From Citrus Waste to Valuable Resources: A Biorefinery Approach
Abstract
1. Introduction
2. Methodology
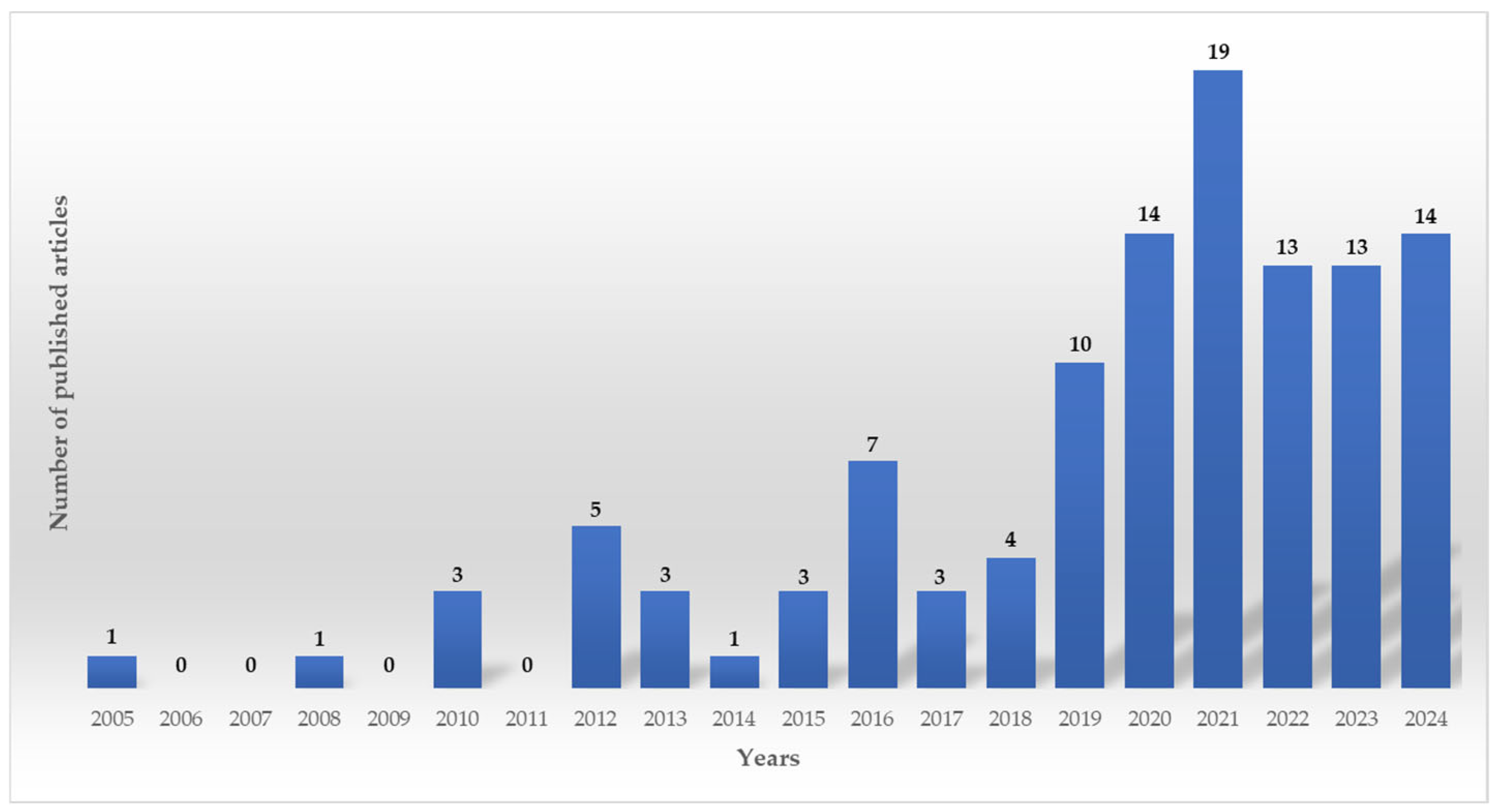
3. Search Results
4. Chemical Composition of Citrus Wastes
5. Phytochemicals Extracted from Citrus Wastes
5.1. Extraction of Essential Oil
5.2. Extraction of Polyphenolic Compounds
5.3. Pectin Extraction
5.4. Micro- and Nano-Cellulose
5.5. Protein and Enzymes
5.6. Other Phytochemicals
6. Added-Value Compounds Produced from Citrus Wastes
6.1. Bioethanol Production
6.2. Biomethane Production
6.3. Production of Volatile Fatty Acids and Biodiesel
6.4. Production of Microbial Enzymes
6.5. Production of Levulinic Acid
6.6. Other Added-Value Compounds
7. Concluding Remarks and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Citrus Fruit Statistical Compendium 2020. Rome. 2021. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/4760a5b5-f3b2-41c7-8713-ccdb1a5f8c08/content (accessed on 26 June 2024).
