Abstract
Concerns about sustainable energy sources arise due to the non-renewable nature of petroleum. Escalating demand for fossil fuels and price inflation negatively impact the energy security and economy of a country. The generation and usage of biofuel could be suggested as a sustainable alternative to fossil fuels. Several studies have investigated the potential of using edible crops for biofuel production. However, the usage of algae as suitable feedstock is currently being promoted due to its ability to withstand adverse environmental conditions, capacity to generate more oil per area, and potential to mitigate energy crises and climate change with no detrimental impact on the environment and food supply. Furthermore, the biorefinery approach in algae-based biofuel production controls the economy of algal cultivation. Hence, this article critically reviews different cultivation systems of algae with critical parameters including harvesting methods, intended algae-based biofuels with relevant processing techniques, other applications of valorized algal biomass, merits and demerits, and limitations and challenges in algae-based biofuel production.
1. Introduction
Biofuels are referred to as liquid, gaseous, and solid fuels generated from biological feedstock through mechanical, biochemical, and thermochemical conversion techniques [1,2]. These biofuels are identified as environmentally friendly low-carbon alternatives to fossil fuels, and they reduce greenhouse gas (GHG) emissions [3,4,5]. Fossil fuel contribution to the world’s energy requirement is approximately 86% [6]. However, the usage of such non-renewable energy sources leads to many detrimental effects on the environment [7]. It is predicted that CO2 emissions from motor vehicles will be tripled by 2030 which will increase the global warming effect significantly [8]. Therefore, biofuels are suggested as one of the sustainable energy sources to replace fossil fuels and as a solution for the transport sector with the reduction of CO2 emissions [9].
Valorizing algae for biofuel has several significant impacts on alleviating food pressure, energy crisis, and climate change [10,11]. Algae-based fuels are renewable sources of energy, which are produced from algae, an oil-rich feedstock. Different types of biofuels can be extracted from microalgae such as bioethanol, biodiesel, bio-oil, biomethane, bio-hydrogen, biobutanol, biomethanol, bioaceton, syngas, bioelectricity, and biochar [7,12]. Among the different renewable alternatives for fossil fuels, bio-based fuels from algae have raised great attention because of their potential capacity to meet the global fuel demand in the long term [4]. The recent discovery of Candidatus Methanoliparum, a bacterium that can directly digest crude oil to produce methane gas [13], has led to an advancement in genetic engineering to modify potential genes to improve desired traits in algae [14] and the development of synthetic biology for the exploitation of genetic manipulation tools, modification of metabolic pathways, and development of high value-added product [15] which can further improve the production of algae-based biofuels.
To critically assess these potentials, the review was conducted through a systematic analysis of the peer-reviewed literature on algal biofuel technologies, identified via comprehensive searches in Scopus, Web of Science, and Google Scholar using targeted keywords. Relevant studies were selected based on their technical merit, publication quality, and relevance to biomass valorization processes. The synthesized findings were critically evaluated to present current advancements, challenges, and future directions in algal biofuel production.
Evolution of Biofuels
Based on the origin of the feedstock, biofuels are broadly categorized into first, second, third, and fourth biofuel generations. The first biofuel generation (G1) is referred to as the production of biofuels from edible feedstock. Both liquid and gaseous stage biofuels are generated via different techniques such as transesterification, anaerobic decomposition, fermentation, and pyrolysis [8]. Zeamays, Helianthus annuus, Cocos Nucifera L., Sesamum indicum L., Arachis hypogea L., Brassica napus, Glycine max, Carthamus tinctorius L., Elaeis guineensis, Corylus avellana, Juglans regia, and Pistacia vera (Figure 1) are some of the feedstocks used in G1 [16,17,18,19,20,21]. The production of ethanol through the fermentation of sugars and biodiesel through the transesterification of triglycerides are some of the G1 biofuels [22,23]. The need for second-generation biofuel sources was raised due to the negative impacts of G1 on global food security [24,25].

Figure 1.
Feedstocks for different generations of biofuel production.
The second-generation biofuels (G2) are produced from lignocellulosic and nonedible triglyceride feedstocks, which have no competition with food and feed supplies [22,23]. Comparatively, higher yield with lower corresponding land area is a noticeable benefit from the G2 feedstock compared to G1 [26]. Different types of waste, wood and plant residues, and nonedible oil crops like Ricinus communis L., Jatropha curcas L., Simmondisa chincnsis, and Azadirachta indica are used as feedstocks in G2 (Figure 1) [1,27,28,29,30,31,32,33,34,35]. Fermentation, gasification, and hydrothermal liquefaction can be indicated as the major technologies practiced in G2. Though G2 fuels are eco-friendly and non-corrosive, the lack of proper technique for commercial applications [25] and the rich content of saturated FA are encountered as demerits [8,36].
The third-generation biofuels (G3) are derived from algae, a sustainable green feedstock with high yield [10,14,37]. Some of the potential algal species [11,38] used for biofuel production are shown in Figure 1. Competition with food production and consumption of large land areas in both G1 and G2 feedstocks have caused a gradual shift toward G3 with the appropriate sources that have no detrimental effects on food production [39]. Though microalgae have been accepted as a promising renewable energy source to replace fossil fuels [1], the requirement of a high amount of nutrients and the presence of a high quantity of unsaturated FA are the major limiting factors for the use of G3 [40].
The fourth-generation biofuels (G4) comprise microscopic organisms (Figure 1) that play a major role in enhancing biofuel production. Additionally, G4 aimed to generate biodiesel in an efficient way to overcome the demerits of all the other three mentioned generations (G1–G3) and reduce fossil fuel usage [26]. Therefore, through genetic engineering, organisms like cyanobacteria, yeast, macroalgae, and microalgae are modified to convert CO2 into fuel directly [14,41]. In addition, modification of the oil-bearing capacity of such organisms is also found to be a special feature of the G4 [42]. In this manner, Rhodococcus, Bacillus subtilis, Cutaneotrichosporon oleaginosus, Yarrowia lipolytica, Chlorella, and Micractinium reisseri are some of the promising oleaginous microbes that can produce high amounts of intracellular lipids through genetic engineering and strain development [43,44,45,46,47,48]. Microalgae are also modified in terms of lipid and carbohydrate metabolism, efficient nutrient use and photosynthesis, hydrogen production, stress tolerance, cell disintegration, and flocculation [49]. More extensive studies on the potential usage of G4 biofuels are needed soon.
2. Algae in Biofuel Production
Algae are simple photosynthetic organisms with rapid reproduction capacity that can grow in both saline and freshwater [2,49]. While nearly one million microalgae species are estimated to exist, only about 44,000 species have been identified so far, and the search for new species continues [10]. Algae can be broadly classified as microalgae and macroalgae based on their morphological appearance [24,50]. Meanwhile, it is also categorized into five main classes such as green, blue-green, red, brown, and golden algae based on their photosynthetic pigments [51]. Algae are considered to be a potential species to withstand extreme environmental conditions [52,53]. The cultivation of microalgae can be carried out with the application of an adequate quantity of CO2, water, light, temperature, and nutrients [54]. They can convert the three major inputs such as water, sunlight, and CO2 into biomass, which consists of nearly 50% of carbon on a dry weight basis [55].
Compared to other feedstock in biofuel production, microalgae are the potential species that can produce more oil per unit area. However, different strains of microalgae differ in their oil productivity, oil content, and co-product generation [56]. Generally, microalgae have 7–65% lipid [54], whereas the cultivation conditions and the growth phase influence the oil content of the algal species [57]. Some of the suitable microalgae species for biofuel production are Chlamydomonas reinhardtii, Chlorella sp., Spirogyra sp., Porphyridium cruentum, Spirulina platensis, Dunaliella salina, Bellerochea sp., Chaetoceros sp., Rhodomonas sp., and Scenedesmus sp. [58]. The production of algae-based biofuels, including the cultivation, harvesting, and processing techniques, are critically reviewed in Section 3 below.
3. Algae-Based Biofuel Production
3.1. Cultivation of Algae
3.1.1. Cultivation Parameters
To optimize the productivity of microalgae, it is necessary to provide the required nutrients and other essential environmental conditions in the cultivation systems. Inorganic nutrients like carbon, nitrogen, and phosphorus are required for the growth of microalgae. Carbon is obtained from CO2, dissolved carbonates, glucose, and acetate for photosynthesis and respiration [59]. Nitrogen content has a greater influence on lipid productivity. According to the literature, a lower concentration of nitrogen reduces the growth rate of microalgae, which decreases lipid productivity consequently [60]. Phosphorus is needed for photosynthesis and some other metabolic activities [61].
Rather than using commercially available nutrient media, it is possible to utilize wastewater (WW) to satisfy the nutrient demand, which reduces the cost of production [62]. On the other hand, the incorporation of WW as nutrient media reduces the quantity of freshwater addition into the system [63]. Domestic WW, municipal WW, and WW from textile industries, meat processing, sugarcane industries, and piggery farms can be used as nutrient sources for algal production [64,65,66,67,68,69,70,71,72]. In contrast, culture instability due to higher organic matter content, inconsistent growth rate with the single source WW, and inhibition of algal growth because of higher ammonia concentration are some of the potential demerits that can result in using WW as a nutrient source for algal cultivation [73,74]. Moreover, the pretreatment or dilution of wastewater is reported as a beneficial practice to improve algae growth [71,72].
pH is an essential factor for algal growth as the solubility and availability of CO2 depend on it. The optimum growth of microalgae occurs at nearly neutral pH [75]. The consumption of carbonic acid, phosphoric acid, and nitric acid makes the system basic, whereas ammonium consumption reduces pH levels [76]. It is important to closely monitor the pH as unfavorable pH level collapses algal cell walls [8]. Algae use sunlight for photosynthesis, and light intensity affects the growth rates of algae [8]. Self-shading is one of the potential problems in high-density cultures, which demand high light intensity [77]. On the contrary, such high intensity of light may lead to photoinhibition and growth inhibition consequently after reaching a saturation point [78]. Depending on the strain, the temperature requirement of microalgae ranges from 15 to 40 °C [79]. It can also differ according to the temperature zones. Algaculture in temperate zones is best at 10–25 °C, whereas in the tropics, it is below 20 °C. Algal growth will be destructive beyond 35 °C [8] since high temperature inhibits the metabolic rate and reduces the CO2 solubility [80].
