scientifically assessing the nutrient composition and effectiveness of these organic fertilizers, this research supports the advancement of eco-friendly, high-quality compost and promotes long-term agricultural sustainability.
2. Methodology
2.1. Composting Process
The composting trial evaluated six organic material combinations (T1–T6,
Table 1) for organic fertilizer production through aerobic decomposition of bagasse, spent wash, boiler ash, and cane tops under controlled conditions. Composting was conducted in single batches due to practical constraints. Standardized 1 m
3 pits were used per formulation with adequate aeration and drainage, where treatment materials were homogenized and layered for uniform decomposition. Throughout the eight-week process, moisture was maintained at 50–60% through periodic watering, while piles were manually turned every 5–7 days to optimize microbial activity. Moisture content was determined using gravimetric analysis with a Humimeter G110 moisture analyzer (Schaller, model G110). The device heats samples in a halogen chamber and measures the weight loss to calculate moisture content. It offers high precision (0.005 g) and a weighing range of 110 g. Measurements were performed at 105 °C and repeated in triplicate for accuracy.
Key parameters, including temperature (measured daily via probe thermometer, with optimal decomposition occurring at 45–55 °C) and pH (monitored weekly), were observed to assess progression. Compost maturity was assessed using a combination of physical, chemical, and biological indicators. The C/N ratio was 15:1, and the pH ranged from 6.5 to 8.0, which are both within the typical range for mature compost. The compost also exhibited a neutral, earthy odor, indicating stability, and a seed germination test showed over 80% germination, confirming biological maturity. These combined factors demonstrated that the compost was mature, stable, and suitable for agricultural use.
2.2. Biochar Preparation and Analysis
Biochar production from paddy husks includes pyrolysis, a thermochemical reaction that takes place under oxygen-deficient conditions. In a laboratory-scale pyrolysis reactor, paddy husks (paddy processing waste) are carbonized at a temperature of 430 °C with a residence time of 40 min. Controlled pyrolysis of organic matter results in biochar, bio-oil, and syngas [
20]. The measured physical and chemical properties, instruments, and procedures are shown in
Table 1.
Table 1.
Measurement of the physical and chemical properties of biochar.
Table 1.
Measurement of the physical and chemical properties of biochar.
| Property | Measurement Method |
|---|
| Particle Size | Sieve Analysis |
| Bulk Density | Mass/Volume Calculation |
| Porosity | Archimedes Principle |
| pH | 1:1 Biochar-to-Water Ratio |
| Cation Exchange Capacity (CEC) | Ammonium Acetate Method |
| Total Organic Carbon (TOC) | Walkley–Black Method |
| Ash Content | Loss on Ignition |
| Elemental Composition | EA3100-Series CHNSP-O analyzer (Manufactured by EuroVector Srl in Pavia, Italy) ASTM E1757 [21] |
| Specific Surface Area | Brunauer–Emmett–Teller (BET) Method
BelSorp Max Analyzer (Manufactured by Bel Japan Inc., Osaka, Japan) ASTM D3663-20 [22] |
2.3. Formulation of Organic Fertilizer
Three biochar proportions (0%, 10%, and 20% by weight) were blended with six distinct compost mixtures. The resulting biochar–compost blends were homogenized with topsoil (collected from a single field station) at a 1:1 ratio (w/w) to prepare the cultivation media.
Growing Medium: Uniform polybags (30 cm height × 15 cm diameter, black polythene) were filled with 50% topsoil and 50% biochar-enriched compost (T7–T18,
Table 1).
Replicates and Planting: Twenty treatment compositions (including controls) were prepared, each with three replicates. One commercially sourced maize seed (Jet 999 (NK 7328) by Syngenta) was sown per polybag under standardized conditions. A summary of the treatment compositions is provided in
Table 2.
2.4. Greenhouse Experimental Study
The experiment was conducted in a greenhouse (
Figure 1) located at the Faculty of Agricultural Sciences, Sabaragamuwa University of Sri Lanka, Belihuloya. The selected experimental design was Randomized Complete Block Design (RCBD). The plants were established as three blocks with respect to the three replicates. The distance between each pair of plants was 30 cm. The plants were grown with a humidity of 70.0% under controlled conditions in the greenhouse. The irrigation water supply was 100 mL per plant per day. Continuous monitoring of the greenhouse micro-climate was implemented, with hourly logging of temperature and relative humidity to minimize uncontrolled growth responses.