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Okino-Delgado, C.H.; Pereira, M.S.; da Silva, J.V.I.; Kharfan, D.; do Prado, D.Z.; Fleuri, L.F. Lipases obtained from orange wastes: Commercialization potential and biochemical properties of different varieties and fractions. Biotechnol. Prog. 2019, 35, e2734. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Remelli, G.; Zanzoni, S.; Bolzonella, D. Valorization of Residual Orange Peels: Limonene Recovery, Volatile Fatty Acids, and Biogas Production. ACS Sustain. Chem. Eng. 2020, 8, 6834–6843. [Google Scholar] [CrossRef]
- Patsalou, M.; Samanides, C.G.; Protopapa, E.; Stavrinou, S.; Vyrides, I.; Koutinas, M. A Citrus Peel Waste Biorefinery for Ethanol and Methane Production. Molecules 2019, 24, 2451. [Google Scholar] [CrossRef] [PubMed]
- Rizzioli, F.; Benedetti, V.; Patuzzi, F.; Baratieri, M.; Bolzonella, D.; Battista, F. Valorization of orange peels in a biorefinery loop: Recovery of limonene and production of volatile fatty acids and activated carbon. Biomass Convers. Biorefin. 2024, 14, 9793–9803. [Google Scholar] [CrossRef]
- Espinosa, E.; Rincón, E.; Morcillo-Martín, R.; Rabasco-Vílchez, L.; Rodríguez, A. Orange peel waste biorefinery in multi-component cascade approach: Polyphenolic compounds and nanocellulose for food packaging. Ind. Crops Prod. 2022, 187, 115413. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Cardona-Alzate, C.A. A comprehensive approach for biorefineries design based on experimental data, conceptual and optimization methodologies: The orange peel waste case. Bioresour. Technol. 2021, 325, 124682. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Inocencio-cruzía, P.J.; Cardona Alzate, C.A. Sustainability analysis of orange peel biorefineries. Enzym. Microb. Technol. 2024, 172, 110327. [Google Scholar] [CrossRef]
- Guarín Manrique, L.D.; Martínez Ardila, H.E.; Becerra Ardila, L.E.; Pastor Llorca, M.J. Technology transfer between university and industry scenarios. In Proceedings of the 21st LACCEI International Multi-Conference for Engineering, Education, and Technology: “Leadership in Education and Innovation in Engineering in the Framework of Global Transformations: Integration and Alliances for Integral Development”, Buenos Aires, Argentina, 17 July 2023. [Google Scholar]
- Santiago, B.; Moreira, M.T.; Feijoo, G.; González-García, S. Identification of environmental aspects of citrus waste valorization into D-limonene from a biorefinery approach. Biomass Bioenergy 2020, 143, 105844. [Google Scholar] [CrossRef]
- García-Cruz, A.; Mtz-Enríquez, A.I.; Díaz-Jiménez, L.; Ramos-González, R.; Valdés, J.A.A.; Flores, M.E.C.; Martínez, J.L.H.; Ilyina, A. Production of fatty acid methyl esters and bioactive compounds from citrus wax. Waste Manag. 2020, 102, 48–55. [Google Scholar] [CrossRef]
- de-Oliveira, K.G.; de Lima, R.R.S.; Moura, H.M.d.A.; Bicudo, T.d.C.; de Carvalho, L.S. Tangerine peel ashes applied as green catalyst: A biorefinery-based approach for biodiesel production. Biofuels Bioprod. Biorefin. 2022, 16, 548–561. [Google Scholar] [CrossRef]
- Bedoya-Betancur, S.; Gil, S.A.; Barrera-Z, R.; Ardila-A, A.N. Technical and Economic Scenario for the Integral Small-Scale Valorization of Orange Waste in Colombia. Ingeniería 2021, 26, 367–380. [Google Scholar] [CrossRef]
- Santra, S.; Das, M.; Karmakar, S.; Banerjee, R. NADES assisted integrated biorefinery concept for pectin recovery from kinnow (Citrus reticulate) peel and strategic conversion of residual biomass to L(+) lactic acid. Int. J. Biol. Macromol. 2023, 250, 126169. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.A.; Bento, H.B.S.; Picheli, F.P.; Paz-Cedeno, F.R.; Mussagy, C.U.; Masarin, F.; Torres Acosta, M.A.; Santos-Ebinuma, V.C. Process development and techno-economic analysis of co-production of colorants and enzymes valuing agro-industrial citrus waste. Sustain. Chem. Pharm. 2023, 35, 101204. [Google Scholar] [CrossRef]
- Biz, A.; Jung Finkler, A.T.; Oliveira Pitol, L.; Schweitzer Medina, B.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Sarkar, R.; Nain, L.; Dutta, A.; Kundu, A.; Saha, S. Unraveling the utilization feasibility of citrus peel solid distillation waste as potential source for antioxidant as well as bioethanol. Biomass Convers. Biorefin. 2022, 1–13. [Google Scholar] [CrossRef]