3.1.2. Cultivation Systems
There are three major methods of algae cultivation, namely open pond systems (OPSs), photobioreactors (PBRs), and hybrid systems [8]. In addition to these three types, a novel technique has been introduced as an offshore membrane enclosure for growing algae (OMEGA) system [81]. The open pond system (OPS) is the most used large-scale algal cultivation method in natural water bodies such as shallow ponds, circular ponds, and raceway ponds [52]. Among these cultivation methods, the raceway ponds (Figure 2) are identified as dominant due to their energy efficiency with less requirement for cost and maintenance [10,56]. However, the operation of the raceway pond can be affected by wind currents, leakage, and animal interference [42].

Figure 2.
Raceway pond system with moving paddles [82].
Photobioreactors (PBRs) are a closed type of algae cultivation in which the environment is kept controlled to ensure optimum productivity [83]. PBRs are designed in different shapes like inclined tubular, flat plate, and horizontal/continuous as shown in Figure 3 [82]. This method can be adapted to overcome limitations such as contamination and evaporation loss encountered with OPS. In addition, a controlled supply of nutrients and the application of artificial light sources for continuous biomass production are also possible with PBRs [37]. However, it requires higher capital investment compared to OPSs [42]. At present, practices of PBRs are confined to laboratories and pilot-scale plants for research purposes [51]. Meanwhile, the commercial success of PBR can be achieved with the understanding of energy consumption in the system [18].

Figure 3.
Photobioreactors for algae cultivation: (A) inclined tubular photobioreactor, (B) flat-plate photobioreactor, and (C) horizontal/continuous photobioreactor [82].
The hybrid system is a combination of OPS and PBR for their synergistic benefits. This system consists of two stages. In the initial stage, algae are cultured in PBR ensuring a higher growth rate with minimal contamination. The culture is then transferred to OPS in the later stage to achieve optimum economic compatibility by reducing the operational cost [8,56]. Offshore membrane enclosures for growing algae (OMEGA) system is a new technique in microalgae cultivation. Floating photobioreactors are used in this system. As they float on the ocean water, uniform temperature is effectively maintained. In addition, the tidal waves from the ocean accomplish the need for mixing. Furthermore, the supply of nutrient-enriched WW from offshore WW outfalls reduces the cost spent on fertilizer inputs [81].
3.2. Harvesting
High biomass concentration at moderate costs of operation, maintenance, and energy is the basic consideration for the harvesting process of microalgae [84]. It is essential to consider the efficiency of the harvesting techniques during the selection process as it accounts for 20–30% of the total costs of production [24]. Mechanical, chemical, biological, and electrical methods are practiced presently in microalgae harvesting while adapting combinations of these methods is advisable to achieve a greater rate of separation in a cost-effective manner [85,86]. Various microalgae harvesting techniques such as flotation, centrifugation, filtration, flocculation, and gravity sedimentation are reviewed briefly below.
Flotation is the process in which air is bubbled through the feed suspension to carry microalgae to the shallow depth of the culture and accumulate as float to be collected easily [10]. Flotation is influenced by the size of both the air bubbles and microalgae [87]. Alternatively, the surface charge and hydrophobic nature of the algal cell also have an impact on the efficiency of this technique [88]. Flotation can be indicated as a less expensive technique compared to centrifugation by excluding the usage of flocculants for the purpose of forming aggregation [56].
Centrifugation is the fastest harvesting method during which centripetal force is used for the sedimentation of heterogeneous mixtures. Thus, the incorporation of homogenizers facilitates the separation of biolipids and other beneficial products from algae [89,90]. Even though the centrifugation technique is recognized as the most efficient method of harvesting, its application is limited as it requires more energy input, time, and high capital and operational costs in large-scale applications [91].
Filtration is practiced forharvesting long-length microalgae during which algae biomass accumulates on filters [92]. According to the pore size of the filters, this method is categorized as macrofiltration, microfiltration, ultrafiltration, and reverse osmosis [18]. Fouling of the filter is the most common demerit of the filtration technique which can be mitigated by adapting tangential flow with respect to the membrane [88]. However, more time consumption, high running costs, and preconcentration requirements remain the demerits of this technique [93].
Flocculation is the process in which the addition of flocculants leads to the formation of algal flocs that settle rapidly [94]. There are two different methods of flocculation: chemical flocculation and physical flocculation [95]. Coagulants used in chemical flocculation can be inorganic metal salts such as aluminum, ferric, and zinc salts [96] or organic biopolymers like chitosan and starch, where the latter is found to be an acceptable practice as organic coagulants reduce the potential contamination that can be caused by chemical salts [95]. However, the flocculation technique is not suitable for small-scale applications [90].
In gravity sedimentation, the feed suspension is separated into two different components, namely slurry and liquid. At natural gravity, the harvesting is performed with sedimentation tanks and lamella separators [97]. The orientation of the plates in lamella separators provides a bigger settling area. This process is accomplished with the continuous pumping of microalgal suspension with an intermittent removal of slurry. Although microalgal separation using sedimentation tanks is cost-effective, the addition of flocculants is necessary to ensure process reliability [90]. Figure 4 summarizes the key parameters required for microalgae cultivation, different cultivation systems, and different harvesting techniques.
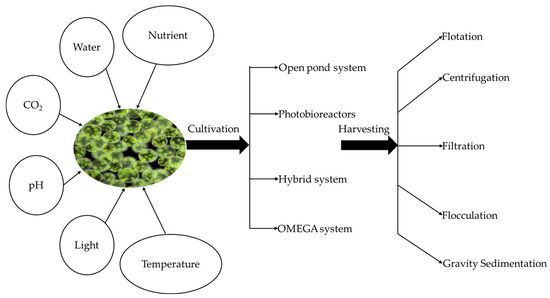
Figure 4.
Algae cultivation and harvesting techniques.
3.3. Processing Techniques
3.3.1. Lipid Extraction
Algae are capable of accumulating large amounts of lipids [98,99,100]. However, lipid productivity and fatty acid composition are markedly influenced by light, temperature, pH, CO2, and nutrient availability in the cultivation medium [101]. Moreover, the extraction of algal oil is not simple compared to other crop seeds due to their rigid cell wall structure [102]. There are various physical, chemical, mechanical, and enzymatic methods used for the extraction of FA and lipids from microalgal cell walls [101,103].
As lipid extraction through physical methods generates excess heat and reduces product quality, it is neither eco-friendly nor economical at a commercial level [104]. Expeller press and bead beating physical methods were reported to be less efficient techniques for lipid extraction [105]. Despite providing high lipid recovery, solvent extraction methods may lead to fire hazards and health and environment-related issues. The combination of mechanical extraction methods like pulsed electric fields with green solvents like ethyl acetate not only replaces the usage of toxic organic solvents but also significantly enhances the rate of lipid recovery [106].
Microwave-assisted extraction (Figure 5) is an alternative green method to obtain lipids through efficient cell disruption. With less energy dispersion and rapid heating, the microwave technique can be considered to be a feasible technique to extract better quality products at high quantities in less extraction time [103,104]. Moreover, algal biomass remaining after lipid extraction can be utilized for combustion and to produce electricity, biomethane, and animal feed [107].
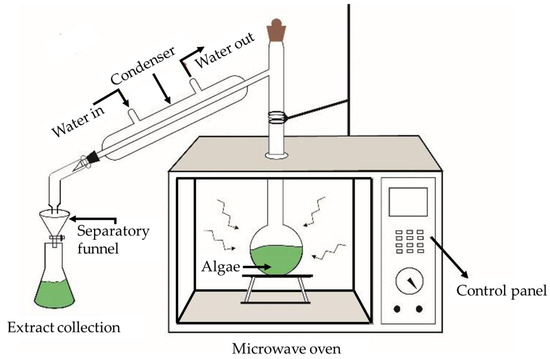
Figure 5.
Microwave-assisted lipid extraction [108].
3.3.2. Transesterification of Lipids
Transesterification is a chemical reaction between triglycerides and alcohol in the presence of a catalyst to produce biodiesel (Figure 6) [109]. After the lipid extraction processes reviewed above, the resulting algal oil can be converted into biodiesel through transesterification [8,57]. The transesterification of oils and lipids extracted from potential algae like Scenedesmus dimorphus, Nannochloropsis sp., and Chlorella vulgaris [52] requires chemical or biological catalysts along with alcohol to produce biodiesel and glycerol as end-products [2,110,111]. The periodic removal of glycerol is important for continuous transesterification while the elimination of methanolic, catalytic, and soap components is crucial to ensure high-quality diesel [112].
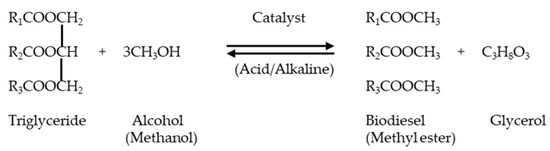
Figure 6.
Biodiesel production through transesterification. Adapted with permission from [109], Copyright {2025}, American Chemical Society.
According to the number of steps involved, the transesterification process can be categorized as in situ and extractive transesterification [113], among which the former is considered feasible, as lipid extraction and transesterification are completed in one step that extends for less processing time with lower utilization of solvent [1]. Regarding the catalyst, less processing time with faster reactions can be obtained with base catalysts [110]. However, to reduce the cost of the downstream separation associated with soap production, it is not recommended to use base catalysts for feedstock with high free fatty acid contents and water [114]. Acid catalysis also has some demerits like susceptibility to high water content, corrosiveness of the catalyst, longer reaction time, and extended downstream separation [113].
3.3.3. Anaerobic Digestion
Anaerobic digestion is the conversion of algal biomass into biogas, a mixture of different gases, majorly CO2 and CH4, through four steps such as hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Figure 7) [115,116], which can be used for domestic cooking and power generation [111]. In addition to biogas production, the mitigation of GHG emissions and organic manure production are some other benefits of this process [117]. As the chemical composition of the algal cell wall influences the yield of anaerobic digestion, it is recommended to cultivate microalgae under adequate nitrogen which makes the cell wall easily digestible [118]. Meanwhile, cell wall degradation can be achieved by adopting thermal, mechanical, and biological pretreatments to enhance biogas yield [119].
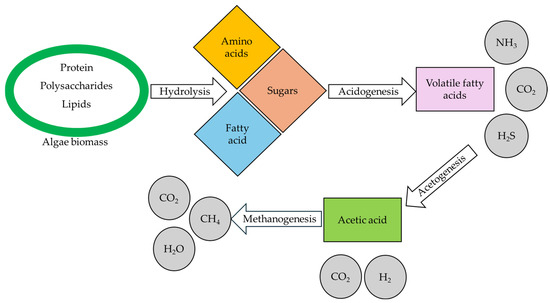
Figure 7.
Methane production through anaerobic digestion.
