1. Introduction
Lignin is one of the most abundant biopolymers on Earth, accounting for about 15 to 40% of the composition of trees, plants, and algae. It is an amorphous polyphenol with a complex three-dimensional structure, formed by the polymerization of three monolignols: coniferyl, sinapyl, and p-coumaryl alcohol [
1,
2,
3]. Technical lignin, which is readily available from industrial pulping processes, particularly the kraft process, is a byproduct with an annual yield of approximately 130 million tons in the paper and pulp industry [
4,
5].
In addition to its wide availability, lignin’s complex structure, rich in phenolic and aliphatic functional groups, provides significant potential for chemical modification, enabling its use in various industrial applications. In recent years, lignin has been used both in the production of epoxy resin and polyurethane [
3,
6,
7,
8,
9], as well as in combination with polymer matrices to promote functional properties such as antioxidant [
10,
11,
12], antiozonant [
13], dielectric [
14], and modification of surface free energy [
15,
16].
Modifying surface free energy is particularly important for applications in packaging and adhesives, especially involving nonpolar matrices like polypropylene (PP), which is widely used in this sector. PP is a nonpolar polymer, resulting in low surface free energy [
17,
18], which consequently leads to poor adhesion properties, often causing coating failures or delamination in laminated films.
Combining PP with renewable-source polymers of a polar nature is not only an important approach to reducing the use of petroleum-based polymers but has also proven to be an effective strategy for modifying surface free energy and enhancing the adhesive properties of PP. Lignin, cellulose, and starch have demonstrated the ability to alter the hydrophobic nature and improve the wettability of PP [
15,
16,
18,
19,
20]. De Sousa et al. [
15] incorporated up to 5 wt% kraft lignin into the polypropylene matrix and performed corona discharge treatment on the sample surfaces. They observed a synergistic effect in increasing surface free energy in samples with lignin subjected to corona discharge, with the sample containing 2 wt% lignin showing the highest surface free energy among those analyzed.
However, a significant challenge remains in achieving adequate dispersions of lignin in nonpolar polymer matrices. At higher lignin concentrations, there is a tendency for large agglomerates to form, deteriorating mechanical and adhesive properties [
14,
21]. Chemical modification of lignin has been employed to improve interaction with the polymer matrix and optimize functional properties [
2]. In a previous work [
16], kraft lignin was hydroxypropylated and subsequently modified with acetic anhydride or maleic anhydride, then combined with a PP matrix. Both modified lignins showed better dispersion in the PP matrix and increased surface free energy of the samples, with the highest value observed in samples with 5 wt% modified lignin.
In this work, we propose to modify kraft lignin (KL) through a simple direct acetylation route, aiming to improve its dispersion in the PP matrix and evaluate the effects of acetylated lignin (AKL) on the wettability and practical adhesion of lignin/PP blends on aluminized biaxially oriented polypropylene (BOPP) substrates. The modification of lignin was confirmed through FTIR and DSC analyses. Subsequently, the combination of PP and acetylated lignin (AKL) and the production of polymer films were carried out in the molten state through a continuous extrusion process. The effects of lignin addition on the thermal and mechanical properties of PP were evaluated, along with the dispersion of lignin and the wettability of the obtained blends. Finally, films made of PP and PP with lignin were bonded to an aluminized BOPP film for practical adhesion analysis through peel tests. The results demonstrated an increase in surface free energy with the addition of lignin to PP, being more effective in samples with AKL due to better dispersion in the polymer matrix. Consequently, there was an improvement in the practical adhesion of the samples. The results indicate that the direct acetylation route not only significantly increases the surface free energy of PP but also promotes the use of lignin, a renewable and low-cost polymer, as a viable alternative to fossil-based materials. This approach offers a promising pathway for developing sustainable materials with improved adhesive properties.
