1. Introduction
The valorization of food waste without generating pollutants represents a significant challenge and a crucial priority for sustainable industrial development aimed at preserving the environment. Biomass waste, which includes materials such as walnut shells, [
1], pecan shells [
2], apple peels [
2], date pits [
3], olive pits [
4], peach pits [
5], corn cobs [
6], coffee beans [
7], coffee grounds [
2,
8,
9,
10,
11], tea waste [
12], bagasse [
13], and coconut shells [
14], presents a promising market for recycling and value addition. These materials are particularly noteworthy due to their high carbon content, which allows them to serve as precursors for the production of modified cellulosic products or activated carbon. Coffee stands out as one of the most widely consumed beverages globally. According to the International Coffee Organization, approximately 680 million tons of coffee was produced in 2008. While some of the coffee grounds generated are repurposed for various applications such as soil remediation or odor adsorption, the majority require carbonization before they can be utilized effectively [
8]. It is important to note that the combustion of 1000 g of coffee grounds is estimated to produce around 538 g of carbon dioxide [
8]. This highlights the environmental impact associated with coffee waste and underscores the need for sustainable management practices.
Coffee grounds are composed of proteins (13–17%), lipids (7–21%), cellulose (8–15%), hemicellulose (30–40%), and lignin (20–30%) [
15]. Due to its chemical composition and high and sustainable annual production [
16], coffee grounds are a valuable raw material for the production of biogas, petroleum, biodiesel, plasticizers, bioplastics, compost, and fertilizers. They can also be used as an adsorbent for pollution control, as well as for the production of food products (e.g., for bakery and meat products) [
17]. Furthermore, coffee grounds can be used to extract cellulose and its derivatives, such as cellulose nanocrystals (CNCs) [
18], cellulose nanospheres (CNSs) [
19], and cellulose nanofibers (CNFs) [
20].
Coffee grounds are an abundant source of cellulose, extracted by chemical, enzymatic, mechanical, or hybrid methods. Chemical approaches are rapid but polluting, while enzymatic ones are ecological but expensive. Mechanical methods improve extraction but are energy-intensive. Combined strategies, such as chemo-enzymatic methods, offer a good compromise between efficiency and sustainability, allowing high yields with reduced impact. Once extracted, cellulose can be modified to optimize its properties, but these transformations must be controlled.
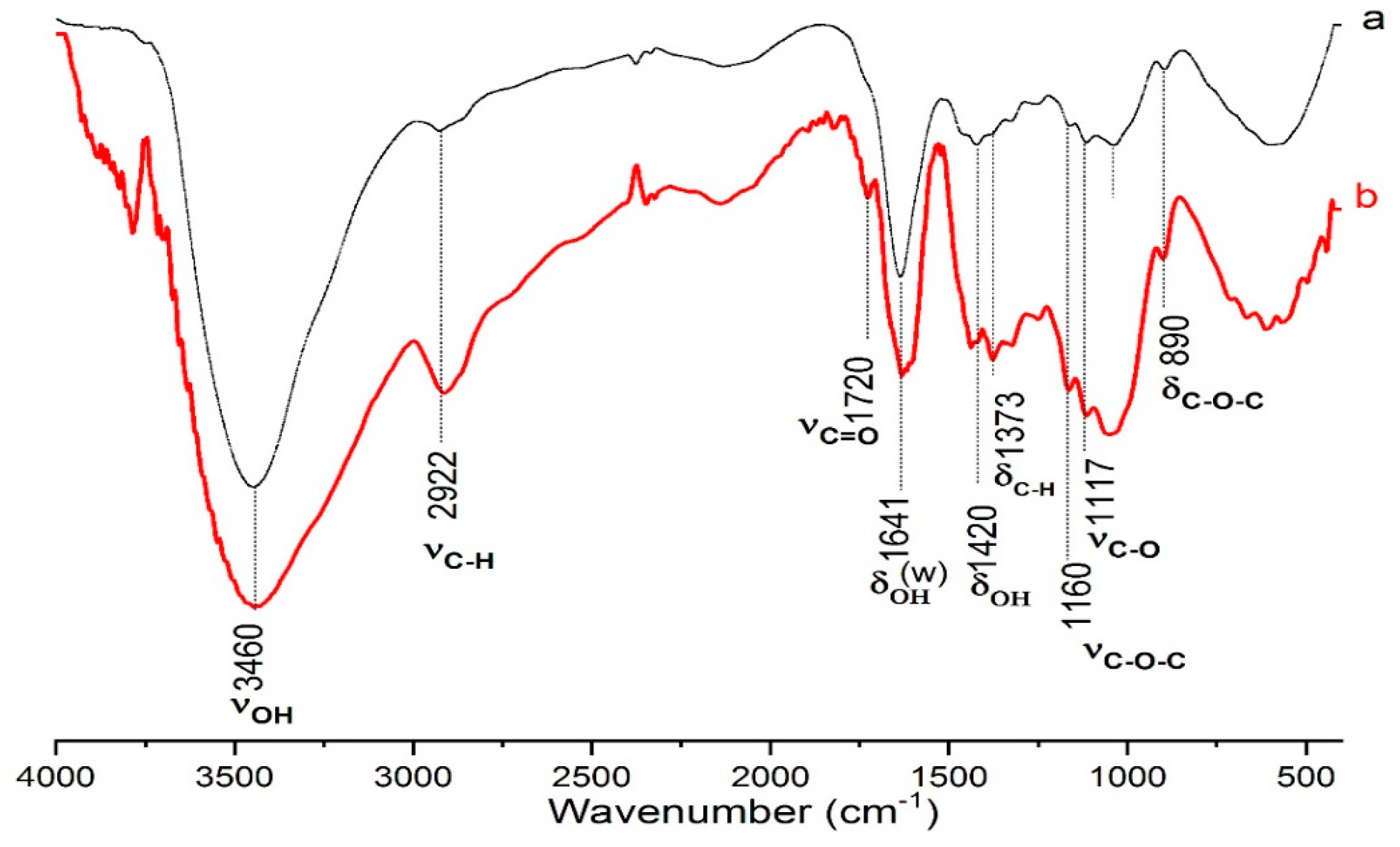
Cellulose is one of the most abundant and versatile biopolymers in the world, used in diverse sectors such as paper, textile, pharmaceutical, and food industries. However, its exploitation in advanced applications often requires chemical modifications to improve its functional properties. Selective oxidation of primary hydroxyl groups to carboxylic groups is one of the most promising methods to increase the reactivity and solubility of cellulose, opening the way to new applications in the fabrication of biocomposites, hydrogels, membranes, and biodegradable packaging. Additionally, emerging industries such as cosmetics and biotechnology are increasingly utilizing these cellulose derivatives. Chemical modification has emerged as one of the most extensively developed methods for enhancing cellulose properties. Among these methods, the oxidation of alcohol groups in cellulose carbohydrates has gained considerable attention in recent years. This growing interest can be attributed to the unique properties exhibited by oxidized polysaccharides and their potential for further modification through processes such as esterification. In our research focused on chemically modifying cellulose extracted from coffee grounds, we specifically selected the oxidation of alcohol groups to aldehydes or carboxylic acids. This oxidation process can be