Catalytic Biomass Gasification for Syngas Production: Recent Progress in Tar Reduction and Future Perspectives
Abstract
1. Introduction
2. Biomass Resources
2.1. Energy Crops
2.2. Agricultural Residues
2.3. Forest Residues
2.4. Wood Processing Residues
2.5. Algae
2.6. Wet Waste
2.7. Influence of Types of Biomass on Gasification Performance and Tar Formation
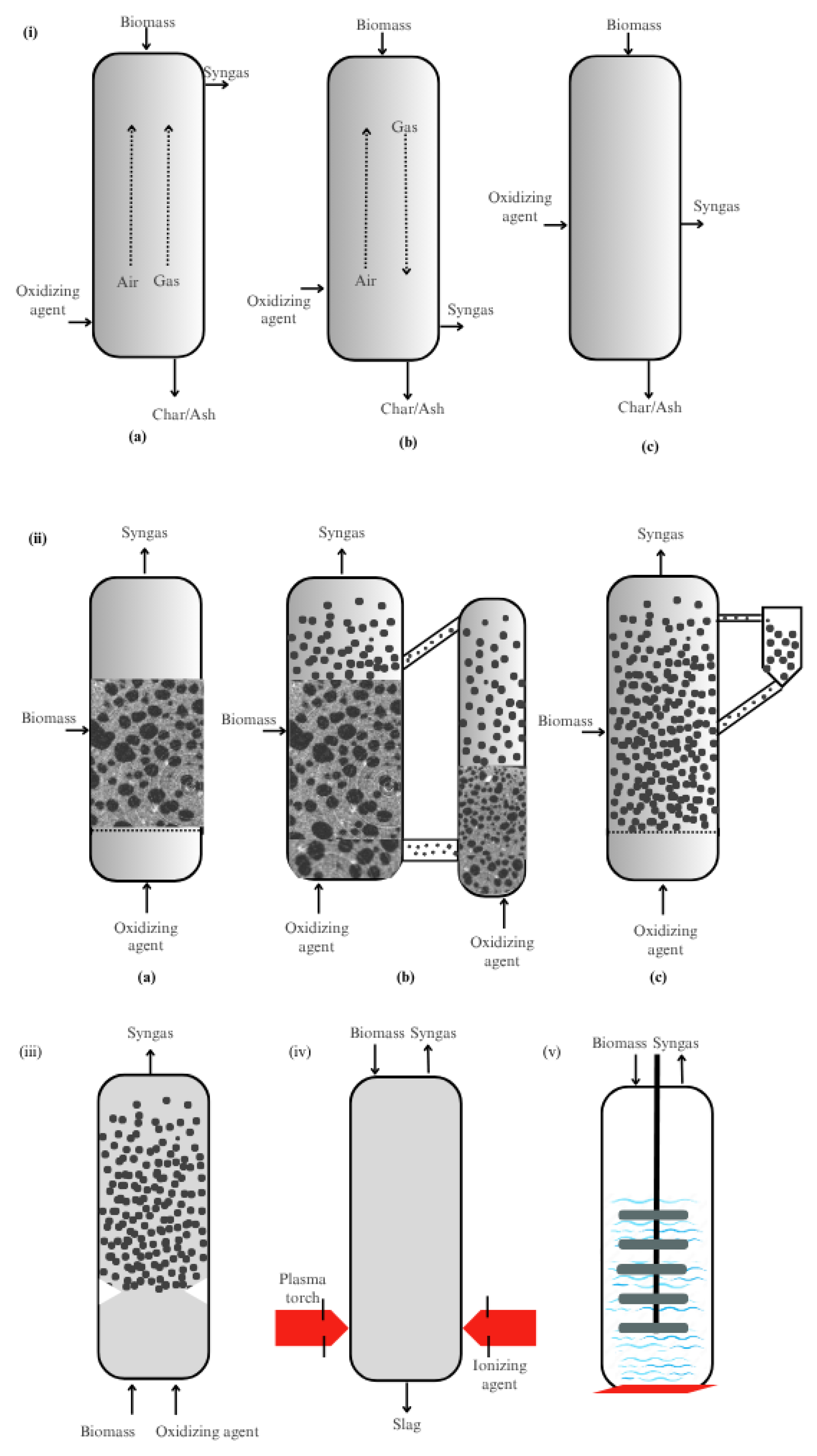
3. Biomass Gasifiers
3.1. Fixed Bed Gasifiers
3.2. Fluidized Bed Gasifiers
3.2.1. Bubbling Fluidized Bed (BFB) Gasifiers
3.2.2. Circulating Fluidized Bed (CFB) Gasifiers
3.2.3. Dual Fluidized Bed (DFB) Gasifiers
3.3. Entrained Flow (EF) Gasifiers
3.4. Plasma Gasifiers
3.5. Hydrothermal Gasifiers
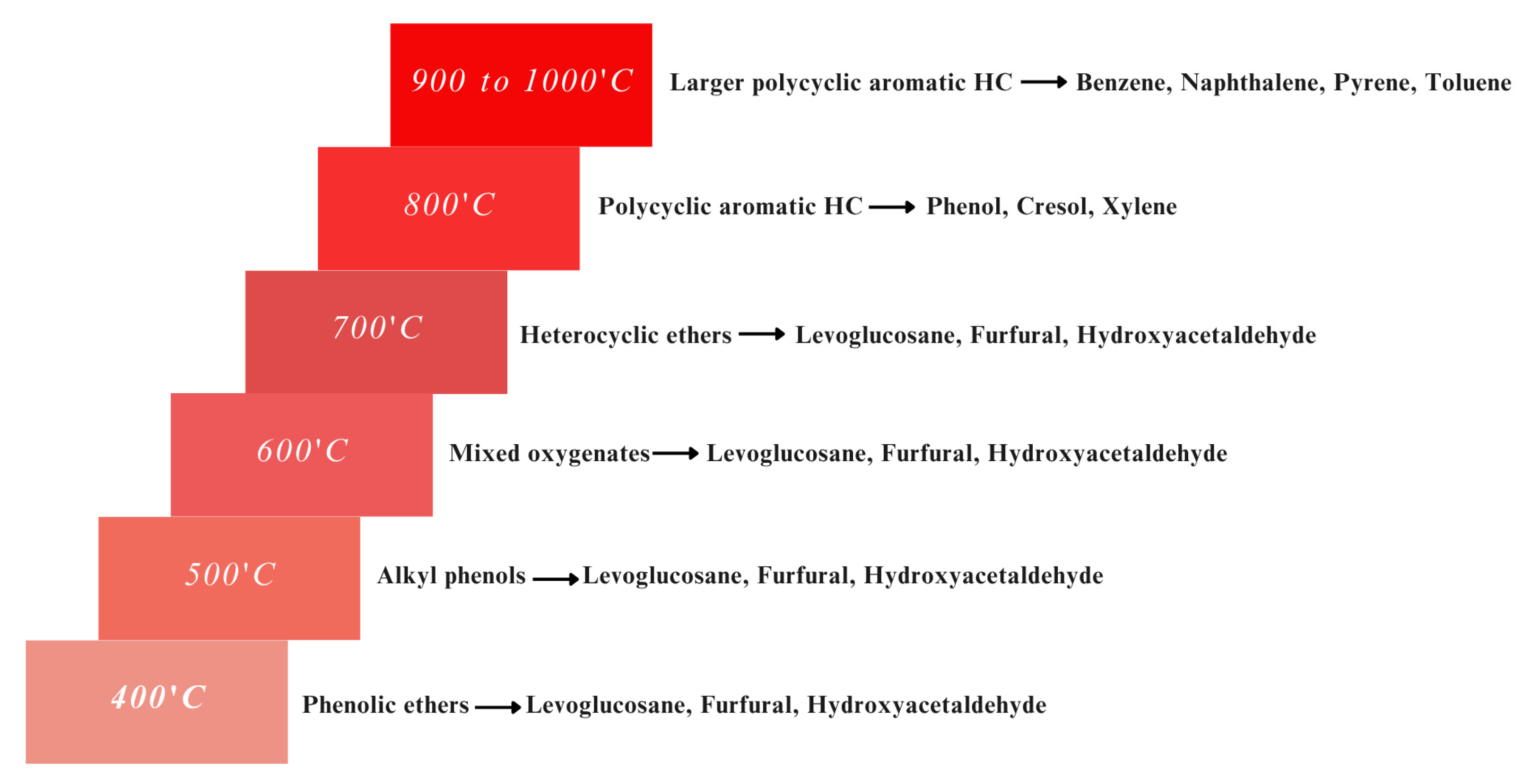
4. Tar Cracking Mechanisms in Catalytic Biomass Gasification
5. Catalytic Biomass Gasification
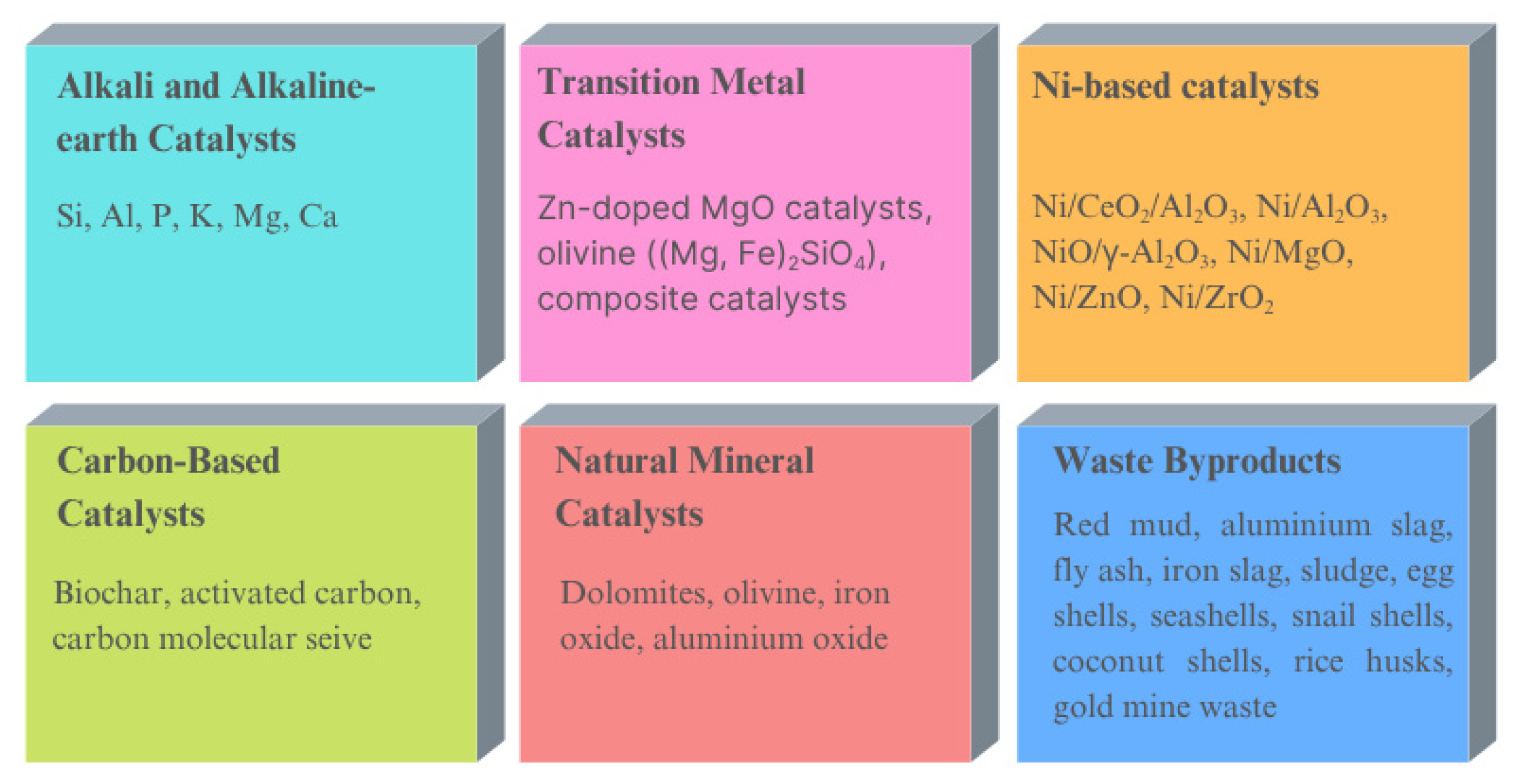
6. Types of Catalysts Used in Biomass Gasification
6.1. Alkali and Alkaline Earth Catalysts
6.2. Transition Metal Catalysts
6.3. Ni-Based Catalysts
6.4. Carbon-Based Catalysts
6.5. Natural Mineral Catalysts
6.6. Catalysts Derived from Waste Byproducts
7. Yield and Composition of Syngas from Catalytic Biomass Gasification
| Biomass | Reactor | Oxidation Agent | Temp, °C | Catalyst | Catalyst Loading | Yield | HV, MJ Nm−3 | Syngas Composition, % | Other Details | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CH4 | CO | CO2 | ||||||||||
| Banana | Fixed bed | Steam | 368 | Ni/Al2O3 | 2.5% w/w | Syngas: 178 mg g−1 | 15.21 (LHV) | 51.8 | 0.44 | 0 | 22.54 | - | [72] |
| Microalgae (C. vulgaris) | Horizontal tubular reactor | Air 20 mL min−1 | 851 | ZnOeNieCaO | 16.4 wt% | Gas: 83.34 wt%; Char: 10.34 wt%; Tar: 6.32 wt% | 14.86 (LHV) | 48.95 | 10.12 | 18.27 | 22.64 | Residence Time: 28.8min | [73] |
| Pine sawdust | Fluidized bed | SBR: 1.0 | 650 | Fe-based composite | CBR: 1.2 | Gas: 60.4% Char: 37.1% Tar: 2.5% | - | 42.2 | 16.5 | 22.5 | 16.4 | - | [65] |
| Citrus peel residues | Fixed bed | SBR: 1.5 wt%/wt% | 750 | Dolomite | - | - | - | 63 | 1 | 16 | 20 | CCG: 54.9% HCE:54.1% | [74] |
| Pine sawdust | Fluidized bed | Air–steam Air: 0.65 Nm3 h−1 Steam: 0.4 ER: 0.3, 0.2 SBR: 0.85, 0.75 | 800 | Dolomite | 65 g | Gas: 1.54 Nm3kg−1; Tar: 19.05 g kg−1 | - | 38.38 | 7.02 | 24.89 | 27.62 | - | [56] |