2. Materials and Methods
2.1. Materials
Technical grade of kraft lignin (KL) from eucalyptus (hardwood) was kindly provided by Suzano S.A. (Limeira, Brazil). According to the supplier, KL has pH 8.1, a solid content of 92.5%, and an ash content of 10%, corresponding to the inorganic matter of the lignin [
22]. Polypropylene (PP), isotactic homopolymer, was obtained from Petroquímica CUYO S.A.I.C. (Buenos Aires, Argentina) and has a density of 0.9 g/cm
3 and melt flow index of 1.8 g/10 min. Acetic anhydride and diiodomethane were purchased from Merck (São Paulo, Brazil) and were used as received. The adhesive used was a bicomponent polyurethane (PU) from Henkel (Jundiaí, Brazil), consisting of the Liofol LA 3752-22 (isocyanate) and Liofol LA 6850-21 (polyol). Flexible biaxially oriented polypropylene (BOPP) films with a thickness of 200 µm, metallized with aluminum, were provided by Vitopel (Mauá, Brazil) and used as substrates in the laminated adhesive composed of PP-KL or AKL, PU, and BOPP.
2.2. Modification of Kraft Lignin
The procedure for acetylation of KL (
Figure 1) was based on the method described by Monteil-Rivera et al. [
23]. Initially, 150 g of KL samples was dried at 70 °C for 24 h to remove moisture and then mixed with 300 mL of acetic anhydride under constant stirring at 70 °C for 24 h. Subsequently, the mixture was transferred into 500 mL of deionized water. The resulting mixture underwent washing at least five times, followed by vacuum filtration and drying in an oven at 80 °C for 24 h. The reaction yielded acetylated kraft lignin (AKL).
2.3. Polymer Processing
The incorporation of KL or AKL into PP was conducted in the melt state using a corotating twin-screw extruder, Coperion ZSK 18 (Stuttgart, Germany). The mixtures were processed at a screw speed of 450 rpm, with temperatures ranging from 160 to 210 °C (from feed to die) across nine-barrel temperature zones. The concentrations of KL or AKL varied between 2.5 and 10 wt%, as presented in
Table 1.
Subsequently, to obtain flat films with a thickness of 200 µm, the samples were reprocessed using a single-screw extruder, AX Plásticos (Diadema, Brazil), with a screw diameter of 16 mm and an L/D ratio of 26. The extruder operated at a screw speed of 30 rpm, with a barrel temperature profile ranging from 160 °C to 190 °C. These flat film samples were then utilized for the characterization of the PP–lignin blends.
2.4. Lignin Characterizations
Lignins were characterized in their powdered form, being dried in a vacuum oven at 80 °C for 24 h before each characterization. Fourier-transform infrared spectroscopy in attenuated total reflection mode (FTIR-ATR) was conducted using a PerkinElmer Spectrum Two instrument (Hopkinton, MA, USA) to compare the chemical profiles of AKL and KL. Spectra were acquired from 3800 to 600 cm−1, with 32 scans and a resolution of 4 cm−1 at room temperature. The obtained data were normalized relative to the peak of the aromatic ring at 1515 cm−1.
Thermogravimetric analysis (TGA) of both modified and unmodified lignins were conducted using Mettler Toledo TGA equipment (Columbus, OH, USA). Approximately 8 mg of each sample was individually placed on the instrument’s balance for analysis. All samples were heated from 40 to 550 °C at a heating rate of 10 °C/min under a nitrogen atmosphere.
Differential scanning calorimetry (DSC) analysis was performed using a TA Instruments DSC Q200 instrument (Waltham, MA, USA). Approximately 8 mg of sample was subjected to a heating ramp up to 200 °C (held isothermally for 3 min) to erase thermal history, followed by cooling to −25 °C and reheating to 200 °C. All heating and cooling cycles were conducted at a rate of 10 °C/min under an inert nitrogen atmosphere. The glass transition temperature (Tg) was determined during the second heating cycle.
2.5. Polymer Characterizations
2.5.1. Optical Microscopy
Optical microscopy was employed to assess the dispersion of KL and AKL in the PP matrix. Micrographs were captured using a Carl Zeiss Axio Scope A1 microscope (Oberkochen, Germany) equipped with a Linkam heating module T95 HS (Salfords, UK). For sample preparation, approximately 4 mg of each sample was placed on a glass slide, covered with a coverslip, heated to 210 °C, and lightly compressed with tweezers. After cooling to room temperature, the samples were examined to obtain detailed images of the lignin dispersion.