achieved through various methods utilizing a wide range of reagents; however, when selectivity or mild operational conditions are required, the selection of suitable reagents becomes more constrained. One effective and selective method for oxidation involves using oxoammonium salts, particularly di-tert-alkyl TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl radical). The TEMPO radical is generated in situ and selectively oxidizes primary and secondary alcohols in a chemoselective and regioselective manner. Among the available oxidants for this purpose, the TEMPO radical has emerged as one of the most widely studied and regenerated in contemporary research. The first documented use of this system was for oxidizing water-soluble polysaccharides such as starch, amylodextrin, and pullulans. This particular system was chosen due to its high selectivity in oxidizing primary alcohols. Over time, several oxidation methods have been developed to selectively modify the hydroxyl groups of various polysaccharides including starch, pullulan (a sugar polymer composed of maltotriose units), amylose [
21], cyclodextrins [
22,
23], and cellulose itself.
TEMPO oxidation improves the wettability and mechanical properties of cellulose but is slower and more expensive. TEMPO-NaClO oxidation is faster, producing more carboxyl groups, which increases solubility and is suitable for applications such as hydrogels. Both methods have advantages, with TEMPO-NaClO being more efficient but requiring careful handling of sodium hypochlorite.
The TEMPO-NaBr-NaClO process is a very efficient method for the oxidation of cellulose, producing a high degree of oxidation with the introduction of carboxyl groups (-COOH), which improves the solubility and wettability of cellulose. This process allows an oxidation degree of 80–90% to be reached [
24], which is higher than that of other methods, such as oxidation by TEMPO alone (30–60%), hydrogen peroxide (H
2O
2) (50–70%), or nitric acid (HNO
3) (50–80%) [
25]. While TEMPO-NaBr-NaClO is particularly effective in creating modified cellulose used in applications such as hydrogels or polyelectrolytes, other methods, such as ozone (30–50%) or N-chlorinated (40–70%) [
26] oxidation, offer lower carboxylation results and produce side reactions. However, the TEMPO-NaBr-NaClO method is very efficient.
Moreover, coffee-derived cellulose presents multiple advantages over conventional sources like wood and cotton, making it a promising sustainable alternative. One of the key benefits lies in its environmental impact, as it is sourced from waste biomass, reducing the need for deforestation and minimizing water and pesticide consumption. Unlike cotton, which requires extensive irrigation and chemical inputs, coffee grounds are an abundant byproduct of the coffee industry, contributing to waste valorization and promoting a circular economy. Economically, coffee cellulose is more cost-effective since it does not require additional cultivation, harvesting, or processing beyond extraction. Its availability as an industrial byproduct ensures a continuous and low-cost supply. Structurally, coffee-derived cellulose features shorter fibers, which provide enhanced flexibility and lightweight properties, making it particularly useful for biodegradable composites and packaging. Additionally, it exhibits superior thermal stability compared to some conventional cellulose sources, likely due to the presence of polyphenols and antioxidants that also confer antimicrobial properties. This unique composition makes coffee cellulose particularly valuable for applications in food packaging, biomedical materials, and filtration membranes. Moreover, its potential for nanocellulose production opens avenues for high-performance applications in coatings, electronics, and reinforced biopolymer composites. Given these benefits, coffee-derived cellulose represents a sustainable, economical, and functionally versatile alternative to traditional sources, offering significant potential for eco-friendly and high-value applications.