- Santos, L.B.; Silva, R.D.; Alonso, J.D.; Brienzo, M.; Silva, N.C.; Perotto, G.; Otoni, C.G.; Azeredo, H.M.C. Bioplastics from orange processing byproducts by an ecoefficient hydrothermal approach. Food Packag. Shelf Life 2023, 38, 101114. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Ahmed, A.; Jeffery, D.M.; Hidayat, S.; Sukri, R.S.; Mahlia, T.M.I.; Jamil, F.; Khurrum, M.S.; Inayat, A.; Moogi, S.; et al. Pyrolysis of solid waste residues from Lemon Myrtle essential oils extraction for bio-oil production. Bioresour. Technol. 2020, 318, 123913. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Baseggio, A.M.; Torres-Mayanga, P.C.; Ávila, P.F.; Tompsett, G.A.; Marostica, M.; Goldbeck, R.; Timko, M.T.; Rostagno, M.; Martinez, J.; et al. Sequential subcritical water process applied to orange peel for the recovery flavanones and sugars. J. Supercrit. Fluids 2020, 160, 104789. [Google Scholar] [CrossRef]
- Vaez, S.; Karimi, K.; Mirmohamadsadeghi, S.; Jeihanipour, A. An optimal biorefinery development for pectin and biofuels production from orange wastes without enzyme consumption. Process Saf. Environ. 2021, 152, 513–526. [Google Scholar] [CrossRef]
- Giannakis, N.; Carmona-Cabello, M.; Makri, A.; Leiva-Candia, D.; Filippi, K.; Argeiti, C.; Pateraki, C.; Dorado, M.P.; Koutinas, A.; Stylianou, E. Spent coffee grounds and orange peel residues based biorefinery for microbial oil and biodiesel conversion estimation. Renew. Energy 2023, 209, 382–392. [Google Scholar] [CrossRef]
- Thulasisingh, A.; Kannaiyan, S.; Pichandi, K. Cellulose nanocrystals from orange and lychee biorefinery wastes and its implementation as tetracycline drug transporter. Biomass Convers. Biorefin. 2023, 13, 1175–1188. [Google Scholar] [CrossRef]
- Baglioni, M.; Fries, A.; Müller, J.M.; Omarini, A.; Müller, M.; Breccia, J.D.; Mazzaferro, L.S. Acremonium sp. diglycosidase-aid chemical diversification: Valorization of industry by-products. Appl. Microbiol. Biotechnol. 2024, 108, 1–10. [Google Scholar] [CrossRef]
- Tsouko, E.; Maina, S.; Ladakis, D.; Kookos, I.K.; Koutinas, A. Integrated biorefinery development for the extraction of value-added components and bacterial cellulose production from orange peel waste streams. Renew. Energy 2020, 160, 944–954. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Orrego-Alzate, C.E.; Acosta-Medina, C.D.; Cardona-Alzate, C.A. Integral use of orange peel waste through the biorefinery concept: An experimental, technical, energy, and economic assessment. Biomass Convers. Biorefin. 2021, 11, 645–659. [Google Scholar] [CrossRef]
- Karanicola, P.; Patsalou, M.; Stergiou, P.Y.; Kavallieratou, A.; Evripidou, N.; Christou, P.; Panagiotou, G.; Damianou, C.; Papamichael, E.M.; Koutinas, M. Ultrasound-assisted dilute acid hydrolysis for production of essential oils, pectin and bacterial cellulose via a citrus processing waste biorefinery. Bioresour. Technol. 2021, 342, 126010. [Google Scholar] [CrossRef]
- Martins, M.; Goldbeck, R. Integrated Biorefinery Development for Xylooligosaccharides, Pectin, and Bioenergy Production from Orange Wastes. SSRN 2023, 17, 1775–1788. [Google Scholar] [CrossRef]
- de-Azevedo, A.R.; dos Santos, M.S.N.; Wancura, J.H.C.; Oro, C.E.D.; Pfeifenberg, R.; Zabot, G.L.; Tres, M.V. Semi-continuous subcritical hydrolysis of orange waste biomasses for integrated production of fermentable sugars and platform chemicals. Chem. Eng. Process. 2024, 197, 109719. [Google Scholar] [CrossRef]
- Kundu, D.; Banerjee, S.; Karmakar, S.; Banerjee, R. Valorization of citrus lemon wastes through biorefinery approach: An industrial symbiosis. Bioresour. Technol. Rep. 2021, 15, 100717. [Google Scholar] [CrossRef]
- Saadatinavaz, F.; Karimi, K.; Denayer, J.F.M. Hydrothermal pretreatment: An efficient process for improvement of biobutanol, biohydrogen, and biogas production from orange waste via a biorefinery approach. Bioresour. Technol. 2021, 341, 125834. [Google Scholar] [CrossRef]
- Patsalou, M.; Chrysargyris, A.; Tzortzakis, N.; Koutinas, M. A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 2020, 113, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Lamine, M.; Hamdi, Z.; Zemni, H.; Rahali, F.Z.; Melki, I.; Mliki, A.; Gargouri, M. From residue to resource: The recovery of high-added values compounds through an integral green valorization of citrus residual biomass. Sustain. Chem. Pharm. 2024, 37, 101379. [Google Scholar] [CrossRef]
- Chit-aree, L.; Unpaprom, Y.; Ramaraj, R.; Thirabunyanon, M. Valorization and biorefinery of kaffir lime peels waste for antifungal activity and sustainable control of mango fruit anthracnose. Biomass Convers. Biorefin. 2023, 13, 10735–10749. [Google Scholar] [CrossRef]
- González-Rivera, J.; Spepi, A.; Ferrari, C.; Duce, C.; Longo, I.; Falconieri, D.; Piras, A.; Tinè, M.R. Novel configurations for a citrus waste based biorefinery: From solventless to simultaneous ultrasound and microwave assisted extraction. Green Chem. 2016, 18, 6482–6492. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Carnaroglio, D.; Tamburino, A.; Cravotto, G.; Grillo, G.; Ilharco, L.M.; Pagliaro, M. Eco-Friendly Extraction of Pectin and Essential Oils from Orange and Lemon Peels. ACS Sustain. Chem. Eng. 2016, 4, 2243–2251. [Google Scholar] [CrossRef]