2.5.2. Contact Angle
Contact angle measurements were performed using the sessile drop method on a SEO Phoenix 300 goniometer (Suwon, Korea). The test methodology was based on previous studies in the literature [
15,
24], in which two different liquids, distilled water and diiodomethane, were used. The droplet volume was set to 10 µL, and measurements were repeated five times for each sample. The surface tensions of both liquids are listed in
Table 2.
The solid surface free energy (
) was calculated using Young’s equation (Equation (1)) in conjunction with the Fowkes model (Equation (2)):
where
θ is the contact angle at the solid–liquid equilibrium condition,
is the surface tension of the liquid, and
is the solid–liquid interfacial energy.
and
are the dispersive and polar components of the surface free energy, respectively, while
and
are the dispersive and polar components of the liquid’s surface tension, respectively.
2.5.3. DSC
DSC analysis was performed using a TA Instruments DSC Q200 instrument (Waltham, MA, USA). Approximately 10 mg of each sample was subjected to a heating ramp up to 210 °C (held isothermally for 5 min) to erase thermal history, followed by cooling to −25 °C and reheating to 210 °C. All heating and cooling cycles were conducted at a rate of 10 °C/min under an inert nitrogen atmosphere. The Tg and melting temperature (Tm) were determined during the second heating cycle, while the crystallization temperature (Tc) was obtained from the cooling scan.
The degree of crystallinity
(%) of a PP-based blend were calculated using Equation (3):
where
is the enthalpy of fusion of the sample,
is the enthalpy of fusion of 100% crystalline PP (207 J/g) [
25], and
f is the concentration of lignin.
2.5.4. Tensile Test
Ultimate tensile strength was determined from tensile tests based on ASTM D882-18, using a universal testing machine, Instron model 3369 (Norwood, MA, USA). Tests were carried out under ambient conditions with a crosshead speed of 5 mm/min, using an optical extensometer with gauge length of 25 mm. Five specimens were tested for each condition.
2.5.5. T-Peel Test
The lamination process to obtain bonded laminates of PP/PU/BOPP was performed manually following the procedure established by Tavares et al. [
26]. PU adhesive (2 mL), a stoichiometric mixture concerning NCO:OH, was dissolved in ethyl acetate at a proportion of 50 wt% and applied to the surface of PP and PP–lignin films using a spiral roller with a controlled thickness of 10 µm. The adhesive-coated films were heated at 50 °C for 15 min to evaporate the excess solvent. Then, a BOPP film was applied over the adhesive-coated film to obtain laminated films. The PU adhesive was cured at room temperature for seven days. After curing, the laminates were cut into samples with dimensions of 2.54 × 10 cm
2 for peel tests.
The average peel strength of the laminates was evaluated using the T-peel test according to ASTM F904-98, with a universal testing machine, Instron model 3369 (Norwood, MA, USA). The T-peel test was conducted by pulling a 50 mm length of the PP film from the BOPP at a 90° angle between the films and the direction of the applied force. Tests were carried out under ambient conditions with a crosshead speed of 0.28 m/min. Five specimens were tested for each condition.
3. Results and Discussion
3.1. Lignin Modification
The spectrum of AKL compared to KL is shown in
Figure 2. The acetylation modification of KL is evidenced in
Figure 2a by a significant decrease in the intensity of the peak at the 3400 cm
−1 band, indicating the partial consumption of hydroxyl (OH) groups [
27]. In the 3050 to 2800 cm
−1 range, an increase in peak intensities is observed, corresponding to the asymmetric and symmetric stretching of CH
2 and CH
3 groups [
16].
In
Figure 2b, two peaks appear at 1765 and 1735 cm
−1, associated with the carbonyl groups of aromatic and aliphatic esters, respectively [
27,
28]. Additionally, the peak intensity around 1370 cm
−1 increases, which is attributed to the vibration of CH
2 and CH
3 groups [
29]. Another indication of successful acetylation is the pronounced peak at 1195 cm
−1, referring to the stretching of the C-O-C bond in aromatic acetyl groups [
16,
29], along with an increase in the intensity of the peak at 1120 cm
−1, related to the stretching of C-O bonds in ether groups [
16].