However, although several studies have been conducted on the modification of cellulose from conventional sources (wood, cotton, algae, etc.), few studies have explored the exploitation of coffee ground residues as an alternative source of cellulose. In addition, the physical and chemical properties of oxidized cellulose extracted from coffee grounds, such as its crystallinity index, degree of oxidation, and structural behavior, remain insufficiently documented.
The main objective of this study is therefore to extract and purify cellulose from coffee grounds and then chemically modify it by TEMPO-NaBr-NaClO oxidation to introduce carboxylic groups and improve its functional properties. A thorough characterization of the oxidized cellulose will be performed using advanced analytical techniques, including scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, and X-ray diffraction (XRD), to assess the impact of oxidation on its structure and performance. This research aims to enhance the value of agro-industrial waste into a functional material with high added value, contributing to the development of sustainable and eco-responsible solutions for the biomaterials industry.
2. Materials and Methods
2.1. Materials
The coffee grounds used in this study were sourced from ZOUHOUR, a renowned commercial establishment specializing in coffee beverages. This establishment is located in the bustling city of Marrakech, Morocco, at the geographic coordinates 31.642792, −7.981603. The coffee used in the research was Amarito M Milagro, a carefully selected blend of Robusta and Arabica beans. This selection was made due to the popularity of the coffee as well as its distinctive qualities that contribute to its appeal to local and international consumers. The Robusta–Arabica blend was chosen for its specific organoleptic properties, which influence not only the taste but also the chemical composition of the coffee residues. The coffee residues were stored in a temperature-controlled oven at 60 °C in order to preserve their chemical composition before use. This storage method helps to minimize the moisture present in the coffee beans, which can impact their chemical composition, particularly the cellulose contained in the grounds. Storing at a constant temperature also helps to reduce microbiological degradation and maintain the properties of the coffee residues until they are transformed into materials. 2,2,6,6-Tetramethylpiperidine 1-oxy radicals (TEMPO, 99.99%), sodium chlorite (NaClO2, 99%), sodium bromide (NaBr, 99%), sodium hydroxide (NaOH, 98.99% ), sodium hypochlorite (NaClO, 99%), and absolute ethanol were obtained from Aldrich. The chemicals used can pose risks to the environment and human health due to their corrosive, toxic nature or the formation of harmful byproducts. However, using them in reduced quantities in the oxidation process can limit these risks while maintaining the efficiency of the reaction.
2.2. Extraction and Purification of Cellulose
The extraction and purification of cellulose from coffee grounds involves a series of chemical processes to remove various contaminants, such as phenolic compounds, lignin, hemicelluloses, and other secondary metabolites, to isolate pure cellulose. Although coffee grounds are a rich source of cellulose, they also contain significant amounts of lignin, hemicelluloses, and other organic compounds that must be efficiently degraded or separated to obtain cellulose in its pure form. In this study, the coffee grounds were first subjected to oven-drying at 105 °C for approximately 30 min to remove any traces of moisture. This is an important step, as the presence of moisture could interfere with subsequent chemical treatments. After drying, the coffee grounds were sieved to remove larger particles, thus ensuring a more uniform sample for subsequent steps.
The next step was to treat the sieved coffee grounds with 1 N sodium hydroxide (NaOH) solution. This treatment was carried out at three successive intervals: 24 h, 12 h, and 6 h. Sodium hydroxide serves as an alkaline agent that breaks down the lignin and hemicelluloses present in the coffee grounds. Lignin, being a complex polymer, is largely responsible for the rigidity and structure of the plant cell wall, and its removal is crucial to isolate cellulose. Hemicellulose, another polysaccharide, is also partially soluble in alkaline solutions, which facilitates its degradation. The treatment time was gradually reduced from 24 h to 6 h, which allowed for complete degradation of the non-cellulosic components. After each treatment, the mixture was thoroughly washed with water to neutralize the alkali and remove dissolved impurities. The solution was then filtered to separate the liquid from the solid cellulosic residue. After the alkaline treatment, the residue was subjected to a bleaching process to remove any remaining color and further degrade the residual lignin. Bleaching was carried out using a sodium chlorite (NaClO2) solution at a concentration of 1.7% (w/v) at 80 °C for 40 min. Sodium chlorite is an effective bleaching agent that targets lignin and phenolic compounds, breaking them down and removing any remaining discoloration. This process produces a cleaner, whiter cellulose suitable for other applications.
Once bleaching was completed, the cellulose underwent triple filtration to ensure complete removal of any residual chemicals, impurities, or contaminants. Each filtration step helped to refine the purity of the cellulose. After filtration, the cellulose was thoroughly washed with water to remove any traces of chemicals from the bleaching process. To ensure complete purification, the cellulose was rinsed with acetone.