| 56 g | Gas: 1.56 Nm3kg−1; Tar: 13.85 g kg−1 | - | 38.13 | 7.48 | 26.06 | 26.20 | |||||||
| Pine sawdust | Downstream fixed bed | Air–steam Air: 0.65 N m3 h−1 Steam: 0.4 ER: 0.3 SBR: 0.85 | 800 | Dolomite | 56 g | Gas: 2.24 Nm3 kg−1 biomass; Tar: 4.29 g kg−1 biomass | - | 50.23 | 4.3 | 12.3 | 32.56 | Residence Time: 28.8 min | |
| Wood residue | Fluidized bed | ER:0.17 SBR: 0.71 wt%/wt% | 823 | Ni/Al2O3 | 40% | Gas: 90.33%; Tar: 4.72%; Char: 04.95% | - | 36.17 | 11.12 | 24.26 | 24.25 | Residence Time: 26 min; CCE: 86.17%; CGE: 56.24% | [19] |
| Wood residue | Fluidized bed | ER: 0.17 SBR: 0.71 wt%/wt% | 823 | Ni/CeO2/Al2O3 | 40% | Gas: 96.84%; Tar: 2.94%; Char: 0.78% | - | 42.52 | 11.47 | 23.04 | 18.10 | Residence Time: 44 min; CCE: 93.65%; CGE: 71.6% | |
| Wood chips/coconut shell | Downdraft gasifier | Air flow rate: 400 L min−1 BR: 70:30 | - | Dolomite | 10% | - | 4.96 (HHV) | 10 | 2 | 22.5 | 10 | - | [75] |
| Enteromorphain testinalis | Fluidized bed | ER: 0.14 SBR: 0.5–1.0 wt% | 800–1000 | Dolomite | - | Gas: 90.2%; Tar: 4.3%; Char: 5.5% | 11.6 (HHV) | 49 | 1 | 27 | 23 | Residence Time: 50 min; CCE: 60.8%; GE: 71.5% | [70] |
| Enteromorphain testinalis | Fluidized bed | ER: 0.14 SBR: 0.5–1.0 wt% | 800–1000 | Olivine | - | Gas: 88.5%; Tar: 5.5%; Char: 6.0% | 12.5 (HHV) | 48 | 1 | 25 | 26 | Residence Time: 50 min; CCE: 58%; GE: 69.1%; | |
| Enteromorphain testinalis | Fluidized bed | ER:0.14 SBR: 0.5–1.0 wt% | 800–1000 | Lime | - | Gas: 85.5%; Tar: 6.1%; Char: 8.4% | 14.2 (HHV) | 49 | 1 | 30 | 20 | Residence Time: 50 min; CCE: 51.8%; GE: 60.5% | |
| Pine sawdust | Tube furnace reactor | - | 850 | Nickel-based catalysts | - | Gas: 2.78 Nm3kg−1 biomass | 9.6 (LHV) 10.9 (HHV) | 2.25 Nm3 kg−1 | 0.54 Nm3 kg−1 | - | - | - | [69] |
| Wood chips of red pine | Two-stage fluidized bed reactor | SBR: 3 mol/mol | 600° C | Ni/Al2O3 | - | Gas: 62 mmol/g-daf | 14 (LHV) | 36.2 | 2.6 | 10.3 | 14 | - | [76] |
| Wood chips of red pine | Two-stage fluidized bed reactor | SBR: 3 mol/mol | 600 | Ni/BCC | - | Gas: 2 Nm3 kg−1; Tar: 60 mg Nm−3 | 14 (LHV) | 46.6 | 4.3 | 21.6 | 16 | - | |