Similarly, the C/N ratio also affects the feasibility of this process, and the acceptable C/N ratio to obtain an improved CH4 yield is 20 to 30 [120]. However, a lower C/N ratio in algal residual biomass requires the incorporation of higher carbonaceous feedstocks to improve the CH4 yield [2,121]. Moreover, the CH4 yield differs according to the algal species and variation in the yield is recorded among the same species [116]. The proportion of CH4 in biogas is influenced by some other factors including temperature, volume and loading rate of the biomass, duration of the digestion process, and bacterial strains [122].
The profitability of biogas production through anaerobic digestion depends on circular economy-related processes like WW treatment, reduction in eutrophication, and the reuse of biomass to generate value-added products. In that sense, methane recovery through the anaerobic digestion of microalgae grown in sewage is a feasible approach for economy and energy balance at WW treatment plants [116,120]. Also, anaerobic digestion is found as a solution to eutrophication caused by algae in waterbodies while generating energy resources [115]. The possibility of using the remaining biomass after lipid extraction as feedstock for biogas production makes this technique more beneficial [123]. Moreover, the residues left after biogas generation can be utilized as fertilizer considering the enrichment of nitrogen and phosphorus in the residue [124].
3.3.4. Microbial Fermentation
Algal biomass is fermented by microbes like yeast, bacteria, and fungi to produce bioethanol [23]. Separate hydrolysis and fermentation, and simultaneous saccharification with fermentation are the two widely performed fermentation methods to produce bioethanol [125]. Bioethanol yield is limited in the former method, as it requires the separation of wet biomass after hydrolysis. In the latter, hydrolyzed biomass is simultaneously converted into bioethanol with a lower enzyme dosage to yield more bioethanol [126].
Microbial fermentation requires pretreatment to remove lignin and the application of suitable microbial strains to produce amylase enzyme which induces saccharification. This step improves the release of polysaccharides and their conversion to monosaccharides [127]. Pretreatment techniques required for the extraction of various compounds are shown in Table 1. The solid residues remaining after microbial fermentation can be used as cattle feed or as substrates for gasification techniques to reduce the total production cost of biofuel production [128]. However, time consumption, the production of photochemical smog, and the high cost of the distillation and production of enzymes are the major challenges encountered in this process [8].

Table 1.
Pretreatment techniques for the extraction of different compounds.
3.3.5. Hydrothermal Liquefaction
Hydrothermal liquefaction (HTL) involves the conversion of wet algal biomass into bio-oil through direct liquefaction at temperatures between 250 °C and 550 °C, and pressures of 5–25 MPa [9] with or without the use of a catalyst. However, the addition of a catalyst improves the conversion rate of algal biomass into bio-oil [140,141]. During the HTL process, the biomass chemical components like proteins, lipids, and carbohydrates are broken down into their respective monomers [8,142]. As the process continues, the lower-molecular weight hydrocarbon will result in the further removal of oxygen, sulfur, phosphorous, and nitrogen [143].
HTL leads to high biocrude production; however, the yield is significantly influenced by the composition of the feedstock [144]. The bio-oil yield further depends on factors like reaction temperature, heating rate, type of reaction and catalyst [145,146], residence time, pressure, and reaction medium [147]. The minimum selling price of biofuel derived from Chlorella through HTL was estimated from USD 6 to USD 22 per gasoline-gallon-equivalent, which can be indicated as the most favorable economic performance [148]. Even though the cost of drying is eliminated in this technique, the higher operating cost associated with the requirement of high temperature and pressure remains a significant drawback [9]. Similarly, lower engine performance with emission of NOX and SOX from the exhaust is also reported as a disadvantage of the application of biocrude from HTL [8].
3.3.6. Pyrolysis
Pyrolysis of algal biomass is a thermochemical process (Figure 8) during which the algal biomass is subjected to moderate temperatures in the absence of oxygen to produce valuable end-products such as bio-oil, biochar, and bio-syngas [149,150,151]. The genus Chlorella is well known to produce bio-oil via pyrolysis [144,152]. The bio-oils produced from algal biomass have higher oxidation stability [153], heating value [154], and aromatics, and lower acidity [155] and calorific value [153] than lignocellulosic biomass-based bio-oil. However, the quantity and quality of end-products in pyrolysis are highly influenced by the residence time, temperature [156,157], reactor type, and characteristics of feedstock [140].
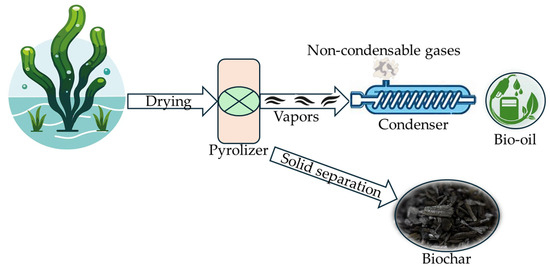
Figure 8.
Pyrolysis of algal biomass [35].
The composition of end-products varies according to the type of pyrolysis [35]. Slow pyrolysis generates carbon-rich solid residue called biochar as the major output at a low heating rate for longer residence time, whereas a fast heating rate with short residence time mainly promotes the formation of bio-oil in fast pyrolysis [2,149]. To avoid the secondary cracking of the primary products, feedstocks are pyrolyzed at a combination of higher heating rates and very short residence time using fluidized beds or entrained-flow reactors [28]. In addition to these conventional methods, several advanced pyrolysis techniques such as microwave-assisted, catalytic, and co-pyrolysis were extensively studied by numerous researchers [158,159,160,161,162,163]. Modes of different pyrolysis techniques reviewed above are shown in Table 2.

Table 2.
Modes of pyrolysis.
3.3.7. Gasification
Gasification is the process of converting biomass into syngas, a mixture of various gases like CO2, CO, H2, N2, CH4, water vapor, and tar [166,167], through partial oxidation at 800–1200 °C [168]. The addition of a catalyst improves H2 production and tar degradation during the gasification process [169,170,171,172]. Syngas can be either burnt directly or used as fuel for gas engines or gas turbines [173,174]. There are possibilities to transform the additional syngas into H2 or lower-range liquid hydrocarbon through water gas shift reaction and Fisher–Tropsch synthesis, respectively [170].
3.3.8. Direct Combustion
Direct combustion is the process of converting algae biomass into hot gasses at 1000 °C with the help of O2 to produce electricity [2]. Direct combustion requires some pretreatments such as drying of algal biomass below 50% moisture content and grinding. As high ash and alkali residue reduce the overall efficiency of this process, the fluidized bed method is reported as an efficient technique to overcome this problem [175]. Furthermore, the additional cost associated with a pretreatment plant can be reduced by utilizing the heat produced during the whole conversion process immediately [52].
4. Other Applications of Algal Biomass
In addition to the production of algae-based biofuels mentioned above, a range of valorized products such as biofertilizers, biosorbents, pigments, and pharmaceuticals can be produced from algal biomass [2,4]. The availability of macro- and micro-nutrients, and plant hormones makes algae an efficient plant growth enhancer [176]. Biochar produced from algal biomass is high in pH and can be utilized to reclaim acidic soil for agriculture purposes. Furthermore, soil health can also be maintained with the application of algal biochar considering its higher nitrogen content and variety of inorganic elements [177].
Algal biomass is a good biosorbent to remove pollutants from water. It has been proven that the cell wall of algae enriched with multifunctional groups that have the potential to remove heavy metals like cobalt, nickel, mercury, chromium, copper, and cadmium [178,179,180,181]; industrial dyes [179,182]; and inorganic nutrients like nitrates, phosphates, and organic compounds [183]. Furthermore, algal biochar can also be used in WW treatment to remove both organic and inorganic contaminants [184].
Algae is a natural producer of bioactive commercial pigments like chlorophylls, phycocyanin, and carotenoids [185]. Generally, these pigments are widely used in biomedical fields for their antioxidant, anti-inflammatory, anticancer, anti-cardiovascular, neuroprotective, and anti-diabetic activity [186]. The application of various pigments derived from algal biomass is shown in Table 3.

Table 3.
Application of pigments in various industries.
Marine polysaccharides present in marine algae have a range of valorization potential for biomedical applications due to their biocompatibility and biodegradability. Other than the pharmaceutical application of pigments shown in Table 3 above, the utilization of a range of biopolymers derived from algal biomass in the biomedical field is shown in Table 4.

Table 4.
Biomedical application of algae-based biopolymers.
Algal biomass is edible and rich in nutrients which make it potentially raw material in the food and feed industries. Carrageenan extracted from certain red seaweeds is used in the dairy and meat-processing industries as thickening agents, binders, and stabilizers to improve the texture and flavor of different products [197,204]. Considering the hydrophilic colloidal properties, biopolymers like alginates and agar are also used for similar purposes in food and feed industries [205].
Cellulose-based aerogels produced from algal biomass are used as absorbent pads for fresh products in food packaging [176]. In addition, cellulose composite films produced from algal biomass are used as a packaging material and edible coating in the food industry [196]. As the usage of plastics for packaging purposes negatively impacts the environment, it is beneficial to adopt these biodegradable packing materials produced from biopolymers derived from algal biomass.
For several decades, algal biomass has been used as feed in both animal and aquatic farming [206]. Brown algae are widely used because of their larger size and simple harvesting technique [207]. Algae are considered to be low-cost and easily available protein sources for free-range ruminants grazing in the coastal areas [208]. However, it is necessary to confirm the quality of the algal feed to prevent the accumulation of pollutants in animal bodies.
5. Merits of Algae-Based Biofuels
Algae grow fast with greater adaptability to a wider range of climatic conditions [3,209], and they can produce more oil per acre compared to other feedstock [97]. Biologically produced algal fuel is a potential alternative energy source to overcome the issues with variations in oil prices and to cope with climate change [210]. Biofuels act as sustainable energy resources to satisfy the fuel demand of the world for several purposes [209]. The utilization of microalgae to produce biofuel creates ecological benefits, especially in the mitigation of eutrophication through pollutant absorption [2,10,211]. Further, the physical removal of nutrients is ensured by harvesting the algae for biofuel production. The residual nutrient concentration in the filtrate that is obtained from algal biomass dewatering is less than that of the original WW [212,213].