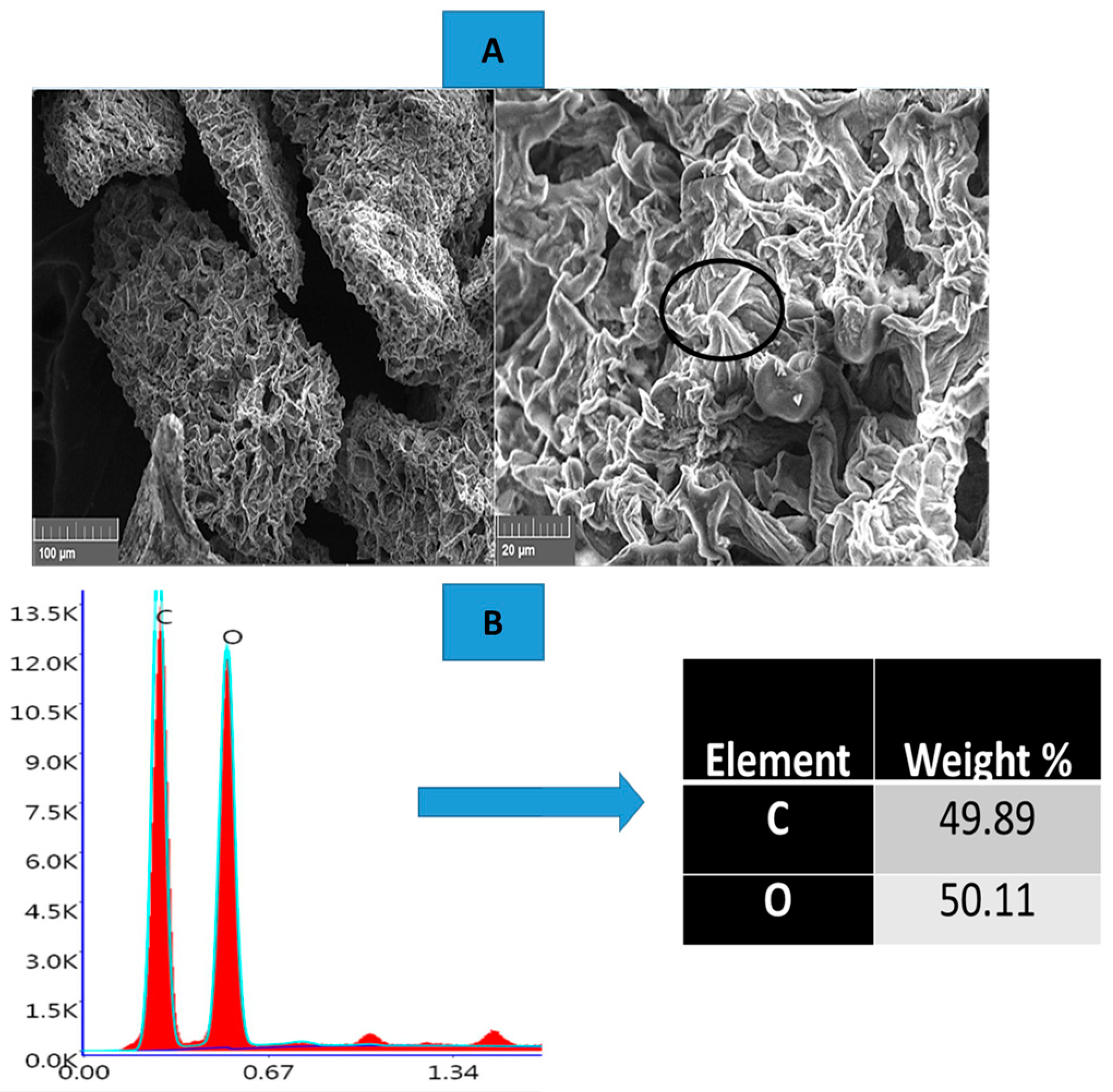
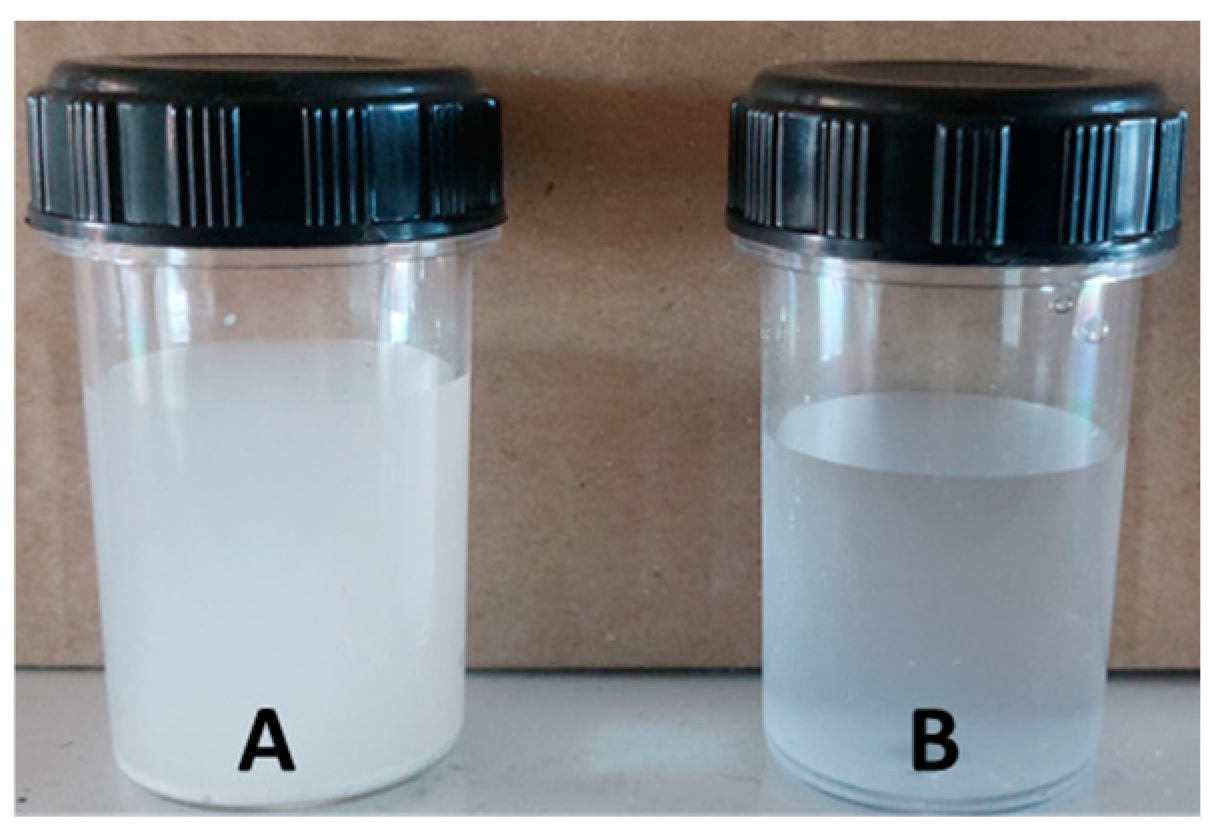
The resulting purified cellulose was a white solid, free of lignin, hemicellulose, and other contaminants, and was recovered for further use (
Figure 1).
2.3. Oxidation of Cellulose Hydroxyl Groups
The TEMPO oxidation process begins with adjusting the pH of the reaction system to 10.5, which is a crucial step to create the optimal conditions for the oxidation reaction. To initiate the process, a carefully prepared mixture containing sodium bromide (NaBr, 1.6 equivalents) and the TEMPO radical (0.0166 equivalents) is dissolved and introduced into the cellulose suspension. In this case, 0.5 g of cellulose was used as the substrate. Sodium bromide plays an important role as a source of bromine ions, which are essential for the oxidation reaction catalyzed by the TEMPO radical. The TEMPO radical itself is a well-known stable oxidizing agent that facilitates the selective oxidation of the primary alcohol groups present in cellulose to form carboxyl groups. Once the mixture is prepared, sodium hypochlorite (NaClO) is gradually added to the reaction system. Sodium hypochlorite serves as the oxidizing agent in this process, and its addition triggers the oxidation of cellulose. Typically, 3.5 equivalents of sodium hypochlorite are used, and it is added gradually to control the rate of the oxidation reaction. Throughout the reaction, it is critical to maintain the pH of the system at 10.5.