| Pig manure compost | Two-stage fluidized bed reactor | SBR: 3 mol/mol | 600 | Ni/BCC | - | Gas: 54 mmol/g-daf | 14 (LHV) | 26 | 5 | 12 | 11 | - | |
| Rice husk | Bubbling fluidized bed | ER: 0.10 | 850 | Calcined dolomite | - | Gas: 80.4 wt%; Char: 11.2 wt%; Tar: 7.5 wt% | 12.2 (LHV) | 34.1 | 4.9 | 35.0 | 22.3 | - | [77] |
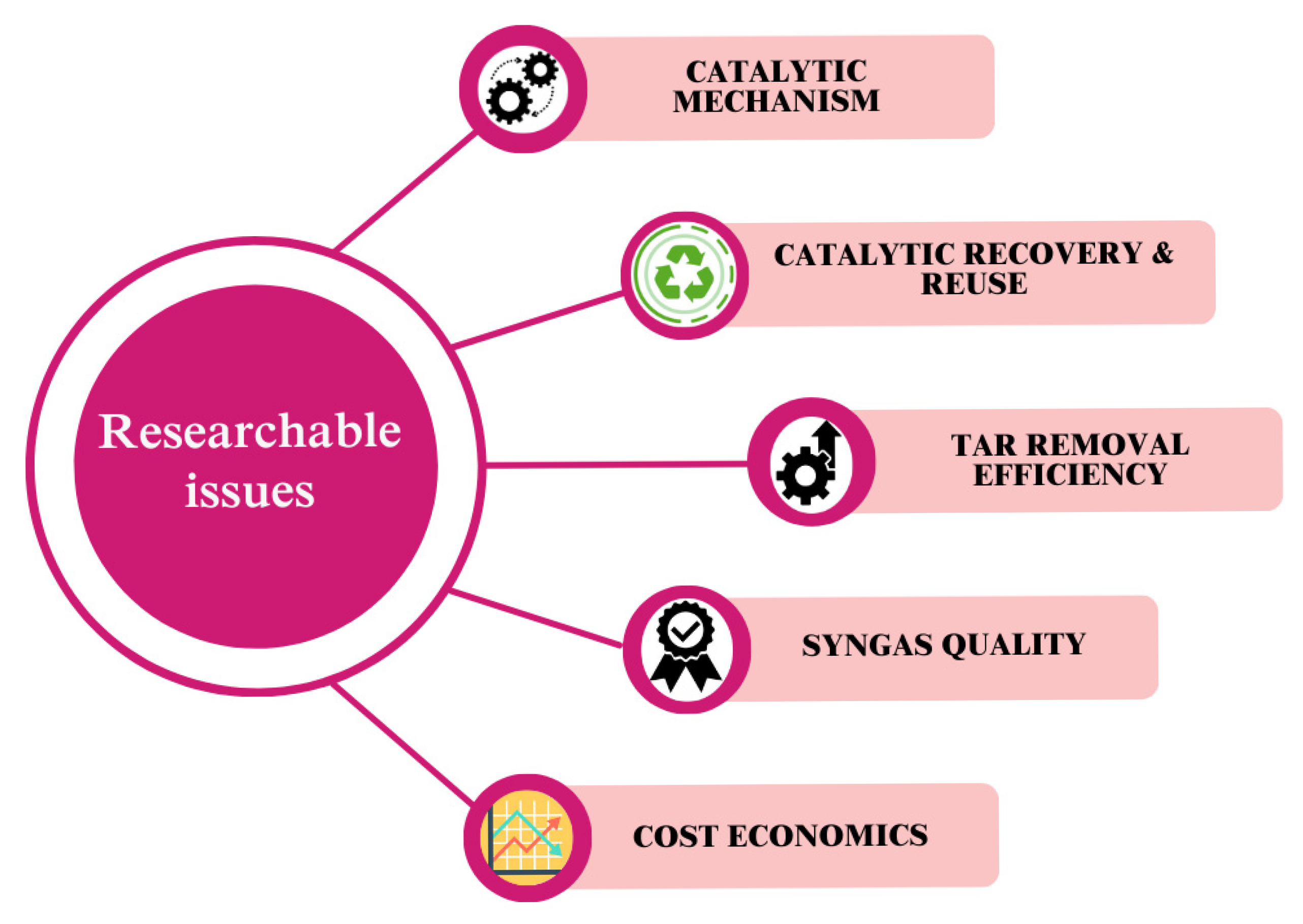
8. Current Status and Future Perspectives
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BFB | Bubbling Fluidized Bed |
| CFB | Circulating Fluidized Bed |
| DFB | Dual Fluidized Bed |
| EF | Entrained Flow |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| CBR | Catalyst-to-Biomass Ratio |
| SBR | Steam-to-Biomass Ratio |
| CBR | Catalyst-to-Biomass Ratio |
| HV | Heating Value |
| LHV | Lower Heating Value |
| HHV | Higher Heating Value |
| CCE | Carbon Conversion Efficiency |
| GE | Gasification Efficiency |
| CGE | Cold Gas Efficiency |
| HCE | Hydrogen Conversion Efficiency |
References
- Wang, D.; Jiang, P.; Zhang, H.; Yuan, W. Biochar production and applications in agro and forestry systems: A review. Sci. Total Environ. 2020, 723, 137775. [Google Scholar] [CrossRef] [PubMed]
- Barot, S. Biomass and bioenergy: Resources, conversion and application. In Renewable Energy for Sustainable Growth Assessment; Wiley: Hoboken, NJ, USA, 2022; pp. 243–262. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Guran, S. Thermochemical conversion of biomass. In Practices and Perspectives in Sustainable Bioenergy: A Systems Thinking Approach; Springer: New Delhi, India, 2020; pp. 159–194. [Google Scholar] [CrossRef]
- Zhang, X.; Brown, R.C. Introduction to thermochemical processing of biomass into fuels, chemicals, and power. In Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power; Wiley: Hoboken, NJ, USA, 2019; pp. 1–16. [Google Scholar] [CrossRef]
- IEA Bioenergy. Gas Analysis in Gasification of Biomass and Waste. In Guideline Report; International Energy Agency: Paris, France, 2018; Volume 1, Available online: https://www.ieabioenergy.com/wp-content/uploads/2018/09/IEA-Bioenergy-Task-33-gas-analysis-report-Document-1-1.pdf (accessed on 11 April 2025).