- Toprakçı, İ.; Balci-Torun, F.; Deniz, N.G.; Ortaboy, S.; Torun, M.; Şahin, S. Recovery of citrus volatile substances from orange juice waste: Characterization with GC-MS, FTIR, 1H- and 13C-NMR spectroscopies. Phytochem. Lett. 2023, 57, 177–184. [Google Scholar] [CrossRef]
- Teke, G.M.; De Vos, L.; Smith, I.; Kleyn, T.; Mapholi, Z. Development of an ultrasound-assisted pre-treatment strategy for the extraction of d-Limonene toward the production of bioethanol from citrus peel waste (CPW). Bioprocess Biosyst. Eng. 2023, 46, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Patsalou, M.; Menikea, K.K.; Makri, E.; Vasquez, M.I.; Drouza, C.; Koutinas, M. Development of a citrus peel-based biorefinery strategy for the production of succinic acid. J. Clean. Prod. 2017, 166, 706–716. [Google Scholar] [CrossRef]
- Hilali, S.; Fabiano-Tixier, A.S.; Ruiz, K.; Hejjaj, A.; Aitnouh, F.; Idlimam, A.; Bily, A.; Mandi, L.; Chemat, F. Green Extraction of Essential Oils, Polyphenols, and Pectins from Orange Peel Employing Solar Energy: Toward a Zero-Waste Biorefinery. ACS Sustain. Chem. Eng. 2019, 7, 11815–11822. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef]
- Bellete, B.S.; Ramin, L.Z.; Porto, D.; Ribeiro, A.I.; Forim, M.R.; Zuin, V.G.; Fernandes, J.B.; Fátima, M.; Silva, G.F. An Environmentally Friendly Procedure to Obtain Flavonoids from Brazilian Citrus Waste. Article J. Braz. Chem. Soc. 2018, 29, 1123–1129. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Integrated Membrane Systems as an Innovative Approach for the Recovery of High Value-Added Compounds from Agro- Food By-Products. Chem. Eng. Trans. 2021, 87, 361–366. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Lamine, M.; Gargouri, M.; Rahali, F.Z.; Mliki, A. Recovering and Characterizing Phenolic Compounds From Citrus By-Product: A Way towards Agriculture of Subsistence and Sustainable Bioeconomy. Waste Biomass Valori. 2021, 12, 4721–4731. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast flavour production by solid state fermentation of orange peel waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, Y.G.; Chang, J.; Bae, H.J. A High-Yield Process for Production of Biosugars and Hesperidin from Mandarin Peel Wastes. Molecules 2020, 25, 4286. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Hernández, I.; Aranda-Ledesma, N.E.; Rojas, R.; Tafolla-Arellano, J.C.; Martínez-Ávila, G.C.G. Antioxidant activity of polyphenolic compounds obtained from Euphorbia antisyphilitica by-products. Heliyon 2021, 7, e06734. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Cedillo, E.E.; González-Chávez, M.M.; Handy, B.E.; Quintana-Olivera, M.F.; López-Mercado, J.; Cárdenas-Galindo, M.G. Acid-catalyzed transformation of orange waste into furfural: The effect of pectin degree of esterifcation. Bioresour. Bioprocess. 2024, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, M.; Patsalou, M.; Xiaris, N.; Tsevis, A.; Koutsokeras, L.; Constantinides, G.; Koutinas, M. Enhancing bioproduction and thermotolerance in Saccharomyces cerevisiae via cell immobilization on biochar: Application in a citrus peel waste biorefinery. Renew. Energy 2020, 155, 53–64. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Rahimifard, S.; Matharu, A.S.; Dugmore, T.I.J. Life-Cycle Assessment of Microwave-Assisted Pectin Extraction at Pilot Scale. ACS Sustain. Chem. Eng. 2019, 7, 5167–5175. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; González-Aguirre, J.A.; Peltonen, K.E.; Richard, P.; Cardona Alzate, C.A. Pre-feasibility analysis of the production of mucic acid from orange peel waste under the biorefinery concept. Biochem. Eng. J. 2020, 161, 107680. [Google Scholar] [CrossRef]
- Sohni, S.; Begum, S.; Hashim, R.; Khan, S.B.; Mazhar, F.; Syed, F.; Khan, S.A. Physicochemical characterization of microcrystalline cellulose derived from underutilized orange peel waste as a sustainable resource under biorefinery concept. Bioresour. Technol. Rep. 2024, 25, 101731. [Google Scholar] [CrossRef]
- Ganatsios, V.; Terpou, A.; Bekatorou, A.; Plessas, S.; Koutinas, A.A. Refining Citrus Wastes: From Discarded Oranges to Efficient Brewing Biocatalyst, Aromatic Beer, and Alternative Yeast Extract Production. Beverages 2021, 7, 16. [Google Scholar] [CrossRef]