Although the TEMPO-NaClO oxidation mechanism may seem simpler at first glance, the choice of the TEMPO-NaBr-NaClO system has significant advantages in terms of efficiency and selectivity. The addition of sodium bromide (NaBr) plays a crucial catalytic role by generating hypobromite (OBr−), a much more powerful oxidant than hypochlorite (ClO−) present in the TEMPO-NaClO system alone. This increased oxidation power significantly accelerates the regeneration of the TEMPO+ oxoammonium ion, the active form of the catalyst, thus allowing faster and more complete oxidation of the substrate. In addition, the TEMPO-NaBr-NaClO system offers superior selectivity in certain applications, notably the selective oxidation of primary hydroxyl groups in polysaccharides. The presence of hypobromite influences the reaction kinetics, favoring the oxidation of primary hydroxyl groups over secondary hydroxyl groups. In contrast, the TEMPO-NaClO system alone can be limited by the oxidation rate, particularly in applications involving polymers, and may be more sensitive to pH and temperature variations. The addition of NaBr overcomes these limitations, enabling faster, more controlled, and more selective oxidation.
In summary, while the TEMPO-NaClO system can work, the TEMPO-NaBr-NaClO system is preferred due to its improved efficiency and selectivity, resulting in faster and more controlled oxidation. This difference is crucial in many applications, particularly those where selectivity and speed are paramount, thus justifying the authors’ choice of the TEMPO-NaBr-NaClO system.