In addition, high-rate algal ponds are being utilized as nutrient removal systems for nitrogen and phosphorus-rich WW [214]. For instance, biogas slurry treatment using a co-cultured Chlorella vulgaris and Ganoderma lucidum showed remarkable removal efficiencies: 92% for chemical oxygen demand, 90% for total nitrogen, 90% for total phosphorus, and 74% for CO2 [215]. Likewise, water pollution control with the removal of heavy metals and other organic impurities is also possible with microalgae [216]. Lands that are unsuited for agriculture can be used for the cultivation of microalgae as the growth of microalgae does not require fertile soil and they can withstand water with different salinity levels and inferior chemical quality as well [12,217]. Further, the extent of land required for cultivation is also less than for other crops used for biofuel production in previous biofuel generations [50]. In addition, microalgae reduce the greenhouse effects through carbon sequestration by biologically fixing the carbon and removing carbon dioxide from industrial emissions while releasing enormous oxygen to the environment during their growing period [3,4,12,50,209]. The merits of algae-based biofuel production and application are illustrated in Figure 9.
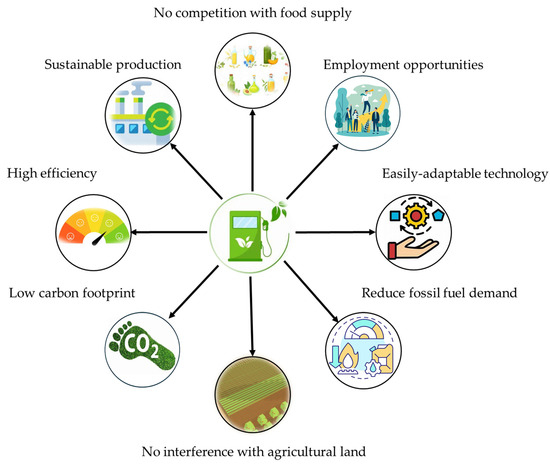
Figure 9.
Merits of algae-based biofuel production and application.
In addition to biofuel extraction, the remaining biomass can be used for the production of methane and organic fertilizers that can be used for agricultural purposes [218]. Moreover, algae are used as cheap resources to generate economic benefits in various sectors such as pharmaceuticals, aquaculture, cosmetics, and construction [219]. As microalgae do not influence the food chains, biofuel production from algal biomass does not violate the ethical concerns regarding the utilization of food sources for fuel production [56,209]. The mitigation of fossil fuel usage, employment opportunities, and reduction in imports of oils from other countries are some other benefits of biofuel production from algae [4,220].
6. Limitations and Challenges in Algae-Based Biofuel Production
Complexity in the conversion methods and the higher production cost for such process are the major negative aspects associated with algae biofuel production [3,52,209]. High capital and operating costs of algae-based biofuels hinder its commercial scale establishment [172,221,222]. Cultivation methods are not always easy, and they require high capital investment [24]. Further, biofuel production is highly dependent on sophisticated infrastructures and management practices [220]. The production of neurotoxins can cause health problems for humans and may affect aquatic life [220]. The excessive growth of algae due to anthropogenic eutrophication reduces the quantity of dissolved oxygen. The subsequent decomposing of such algal material further reduces the available oxygen and leads to the creation of anoxic conditions, which are not suited for the survival of aquatic species [223].
The chemical composition and properties of algae compared to the terrestrial biomass itself limit the production and application of algae-based biofuels. In detail, the high moisture content (70–90%), ash content (27%), bulk density (954 kg/m3), major elements, alkaline and halogen, and a range of trace elements; water-soluble fraction (15%); electrical conductivity of leachates (19.8 mS/cm); and combustion temperature (200–800 °C) negatively impact on algal biofuel production. Similarly, lower values of cellulose crystallinity index, heating value, organic matter, reactivity, ash-fusion temperature, carbohydrates (30%), cellulose (14%), initial ignition temperature (200 °C), volatile matter (52%), and hydrogen (7%) affect the efficiency of production [4].
The high energy requirements for algal biofuel production make it energetically unviable [224]. Also, the demand for huge quantities of nutrient sources and water for algal cultivation affects the sustainability of this technology [225,226]. Moreover, harvesting and downstream processes add extra costs, which makes the process further challenging [227]. Large-scale algae cultivation affects the coastal biodiversity through invasion by algal species of coastal shallow ecosystems [228]. The lack of both government policies and technological infrastructures on algae-based fuel production can also be indicated as considerable limitations [220,229]. Therefore, it is recommended to adopt new strategies including genetic engineering and the utilization of waste materials (Figure 10) to maximize production along with suitable biorefinery approaches [230,231].
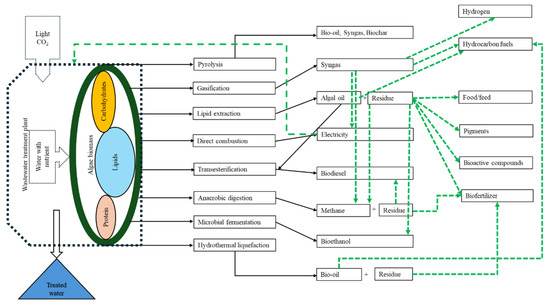
Figure 10.
Processing techniques of algae with biorefinery approach (in broken green lines) [2,50,51,227].
7. Recent Advancements and Future Research Directions
Recent years have witnessed significant advancements in algal biofuel technology, particularly in genetic modification, cultivation systems, nutrient supply, harvesting methods, conversion techniques, and safe biofuel applications. Notably, the targeted knockout of phospholipase A2 using genome editing has enhanced lipid productivity in Chlamydomonas reinhardtii [232], demonstrating the potential of genetic engineering for strain improvement. Concurrently, studies have revealed that controlled nutrient deficiencies can stimulate lipid accumulation by altering the cellular biochemical pathways [233,234,235,236,237]. In algae cultivation practices, WW from various industrial sources (such as meat processing) has proven an alternative nutrient medium [68].
Conversion technologies have also progressed, with plasma gasification and supercritical water gasification emerging as efficient methods for syngas production while minimizing tar formation [238,239]. Modeling studies have further validated the feasibility of electrical power generation from marine algae through gasification processes [36]. Regarding fuel performance, comprehensive analyses confirm that algal biofuels exhibit superior combustion characteristics, emitting significantly lower levels of carbon monoxide, hydrocarbons, particulate matter, and smoke compared to conventional fuels [228,229,230,231,232,233], solidifying their status as sustainable alternatives for internal combustion engines.
Despite these advances, critical knowledge gaps remain. The environmental implications of genetically modified algal strains require urgent investigation, particularly regarding potential lateral gene transfer during energy extraction and wastewater treatment [39]. Future research should prioritize (1) the identification of robust algae strains capable of thriving in wastewater with high toxicant loads without compromising productivity [54], and (2) the development of scalable solutions to overcome persistent technological bottlenecks in commercial-scale operations.
8. Conclusions
Algal biofuel production represents a viable renewable energy solution that addresses both environmental concerns and growing energy demands. The integration of wastewater treatment with algal cultivation offers the dual benefits of nutrient recycling and biomass production, while biorefinery approaches enable the simultaneous generation of biofuels and high-value co-products such as biofertilizers, pharmaceuticals, and pigments. However, commercialization faces three major hurdles: high production costs associated with cultivation and harvesting, technical limitations in conversion processes, and unresolved environmental concerns regarding genetically modified strains. For successful implementation, future work must focus on developing cost-effective cultivation systems, optimizing conversion technologies, and conducting rigorous environmental risk assessments. Government support through policy incentives and research funding will be crucial to overcome these barriers. When these challenges are addressed, algal biofuel systems can evolve from laboratory-scale promise to large-scale, sustainable energy solutions that contribute meaningfully to global decarbonization efforts while supporting circular economy principles through wastewater remediation and co-product development.
Author Contributions
Conceptualization, investigation, writing—original draft preparation, and validation, V.A.; writing—review and editing, V.A., P.A.O. and L.A.; visualization, V.A.; supervision, L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S. Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review: Environmental sustainability of biofuels. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 20200351. [Google Scholar] [CrossRef]
- Dong, K.; Dong, X.; Jiang, Q. How renewable energy consumption lower global CO2 emissions? Evidence from countries with different income levels. World Econ. 2020, 43, 1665–1698. [Google Scholar] [CrossRef]
- Kosmela, P.; Kazimierski, P.; Formela, K.; Haponiuk, J.; Piszczyk, Ł. Liquefaction of macroalgae Enteromorpha biomass for the preparation of biopolyols by using crude glycerol. J. Ind. Eng. Chem. 2017, 56, 399–406. [Google Scholar] [CrossRef]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Chi, N.T.L.; Duc, P.A.; Pugazhendhi, A. A review on prospective production of biofuel from microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef]
- Ou, L.; Thilakaratne, R.; Brown, R.C.; Wright, M.M. Techno-economic analysis of transportation fuels from defatted microalgae via hydrothermal liquefaction and hydroprocessing. Biomass Bioenergy 2015, 72, 45–54. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal biomass valorization for biofuel production and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae biofuels: Illuminating the path to a sustainable future amidst challenges and opportunities. Biotechnol. Biofuels Bioprod. 2024, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Zaini, J.; Mahlia, T.M.I.; Azad, A.K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, C.-J.; Liu, P.-F.; Fu, L.; Laso-Pérez, R.; Yang, L.; Bai, L.-P.; Li, J.; Yang, M.; Lin, J.-Z.; et al. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species. Nature 2022, 601, 257–262. [Google Scholar] [CrossRef]