- Ramesh, D.; Muniraj, I.K.; Thangavelu, K.; Karthikeyan, S. Chemicals and fuels production from agro residues: A biorefinery approach. In Sustainable Approaches for Biofuels Production Technologies: From Current Status to Practical Implementation; Springer: Cham, Switzerland, 2019; Volume 7, pp. 47–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghaly, A.E.; Li, B. Availability and Physical Properties of Residues from Major Agricultural Crops for Energy Conversion through Thermochemical Processes. Am. J. Agric. Biol. Sci. 2012, 7, 312–321. [Google Scholar] [CrossRef]
- Koçar, G.; Civaş, N. An overview of biofuels from energy crops: Current status and future prospects. Renew. Sustain. Energy Rev. 2013, 28, 900–916. [Google Scholar] [CrossRef]
- López-Bellido, L.; Wery, J.; López-Bellido, R.J. Energy crops: Prospects in the context of sustainable agriculture. Eur. J. Agron. 2014, 60, 1–12. [Google Scholar] [CrossRef]
- Porichha, G.K.; Hu, Y.; Rao, K.T.V.; Xu, C.C. Crop residue management in India: Stubble burning vs. other utilizations including bioenergy. Energies 2021, 14, 4281. [Google Scholar] [CrossRef]
- Panwar, N.L.; Paul, A.S. An overview of recent development in bio-oil upgrading and separation techniques. Environ. Eng. Res. 2021, 26, 200382. [Google Scholar] [CrossRef]
- Desikan, R.; Rakesh, S.; Subburamu, K. Algae-derived materials and pathways for applications in the automobile industries. In Algae Mater; Academic Press: Cambridge, MA, USA, 2023; pp. 189–202. [Google Scholar] [CrossRef]
- Raheem, A.; Abbasi, S.A.; Mangi, F.H.; Ahmed, S.; He, Q.; Ding, L.; Memon, A.A.; Zhao, M.; Yu, G. Gasification of algal residue for synthesis gas production. Algal Res. 2021, 58, 102411. [Google Scholar] [CrossRef]
- Prasad, S.; Ingle, A.P. Impacts of sustainable biofuels production from biomass. In Sustainable Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–346. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Li, Z.; Chen, D. Characteristics of tar formation during cellulose, hemicellulose and lignin gasification. Fuel 2014, 118, 250–256. [Google Scholar] [CrossRef]
- Naryanto, R.F.; Enomoto, H.; Vo Cong, A.; Fukadu, K.; Zong, Z.; Delimayanti, M.K.; Chunti, C.; Noda, R. The effect of moisture content on the tar characteristic of wood pellet feedstock in a downdraft gasifier. Appl. Sci. 2020, 10, 2760. [Google Scholar] [CrossRef]