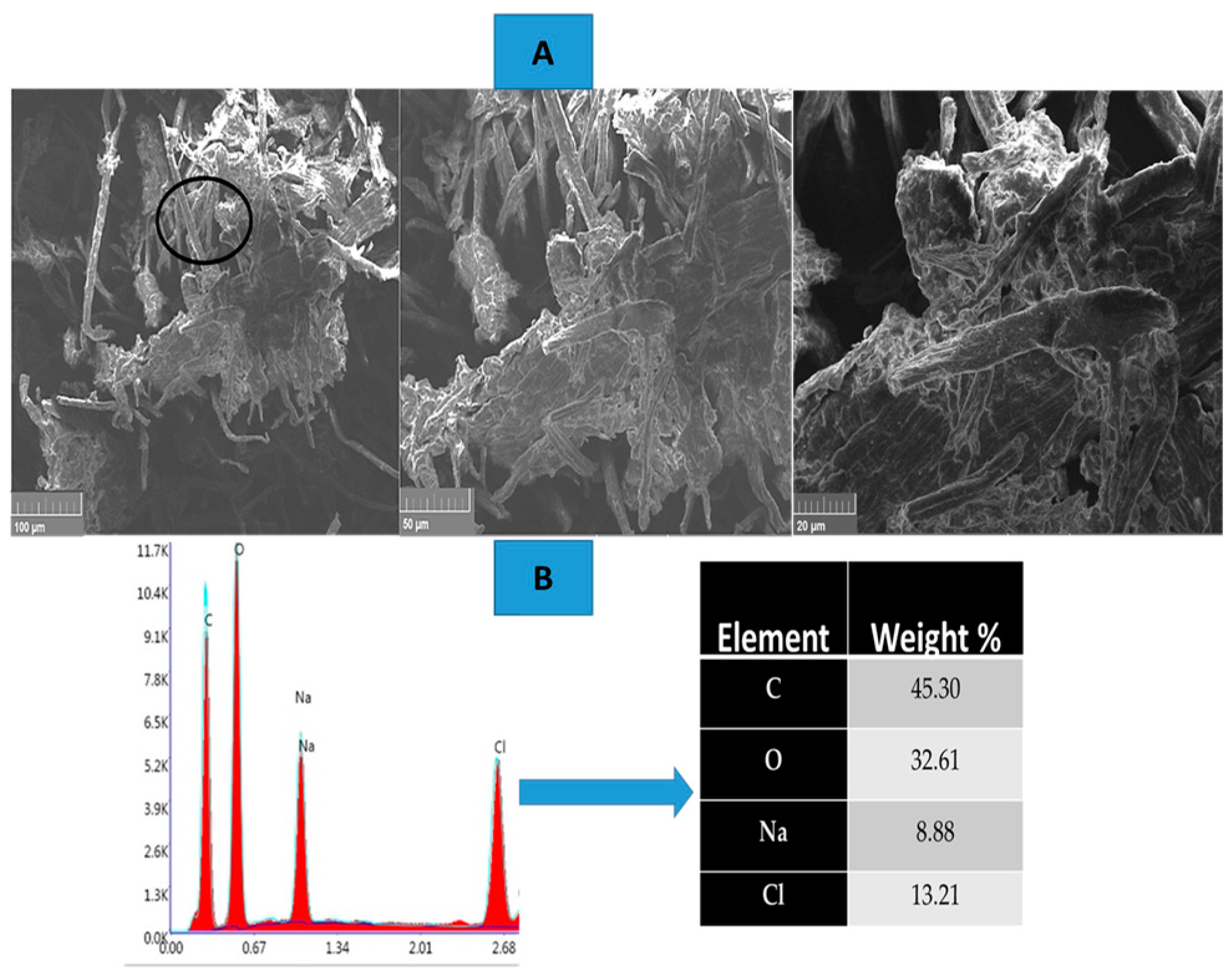
This is carried out by the periodic addition of a 1 M sodium hydroxide (NaOH) solution, which ensures that the environment remains basic, a condition necessary for the effective operation of TEMPO-mediated oxidation. Maintaining a stable pH is essential to avoid side reactions and to ensure that the cellulose is oxidized at the desired rate. The oxidation reaction is allowed to proceed for 120 min. During this time, the TEMPO radical catalyzes the oxidation of the cellulose, selectively converting the hydroxyl groups at the C6 position of the glucose units into carboxyl groups. This modification significantly enhances the reactivity and surface charge of the cellulose, which can improve its dispersion and compatibility in various applications, such as nanocellulose production. After the 120 min reaction period, the oxidation is terminated by adding 1.25 mL of absolute methanol. Methanol acts as a quenching agent, effectively stopping the reaction and preventing further oxidation. Once the reaction is stopped, the pH of the mixture is adjusted to neutral (pH 7) to stabilize the oxidized cellulose and ensure it remains in its desired form. This neutralization is important for preventing any continued reactivity of the oxidized cellulose and for ensuring that the final product can be safely handled and processed. To isolate the purified oxidized cellulose, the reaction mixture is subjected to centrifugation at 11,000 rpm for 30 min. This high-speed centrifugation step serves to separate the solid phase (oxidized cellulose) from the liquid phase, which contains residual reactants and byproducts. After centrifugation, the oxidized cellulose is washed thoroughly with distilled water in three successive cycles. This washing process is critical for removing any remaining sodium bromide, sodium hypochlorite, and other reaction byproducts, ensuring that the final product is free from contaminants. The resulting material is purified, oxidized cellulose that has undergone selective modification of its hydroxyl groups into carboxyl groups. The degree of oxidation of the cellulose is reported to be approximately 62%, which reflects the extent to which the primary alcohol groups have been converted to carboxyl groups during the oxidation process
Figure 2.