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Brück, T. The potential of biofuels from first to fourth generation. PLoS Biol. 2023, 21, e3002063. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, L.; Ma, L.; Amin, F.R.; Zhai, Y.; Chen, G.; Li, D. From lignocellulosic biomass to single cell oil for sustainable biomanufacturing: Current advances and prospects. Biotechnol. Adv. 2024, 77, 108460. [Google Scholar] [CrossRef]
- Kolakoti, A.; Rao, B.V.A. Effect of fatty acid composition on the performance and emission characteristics of an IDI supercharged engine using neat palm biodiesel and coconut biodiesel as an additive. Biofuels 2019, 10, 591–605. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Patel, C.; Tiwari, N.; Agarwal, A.K. Experimental investigations of Soyabean and Rapeseed SVO and biodiesels on engine noise. vibrations, and engine characteristics. Fuel 2019, 238, 86–97. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks. production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Gunawan, M.L.; Novita, T.H.; Aprialdi, F.; Aulia, D.; Nanda, A.S.F.; Rasrendra, C.B.; Addarojah, Z.; Mujahidin, D.; Kadja, G.T.M. Palm-oil transformation into green and clean biofuels: Recent advances in the zeolite-based catalytic technologies. Bioresour. Technol. Rep. 2023, 23, 101546. [Google Scholar] [CrossRef]
- Shaah, M.A.H.; Hossain, M.S.; Allafi, F.A.S.; Alsaedi, A.; Ismail, N.; Kadir, M.O.A.; Ahmad, M.I. A review on non-edible oil as a potential feedstock for biodiesel: Physicochemical properties and production technologies. RSC Adv. 2021, 11, 25018–25037. [Google Scholar] [CrossRef] [PubMed]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Aro, E.M. From first generation biofuels to advanced solar biofuels. Ambio 2016, 45, 24–31. [Google Scholar] [CrossRef]
- Guerrero, A.B.; Muñoz, E. Life cycle assessment of second generation ethanol derived from banana agricultural waste: Environmental impacts and energy balance. J. Clean. Prod. 2018, 174, 710–717. [Google Scholar] [CrossRef]
- Maliutina, K.; Tahmasebi, A.; Yu, J.; Saltykov, S.N. Comparative study on flash pyrolysis characteristics of microalgal and lignocellulosic biomass in entrained-flow reactor. Energy Convers. Manag. 2017, 151, 426–438. [Google Scholar] [CrossRef]
- Acharya, N.; Nanda, P.; Panda, S.; Acharya, S. A comparative study of stability characteristics of mahua and jatropha biodiesel and their blends. J. King Saud. Univ.-Eng. Sci. 2019, 31, 184–190. [Google Scholar] [CrossRef]
- Sandouqa, A.; Al-Hamamre, Z. Energy analysis of biodiesel production from jojoba seed oil. Renew. Energy 2019, 130, 831–842. [Google Scholar] [CrossRef]
- Mallah, T.A.; Sahito, A.R. Optimization of castor and neem biodiesel blends and development of empirical models to predicts its characteristics. Fuel 2020, 262, 116341. [Google Scholar] [CrossRef]
- Dhiman, S.; Mukherjee, G. Utilization of food waste for biofuel production: A biorefining perspective. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Manikandan, G.; Kanna, P.R.; Taler, D.; Sobota, T. Review of Waste Cooking Oil (WCO) as a Feedstock for Biofuel—Indian Perspective. Energies 2023, 16, 1739. [Google Scholar] [CrossRef]
- Putra, N.R.; Veza, I.; Irianto, I. Harnessing wood waste for sustainable biofuel: A bibliometric analysis and review of valorisation strategies. Waste Manag. Bull. 2024, 2, 209–222. [Google Scholar] [CrossRef]
- Banerjee, N. Biomass to Energy—An Analysis of Current Technologies. Prospects, and Challenges. Bioenergy Res. 2023, 16, 683–716. [Google Scholar] [CrossRef]
- Chowdhury, H.; Loganathan, B. Third-generation biofuels from microalgae: A review. Curr. Opin. Green. Sustain. Chem. 2019, 20, 39–44. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Badawi, M.; Mohanakrishna, G.; Aminabhavi, T.M. Valorization of micro-algae biomass for the development of green biorefinery: Perspectives on techno-economic analysis and the way towards sustainability. Chem. Eng. J. 2023, 453, 139754. [Google Scholar] [CrossRef]
- de Queiróz Lamas, W. Algae’s potential as a bio-mass source for bio-fuel production: MLR vs. ANN models analyses. Fuel 2025, 395, 134853. [Google Scholar] [CrossRef]
- Mishra, A.; Medhi, K.; Maheshwari, N.; Srivastava, S.; Thakur, I.S. Biofuel production and phycoremediation by Chlorella sp. ISTLA1 isolated from landfill site. Bioresour. Technol. 2018, 253, 121–129. [Google Scholar] [CrossRef]
- Miranda, C.T.; de Lima, D.V.N.; Atella, G.C.; de Aguiar, P.F.; Azevedo, S.M.F.O.; Nitrogen, O.O. Phosphorus and Salt for Lipid Accumulation of Microalgae: Towards the Viability of Microalgae Biodiesel. Nat. Sci. 2016, 8, 557–573. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.A.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Shokravi, H.; Heidarrezaei, M.; Shokravi, Z.; Ong, H.C.; Lau, W.J.; Din, M.F.M.; Ismail, A.F. Fourth generation biofuel from genetically modified algal biomass for bioeconomic development. J. Biotechnol. 2022, 360, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Stellner, N.I.; Rerop, Z.S.; Mehlmer, N.; Masri, M.; Ringel, M.; Brück, T.B. Expanding the genetic toolbox for Cutaneotrichosporon oleaginosus employing newly identified promoters and a novel antibiotic resistance marker. BMC Biotechnol. 2023, 23, 40. [Google Scholar] [CrossRef]
- Di Fidio, N.; Minonne, F.; Antonetti, C.; Galletti, A.M.R. Cutaneotrichosporon oleaginosus: A versatile whole-cell biocatalyst for the production of single-cell oil from agro-industrial wastes. Catalysts 2021, 11, 1291. [Google Scholar] [CrossRef]
- Shoff, C.J.; Perfect, J.R. Uncommon Yeasts and Molds Causing Human Disease. Encycl. Mycol. 2021, 1, 813–834. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Zhang, C.; Liu, J.; Fu, H.; Zhou, W.; Gong, Z. Highly-efficient lipid production from hydrolysate of Radix paeoniae alba residue by oleaginous yeast Cutaneotrichosporon oleaginosum. Bioresour. Technol. 2024, 391, 129990. [Google Scholar] [CrossRef]
- Yook, S.; Deewan, A.; Ziolkowski, L.; Lane, S.; Tohidifar, P.; Cheng, M.H.; Singh, V.; Stasiewicz, M.J.; Rao, C.V.; Jin, Y.S. Engineering and evolution of Yarrowia lipolytica for producing lipids from lignocellulosic hydrolysates. Bioresour. Technol. 2025, 416, 131806. [Google Scholar] [CrossRef]
- Yang, Y.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A.; Thakur, N.; Zheng, Y.; Alalawy, A.I.; Koutb, M.; Salama, E.S. Potential of oleaginous microbes for lipid accumulation and renewable energy generation. World J. Microbiol. Biotechnol. 2024, 40, 337. [Google Scholar] [CrossRef]
- Bharadwaj, S.V.V.; Ram, S.; Pancha, I.; Mishra, S. Recent trends in strain improvement for production of biofuels from microalgae. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–225. [Google Scholar] [CrossRef]
- Raheem, A.; Azlina, W.A.K.G.W.; Yap, Y.H.T.; Danquah, M.K.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Pérez-Alva, A.; MacIntosh, A.J.; Baigts-Allende, D.K.; García-Torres, R.; Ramírez-Rodrigues, M.M. Fermentation of algae to enhance their bioactive activity: A review. Algal Res. 2022, 64, 102684. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Yunus Khan, T.M.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel. biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost. energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Dickinson, S.; Mientus, M.; Frey, D.; Amini-Hajibashi, A.; Ozturk, S.; Shaikh, F.; Sengupta, D.; El-Halwagi, M.M. A review of biodiesel production from microalgae. Clean Technol. Environ. Policy 2017, 19, 637–668. [Google Scholar] [CrossRef]
- El Maghraby, D.M.; Fakhry, E.M. Lipid content and fatty acid composition of Mediterranean macro-algae as dynamic factors for biodiesel production. Oceanologia 2015, 57, 86–92. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.M.I.; Saidur, R. Latest development in microalgae-biofuel production with nano-additives. Biotechnol. Biofuels 2019, 12, 125. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Pires, J.C.M. COP21: The algae opportunity? Renew. Sustain. Energy Rev. 2017, 79, 867–877. [Google Scholar] [CrossRef]
- Palanisami, S. Blended wastewater as a source of nutrients and biosynthetic elicitors for microalgal biorefinery. Green. Technol. Sustain. 2024, 2, 100098. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation. CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Ayre, J.M.; Moheimani, N.R.; Ubi, B.E.; Ogbonna, J.C. Growth comparison of microalgae in tubular photobioreactor and open pond for treating anaerobic digestion piggery effluent. Algal Res. 2016, 17, 268–276. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Yen, H.W.; Ho, S.H.; Lo, Y.C.; Cheng, C.L.; Ren, N.; Chang, J.S. Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour. Technol. 2015, 198, 619–625. [Google Scholar] [CrossRef]
- Sipaúba-Tavares, L.H.; Tedesque, M.G.; Scardoeli-Truzzi, B. Evaluation of the effects of sugarcane molasses as a carbon source for Ankistrodesmus gracilis and Haematococcus pluvialis (Chlorophyceae). Braz. J. Biol. 2020, 80, 594–600. [Google Scholar] [CrossRef]
- Magalhães, I.B.; Ferreira, J.; Castro, J.d.S.; de Assis, L.R.; Calijuri, M.L. Agro-industrial wastewater-grown microalgae: A techno-environmental assessment of open and closed systems. Sci. Total Environ. 2022, 834, 155282. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- Yao, L.; Shi, J.; Miao, X. Mixed wastewater coupled with CO2 for microalgae culturing and nutrient removal. PLoS ONE 2015, 10, e0139117. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, D.; Liu, W.; Cobb, K.; Zhou, N.; Liu, Y.; Liu, H.; Wang, Q.; Chen, P.; Zhou, C.; et al. Auto-flocculation microalgae species Tribonema sp. and Synechocystis sp. with T-IPL pretreatment to improve swine wastewater nutrient removal. Sci. Total Environ. 2020, 725, 138263. [Google Scholar] [CrossRef]