The presence of carbonyl peaks associated with aliphatic and aromatic esters confirms the reaction of the corresponding -OH groups during acetylation reaction [
27]. Consequently, FTIR spectroscopy enables a semiquantitative analysis of this reaction through the hydroxyl conversion (%Δ
OH), as shown in Equation (4):
where A
KL−OH and A
AKL−OH represent the areas of the -OH absorbance peaks for the KL and AKL samples, respectively. The areas of the -OH groups were determined with the baseline set in the range of 3740 cm
−1 to 3040 cm
−1, from spectra normalized with respect to the -C=C stretching of the aromatic ring, which remains unchanged during the acetylation reaction.
Thus, the acetylation conversion value was 75%, indicating partial acetylation, which is consistent with the findings of Gouveia et al. [
27] for similar reaction times.
TGA analysis was used to investigate the thermal degradation behavior of both unmodified KL and AKL.
Figure 3a displays the TGA curves. The TGA results show that both KL and AKL samples exhibit an initial mass loss due to water adsorbed in the polyphenols. The derivatives of the weight loss (DTG) curves reveal maximum degradation temperatures (T
d) of 353 °C and 380 °C for KL and AKL, respectively.
Figure 3b presents the DSC curves for KL and AKL. The T
g of KL is observed at 164 °C, which is consistent with values reported in the literature [
16,
30]. In contrast, the T
g of the modified lignin (AKL) is reduced to 128 °C. This reduction in T
g is primarily attributed to the acetylation process, where hydroxyl groups are replaced by acetyl groups, as indicated by the FTIR spectrum (
Figure 2). These acetyl groups exhibit lower polarity, resulting in reduced hydrogen bonding. This reduction in hydrogen bonding significantly affects the macromolecular dynamics of lignin, leading to the observed decrease in T
g [
16,
29].
3.2. Dispersion of Lignin
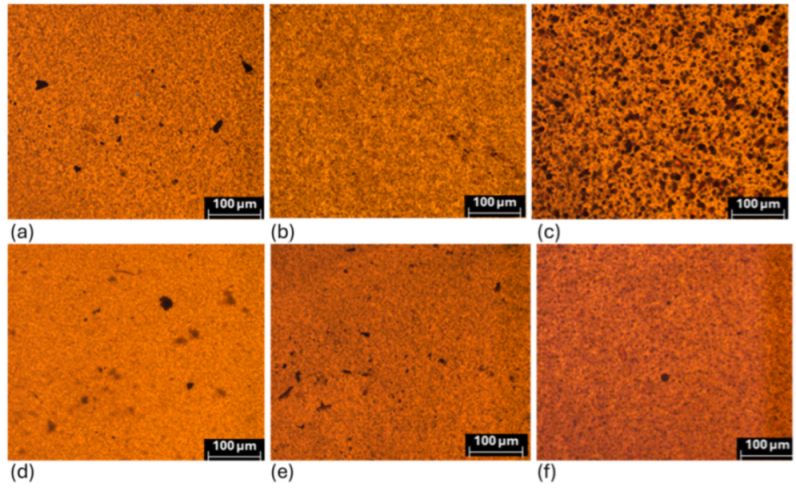
Figure 4 shows optical micrographs of samples of PP-KL (a–c) and PP-AKL (d–f). The difference in dispersion between the types of lignin is evident in the micrographs, especially at a concentration of 10 wt% KL, where large agglomerates form in the PP matrix. Achieving good dispersion levels of KL in nonpolar matrices like PP is challenging due to the polar nature of lignin [
21,
31,
32]. However, the reduction of hydroxyl groups in AKL improves the PP-AKL interaction, resulting in a better degree of dispersion compared to PP-KL.
3.3. Surface Free Energy
The wettability of films based on PP/KL and PP/AKL was assessed through contact angle measurements using water and diiodomethane. The aim was to determine whether the addition of KL and AKL, with their polar structures, increases the wettability of PP, which is intrinsically low due to its nonpolar structure.
Table 3 presents the contact angle values obtained with water and diiodomethane for the PP–lignin samples. Neat PP showed the highest contact angle with water, while the addition of lignin, regardless of the type, resulted in a reduction of the contact angle. This decrease is attributed to lignin’s polar functional groups, such as hydroxyl and carboxyl, which increase the material’s hydrophilicity.