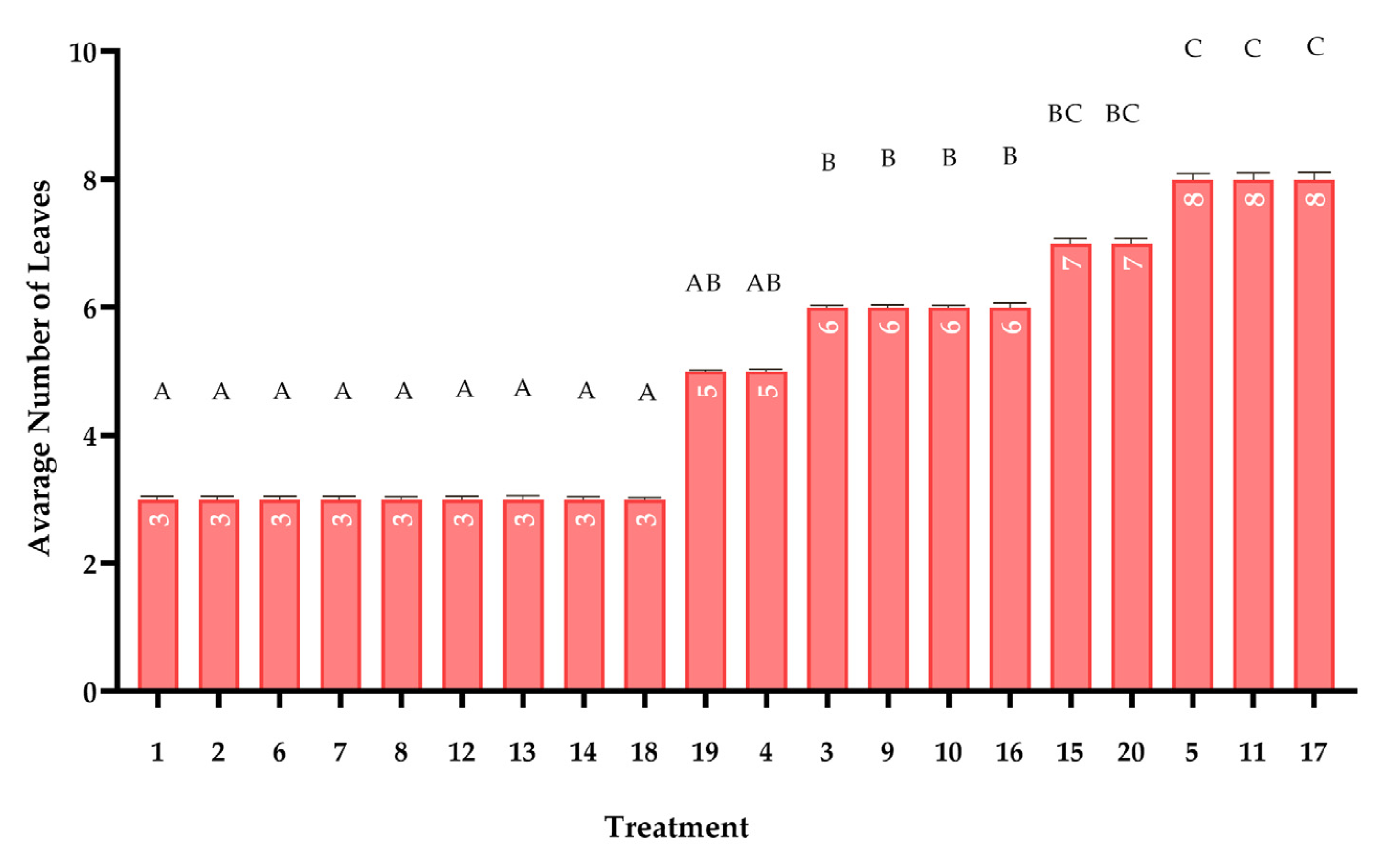
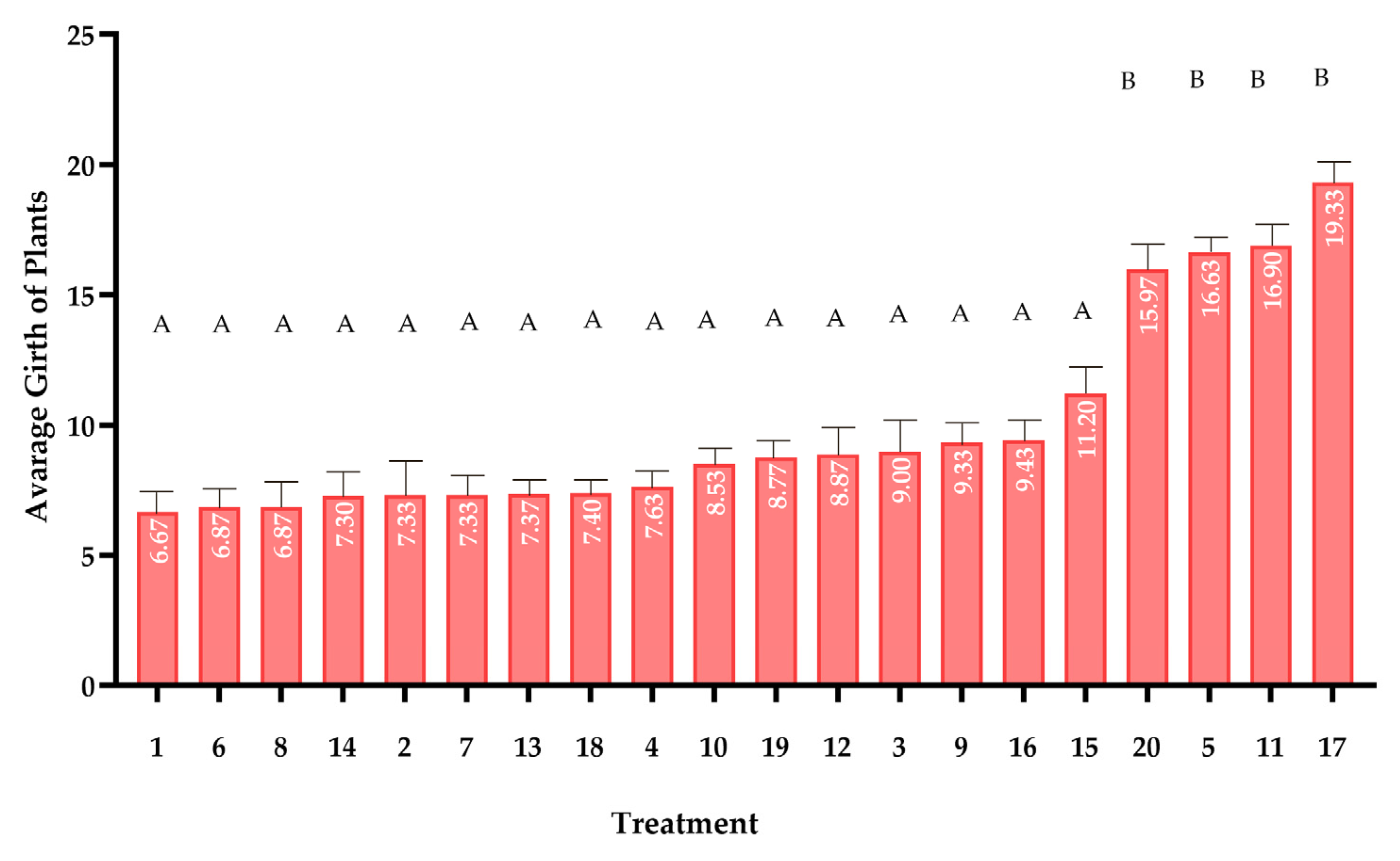
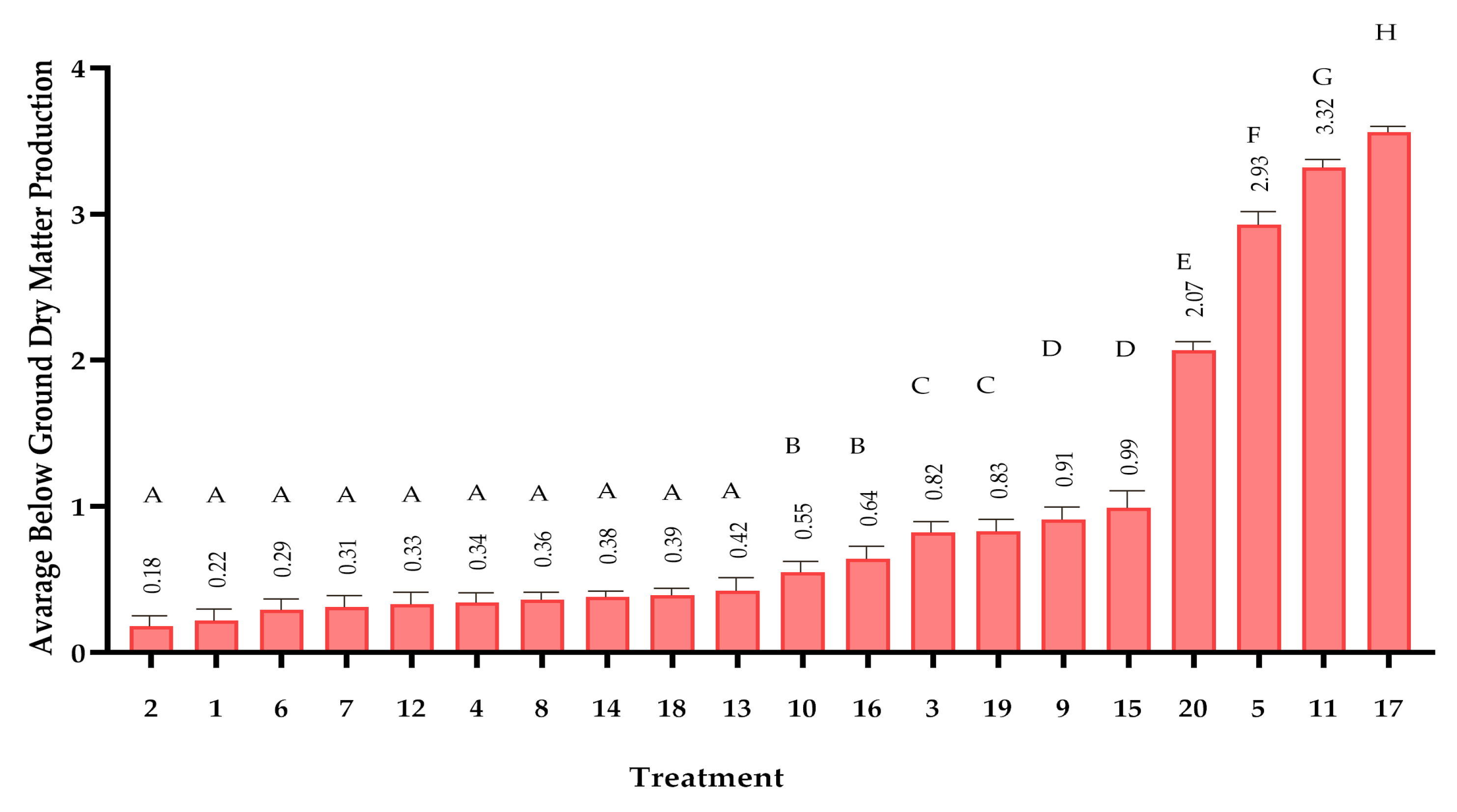
2.5. Plant Growth Study
The factors studied were height, girth, number of leaves, and dry matter content. For convenient data acquisition, some data were obtained every other day, and some data were obtained once every several days. The growth of maize plants was studied for a period of 45 days, which corresponds to early vegetative growth of maize [
23].
Plant Height Measurement: Plant height was measured every 48 h throughout the 45-day study period using a precision graduated scale. Measurements were taken from the soil surface to the apical meristem (highest point of the crown), with an accuracy of ±0.5 mm. This frequent measurement interval was implemented to accurately capture the rapid vertical growth characteristic of maize (
Zea mays L.) during early developmental stages. All measurements were conducted at a consistent daylight hour (08:00–10:00) to minimize diurnal variation effects [
24].
Stem Girth Measurement: Stem diameter growth was monitored at 4-day intervals using a digital caliper with 0.01 mm resolution. Circumference measurements were standardized at 2 cm above the soil surface, corresponding to the first visible node position in juvenile plants. The 4-day interval was empirically determined to be optimal for detecting significant diametric growth while minimizing measurement-induced stress. Each measurement was performed by gently wrapping a non-stretchable thread around the stem and subsequently measuring its length against a precision ruler [
25].