- Javed, F.; Rehman, F.; Khan, A.U.; Fazal, T.; Hafeez, A.; Rashid, N. Real textile industrial wastewater treatment and biodiesel production using microalgae. Biomass Bioenergy 2022, 165, 106559. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: A critical review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Singh, R.; Balagurumurthy, B.; Prakash, A.; Bhaskar, T. Catalytic hydrothermal liquefaction of water hyacinth. Bioresour. Technol. 2015, 178, 157–165. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth. lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Pruvost, J. Cultivation of Algae in Photobioreactors for Biodiesel Production. In Biofuels; Elsevier: Amsterdam, The Netherlands, 2011; pp. 439–464. [Google Scholar] [CrossRef]
- Jain, A.; Voulis, N.; Jung, E.E.; Doud, D.F.R.; Miller, W.B.; Angenent, L.T.; Erickson, D. Optimal intensity and biomass density for biofuel production in a thin-light-path photobioreactor. Environ. Sci. Technol. 2015, 49, 6327–6334. [Google Scholar] [CrossRef]
- Judd, S.; van den Broeke, L.J.P.; Shurair, M.; Kuti, Y.; Znad, H. Algal remediation of CO2 and nutrient discharges: A review. Water Res. 2015, 87, 356–366. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef]
- Wiley, P.; Harris, L.; Reinsch, S.; Tozzi, S.; Embaye, T.; Clark, K.; McKuin, B.; Kolber, Z.; Adams, R.; Kagawa, H.; et al. Microalgae Cultivation Using Offshore Membrane Enclosures for Growing Algae (OMEGA). J. Sustain. Bioenergy Syst. 2013, 3, 18–32. [Google Scholar] [CrossRef]
- Bitog, J.P.; Lee, I.B.; Lee, C.G.; Kim, K.S.; Hwang, H.S.; Hong, S.W.; Seo, I.H.; Kwon, K.S.; Mostafa, E. Application of computational fluid dynamics for modeling and designing photobioreactors for microalgae production: A review. Comput. Electron. Agric. 2011, 76, 131–147. [Google Scholar] [CrossRef]
- Kandiyoti, R.; Herod, A.; Bartle, K.; Morgan, T. Fossil fuels and renewables. In Solid Fuels and Heavy Hydrocarbon Liquids, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Gautam, R.; Patial, S.K.; Singh, S. Algae Biomass: Importance, Harvesting Techniques, Extraction Methods, and Associated Challenges. In Value Added Products From Bioalgae Based Biorefineries: Opportunities and Challenges; Arya, S.K., Khatri, M., Singh, G., Eds.; Springer: Singapore, 2024; pp. 67–94. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Ijaola, A.O.; Akamo, D.O.; George, T.T.; Sengul, A.; Adediji, M.Y.; Asmatulu, E. Algae as a potential source of protein: A review on cultivation. harvesting, extraction, and applications. Algal Res. 2024, 77, 103329. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Biotechnol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Hu, X.; Su, W.; Zhong, M. Combined enzymatic and mechanical cell disruption and lipid extraction of green alga Neochloris oleoabundans. Int. J. Mol. Sci. 2015, 16, 7707–7722. [Google Scholar] [CrossRef]
- Chutia, S.; Gohain, M.; Deka, D.; Kakoty, N.M. A Review on the Harvesting Techniques of Algae for Algal Based Biofuel Production. J. Energy Res. Environ. Technol. 2017, 4, 58–62. [Google Scholar]
- Min, K.H.; Kim, D.H.; Ki, M.R.; Pack, S.P. Recent progress in flocculation. dewatering, and drying technologies for microalgae utilization: Scalable and low-cost harvesting process development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef]
- Salim, S.; Bosma, R.; Vermuë, M.H.; Wijffels, R.H. Harvesting of microalgae by bio-flocculation. J. Appl. Phycol. 2011, 23, 849–855. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Visigalli, S.; Barberis, M.G.; Turolla, A.; Canziani, R.; Zrimec, M.B.; Reinhardt, R.; Ficara, E. Electrocoagulation–flotation (ECF) for microalgae harvesting–A review. Sep. Purif. Technol. 2021, 271, 118684. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, J.; Zheng, Y.; Hou, M.; Song, L.; Na, J.; Jiang, Y.; Huang, Y.; Liu, T.; Wei, H. Insight into coagulation/flocculation mechanisms on microalgae harvesting by ferric chloride and polyacrylamide in different growth phases. Bioresour. Technol. 2024, 393, 130082. [Google Scholar] [CrossRef] [PubMed]
- Al Hattab, M. Microalgae Harvesting Methods for Industrial Production of Biodiesel: Critical Review and Comparative Analysis. J. Fundam. Renew. Energy Appl. 2015, 5, 1000154. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from microalgae biomass: A review of conversion processes and procedures. Arab. J. Chem. 2022, 15, 103591. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Zabed, H.M.; Qi, X.; El-Shenody, R.A. Enhancing biomass and lipid productivity of a green microalga Parachlorella kessleri for biodiesel production using rapid mutation of atmospheric and room temperature plasma. Biotechnol. Biofuels Bioprod. 2022, 15, 122. [Google Scholar] [CrossRef]
- Lopes, D.; Aveiro, S.S.; Conde, T.; Rey, F.; Couto, D.; Melo, T.; Moreira, A.S.P.; Domingues, M.R. Algal lipids: Structural diversity; analysis; applications. In Functional Ingredients from Algae for Foods and Nutraceuticals, 2nd ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 335–396. [Google Scholar] [CrossRef]
- Shin, Y.S.; Choi, H.I.; Choi, J.W.; Lee, J.S.; Sung, Y.J.; Sim, S.J. Multilateral approach on enhancing economic viability of lipid production from microalgae: A review. Bioresour. Technol. 2018, 258, 335–344. [Google Scholar] [CrossRef]
- Theegala, C.S. Algal Cell Disruption and Lipid Extraction: A Review on Current Technologies and Limitations. In Algal Biorefineries; Prokop., A., Bajpai, R., Zappi, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 419–441. [Google Scholar] [CrossRef]
- de Moura, R.R.; Etges, B.J.; Santos, E.O.D.; Martins, T.G.; Roselet, F.; Abreu, P.C.; Primel, E.G.; D’Oca, M.G.M. Microwave-Assisted Extraction of Lipids from Wet Microalgae Paste: A Quick and Efficient Method. Eur. J. Lipid Sci. Technol. 2018, 120, 1700419. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-assisted extraction for microalgae: From biofuels to biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Kumar, R.R.; Rao, P.H.; Arumugam, M. Lipid extraction methods from microalgae: A comprehensive review. Front. Energy Res. 2015, 3, 61. [Google Scholar] [CrossRef]
- Antezana, M.D.; Sturm, B.S.M.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed Electric Field (PEF) as an Intensification Pretreatment for Greener Solvent Lipid Extraction From Microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal biofuels: Current status and key challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef] [PubMed]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Kumar, M.; Morya, R.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Characterization of carbon dioxide concentrating chemolithotrophic bacterium Serratia sp. ISTD04 for production of biodiesel. Bioresour. Technol. 2017, 243, 893–897. [Google Scholar] [CrossRef]
- Lee, O.K.; Lee, E.Y. Sustainable production of bioethanol from renewable brown algae biomass. Biomass Bioenergy 2016, 92, 70–75. [Google Scholar] [CrossRef]
- Manzoor, F.; Munir, N.; Sharif, N.; Naz, S. Harvesting and processing of microalgae biomass fractions for biodiesel production (a review). Sci. Tech. Dev. 2013, 32, 235–243. [Google Scholar]
- Saifuddin, N.; Samiuddin, A.; Kumaran, P. A Review on Processing Technology for Biodiesel Production. Trends Appl. Sci. Res. 2015, 10, 1–37. [Google Scholar] [CrossRef]
- Kumar, M.; Thakur, I.S. Municipal secondary sludge as carbon source for production and characterization of biodiesel from oleaginous bacteria. Bioresour. Technol. Rep. 2018, 4, 106–113. [Google Scholar] [CrossRef]
- McKennedy, J.; Sherlock, O. Anaerobic digestion of marine macroalgae: A review. Renew. Sustain. Energy Rev. 2015, 52, 1781–1790. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Maneein, S.; Harvey, P.J. A brief review of anaerobic digestion of algae for bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef]
- Paolini, V.; Petracchini, F.; Segreto, M.; Tomassetti, L.; Naja, N.; Cecinato, A. Environmental impact of biogas: A short review of current knowledge. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2018, 53, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Klassen, V.; Blifernez-Klassen, O.; Wibberg, D.; Winkler, A.; Kalinowski, J.; Posten, C.; Kruse, O. Highly efficient methane generation from untreated microalgae biomass. Biotechnol. Biofuels 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ometto, F.; Quiroga, G.; Pšenička, P.; Whitton, R.; Jefferson, B.; Villa, R. Impacts of microalgae pre-treatments for improved anaerobic digestion: Thermal treatment. thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014, 65, 350–361. [Google Scholar] [CrossRef]
- Hidaka, T.; Inoue, K.; Suzuki, Y.; Tsumori, J. Growth and anaerobic digestion characteristics of microalgae cultivated using various types of sewage. Bioresour. Technol. 2014, 170, 83–89. [Google Scholar] [CrossRef]
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from microalgae: Review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew. Sustain. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A review on the valorization of macroalgal wastes for biomethane production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, B.; Trably, E.; Steyer, J.P. Integrating microalgae production with anaerobic digestion: A biorefinery approach. Biofuels Bioprod. Biorefining 2014, 8, 516–529. [Google Scholar] [CrossRef]
- Macura, B.; Johannesdottir, S.L.; Piniewski, M.; Haddaway, N.R.; Kvarnström, E. Effectiveness of ecotechnologies for recovery of nitrogen and phosphorus from anaerobic digestate and effectiveness of the recovery products as fertilisers: A systematic review protocol. Environ. Evid. 2019, 8, 120. [Google Scholar] [CrossRef]
- Chandrasekhar, T.; Varaprasad, D.; Gnaneswari, P.; Swapna, B.; Riazunnisa, K.; Prasanna, V.A.; Korivi, M.; Wee, Y.J.; Lebaka, V.R. Algae: The Reservoir of Bioethanol. Fermentation 2023, 9, 712. [Google Scholar] [CrossRef]
- Nguyen, T.Y.; Cai, C.M.; Kumar, R.; Wyman, C.E. Overcoming factors limiting high-solids fermentation of lignocellulosic biomass to ethanol. Proc. Natl. Acad. Sci. USA 2017, 114, 11673–11678. [Google Scholar] [CrossRef]