- Peng, W.X.; Wang, L.S.; Mirzaee, M.; Ahmadi, H.; Esfahani, M.J.; Fremaux, S. Hydrogen and syngas production by catalytic biomass gasification. Energy Convers. Manag. 2017, 135, 270–273. [Google Scholar] [CrossRef]
- Bonilla, J.; Gordillo, G.; Cantor, C. Experimental gasification of coffee husk using pure oxygen-steam blends. Front. Energy Res. 2019, 7, 127. [Google Scholar] [CrossRef]
- Pandey, B.; Sheth, P.N.; Prajapati, Y.K. Tar cracking enhancement by air sparger installation in the combustion zone of the downdraft gasifier. Biomass Bioenergy 2020, 161, 106470. [Google Scholar] [CrossRef]
- James, A.M.R.; Castorena, C.; Yuan, W. Modeling product distribution of top-lit updraft gasification. BioResources 2021, 16, 3245–3263. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Haridasan, T.M.; Reed, T.B.; Bai, R.K. Experimental investigation and modelling study of long stick wood gasification in a top lit updraft fixed bed gasifier. Fuel 2007, 86, 2846–2856. [Google Scholar] [CrossRef]
- Sarker, S.; Nielsen, H.K. Assessing the gasification potential of five woodchips species by employing a lab-scale fixed-bed downdraft reactor. Energy Convers. Manag. 2015, 103, 801–813. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Decomposition of biomass gasification tar model compounds over waste tire pyrolysis char. Waste Dispos. Sustain. Energy 2022, 4, 75–89. [Google Scholar] [CrossRef]
- Lan, W.; Ding, H.; Jin, X.; Yin, D.; Wang, Y.; Ji, J. Catalytic biomass gasification of sawdust: Integrated experiment investigation with process modeling and analysis. Int. J. Low-Carbon. Technol. 2022, 17, 482–487. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2010; p. 364. Available online: https://www.academia.edu/36068327/Biomass_Gasification_and_Pyrolysis_Practical_Design_and_Theory (accessed on 18 May 2025).
- Subramanian, P.; Sampathrajan, A.; Venkatachalam, P. Fluidized bed gasification of select granular biomaterials. Bioresour. Technol. 2011, 102, 1914–1920. [Google Scholar] [CrossRef]