- Kammoun, M.; Ayeb, H.; Bettaieb, T.; Richel, A. Chemical characterisation and technical assessment of agri-food residues, marine matrices, and wild grasses in the South Mediterranean area: A considerable inflow for biorefineries. Waste Manag. 2020, 118, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Machin-Ferrero, L.M.; Wheeler, J.; Mele, F.D. Life cycle assessment of the Argentine lemon and its derivatives in a circular economy context. Sustain. Prod. Consum. 2022, 29, 672–684. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Chen, Y. The environmental impacts of citrus residue management in China: A case study in The Three Gorges Reservoir Region. Waste Manag. 2021, 133, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.A.; Hamelin, L.; Tiruta-Barna, L.; Ahmadi, A.; Thomsen, M. Circular bioeconomy: Life cycle assessment of scaled-up cascading production from orange peel waste under current and future electricity mixes. Sci. Total Environ. 2022, 812, 152574. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; Serrano, A.; Alonso-Fariñas, B.; Siles, J.A.; Martín, M.A. Impact of D-Limonene in the technical and economic feasibility of the anaerobic digestion applied in biorefineries. Biomass Convers. Biorefin. 2022, 14, 14877–14888. [Google Scholar] [CrossRef]
- Joglekar, S.N.; Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Process of fruit peel waste biorefinery: A case study of citrus waste biorefinery, its environmental impacts and recommendations. Environ. Sci. Pollut. R. 2019, 26, 34713–34722. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Cardona Alzate, C.A. Comparative environmental life cycle assessment of orange peel waste in present productive chains. J. Clean. Prod. 2021, 322, 128814. [Google Scholar]
- Satpathy, A.; Mukherjee, K.; Nigam, V.K. Batch cultivation and optimization of pectinase production using Bacillus sp. (BIOSMNF02) through RSM-D-optimal quadratic model. Biomass Convers. Biorefin. 2023, 1–12. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Corsini, A.; Boschi, N.; Galletti, A.M.R. Smart Valorization of Waste Biomass: Exhausted Lemon Peels, Coffee Silverskins and Paper Wastes for the Production of Levulinic Acid. Chem. Eng. Trans. 2018, 65, 637–642. [Google Scholar] [CrossRef]
- Jeong, G.T. Statistical approach for the optimization of levulinic acid production from orange peel as agricultural waste. Biofuels Bioprod. Biorefin. 2023, 17, 1303–1314. [Google Scholar] [CrossRef]
- Zhou, T.; Ju, X.; Yan, L.; Fang, R.; Xu, X.; Li, L. Production of mannooligosaccharides from orange peel waste with β-mannanase expressed in Trichosporonoides oedocephalis. Bioresour. Technol. 2024, 395, 130373. [Google Scholar] [CrossRef]

| Citrus Waste | Moisture (%) | Ash (%) | Non-Polar Solvent Extractables (%) | Polar Solvent Extractables | Cellulose/Glucan (%) | Hemicellulose (%) | Lignin (%) | Fat | Protein | Free Sugars | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Citrus by-products | – | 6.58 ± 0.11 | – | 25.04 ± 2.14 | 11.94 ± 0.16 | 6.50 ± 0.09 ** | 21.58 ± 1.45 | 2.08 ± 0.19 | 6.56 ± 0.31 | – | [16] |
| Citrus pulp | – | 1.7 | 3.9 | – | 16.2 | 13.8 | 1 | – | 7.9 | 41 | [17] |
| Distilled kinnow peels | – | – | – | – | 13 ± 0.8 | 8.3 ± 0.8 | 3.6 ± 0.4 | – | – | – | [18] |
| Distilled mosambi peels | – | – | – | – | 21.2 ± 1.1 | 12.5 ± 0.6 | 5.1 ± 0.5 | – | – | – | |
| Distilled orange peels | – | – | – | – | 24.6 ± 0.9 | 13.4 ± 0.7 | 6.5 ± 0.4 | – | – | – | |
| Finisher pulp | – | 2.81 ± 0.03 | 1.1 ± 0.1 | 56 ± 1 | 8 ± 0.9 | 5.74 ± 0.08 | 1.2 ± 0.5 | – | – | – | [19] |
| Lemon Myrtle | 7.41 | 4.88 | – | – | – | – | – | – | – | – | [20] |
| Orange peel | 8.71 ± 0.0 | 3.99 ± 0.04 | 3.79 ± 1.04 | 17.15 ± 1.73 | 34.22 ± 4.68 | 6.32 | 16.8 | – | 6.85 | – | [21] |
| Orange peel | – | 2.3–3.1 * | – | – | 55.4–67.1 * | * 0.3–2.5 ** | 8.5–25.4 * | – | – | – | [22] |
| Orange peel | – | 4.3 | – | – | 25.3 | 5.3 | 5.4 | 2.1 | 5.2 | 31.3 | [23] |
| Orange peel | – | 4.4 ± 0.1 | 4.1 ± 0.2 | 43.8 ± 0.7 | 8.8 ± 0.1 | 7.6 ± 0.4 | 2.4 | – | – | – | [19] |
| Orange peel waste | 14.28 | – | – | 9.32 | 32.7 | 13.68 | – | – | – | [24] | |
| Orange peel waste | – | – | – | – | 20.45 ± 0.45 | 26 ± 2.82 | 2.75 | 12.02 ± 0.23 | 8.11 ± 0.13 | – | [25] |
| Orange peel waste | 79.1 ± 0.01 | 3.3 ± 0.20 | – | – | 9.2 ± 0.21 | 5.4 ± 0.19 | 1.2 ± 0.5 | – | 6.6 ± 0.30 | 50 ± 2.01 | [26] |
| Orange peel waste | 77.38 ± 0.36 | 2.1 ± 0.20 | – | 26.56 | 23.88 ± 0.50 | 14.15 ± 2.01 | 5.1 ± 2.44 | 4.6 ± 1.91 | 4.96 ± 0.20 | – | [8] |
| Orange peel waste | 78.53 ± 0.15 | 3.61 ± 0.20 | – | – | 30.17 ± 0.50 | 9.35 ± 4.36 | 5.07 ± 2.14 | 5.18 ± 1.91 | 4.68 ± 0.20 | – | [27] |
| Orange peel waste | 80 | 4.5 | – | – | 21.3 | 4.8 | 0.5 | – | – | – | [28] |
| Orange peel waste | – | 2.53 ± 0.19 | – | 27.61 | 28.78 ± 4.61 | 14.89 ± 4.45 | 4.62 ± 1.25 | 4.72 ± 0.10 | – | – | [9] |
| Orange pomace | – | 3.12 ± 0.01 | 3.6 ± 0.4 | 39 ± 4 | 13 ± 2 | 8.6 ± 0.4 | 2.62 | – | – | – | [19] |