- Behera, S.; Singh, R.; Arora, R.; Sharma, N.K.; Shukla, M.; Kumar, S. Scope of Algae as Third Generation Biofuels. Front. Bioeng. Biotechnol. 2015, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Gurav, R.; Bhatia, R.K.; Govarthanan, M.; Tyagi, V.K.; Kumar, V.; Pugazendhi, A.; Banu, J.R.; et al. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Bioresour. Technol. 2022, 358, 127437. [Google Scholar] [CrossRef]
- Juárez, J.M.; Hernando, A.L.; Torre, R.M.; Lanza, S.B.; Rodríguez, S.B. Saccharification of microalgae biomass obtained from wastewater treatment by enzymatic hydrolysis. Effect of alkaline-peroxide pretreatment. Bioresour. Technol. 2016, 218, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Castro, Y.A.; Ellis, J.T.; Miller, C.D.; Sims, R.C. Optimization of wastewater microalgae saccharification using dilute acid hydrolysis for acetone. butanol, and ethanol fermentation. Appl. Energy. 2015, 140, 14–19. [Google Scholar] [CrossRef]
- Postma, P.R.; Suarez-Garcia, E.; Safi, C.; Olivieri, G.; Olivieri, G.; Wijffels, R.H.; Wijffels, R.H. Energy efficient bead milling of microalgae: Effect of bead size on disintegration and release of proteins and carbohydrates. Bioresour. Technol. 2017, 224, 670–679. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Acién, F.G.; Alarcón, F.J. Differential hydrolysis of proteins of four microalgae by the digestive enzymes of gilthead sea bream and Senegalese sole. Algal Res. 2019, 37, 145–153. [Google Scholar] [CrossRef]
- Tan, H.T.; Khong, N.M.H.; Khaw, Y.S.; Ahmad, S.A.; Yusoff, F.M. Optimization of the freezing-thawing method for extracting phycobiliproteins from Arthrospira sp. Molecules 2020, 25, 3894. [Google Scholar] [CrossRef]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild disintegration of the green microalgae Chlorella vulgaris using bead milling. Bioresour. Technol. 2015, 184, 297–304. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Orr, V.C.A.; Plechkova, N.V.; Seddon, K.R.; Rehmann, L. Disruption and Wet Extraction of the Microalgae Chlorella vulgaris Using Room-Temperature Ionic Liquids. ACS Sustain. Chem. Eng. 2016, 4, 591–600. [Google Scholar] [CrossRef]
- Cho, H.S.; Oh, Y.K.; Park, S.C.; Lee, J.W.; Park, J.Y. Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris. Renew. Energy 2013, 54, 156–160. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Rizzo, A.M.; Pari, L. Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl. Energy 2017, 185, 963–972. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Chauhan, P.K.; Verma, M.; Bahuguna, V.; Joshi, H.C.; Ahmad, W.; Negi, P.; Sharma, N.; Ramola, B.; et al. Low-temperature catalyst based Hydrothermal liquefaction of harmful Macroalgal blooms, and aqueous phase nutrient recycling by microalgae. Sci. Rep. 2019, 9, 11384. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.H.; Nguyen, D.D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res. 2013, 2, 445–454. [Google Scholar] [CrossRef]
- Xu, X.; Tu, R.; Sun, Y.; Li, Z.; Jiang, E. Influence of biomass pretreatment on upgrading of bio-oil: Comparison of dry and hydrothermal torrefaction. Bioresour. Technol. 2018, 262, 261–270. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhang, Y.; Zhang, J.; Yu, G.; Schideman, L.C.; Zhang, P.; Minarick, M. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil. Bioresour. Technol. 2014, 152, 130–139. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Feng, S.; Xu, C.C.; Bassi, A. A review of recent developments of pre-treatment technologies and hydrothermal liquefaction of microalgae for bio-crude oil production. Renew. Sustain. Energy Rev. 2019, 101, 476–492. [Google Scholar] [CrossRef]
- Cheng, F.; Luo, H. Evaluating the minimum fuel selling price of algae-derived biofuel from hydrothermal liquefaction. Bioresour. Technol. Rep. 2022, 17, 100901. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar. bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Yu, J.; Maliutina, K.; Tahmasebi, A. A review on the production of nitrogen-containing compounds from microalgal biomass via pyrolysis. Bioresour. Technol. 2018, 270, 689–701. [Google Scholar] [CrossRef]
- Li, F.; Srivatsa, S.C.; Bhattacharya, S. A review on catalytic pyrolysis of microalgae to high-quality bio-oil with low oxygeneous and nitrogenous compounds. Renew. Sustain. Energy Rev. 2019, 108, 481–497. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Scale-Up and Commercialization of Algal Cultivation and Biofuel Production. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2014; pp. 261–286. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Jiang, X.; Han, X.; Ji, H. Compositional analysis of bio-oil derived from pyrolysis of seaweed. Energy Convers. Manag. 2013, 68, 273–280. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Morgan, T.; Wilson, M.; Crocker, M.; Zhang, J.; Liu, K.; Stork, J.; Debolt, S. Microalgae as a renewable fuel source: Fast pyrolysis of Scenedesmus sp. Renew. Energy 2013, 60, 625–632. [Google Scholar] [CrossRef]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic pyrolysis of microalgae to high-quality liquid bio-fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Cho, D.W.; Wang, D.; Tsang, D.C.W.; Zhang, S.; Ding, S.; Wang, L.; Ok, Y.S. Microwave-assisted low-temperature hydrothermal treatment of red seaweed (Gracilaria lemaneiformis) for production of levulinic acid and algae hydrochar. Bioresour. Technol. 2019, 273, 251–258. [Google Scholar] [CrossRef]
- Xu, W.; Ding, K.; Hu, L. A mini review on pyrolysis of natural algae for bio-fuel and chemicals. Processes 2021, 9, 2042. [Google Scholar] [CrossRef]
- Norouzi, O.; Tavasoli, A.; Jafarian, S.; Esmailpour, S. Catalytic upgrading of bio-products derived from pyrolysis of red macroalgae Gracilaria gracilis with a promising novel micro/mesoporous catalyst. Bioresour. Technol. 2017, 243, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, K.; Xia, M.; Yang, H.; Chen, Y.; Chen, X.; Che, Q.; Chen, H. Catalytic deoxygenation co-pyrolysis of bamboo wastes and microalgae with biochar catalyst. Energy 2018, 157, 472–482. [Google Scholar] [CrossRef]
- Wang, S.; Cao, B.; Liu, X.; Xu, L.; Hu, Y.; Afonaa-Mensah, S.; Abomohra, A.E.F.; He, Z.; Wang, Q.; Xu, S. A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM-5 catalysts. Bioresour. Technol. 2018, 256, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Chetpattananondh, P.; Ratanawilai, S. Application of extracted marine Chlorella sp. residue for bio-oil production as the biomass feedstock and microwave absorber. Energy Convers. Manag. 2019, 195, 819–829. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, W.; Chen, Y.; Yang, H.; Chen, H. Co-pyrolysis of microalgae and plastic: Characteristics and interaction effects. Bioresour. Technol. 2019, 274, 145–152. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y. Co-pyrolysis of algae with other carbon-based materials: A review on synergistic products and molecular reaction pathway. J. Therm. Anal. Calorim. 2023, 148, 2233–2249. [Google Scholar] [CrossRef]
- Bae, Y.J.; Ryu, C.; Jeon, J.K.; Park, J.; Suh, D.J.; Suh, Y.W.; Chang, D.; Park, Y.K. The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 2011, 102, 3512–3520. [Google Scholar] [CrossRef]
- Campanella, A.; Harold, M.P. Fast pyrolysis of microalgae in a falling solids reactor: Effects of process variables and zeolite catalysts. Biomass Bioenergy 2012, 46, 218–232. [Google Scholar] [CrossRef]
- Duman, G.; Uddin, M.A.; Yanik, J. Hydrogen production from algal biomass via steam gasification. Bioresour. Technol. 2014, 166, 24–30. [Google Scholar] [CrossRef]
- Rizwan, M.; Lee, J.H.; Gani, R. Optimal design of microalgae-based biorefinery: Economics, opportunities and challenges. Appl. Energy 2015, 150, 69–79. [Google Scholar] [CrossRef]
- Abioye, K.J.; Rajamanickam, R.; Ogunjinmi, T.; Paul, S.; Selvasembian, R.; Ighalo, J.O. Advancements in biomass waste conversion to sustainable biofuels via gasification. Chem. Eng. J. 2025, 505, 159151. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Raheem, A.; Abbasi, S.A.; Mangi, F.H.; Ahmed, S.; He, Q.; Ding, L.; Memon, A.A.; Zhao, M.; Yu, G. Gasification of algal residue for synthesis gas production. Algal Res. 2021, 58, 102411. [Google Scholar] [CrossRef]
- Raheem, A.; Ji, G.; Memon, A.; Sivasangar, S.; Wang, W.; Zhao, M.; Taufiq-Yap, Y.H. Catalytic gasification of algal biomass for hydrogen-rich gas production: Parametric optimization via central composite design. Energy Convers. Manag. 2018, 158, 235–245. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.S.; Saini, A.K.; Thakur, V.K. Recent update on gasification and pyrolysis processes of lignocellulosic and algal biomass for hydrogen production. Fuel 2023, 332, 126169. [Google Scholar] [CrossRef]
- Yang, M.; Yang, H.; Zhou, H.; Yang, Q.; Zhao, H.; Gul, E.; Khan, M.A.; Skreiberg, Ø.; Wang, L.; Chao, H.; et al. Syngas Production. Storage, Compression and Use in Gas Turbines. In Production of Biofuels and Chemicals with Pyrolysis, Biofuels and Biorefineries; Fang, Z., Smith, R.L., Jr., Xu, L., Eds.; Springer: Singapore, 2020; Volume 10, pp. 323–371. [Google Scholar] [CrossRef]
- Fiore, M.; Magi, V.; Viggiano, A. Internal combustion engines powered by syngas: A review. Appl. Energy 2020, 276, 115415. [Google Scholar] [CrossRef]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Rudovica, V.; Rotter, A.; Gaudêncio, S.P.; Novoveská, L.; Akgül, F.; Akslen-Hoel, L.K.; Alexandrino, D.A.M.; Anne, O.; Arbidans, L.; Atanassova, M.; et al. Valorization of Marine Waste: Use of Industrial By-Products and Beach Wrack Towards the Production of High Added-Value Products. Front. Mar. Sci. 2021, 8, 723333. [Google Scholar] [CrossRef]
- Gurau, S.; Imran, M.; Ray, R.L. Algae: A cutting-edge solution for enhancing soil health and accelerating carbon sequestration—A review. Environ. Technol. Innov. 2025, 37, 103980. [Google Scholar] [CrossRef]
- Zhong, Q.Q.; Zhao, Y.Q.; Shen, L.; Hao, B.; Xu, X.; Gao, B.Y.; Shang, Y.N.; Chu, K.Z.; Zhang, X.H.; Yue, Q.Y. Single and Binary Competitive Adsorption of Cobalt and Nickel onto Novel Magnetic Composites Derived from Green Macroalgae. Environ. Eng. Sci. 2020, 37, 188–200. [Google Scholar] [CrossRef]
- Saldarriaga-Hernandez, S.; Hernandez-Vargas, G.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef] [PubMed]