The economic feasibility of this process is supported by the use of low-cost coffee grounds, efficient oxidation, scalability potential, and growing market demand for sustainable materials. Environmentally, the use of a renewable resource like coffee grounds and the biodegradability of oxidized cellulose contribute to waste reduction and provide a more environmentally friendly alternative to synthetic polymers.
2.4. Analysis
2.4.1. Weight Loss Determination
The weight loss of the extracted cellulose was determined gravimetrically at each step of the extraction procedure. After oven-drying the samples and ensuring a constant weight, measurements were taken to calculate the mass lost during each stage of the process.
2.4.2. Degree of Oxidation
The degree of oxidation (OD) of cellulose was evaluated by conductometric titration with sodium hydroxide (NaOH). Conductimetry allows for the determination of the degree of oxidation and esterification of the samples. A conductimeter of the EUTECH con 510 type was used. The mass of the sample to be assayed was placed in a beaker in which 15 mL of 0.01 M hydrochloric acid was added in order to acidify the substrate. The mixture was stirred for 30–100 min and the titration was carried out with a solution of sodium hydroxide NaOH 0.01 M, following the variation in conductivity. The degree of oxidation was calculated by the following equation:
Herein, C is the concentration of NaOH (mol/L). V1 and V2 are the volumes of NaOH (L) used in the titration of oxidized cellulose and oxidized cellulose, respectively.
The molecular weight of the cellulose monomer was 162 g/mol. w is the dry mass of oxidized cellulose (g) [
27].
2.4.3. Degree of Polymerization
Oxidized cellulose (50 mg) was cut into small pieces and mixed with 5 mL of distilled water under stirring for 30 min. Then, 5 mL of cupriethylenediamine (Sigma-Aldrich) (Company SOMAPROL, Casablanca, Morocco) saturated with copper(II) hydroxide was added and the mixture was stirred for 60 min and then centrifuged for 5 min at 7000 rcf. Finally, the intrinsic viscosities of cellulose were obtained using a Rheotek Ubbelohde capillary tube viscometer placed in a water bath at 25 °C. The intrinsic viscosity as a function of the average molecular weight is generally represented by the empirical Mark–Houwink–Sakurada equation shown in Equation (2) [
28].
where K = 0.00015 and α = 1.9325.
Finally, the molecular mass of cellulose was calculated using the following relation:
where M is the molecular mass of the cellulose and 162 (g/mol) is the molecular mass of an anhydroglucose unit. OD is the degree of oxidation. All samples were analyzed in triplicate.
2.4.4. Crystallinity and Chemical Bonding of Constituents
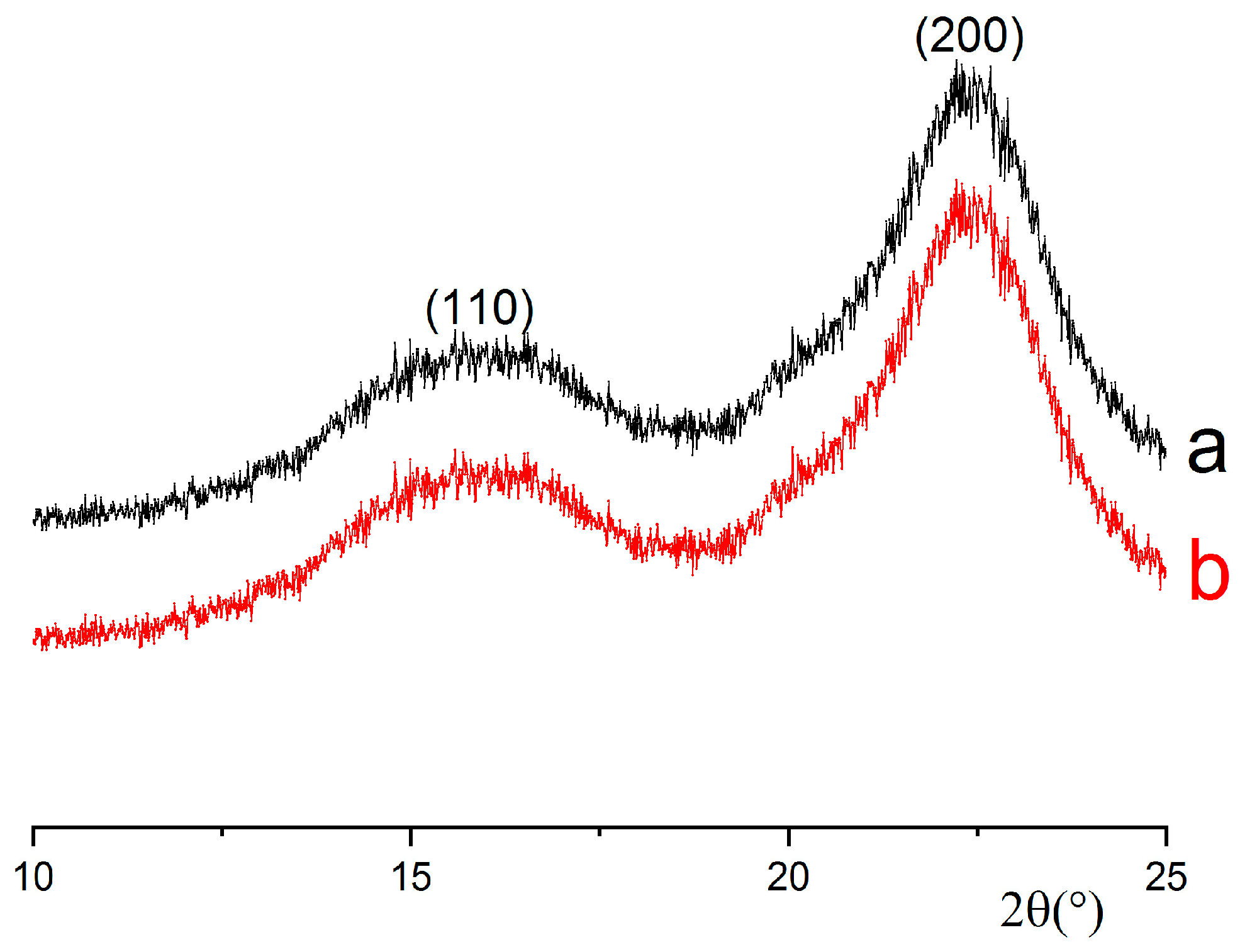
The XRD analysis was performed on powder samples using a Rigaku Smart Lab diffractometer operating with a copper anode (λKα = 1.5418 Å). The XRD traces were recorded under the following conditions: generator voltage: 40 kV; tube current: 30 mA. The diffraction angle (2θ) varied from 10 to 40°, with a scanning speed of 1.5° per minute and a step size of 0.05°. Circular samples were fixed on zero-background supports fabricated from 9 N semiconductor-grade silicon.
The crystallinity index (CI) was determined by using data obtained from the X-ray diffraction (XRD) trace of cellulose and Equation (4) [
29]:
where I
200 is the maximum intensity of the (200) lattice diffraction and I
am is the intensity diffraction at 18°.
Crystal sizes were determined according to the Scherrer equation (Equation (5)), where D is the average crystal size (nm), λ is the X-ray wavelength (1.54059 Å), 0.9 is the Scherrer constant, θ is the half diffraction angle for the I