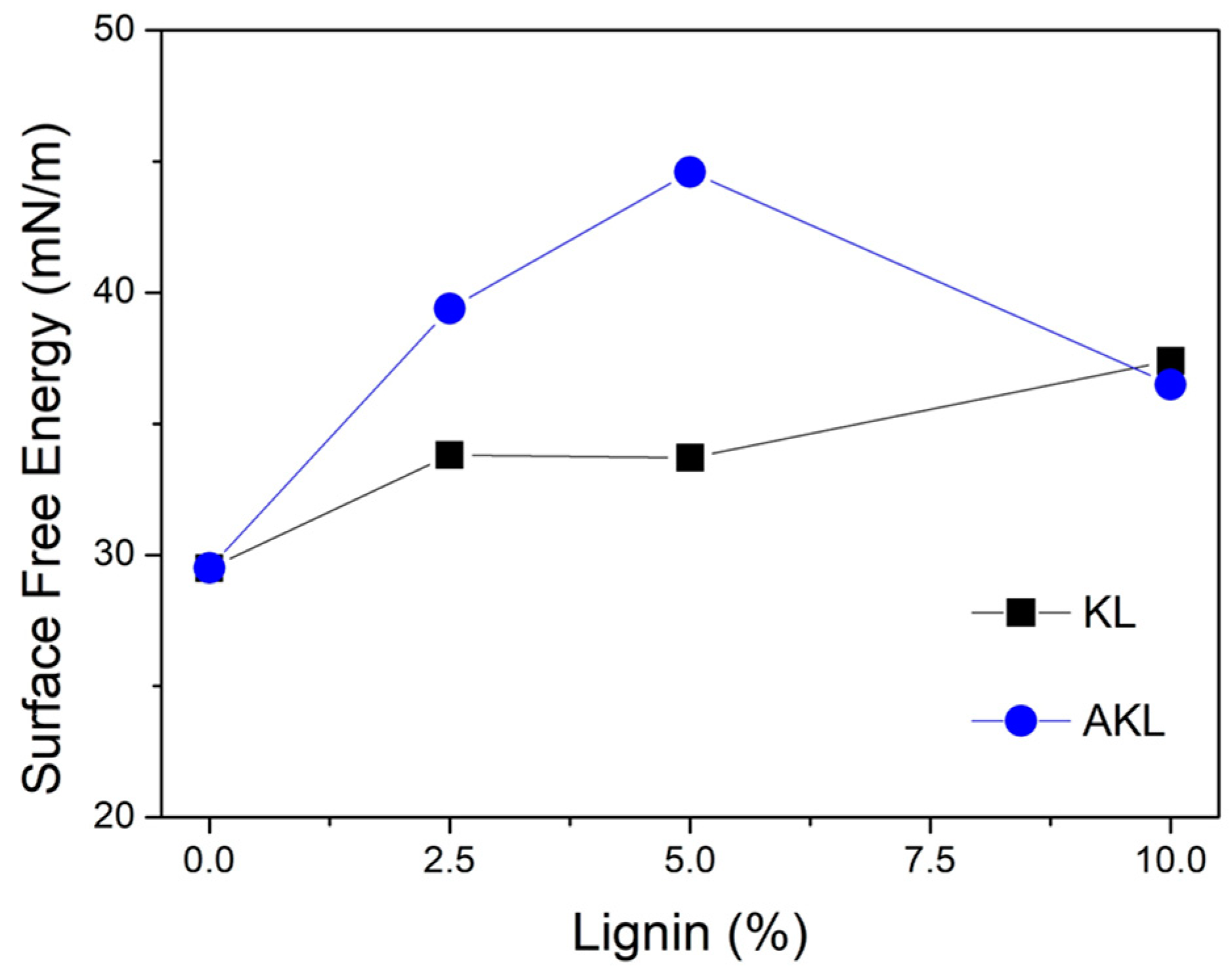
The total surface free energy, calculated from the contact angle data, is shown in
Figure 5, while the values of its polar and dispersive contributions are presented in
Table S1. As expected, adding lignin increased the surface free energy of PP. Comparing different types of lignin, AKL caused a more significant increase in surface energy compared to KL. This is likely due to better dispersion in the polymer matrix, as seen in optical microscopy (
Figure 4). Specifically, the PP-AKL 5 sample had the highest surface free energy at 44.6 mN/m, a 51% increase over neat PP.