Leaf Count Assessment: Leaf emergence was recorded every 72 h following a standardized protocol specific to maize morphology. Only fully emerged leaves (those completely visible above the ligule of the preceding leaf) were counted, excluding any partially unfurled or senescing leaves. This conservative approach accounted for maize’s distinct leaf development pattern, where new leaves emerge from the whorl gradually. Counting was performed during morning hours when leaves were fully turgid and clearly distinguishable [
26].
Dry Matter Determination: Destructive biomass sampling was conducted at study termination following a 6-hour oven-drying protocol at 105 °C (±2 °C). Entire plants were carefully excavated, with roots gently washed to remove soil particles. After separation into root and shoot components, samples were dried to constant mass (verified by consecutive weighing at 2-hour intervals). Dry weights were measured using an analytical balance (0.0001 g precision) in a temperature-controlled laboratory environment [
27].
2.6. Statistical Analysis
In this study, the effects of 20 compost treatments on maize growth were analyzed using Repeated Measures ANOVA, with alpha (α) = 0.05 to determine statistical significance. The analysis considered multiple growth measurements taken overtime. To ensure the validity of the tests, Shapiro–Wilk test was used to check the normality of the data, and Levene’s test was used to assess the homogeneity of variance. If the assumptions were met, Tukey’s HSD test was conducted for pairwise comparisons to identify which compost treatments significantly differed from each other. All analyses were performed using R software (version 4.5.0), ensuring the results were both robust and reproducible.
2.7. Nutrient Composition Assessment
After evaluating maize plant growth, the compost mixture yielding the highest agricultural output was selected, and its nutrient composition was analyzed to assess its suitability for crop production. The analysis included quantification of macro and micronutrients—nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), manganese (Mn), iron (Fe), zinc (Zn), boron (B), and chlorine (Cl)—along with critical chemical properties such as pH, electrical conductivity (EC), and carbon-to-nitrogen (C/N) ratio. The analysis was performed by the Sugarcane Research Institute Main Research Center, Dakunu Ela Road, Uda Walawe (70190), Sri Lanka. To ensure precise results, various laboratory techniques and instruments were used, as mentioned in
Table 3.
Soil testing was conducted to assess the nutrient and micronutrient levels before and after the addition of biochar. The soil samples were collected from the experimental field at a depth of 0–20 cm, which is the root zone for most crops. The pH of the soil was measured using a 1:1 soil-to-water ratio with a pH meter to determine the soil’s acidity or alkalinity. Cation exchange capacity (CEC), which indicates the soil’s ability to hold onto essential nutrients, was determined using the ammonium acetate method. Organic carbon content was estimated using the Walkley–Black method, which involves oxidation of organic matter in the soil. For nutrient analysis, the concentrations of nitrogen (N), phosphorus (P), potassium (K), sodium (Na), magnesium (Mg), calcium (Ca), and zinc (Zn) were quantified using Inductively Coupled Plasma (ICP) Spectrometry after digesting the soil samples with nitric acid. This method ensures precise and reliable measurement of nutrient levels in the soil. The data obtained from the pre- and post-biochar soil samples were compared to evaluate the impact of biochar addition on soil fertility.
2.8. Cost–Benefit Analysis of Traditional Waste Management vs. Composting
This study evaluates the economic feasibility of composting with biochar enrichment compared to traditional waste management in the Sri Lankan sugar industry. The methodology consists of data collection, cost analysis, revenue estimation, sensitivity analysis, and return-on-investment (ROI) calculation over a 5-year period. The cost comparison considers collection, transportation, processing, labor, storage, pollution control, and revenue generation.
The ROI analysis uses an 8% annual discount rate, reflecting the industry’s average cost of capital, to account for inflation and opportunity costs. The composting infrastructure’s salvage value is estimated at 10% of its initial cost by Year 5, based on industry-standard depreciation. Sensitivity analysis shows labor costs may vary by ±15%, due to wage changes and operational efficiencies, while compost prices could range from
$46 to
$108 per ton, reflecting market volatility and demand fluctuations [
34].