| Orange pulp | – | 1.7–4 * | – | – | 49.4–60.9 * | * 0.3–3.2 ** | 2.7–26.3 * | – | – | – | [22] |
| Orange wastes | 81 ± 1.9 | 1 | – | – | 39 ± 2.4 *** | 14.8 ± 0.2 | 1.2 | 2.3 ± 0.1 | 3.2 ± 0.1 | 50 ± 4.3 | [29] |
| Undistilled kinnow peels | – | – | – | – | 13.8 ± 0.9 | 8.4 ± 1.6 | 3.8 ± 0.3 | – | – | – | [18] |
| Undistilled mosambi peels | – | – | – | – | 21.6 ± 1.1 | 12.9 ± 0.8 | 5.2 ± 0.5 | – | – | – | |
| Undistilled orange peels | – | – | – | – | 24.6 ± 1.3 | 13.7 ± 0.7 | 6.8 ± 0.3 | – | – | – |
| Citrus By-Product | Essential Oil Yield (%) | Main Components | Percentage of Main Components | Extraction Methods | References |
|---|---|---|---|---|---|
| Assam lemon | 4.19 ± 0.11 | Limonene | 94.47 | Distillation | [31] |
| β-bisabolene | 1.26 | ||||
| Minor components (i.e., α-pinene, among others) | 0.1–0.9 | ||||
| C. aurantium pruning leftovers | 0.66 | Linalyl acetate | 33.23 | Hydro-distillation | [34] |
| Linalool | 26.3 | ||||
| β-fenchyl alcohol | 7.7 | ||||
| β-pinene | 6.14 | ||||
| β-ocimene | 4.7 | ||||
| C. limon pruning leftovers | 0.85 | Limonene | 25.52 | Hydro-distillation | |
| (E)-citral | 15.55 | ||||
| β-pinene | 11.21 | ||||
| Sabinene | 8.42 | ||||
| Neryl acetate | 8.81 | ||||
| C. reticulata pruning leftovers | 1.5 | Methyl N-methyl anthranilate | 78.34 | Hydro-distillation | |
| γ-terpinolene | 12.91 | ||||
| Limonene | 4.36 | ||||
| p-cymene | 2.24 | ||||
| β-pinene | 1.29 | ||||
| Citrus household kitchen residues | 0.24 | Limonene | 94.41 ± 0.2 | Distillation | [33] |
| β-myrcene | 1.32 ± 0.04 | ||||
| Linalool | 1.30 ± 0.04 | ||||
| Citrus peel wastes | 0.43 | Limonene | – | Distillation | [40] |
| Citrus wax | – | Limonene | 4 | – | [12] |
| Flavedo orange peels | 0.42 | α-pinene | 80–85 | Steam distillation | [14] |
| β-myrcene | – | ||||
| Linalool | – | ||||
| Ground mandora residues | 0.43 | Limonene | 96.36 ± 0.09 | Distillation | [33] |
| β-myrcene | 1.35 ± 0.02 | ||||
| Decanol | 0.58 ± 0.01 | ||||
| Non-ground mandora residues | 0.19 | Limonene | 96.70 ± 0.02 | Distillation | |
| β-myrcene | 1.53 ± 0.02 | ||||
| α-pinene | 0.34 ± 0.01 | ||||
| Orange flavedo | 0.7 ± 0.01 | – | – | Steam distillation | [26] |
| Orange juice waste | – | Limonene | 89.65 | Solid/liquid (hexane) extraction | [38] |
| linalyl acetate | 4.51 | ||||
| α-pinene | 1.4 | ||||
| Orange peel | 1.03 ± 0.015 | Limonene | 95.96 | Solar hydro-distillation | [41] |
| Myrcene | 1.7 | ||||
| Orange peel | 1.05 ± 0.011 | Limonene | 95.24 | Conventional steam hydro-distillation | |
| Myrcene | 1.73 | ||||
| Orange peel | 1.16 ± 0.01 | Limonene | 95.2 | Coaxial solventless MW-assisted extraction | [36] |
| Valencene | 0.2 | ||||
| Orange peel | 1.17 | Limonene | 91.62 | distillation | [9] |
| Orange peel | 1.2 * | – | – | Solid/liquid (hexane) extraction | [6] |
| Orange peel | 1.31 | Limonene | – | Solid/liquid (hexane) extraction | [4] |
| Orange peel | 1.53 ± 0.04 | Limonene | 95 | Ultrasound coaxial MW-assisted hydro-distillation | [36] |
| Valencene | 0.4 | ||||
| Orange peel | 1.55 ± 0.05 | Limonene | 94.4 | Hydro-distillation | |
| Valencene | 0.3 | ||||
| Orange peel | 1.57 ± 0.04 | Limonene | 94.7 | Microwave-assisted hydro-distillation | |
| Valencene | 0.3 | ||||
| Orange peel waste | 0.12 * | – | – | Solid/liquid (hexane) extraction | [28] |
| Orange peel waste | 0.66 ± 0.05 | Limonene | 88.39 ± 1.33 | Steam distillation | [8] |
| β-myrcene | 2.28 ± 0.37 | ||||
| γ-terpinolene | 4.96 ± 0.83 | ||||
| Linalool | 3.51 ± 0.64 | ||||
| Orange peel waste | 0.84 ± 0.01 | Limonene | 88.39 | Steam distillation | [27] |
| γ-terpinolene | 4.96 | ||||
| Linalool | 3.51 | ||||
| Orange peel waste | 1.14 ± 0.11 | Limonene | 91.27 ± 1.26 | Steam distillation | [8] |
| β-myrcene | 1.82 ± 0.19 | ||||
| γ-terpinolene | 2.76 ± 0.75 | ||||
| Linalool | 2.23 ± 0.35 | ||||
| Ripe kaffir lime peels | – | Limonene | 24.62 | Hydro-distillation | [35] |
| β-pinene | 16.71 | ||||
| β-citronellol | 8 | ||||
| Unripe kaffir lime peels | – | β-pinene | 23.67 | Hydro-distillation | |
| β-citronellol | 13.96 | ||||
| 4-terpineol | 11.92 | ||||
| Waste lemon peel | ≈0.05 | Limonene | – | Microwave-assisted hydro-distillation | [37] |
| Citrus By-Product | Total Polyphenolic Content (GAE/g) | Main Components | Predominance (%) or Concentration | Extraction Method | References |
|---|---|---|---|---|---|
| C. aurantium post-hydrodistillation wastewater | 26.30 ± 0.15 | Hesperetin | 0.66 mg/g | – | [34] |
| Benzoic acid | 0.08 mg/g | ||||
| C. aurantium pruning leftovers | 350 ± 0.85 | Flavonoids | 30.10 ± 0.45 ** | Homogenization | |
| C. limon post-hydrodistillation wastewater | 45.79 ± 0.34 | 4-coumaric acid | 2.83 mg/g | – | |