Samples with KL showed an increase in surface free energy, stabilizing around 33.8 mN/m for concentrations of 2.5 wt% and 5 wt%, with the highest value observed at the 10 wt% concentration, 37.4 mN/m. This sudden rise in surface free energy may be attributed to the formation of significantly larger agglomerates due to the high KL concentration. These agglomerates promote greater phase segregation, increasing the availability of lignin on the sample surface and enhancing wettability.
On the other hand, it is noteworthy that the PP-AKL 10 sample shows a decrease in surface free energy, although it remains higher than the PP matrix and similar to the KL sample. As discussed, obtaining proper dispersion of lignin in non-polar matrices such as PP is a major technological challenge, particularly at high lignin concentrations. In this context, the PP-AKL 5 sample demonstrated the most favorable balance between lignin dispersion and the enhancement of PP’s surface free energy.
3.4. Thermal Properties
DSC analysis was performed to evaluate the effects of KL and AKL incorporation on the degree of crystallinity of the PP matrix, as well as the influence of KL’s chemical modification and concentration on the thermal behavior of KL and AKL-PP.
Table 4 presents the main thermal events as well as the degree of crystallinity. DSC thermograms from the cooling and second heating cycle are presented in
Figure S1.
The addition of KL and AKL did not induce significant changes in the crystallinity of the materials compared to neat PP. PP-AKL exhibited slightly higher crystallinity levels than those based on KL. Interestingly, higher crystallinity degrees were observed at elevated concentrations of KL and AKL, which contrasts the results reported in other studies [
15,
33,
34]. Previous studies in the literature have suggested that at low concentrations, KL can enhance the crystallinity of thermoplastics by serving as nucleation sites [
15,
33,
35]. At higher concentrations of lignin, it is expected that the amorphous phase becomes more pronounced, thereby reducing the overall crystallinity of the blends. However, several studies have reported no significant changes in the degree of crystallinity with the incorporation of lignins [
15,
16,
35,
36], which supports the findings of the present work.
The thermograms showed that the addition of KL and AKL to PP resulted in slight reductions in TC, with this reduction being more significant at higher concentrations of KL and AKL.
Furthermore, the T
g of lignin, KL or AKL, was not detectable in the blend with PP. Previous studies have also reported a similar trend [
15,
16]. This phenomenon is hypothesized to arise from the overlap of the lignin Tg with the crystalline melting region of PP.
3.5. Ultimate Tensile Strength
The analysis of ultimate tensile strength (UTS) provides an indication of the interaction between components in a polymer blend [
21].
Figure 6 presents the UTS results for the PP-KL and PP-AKL samples.
Ultimate tensile strength results showed that the addition of KL and AKL caused a reduction in ultimate tensile strength at all concentrations compared to neat PP. Nevertheless, there was an increase in tensile strength as the content of KL and AKL increased from 2.5% to 5%, with 5% being the optimal concentration for both acetylated and unmodified lignin blends. Recently, Tahir et al. [
36] evaluated the influence of incorporating tobacco-extracted lignin on the mechanical properties of PP blends based on lignin. The authors also obtained higher values of ultimate tensile strength for the blend with 5% lignin. In this study, at the concentration of 10%, ultimate tensile strength decreased for both KL and AKL blends, with this reduction being more pronounced in the unmodified lignin blends.
Other studies in the literature have reported a decrease in the tensile strength of PP and other thermoplastics with the addition of lignin, with the decline in mechanical properties becoming more prominent when the lignin content in the blend exceeds 10 wt% [
14,
21,
37,
38]. Johansson et al. [
37] observed that the incorporation of acetylated lignin into PLA progressively reduced the tensile strength of the blends. The authors evaluated blends based on PLA and acetylated lignin (via reaction with acetic anhydride and pyridine as a catalyst) at concentrations ranging from 5 wt% to 60 wt%. The blend with 5 wt% of acetylated lignin exhibited the highest tensile strength and impact strength among the concentrations analyzed.