- González-Vázquez, M.D.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Comparison of the gasification performance of multiple biomass types in a bubbling fluidized bed. Energy Convers. Manag. 2018, 176, 309–323. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-gasification of biomass blends: Performance evaluation in circulating fluidized bed gasifier. Energy 2020, 192, 116682. [Google Scholar] [CrossRef]
- Fuchs, J.; Schmid, J.C.; Müller, S.; Hofbauer, H. Dual fluidized bed gasification of biomass with selective carbon dioxide removal and limestone as bed material: A review. Renew. Sustain. Energy Rev. 2019, 107, 212–231. [Google Scholar] [CrossRef]
- Legonda, I.A. Biomass Gasification Using a Horizontal Entrained-Flow Gasifier and Catalytic Processing of the Product Gas. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2012. Available online: https://orca.cardiff.ac.uk/id/eprint/39280/ (accessed on 19 May 2025).
- Gitanjali, J.; Pugalendhi, S.; Kamaraj, S.; Karthikeyan, S.; Kumar, V.J.F. Feasibility test of agricultural residues through characterization for utilization in plasma gasification. Indian. J. Agric. Sci. 2015, 85, 1534–1539. [Google Scholar] [CrossRef]
- Erdogan, A.A.; Yilmazoglu, M.Z. Plasma gasification of the medical waste. Int. J. Hydrogen Energy 2021, 46, 29108–29125. [Google Scholar] [CrossRef]
- Jarana, M.G.; Sánchez-Oneto, J.; Portela, J.R.; Sanz, E.N.; De La Ossa, E.M. Supercritical water gasification of industrial organic wastes. J. Supercrit. Fluids 2008, 46, 329–334. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; Vo, D.V.N.; SundarRajan, P.; Swathi, M. Co-hydrothermal gasification of Scenedesmus sp. with sewage sludge for bio-hydrogen production using novel solid catalyst derived from carbon-zinc battery waste. Bioresour. Technol. Rep. 2020, 11, 100459. [Google Scholar] [CrossRef]
- Rios, M.L.V.; González, A.M.; Lora, E.E.S.; del Olmo, O.A.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 171, 1–13. [Google Scholar] [CrossRef]
- Pal, A.; Dixit, A.; Srivastava, A.K. Design and optimization of the shape of electrostatic precipitator system. Mater. Today Proc. 2021, 47, 3871–3876. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J.; Abatzaglou, N. Biomass Gasifier ”Tars”: Their Nature, Formation, and Conversion; NREL: Golden, CO, USA, 1998; p. 204. Available online: https://docs.nrel.gov/docs/fy99osti/25357.pdf (accessed on 19 May 2025).