| Ferulic acid | 1.15 mg/g | ||||
| Sinapinic acid | 0.51 mg/g | ||||
| Isovanillic acid | 0.28 mg/g | ||||
| C. limon pruning leftovers | 25.5 ± 0.5 | Flavonoids | 38.20 ± 0.80 ** | Homogenization | |
| C. reticulata post-hydrodistillation wastewater | 13.45 ± 0.45 | Ferulic acid | 1.83 mg/g | – | |
| Caffeic acid | 0.71 mg/g | ||||
| Benzoic acid | 0.61 mg/g | ||||
| Protocatechoic acid | |||||
| C. reticulata pruning leftovers | 340 ± 0.95 | Flavonoids | 45.50 ± 0.09 ** | Homogenization | |
| Citrus fruit processing wastes | – | Hesperitin | 8.11 mg/g | Homogenization | [43] |
| Naringenin | 5.76 mg/g | ||||
| Nobiletin | 3.25 mg/g | ||||
| 3,5,6,7,3′,4′-hexamethoxyflavone | 3.0 mg/g | ||||
| 3,5,6,7,4′-pentamethoxyflavone | 2.73 mg/g | ||||
| Citrus paradisi L. peel | – | Neohesperidin | 0.03–0.09 mg/g # | Conventional solid-liquid extraction | [45] |
| Neoeritrocin | 0.03–0.16 mg/g # | ||||
| Narirutin | 0.28–0.70 mg/g # | ||||
| Naringin | 18–28 mg/g # | ||||
| Hesperidin | 0.23–0.74 mg/g # | ||||
| Tangeritin | 0.09–0.07 mg/g # | ||||
| Citrus paradisi L. peel | – | Neohesperidin | 0.04–0.16 mg/g # | Ultrasound assisted extraction | |
| Neoeritrocin | 0.05–0.18 mg/g # | ||||
| Narirutin | 0.42–0.98 mg/g # | ||||
| Naringin | 24–33 mg/g # | ||||
| Hesperidin | 0.72–1.14 mg/g # | ||||
| Tangeritin | 0.01–0.02 mg/g # | ||||
| Citrus wax | – | 3,7-dimethylquercetin | 1.05% | – | [12] |
| 5,6-dihydroxy-7,8,3′,4′-tetramethoxyflavone | 0.89% | ||||
| 5-5′-dehydrodiferulic acid | 0.58% | ||||
| Tangeretin | 0.39% | ||||
| Distilled kinnow peels | 98.7 | Flavonoids | 32.8 ** | Ultrasonic-assisted extraction | [18] |
| Distilled mosambi peels | 78.1 | Flavonoids | 22.9 ** | Ultrasonic-assisted extraction | |
| Distilled orange peels | 97 | Flavonoids | 32.6 ** | Ultrasonic-assisted extraction | |
| Kinnow peels | 25.96–41.24 $ | – | – | – | [15] |
| Lemon myrtle oil | – | Catechol | 21.67% | Pyrolisis | [20] |
| p-cresol | 4.17% | ||||
| Gayacol | 2.77% | ||||
| Syringol | 2.69% | ||||
| Mandarin waste | 0.015 | Rutin | 12 mg/g | Maceration | [46] |
| Catechin hydrate | 6.56 mg/g | ||||
| p-coumaric acid | 6.12 mg/g | ||||
| Isorhamnetin 3-O-rutoside | 5.29 mg/g | ||||
| Gallic acid | 4.8 mg/g | ||||
| Orange peel waste | – | Hesperidin | 3.50% | – | [25] |
| Orange peel waste | 9–11 | – | – | Ultrasonic-assisted extraction | [7] |
| Orange peels | – | Hesperidin | 4.42–7.88 mg/g Φ | Homogenization | [9] |
| Apigenin | 0.97–0.48 mg/g Φ | ||||
| Naringenin | 0–0.675 mg/g Φ | ||||
| Orange peels | 1.96–9.4 ± 0.35 | Flavonoids | 0.12–3.70 ** @ | Conventional steam hydro-distillation | [41] |
| Hesperidin | 0.08–1.21 mg/g @ | ||||
| Naruritin | 0.08–0.24 mg/g @ | ||||
| Orange peels | 2.13–9.7 ± 0.37 | Flavonoids | 0.14–3.81 ** @ | Solar hydro-distillation | |
| Hesperidin | 0.1–1.9 mg/g @ | ||||
| Naruritin | 0.08–0.26 mg/g @ | ||||
| Orange peels | 3.009 ± 0.245 | – | – | Fermentation | [47] |
| Orange peels | 3.4 ± 0.73–5.53 ± 0.43 *** | Hesperidin | 9.82–22.9 | Sequential subcritical water process | [21] |
| Narirutin | 0.99–22.99 mg/g | ||||
| Orange peels | 5.5–6.0 * | Quinic acid | 72.50% | Ultrasonic-assisted extraction | [26] |
| Hesperidin | 14.50% | ||||
| Hesperetin | 4.90% | ||||
| Oranges waste | 67–79 mM GAE ^ | Tannins | – | High pressure | [32] |
| 2.17–2.47 mM GAE ^ | Other phenolics | – | |||
| Ripe kaffir lime peel oil | 15.76 ± 1.74 | – | – | Hydro-distillation | [35] |
| Sour orange waste | 0.021 | Luteolin 7-O glucoside | 8.58 mg/g | Maceration | [46] |
| Ferulic acid | 7.5 mg/g | ||||
| Kaempferol 3-rutinoside-7-galactoside | 5.11 mg/g | ||||
| Myrcetin | 4.53 mg/g | ||||
| Isorhamnetin | 3.54 mg/g | ||||
| Sweet orange waste | 0.0423 | Myrcetin | 9.5 mg/g | Maceration | |
| Sinapic acid | 7.64 mg/g | ||||
| Apigenin | 6.28 mg/g | ||||
| Ferulic acid | 3.75 mg/g | ||||
| Amentoflavone | 2.67 mg/g | ||||
| Undistilled kinnow peels | 132 | Flavonoids | 47.4 ** | Ultrasonic-assisted extraction | [18] |
| Undistilled mosambi peels | 95.4 | Flavonoids | 34.1 ** | Ultrasonic-assisted extraction | |
| Undistilled orange peels | 12.48 ± 0.14 | Flavonoids | 4.84 ± 0.026 ** | – | [41] |
| Hesperidin | 2.44 ± 1.4 10−3 mg/g | ||||
| Naruritin | 0.041 ± 6.4 10−4 mg/g | ||||
| Undistilled orange peels | 118.7 | Flavonoids | 41.7 ** | Ultrasonic-assisted extraction | [18] |
| Unripe kaffir lime peel oil | 16.17 ± 1.81 | – | – | Hydro-distillation | [35] |
| Citrus Waste | Yield (%) | Extraction Method | References |
|---|---|---|---|
| Assam lemon | 12.67 ± 0.46 | Enzymatic hydrolysis | [31] |
| Citrus peel wastes | 30.5 | Sulfuric acid hydrolysis | [51] |
| Citrus peel wastes | 23.25 | Dilute-acid hydrolysis | [40] |