The increase in KL concentration results in a higher number of lignin aggregates, which act as stress concentration points and degrade the mechanical performance of the blend. However, it was noteworthy that acetylated lignin consistently provided superior tensile strengths compared to unmodified lignin across all concentrations used in the blends. The conversion of hydroxyl groups into ester groups through acetylation reduces polarity and increases the size of the lateral groups of lignin macromolecules (increase in molecular free volume), thereby enhancing intermolecular interactions with PP chains [
37,
39,
40]. The improved interaction between AKL particles and the PP matrix enhances their dispersion compared to KL. This enhancement not only improves the mechanical properties due to better blend homogeneity but also facilitates interfacial stress transfer between the PP matrix and the dispersed AKL phase (agglomerates and clusters). Indeed, the results from optical microscopy showed that blends based on AKL exhibited better dispersion than those based on KL, which may be directly related to their superior mechanical properties.
3.6. Peel Strength
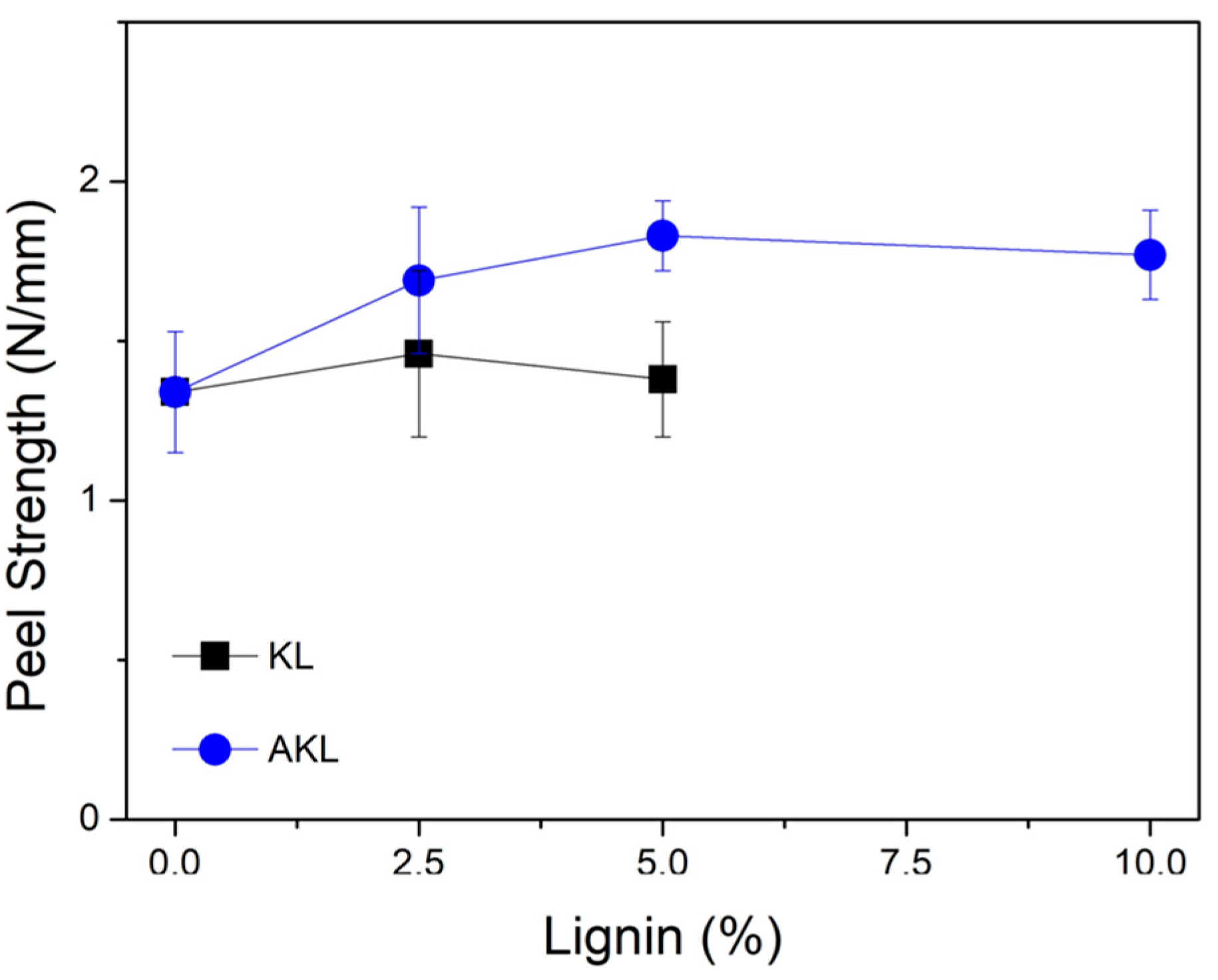
Figure 7 shows the results of the average peel strength for the PP-KL and PP-AKL samples. The addition of AKL results in higher peel strength values, and thus better practical adhesion was observed for BOPP/PU/PP-AKL laminates compared to BOPP/PU/PP laminates. In the peel test, samples based on AKL also exhibited better performance, particularly at the 5 wt% concentration.