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.D.R.A.; Zhang, L.; Wang, R.; Bi, X.; Olazar, M. A comprehensive review of primary strategies for tar removal in biomass gasification. Energy Convers. Manag. 2023, 276, 116496. [Google Scholar] [CrossRef]
- Mandal, S.; Daggupati, S.; Majhi, S.; Thakur, S.; Bandyopadhyay, R.; Das, A.K. Catalytic gasification of biomass in dual-bed gasifier for producing tar-free syngas. Energy Fuels 2019, 33, 2453–2466. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, Y.; Zhao, Y.; Sun, S. Mechanism of in-situ catalytic cracking of biomass tar over biochar with multiple active sites. In Applications of Biochar for Environmental Safety; IntechOpen: Rijeka, Croatia, 2020; pp. 57–72. [Google Scholar] [CrossRef]
- Hu, M.; Cui, B.; Xiao, B.; Luo, S.; Guo, D. Insight into the ex situ catalytic pyrolysis of biomass over char supported metals catalyst: Syngas production and tar decomposition. Nanomaterials 2020, 10, 1397. [Google Scholar] [CrossRef]
- Forsberg, O. Catalytic Tar Reforming in Biomass Gasification: Tungsten Bronzes and the Effect of Gas Alkali During Tar Steam Reforming. Master’s Thesis, KTH Royal institute of Technology, Stockholm, Sweden, 2014. Available online: https://www.diva-portal.org/smash/get/diva2:846859/FULLTEXT01.pdf (accessed on 20 May 2025).
- Neubauer, Y. Strategies for tar reduction in fuel-gases and synthesis-gases from biomass gasification. J. Sustain. Energy Environ. Spec. Issue 2011, 67, 71. [Google Scholar]
- Haryanto, A.; Fernando, S.D.; Adhikari, S. Catalytic biomass gasification to produce sustainable hydrogen. In 2006 ASAE Annual Meeting; American Society of Agricultural and Biological Engineers: Joseph, MI, USA, 2006; p. 1. Available online: https://elibrary.asabe.org/abstract.asp?aid=21553 (accessed on 20 May 2025).
- Deepanraj, S.R. Recent advances in direct catalytic thermochemical gasification of biomass to biofuels. Thermochem. Catal. Convers. Technol. Future Biorefineries 2022, 1, 241–295. [Google Scholar] [CrossRef]
- Alptekin, F.M.; Celiktas, M.S. Review on catalytic biomass gasification for hydrogen production as a sustainable energy form and social, technological, economic, environmental, and political analysis of catalysts. ACS Omega 2022, 7, 24918–24941. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Grilc, M.; Likozar, B. Catalytic cracking of biomass-derived hydrocarbon tars or model compounds to form biobased benzene, toluene, and xylene isomer mixtures. Ind. Eng. Chem. Res. 2019, 58, 7690–7705. [Google Scholar] [CrossRef]
- Baker, E.G.; Mudge, L.K. Mechanisms of catalytic biomass gasification. J. Anal. Appl. Pyrolysis 1984, 6, 285–297. [Google Scholar] [CrossRef]
- Arnold, R.A.; Hill, J.M. Catalysts for gasification: A review. Sustain. Energy Fuels 2019, 3, 656–672. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Su, W.; Cai, C.; Liu, P.; Lin, W.; Liang, B.; Zhang, H.; Liu, W. Supercritical water gasification of food waste: Effect of parameters on hydrogen production. Int. J. Hydrogen Energy 2020, 45, 14744–14755. [Google Scholar] [CrossRef]
- Lv, P.; Yuan, Z.; Wu, C.; Ma, L.; Chen, Y.; Tsubaki, N. Bio-syngas production from biomass catalytic gasification. Energy Convers. Manag. 2007, 48, 1132–1139. [Google Scholar] [CrossRef]
- Heberlein, S.; Chan, W.P.; Veksha, A.; Giannis, A.; Hupa, L.; Lisak, G. High temperature slagging gasification of municipal solid waste with biomass charcoal as a greener auxiliary fuel. J. Hazard. Mater. 2022, 423, 127057. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhou, N.; Dai, L.; Deng, W.; Liu, C.; Kirk, C.; Paul, C.; Ruan, R. Applications of calcium oxide–based catalysts in biomass pyrolysis/gasification–a review. J. Clean. Prod. 2021, 291, 125826. [Google Scholar] [CrossRef]
- Shang, S.; Qin, Z.; Lan, K.; Wang, Y.; Zhang, J.; Xiong, T.; He, W.; Li, J. Hydrogen-rich syngas production via catalytic gasification of biomass using Ni/Zr-MOF catalyst. Bioresources 2020, 15, 1716. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, X.; Gu, J.; Shi, J. Co-gasification of coal and biomass blends using dolomite and olivine as catalysts. Renew. Energy 2019, 132, 509–514. [Google Scholar] [CrossRef]
- Agarwal, M.; Sudharsan, J. A comprehensive review on scavenging H2S compounds in oil and gas industry by using nanomaterials. Mater. Today Proc. 2021, 44, 1504–1510. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Nickel-based catalysts for tar reduction in biomass gasification. Biofuels 2014, 2, 451–464. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef]
- Ten Have, I.C.; van den Brink, R.Y.; Marie-Rose, S.C.; Meirer, F.; Weckhuysen, B.M. Using biomass gasification mineral residue as catalyst to produce light olefins from CO, CO2, and H2 mixtures. ChemSusChem 2022, 15, e202200436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, Z.; Wei, D.; Zhang, H.; Lu, W. Application of Fe based composite catalyst in biomass steam gasification to produce hydrogen rich gas. Front. Chem. 2022, 10, 882787. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Yusup, S.; Shahbaz, M. Utilization of bottom ash as catalyst in biomass steam gasification for hydrogen and syngas production. Chem. Eng. Trans. 2016, 52, 1249–1254. [Google Scholar] [CrossRef]