| Citrus pulp | 14.4 | – | [17] |
| Distilled innow peels | 11.6 ± 0.5 | HCl acid hydrolysis | [18] |
| Distilled mosambi peels | 14.9 ± 0.3 | ||
| Distilled orange peels | 15.3 ± 0.6 | ||
| Finisher pulp | 17.1 ± 0.6 | Citric acid hydrolysis | [19] |
| Kinnow peels | 16.93–35.66 ** | Natural deep eutectic solvents | [15] |
| Orange albedo | 19.36 ± 0.44 | HCl acid hydrolysis | [14] |
| Orange peel | <1–24.7 ^ | Sulfuric acid hydolisis | [22] |
| Orange peel | 1.9 | Fermentation | [47] |
| Orange peel | 14.2 ± 1.3 | Ultrasound coaxial MW-assisted hydro-distillation | [36] |
| Orange peel | 15.7 ± 2.5 | Coaxial solventless MW-assisted extraction | |
| Orange peel | 15.9 ± 3.0 | Microwave-assisted hydro-distillation | |
| Orange peel | 17.4 ± 3.3 | Hydro-distillation | |
| Orange peel | 3.05 | HCl acid hydrolysis | [52] |
| Orange peel | 5 | Microwave-assisted extraction | |
| Orange peel | 0.47–8.26 # | Solar hydro-distillation | [41] |
| Orange peel | 0.51–7.69 # | Conventional steam hydro-distillation | |
| Orange peel | 19.62 ± 3.24 | Subcritical water extraction | [21] |
| Orange peel | 23.9 ± 0.3 | Citric acid hydrolysis | [19] |
| Orange peel residues | 17.6 | HCl acid hydrolysis | [23] |
| Orange peel residues | ≈19 | Citric acid hydrolysis | |
| Orange peel residues | ≈44 | Sulfuric acid hydrolysis | |
| Orange peel residues | 46.7 | Oxalic acid hydrolysis | |
| Orange peel waste | 15.85 ± 0.6 | Citric acid hydrolysis | [53] |
| Orange peel waste | 10.35 | Citric acid hydrolysis | [27] |
| Orange peel waste (dry) | 2.24 | Citric acid hydrolysis | [8] |
| Orange peel waste (wet) | 14.6 | ||
| Orange peels | NI | Sulfuric acid hydrolysis | [5] |
| Orange pomace | 25.2 ± 0.6 | Citric acid hydrolysis | [19] |
| Orange pulp | <1–23.7 ^ | Sulfuric acid hydrolysis | [22] |
| Orange residues | 43 (lab-scale) | sulfuric UADAH | [28] |
| Orange residues | 45 (pilot-scale) | ||
| Orange wastes | 6–42 *** | Citric acid hydrolysis | [29] |
| Orange peel waste | ≈8–22 * | HCl hydrolysis | [26] |
| Orange peel waste | ≈19–32.6 * | Citric acid hydrolysis | |
| Undistilled orange peels | 12.08 ± 0.7 | – | [41] |
| Undistilled kinnow peels | 17.8 ± 0.6 | HCl acid hydrolysis | [18] |
| Undistilled mosambi peels | 20.9 ± 0.4 | ||
| Undistilled orange peels | 23.3 ± 0.8 | ||
| Waste lemon peel | 15 | Microwave-assisted hydro-distillation | [37] |
| Citrus Waste | Pretreatment | Microorganism | Fermentation Type | Temperature Range (°C) | Yield | References |
|---|---|---|---|---|---|---|
| Assam lemon waste | Enzyme hydrolysis | Saccharomyces cerevisiae and Pichia kudriavzevii | Partial simultaneous saccharification and co-fermentation | 35 | 12.16 (%) | [31] |
| Citrus peel waste | Acid and enzyme hydrolysis | Pichia kudriavzevii | Batch | 42 | 30.7 g·L−1 | [5] |
| Citrus peel waste | - | Saccharomyces cerevisiae | Batch | 37 | 63 g·L−1 | [51] |
| Clementine peel waste | Ultrasound-assisted extraction | Saccharomyces cerevisiae | Simultaneous saccharification and sermentation | 37 | 1.97 g·L−1 | [39] |
| Clementine peel waste | Ultrasound-assisted extraction | Saccharomyces cerevisiae | Sequential hydrolysis and fermentation | 37 | 1.39 g·L−1 | |
| Kinnow peels | Hydro-distillation | Saccharomyces cerevisiae | Batch | 30 | 5.08 g·L−1 | [18] |
| Mosambi peels | 30 | 7.16 g·L−1 | ||||
| Orange peel | 30 | 7.89 g·L−1 | ||||
| Orange peel | Dilute acid | Saccharomyces cerevisiae | Batch | 32 | 81.5 (%) | [22] |
| Orange pulp | Dilute acid | Saccharomyces cerevisiae | Batch | 32 | 82.9 (%) | |
| Orange waste | Hydrothermal | Clostridium acetobutylicum | Batch | 37 | 42.3 (g·kg−1) | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Herrera, N.; Martínez-Ávila, G.C.G.; Robledo-Jiménez, C.L.; Rojas, R.; Orozco-Zamora, B.S. From Citrus Waste to Valuable Resources: A Biorefinery Approach. Biomass 2024, 4, 784-808. https://doi.org/10.3390/biomass4030044
Medina-Herrera N, Martínez-Ávila GCG, Robledo-Jiménez CL, Rojas R, Orozco-Zamora BS. From Citrus Waste to Valuable Resources: A Biorefinery Approach. Biomass. 2024; 4(3):784-808. https://doi.org/10.3390/biomass4030044
Chicago/Turabian StyleMedina-Herrera, Nancy, Guillermo Cristian Guadalupe Martínez-Ávila, Claudia Lizeth Robledo-Jiménez, Romeo Rojas, and Bianca Sherlyn Orozco-Zamora. 2024. "From Citrus Waste to Valuable Resources: A Biorefinery Approach" Biomass 4, no. 3: 784-808. https://doi.org/10.3390/biomass4030044
APA StyleMedina-Herrera, N., Martínez-Ávila, G. C. G., Robledo-Jiménez, C. L., Rojas, R., & Orozco-Zamora, B. S. (2024). From Citrus Waste to Valuable Resources: A Biorefinery Approach. Biomass, 4(3), 784-808. https://doi.org/10.3390/biomass4030044