200 plane in degrees, and β is the peak width at half its maximum in radians [
30]:
For the Fourier transform infrared (FTIR) spectroscopy investigation, to analyze chemical functional groups, samples were mixed with KBr (~1:100, w/w) and pressed into transparent pellets. FTIR spectra were recorded, shaped, and analyzed using a Perkin Elmer 1725-x spectrophotometer functioning in the range of 4000–400 cm−1.
2.4.5. Scanning Electron Microscopy and EDX Analysis
The microstructure of the carbon-coated cured samples was meticulously investigated using a TESCAN VEGA3 scanning electron microscope (SEM), offering high-resolution imaging capabilities. This SEM was equipped with a 20 mm2 X-Max diffusion silicon detector, which significantly enhances energy-dispersive X-ray (EDX) analysis, enabling precise elemental mapping of the sample. The morphological examination provided detailed insights into the surface features and texture of the carbon coatings. Additionally, EDX spectroscopy was employed to analyze the elemental composition of the carbon-coated surfaces, allowing for the identification and quantification of specific elements present in the sample.
4. Conclusions
This study underscores the sustainable valorization of coffee grounds by extracting and chemically modifying the cellulose content. The TEMPO-NaBr-NaClO oxidation system was employed to efficiently convert hydroxyl groups into carboxyl groups (-COOH), significantly enhancing the chemical reactivity and physical properties of the cellulose. Detailed analysis through FTIR spectroscopy, SEM, and EDX spectroscopy confirmed the successful introduction of new functionalities, such as carboxyl groups, and highlighted structural modifications that increase the material’s potential for various applications. This transformation not only offers a sustainable alternative to traditional materials but also contributes to the ecological management of organic waste, addressing the pressing demand for renewable and environmentally friendly materials. Moreover, the solubility of oxidized cellulose in water enhances its versatility, allowing it to be used in the production of polyelectrolytes, gels, and other functional intermediates, which are of high value in a variety of industries such as biomedicine, water treatment, and electronics. The incorporation of such materials could significantly affect the development of green technologies, paving the way for more sustainable innovations across multiple sectors. Overall, this research presents an exciting opportunity not only to valorize coffee grounds—a commonly discarded waste product—but also to develop functionalized cellulose with enhanced performance characteristics, contributing to the advancement of sustainable materials and the circular economy. TEMPO-oxidized cellulose improves paper strength and wettability, making it suitable for applications such as packaging and tissue paper. Its economic feasibility depends on processing costs and market demand, while environmental benefits include waste reduction. However, the use of chemicals in the oxidation process requires careful management to ensure the durability and biodegradability of the final product. Moreover, future research could focus on optimizing the solubility and functionality of TEMPO-oxidized cellulose by exploring various reaction conditions, such as temperature, reaction time, and reactant concentrations. Studies of molecular weight and oxidation degree are needed to improve their applications.