On the other hand, the addition of KL led to a slight increase in peel strength only at low concentration, 2.5 wt%, followed by a decrease from the 5 wt% concentration. Additionally, due to the high concentration of agglomerates in the PP-KL 10.0 sample, it was not possible to obtain laminated films under these conditions for the T-peel test.
In the literature, two primary hypotheses are proposed to explain the improved adhesion provided by lignin when incorporated into thermoplastics. The first hypothesis is based on the reduction in PP crystallinity: lower crystallinity implies a greater amorphous phase, and the larger the amorphous phase, the higher the mobility of the thermoplastic polymer chains due to reduced blocking by crystalline domains [
41,
42]. This increased mobility enhances the diffusion of polymer chains into the substrate’s surface, thereby increasing interfacial adhesion. The second mechanism relates to improved wettability, which arises from the increase in surface energy caused by the incorporation of lignin. It is known that the addition of lignin to polymers tends to increase their surface energy, thereby enhancing the adhesion of lignin-based materials to various substrates [
15,
16,
43].
Since the crystallinity of the blends based on KL and AKL was not significantly altered, it is not feasible to attribute the improvement in practical adhesion to the slightly lower crystallinity degrees (as observed in the results of the DSC analysis). The superior performance of PP-AKL over neat PP is hypothetically due to the increase in surface energy that these polyphenols provide when incorporated into neat PP.
Similar results have been reported in previous studies. De Sousa et al. [
15] investigated the impact of KL incorporation on the practical adhesion of BOPP/PU/PP-KL laminates, utilizing both standard PP-KL and corona-treated PP-KL films. The authors observed an increase in surface energy for both untreated PP and corona-treated PP laminates with KL incorporation.
Bisneto et al. [
16] also investigated the practical adhesion of laminates comprising layers of BOPP, PU, and blends of PP with KL and esterified lignins (synthesized via two methods, one involving maleic anhydride and the other involving acetic anhydride reactions). The authors also reported that lignin incorporation did not affect the degree of crystallization of PP. Modifications to lignin improved compatibility with the PP matrix, leading to higher surface energies than those of neat PP. Additionally, they observed increases in peel strength, further corroborating the findings of the present study. It is noteworthy that in the work by Bisneto et al. [
16], KL was previously oxypropylated before the esterification reactions. Our results suggest that a direct and simplified modification route of KL, combined with PP, enhances the surface free energy of the polymeric matrix and improves practical adhesion.
4. Conclusions
In this study, KL was acetylated via direct reaction with acetic anhydride. The modification was confirmed by FTIR and DSC analyses. PP with KL and AKL was prepared in the molten state through a continuous extrusion process. The degree of lignin dispersion in the polymer and the mechanical and thermal properties and surface free energy were evaluated. The influence of KL and AKL on the practical adhesion of PP was assessed using T-peel tests. Laminates containing PP and PP–lignin films were prepared with PU adhesive and BOPP. The results demonstrated that AKL significantly improved dispersion within the PP matrix compared to KL, leading to increased surface free energy and enhanced practical adhesion. Notably, samples containing AKL exhibited superior performance in these aspects compared to unmodified KL. This study highlights the potential of direct acetylation of lignins to enhance their compatibility with PP, paving the way for the increased use of lignin, a renewable biopolymer, in a wide range of technological applications.