- Shahbaz, M.; Inayat, A.; Patrick, D.O.; Ammar, M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: A review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Mandal, S.; Jena, P.C.; Gangil, S.; Pal, S.; Haydary, J. Ex-situ catalytic cracking of gasifier tar using Ni-loaded pigeonpea stalk char catalyst. Res. Sq. 2022, 1, 21203. [Google Scholar] [CrossRef]
- Xie, Q.; Kong, S.; Liu, Y.; Zeng, H. Syngas production by two-stage method of biomass catalytic pyrolysis and gasification. Bioresour. Technol. 2012, 110, 603–609. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, X.; Lin, S.; Ji, X.; Bai, J.; Xu, M. Syngas production from air-steam gasification of biomass with natural catalysts. Sci. Total Environ. 2018, 645, 518–523. [Google Scholar] [CrossRef]
- Baratieri, M.; Pieratti, E.; Nordgreen, T.; Grigiante, M. Biomass gasification with dolomite as catalyst in a small fluidized bed experimental and modelling analysis. Waste Biomass Valorization 2010, 1, 283–291. [Google Scholar] [CrossRef]
- Tacuri, D.; Andrade, C.; Álvarez, P.; Abril-González, M.; Zalamea, S.; Pinos-Vélez, V.; Montero-Izquierdo, A. Design and development of a catalytic fixed-bed reactor for gasification of banana biomass in hydrogen production. Catalysts 2022, 12, 395. [Google Scholar] [CrossRef]
- Raheem, A.; Ji, G.; Memon, A.; Sivasangar, S.; Wang, W.; Zhao, M.; Taufiq-Yap, Y.H. Catalytic gasification of algal biomass for hydrogen-rich gas production: Parametric optimization via central composite design. Energy Convers. Manag. 2018, 158, 235–245. [Google Scholar] [CrossRef]
- Chiodo, V.; Urbani, F.; Zafarana, G.; Prestipino, M.; Galvagno, A.; Maisano, S. Syngas production by catalytic steam gasification of citrus residues. Int. J. Hydrogen Energy 2017, 42, 28048–28055. [Google Scholar] [CrossRef]
- Sulaiman, S.A.; Roslan, R.; Inayat, M.; Naz, M.Y. Effect of blending ratio and catalyst loading on co-gasification of wood chips and coconut waste. J. Energy Inst. 2018, 91, 779–785. [Google Scholar] [CrossRef]
- Xiao, X.; Meng, X.; Le, D.D.; Takarada, T. Two-stage steam gasification of waste biomass in fluidized bed at low temperature: Parametric investigations and performance optimization. Bioresour. Technol. 2011, 102, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, H.; Wang, J.; Wu, B. Catalytic gasification characteristics of rice husk with calcined dolomite. Energy 2018, 165, 1173–1177. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Wong, P.; Marliza, T.S.; Nurul Suziana, N.M.; Tang, L.H.; Sivasangar, S. Hydrogen production from wood gasification promoted by waste eggshell catalyst. Int. J. Energy Res. 2013, 37, 1866–1871. [Google Scholar] [CrossRef]
- Raheem, A.; Liu, H.; Ji, G.; Zhao, M. Gasification of lipid-extracted microalgae biomass promoted by waste eggshell as CaO catalyst. Algal Res. 2019, 42, 101601. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Wang, L.; Huang, J.; Williams, P.T. Catalytic steam gasification of biomass for a sustainable hydrogen future: Influence of catalyst composition. Waste Biomass Valorization 2014, 5, 175–180. [Google Scholar] [CrossRef]
- Singh, M.M.; Chaudhari, M.J.; Solanki, M.B.; Bhatt, M.J.; Mahida, M.H. Qualitative and Cost Effective Producer gas production system. In Proceedings of the Second International Conference on Current Research Trends in Engineering and Technology, Okinawa, Japan, 2–4 July 2018; Volume 4, pp. 646–650. Available online: https://www.researchgate.net/profile/Hirenkumar-Mahida/publication/356128603_Qualitative_and_Cost_Effective_Producer_gas_production_system/links/618cdb24d7d1af224bd54491/Qualitative-and-Cost-Effective-Producer-gas-production-system.pdf (accessed on 21 May 2025).
- Narnaware, S.L.; Panwar, N.L. Catalysts and their role in biomass gasification and tar abetment: A review. Biomass Convers. Biorefin. 2021, 1–31. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jothiprakash, G.; Balasubramaniam, P.; Sundaram, S.; Ramesh, D. Catalytic Biomass Gasification for Syngas Production: Recent Progress in Tar Reduction and Future Perspectives. Biomass 2025, 5, 37. https://doi.org/10.3390/biomass5030037
Jothiprakash G, Balasubramaniam P, Sundaram S, Ramesh D. Catalytic Biomass Gasification for Syngas Production: Recent Progress in Tar Reduction and Future Perspectives. Biomass. 2025; 5(3):37. https://doi.org/10.3390/biomass5030037
Chicago/Turabian StyleJothiprakash, Gitanjali, Prabha Balasubramaniam, Senthilarasu Sundaram, and Desikan Ramesh. 2025. "Catalytic Biomass Gasification for Syngas Production: Recent Progress in Tar Reduction and Future Perspectives" Biomass 5, no. 3: 37. https://doi.org/10.3390/biomass5030037
APA StyleJothiprakash, G., Balasubramaniam, P., Sundaram, S., & Ramesh, D. (2025). Catalytic Biomass Gasification for Syngas Production: Recent Progress in Tar Reduction and Future Perspectives. Biomass, 5(3), 37. https://doi.org/10.3390/biomass5030037