- Fabre, E.; Dias, M.; Costa, M.; Henriques, B.; Vale, C.; Lopes, C.B.; Pinheiro-Torres, J.; Silva, C.M.; Pereira, E. Negligible effect of potentially toxic elements and rare earth elements on mercury removal from contaminated waters by green. brown and red living marine macroalgae. Sci. Total Environ. 2020, 724, 138133. [Google Scholar] [CrossRef]
- Shobier, A.H.; El-Sadaawy, M.M.; El-Said, G.F. Removal of hexavalent chromium by ecofriendly raw marine green alga Ulva fasciata: Kinetic. thermodynamic and isotherm studies. Egypt. J. Aquat. Res. 2020, 46, 325–331. [Google Scholar] [CrossRef]
- Bouzikri, S.; Ouasfi, N.; Benzidia, N.; Salhi, A.; Bakkas, S.; Khamliche, L. Marine alga “Bifurcaria bifurcata”: Biosorption of Reactive Blue 19 and methylene blue from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 33636–33648. [Google Scholar] [CrossRef]
- Navarro, A.E.; Lim, H.; Chang, E.; Lee, Y.; Manrique, A.S. Uptake of Sulfa Drugs from Aqueous Solutions by Marine Algae. Sep. Sci. Technol. 2014, 49, 2175–2181. [Google Scholar] [CrossRef]
- Filote, C.; Volf, I.; Santos, S.C.R.; Botelho, C.M.S. Bioadsorptive removal of Pb(II) from aqueous solution by the biorefinery waste of Fucus spiralis. Sci. Total Environ. 2019, 648, 1201–1209. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Premaratne, M.; Liyanaarachchi, V.C.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Malik, A.; Attalage, R.A. Co-production of fucoxanthin, docosahexaenoic acid (DHA) and bioethanol from the marine microalga Tisochrysis lutea. Biochem. Eng. J. 2021, 176, 108160. [Google Scholar] [CrossRef]
- Stanic-Vucinic, D.; Minic, S.; Nikolic, M.R.; Velickovic, T.C. Spirulina Phycobiliproteins as Food Components and Complements. In Microalgal Biotechnology; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Boonpok, S.; Pornpukdeewattana, S. Combination of mechanical and chemical extraction of astaxanthin from Haematococcus pluvialis and its properties of microencapsulation. Biocatal. Agric. Biotechnol. 2021, 33, 101979. [Google Scholar] [CrossRef]
- Gayathri, S.; Rajasree, S.R.R.; Kirubagaran, R.; Aranganathan, L.; Suman, T.Y. Spectral characterization of β, ε-carotene-3, 3′-diol (lutein) from marine microalgae Chlorella salina. Renew. Energy 2016, 98, 78–83. [Google Scholar] [CrossRef]
- Bourdon, L.; Jensen, A.A.; Kavanagh, J.M.; McClure, D.D. Microalgal production of zeaxanthin. Algal Res. 2021, 55, 102266. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Sapalidis, A.; Kikionis, S.; Aggelidou, E.; Demiri, E.; Kritis, A.; Ioannou, E.; Roussis, V. Hybrid Sponge-Like Scaffolds Based on Ulvan and Gelatin: Design, Characterization and Evaluation of Their Potential Use in Bone Tissue Engineering. Materials 2020, 13, 1763. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong, E.W.N.; Fazita, M.R.N.; Hafiidz, J.M.; Banerjee, A.; Syakir, M.I. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Kwon, M.J.; Nam, T.J. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006, 79, 1956–1962. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Oh, Y.O.; Seo, H.; Oh, J. Marine Biopolymer-Based Nanomaterials as a Novel Platform for Theranostic Applications. Polym. Rev. 2017, 57, 631–667. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application: A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; López-García, M.; González-Muñoz, M.J.; Vilariño, J.M.L.; Domínguez, H. Ultrasound-assisted extraction of fucoidan from Sargassum muticum. J. Appl. Phycol. 2017, 29, 1553–1561. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Ebadi, S.; Nafchi, A.M.; Karim, A.A.; Kiahosseini, S.R. Functional properties of dually modified sago starch/κ-carrageenan films: An alternative to gelatin in pharmaceutical capsules. Carbohydr. Polym. 2017, 160, 43–51. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication. characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed. Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Bay-Larsen, I.; Risvoll, C.; Vestrum, I.; Bjørkhaug, H. Local protein sources in animal feed—Perceptions among arctic sheep farmers. J. Rural. Stud. 2018, 59, 98–110. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J.; Kumar, R.P.; Palani, S. Aquatic biomass (algae) as a future feed stock for bio-refineries: A review on cultivation. processing and products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Jha, S.; Singh, R.; Pandey, B.K.; Tiwari, A.K.; Shukla, S.; Dikshit, A.; Bhardwaj, A.K. Recent aspects of algal biomass for sustainable fuel production: A review. Discov. Sustain. 2024, 5, 300. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Chen, C.Y.; Chang, J.S. Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresour. Technol. 2020, 302, 122817. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Sharma, G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2022, 311, 122543. [Google Scholar] [CrossRef]
- Udayan, A.; Sirohi, R.; Sreekumar, N.; Sang, B.I.; Sim, S.J. Mass cultivation and harvesting of microalgal biomass: Current trends and future perspectives. Bioresour. Technol. 2022, 344, 126406. [Google Scholar] [CrossRef]
- Magalhães, I.B.; Pereira, A.S.A.d.P.; Silva, T.A.; Ferreira, J.; Braga, M.Q.; Couto, E.A.; Assemany, P.P.; Calijuri, M.L. Advancements in high-rate algal pond technology for enhanced wastewater treatment and biomass production: A review. J. Water Process Eng. 2024, 66, 105929. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, G.; Sun, S.; Hu, C.; Liu, J. Co-pelletization of microalgae and fungi for efficient nutrient purification and biogas upgrading. Bioresour. Technol. 2019, 289, 121656. [Google Scholar] [CrossRef]
- Banerjee, I.; Pohrmen, C.B.; Verma, R.; Dutta, S.; Singh, D. Microalgae-based carbon sequestration to mitigate climate change and application of nanomaterials in algal biorefinery. Octa J. Biosci. 2020, 8, 129–136. [Google Scholar]
- Pikula, K.; Zakharenko, A.; Stratidakis, A.; Razgonova, M.; Nosyrev, A.; Mezhuev, Y.; Tsatsakis, A.; Golokhvast, K. The advances and limitations in biodiesel production: Feedstocks. Oil extraction methods, production, and environmental life cycle assessment. Green Chem. Lett. Rev. 2020, 13, 11–30. [Google Scholar] [CrossRef]
- Chernova, N.I.; Kiseleva, S.V.; Larina, O.M.; Sytchev, G.A. Manufacturing gaseous products by pyrolysis of microalgal biomass. Int. J. Hydrog. Energy 2020, 45, 1569–1577. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef]
- Ziolkowska, J.R.; Simon, L. Recent developments and prospects for algae-based fuels in the US. Renew. Sustain. Energy Rev. 2014, 29, 847–853. [Google Scholar] [CrossRef]
- Merlo, S.; Durany, X.G.; Tonon, A.P.; Rossi, S. Marine microalgae contribution to sustainable development. Water 2021, 13, 1373. [Google Scholar] [CrossRef]
- Culaba, A.B.; Ubando, A.T.; Ching, P.M.L.; Chen, W.H.; Chang, J.S. Biofuel from microalgae: Sustainable pathways. Sustainability 2020, 12, 8009. [Google Scholar] [CrossRef]
- Lill, J.O.; Salovius-Laurén, S.; Harju, L.; Rajander, J.; Saarela, K.E.; Lindroos, A.; Heselius, S.J. Temporal changes in elemental composition in decomposing filamentous algae (Cladophora glomerata and Pilayella littoralis) determined with PIXE and PIGE. Sci. Total Environ. 2012, 414, 646–652. [Google Scholar] [CrossRef]
- Pragya, N.; Pandey, K.K. Life cycle assessment of green diesel production from microalgae. Renew. Energy 2016, 86, 623–632. [Google Scholar] [CrossRef]
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Allahverdiyeva, Y.; Funk, C. Wastewater treatment by microalgae. Physiol. Plant 2021, 173, 568–578. [Google Scholar] [CrossRef]
- Herrera, A.; D’Imporzano, G.; Fernandez, F.G.A.; Adani, F. Sustainable production of microalgae in raceways: Nutrients and water management as key factors influencing environmental impacts. J. Clean. Prod. 2021, 287, 125005. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Zhang, F.; Yun, J.; Shen, Z. The impact of biofuel plantation on biodiversity: A review. Chin. Sci. Bull. 2014, 59, 4639–4651. [Google Scholar] [CrossRef]
- Noraini, M.Y.; Ong, H.C.; Badrul, M.J.; Chong, W.T. A review on potential enzymatic reaction for biofuel production from algae. Renew. Sustain. Energy Rev. 2014, 39, 24–34. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrogen Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technol. Sustain. 2024, 2, 100060. [Google Scholar] [CrossRef]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.S.; Sim, S.J. Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Timiryazev, A.; Khmelnytskyi, B.; Maltseva, S.; Kociolek, J.P.; Kulikovskiy, M. A New Species of Freshwater Algae Nephrochlamys yushanlensis sp. nov. (Selenastraceae, Sphaeropleales) and Its Lipid Accumulation during Nitrogen and Phosphorus Starvation. J. Phycol. 2021, 57, 606–618. [Google Scholar] [CrossRef]
- Maltsev, Y.; Krivova, Z.; Maltseva, S.; Maltseva, K.; Gorshkova, E.; Kulikovskiy, M. Lipid accumulation by Coelastrella multistriata (Scenedesmaceae, Sphaeropleales) during nitrogen and phosphorus starvation. Sci. Rep. 2021, 11, 19818. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Xu, Z.; Qin, L.; Alam, M.A.; Wang, Z.; Zhu, S. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef]
- Li, X.; Chen, K.; He, Y. In situ and non-destructive detection of the lipid concentration of Scenedesmus obliquus using hyperspectral imaging technique. Algal Res. 2020, 45, 101680. [Google Scholar] [CrossRef]
- Shrestha, N.; Dandinpet, K.K.; Schneegurt, M.A. Effects of nitrogen and phosphorus limitation on lipid accumulation by Chlorella kessleri str. UTEX 263 grown in darkness. J. Appl. Phycol. 2020, 32, 2795–2805. [Google Scholar] [CrossRef]
- Mathieu, S.; Harding, J.; Tu, X. Plasma technology for syngas cleaning. In Advances in Synthesis Gas: Methods, Technologies and Applications: Volume 2: Syngas Purification and Separation; Elsevier: Amsterdam, The Netherlands, 2023; pp. 389–417. [Google Scholar] [CrossRef]
- Khandelwal, K.; Nanda, S.; Boahene, P.; Dalai, A.K. Conversion of biomass into hydrogen by supercritical water gasification: A review. Environ. Chem. Lett. 2023, 21, 2619–2638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).