Yield and Toxin Analysis of Leaf Protein Concentrate from Common North American Coniferous Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- Western Cedar (WC) is a native tree in North America [10]. This tree is widespread from northern California to southeastern Alaska, and from McGregor River to western Montana and northern Idaho [20]. Western cedar represented about 750 million cubic meters stock in British Columbia, 5 million seedlings were planted, and 1 percent of the stock was harvested annually in 2003 [21].
- (2)
- (3)
- (4)
- (5)
2.2. Material Processing
2.3. LC/MS Instrumentation
2.4. Data Analysis
3. Results
3.1. Western Cedar
Western Cedar and Viscose Liquid (WC #2 LPC (+))
3.2. Douglas Fir (DF)
3.3. Ponderosa Pine (PP)
3.4. Western Hemlock (WHL)
3.5. LodgePole Pine (LPP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
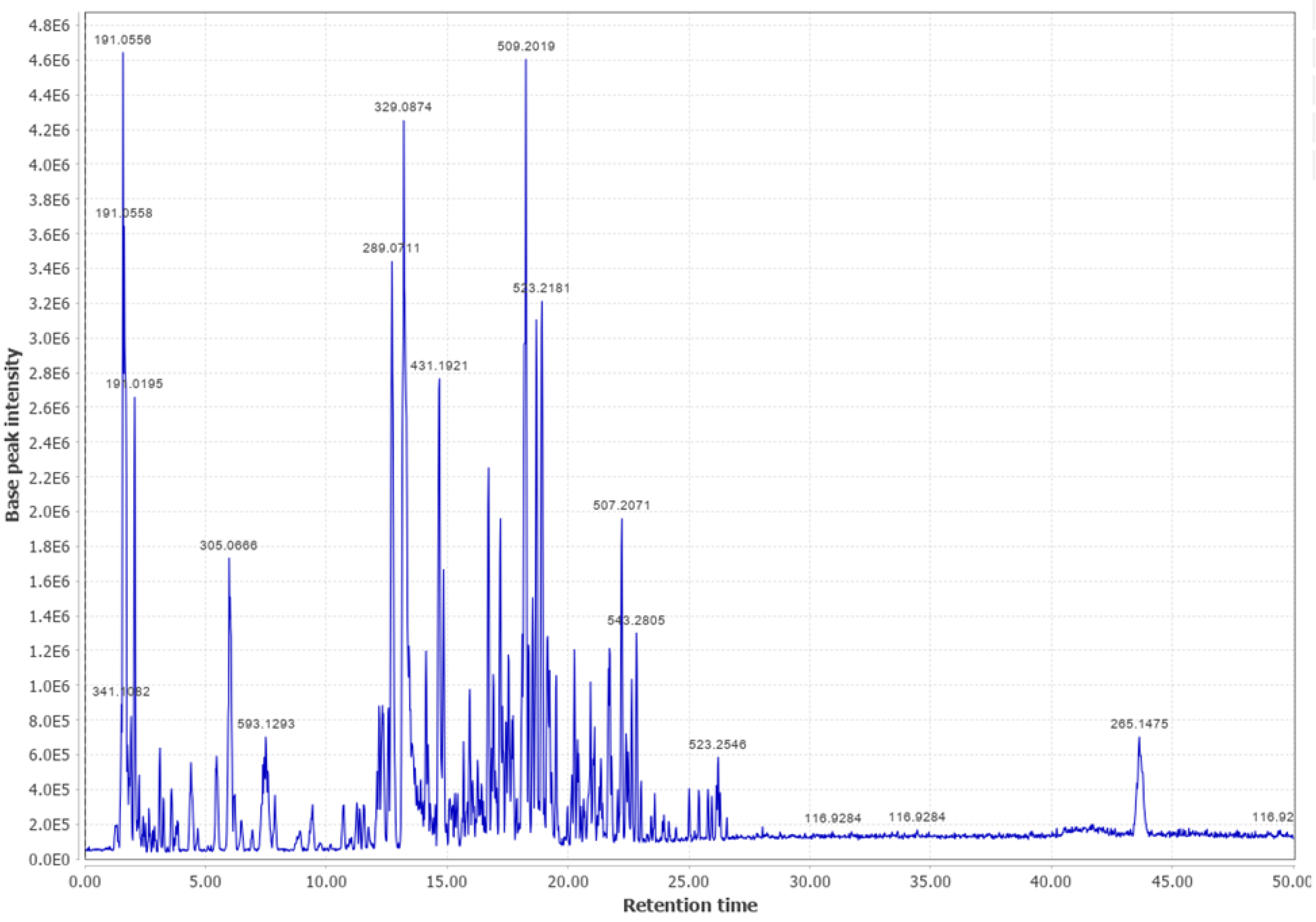
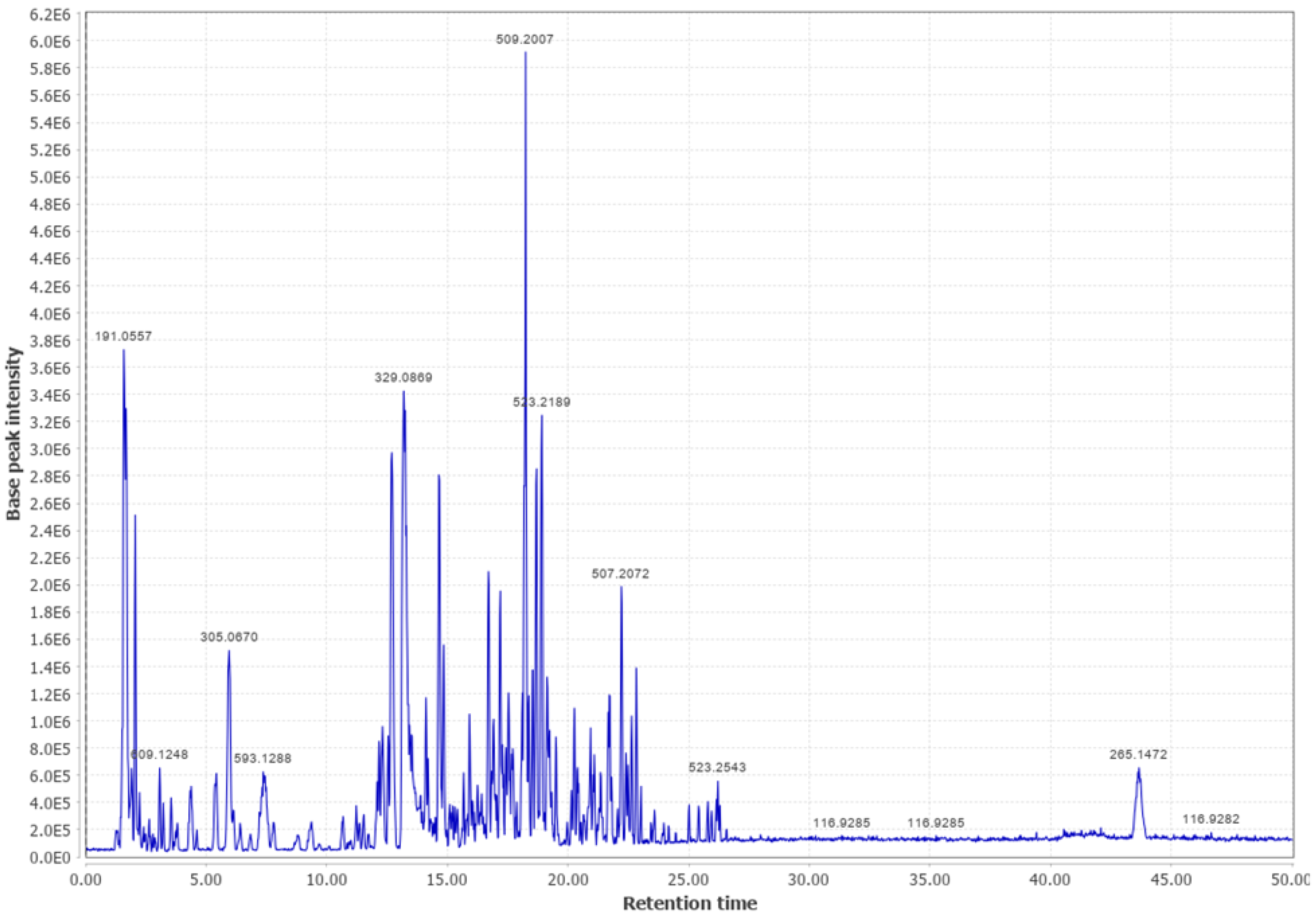

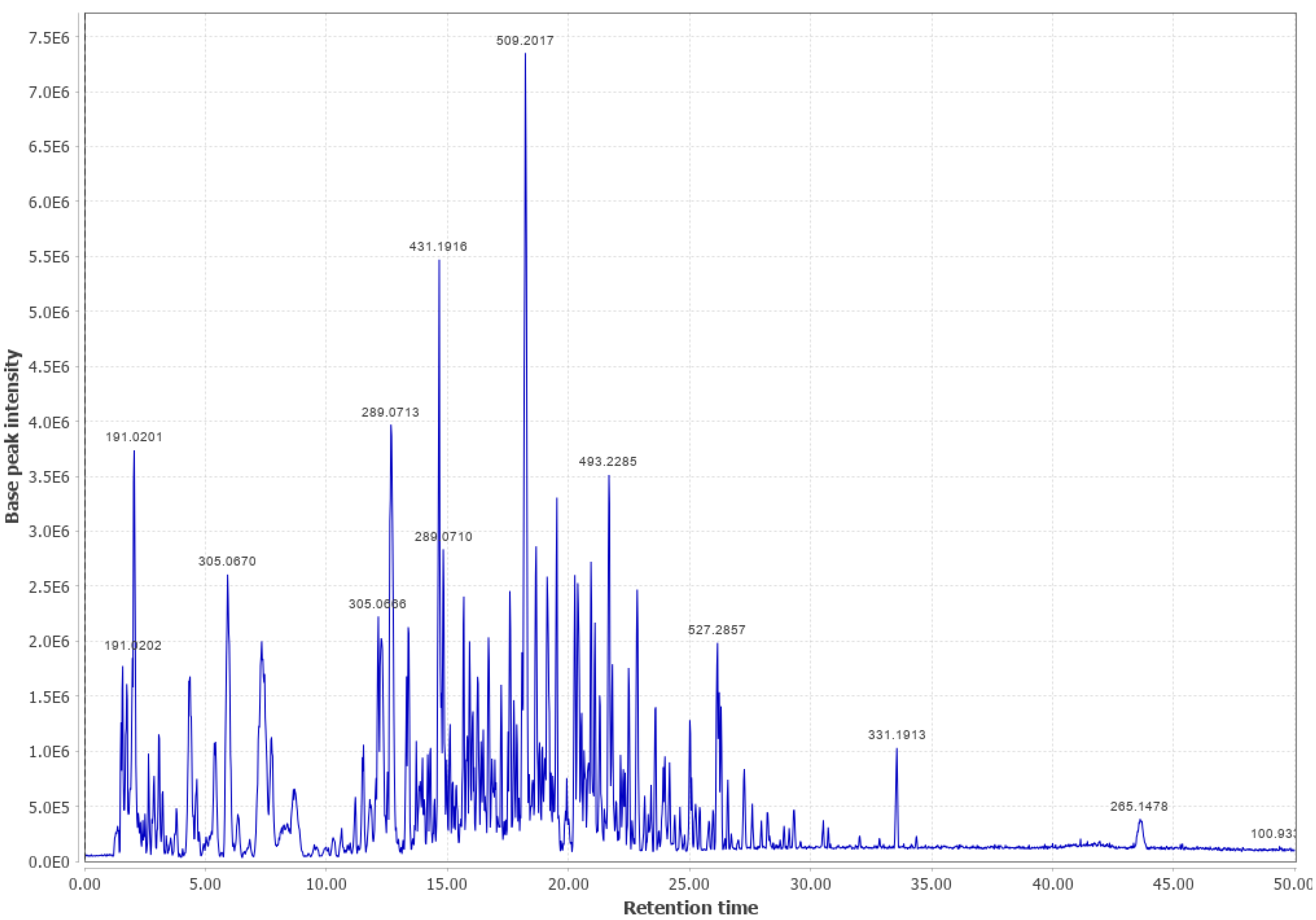
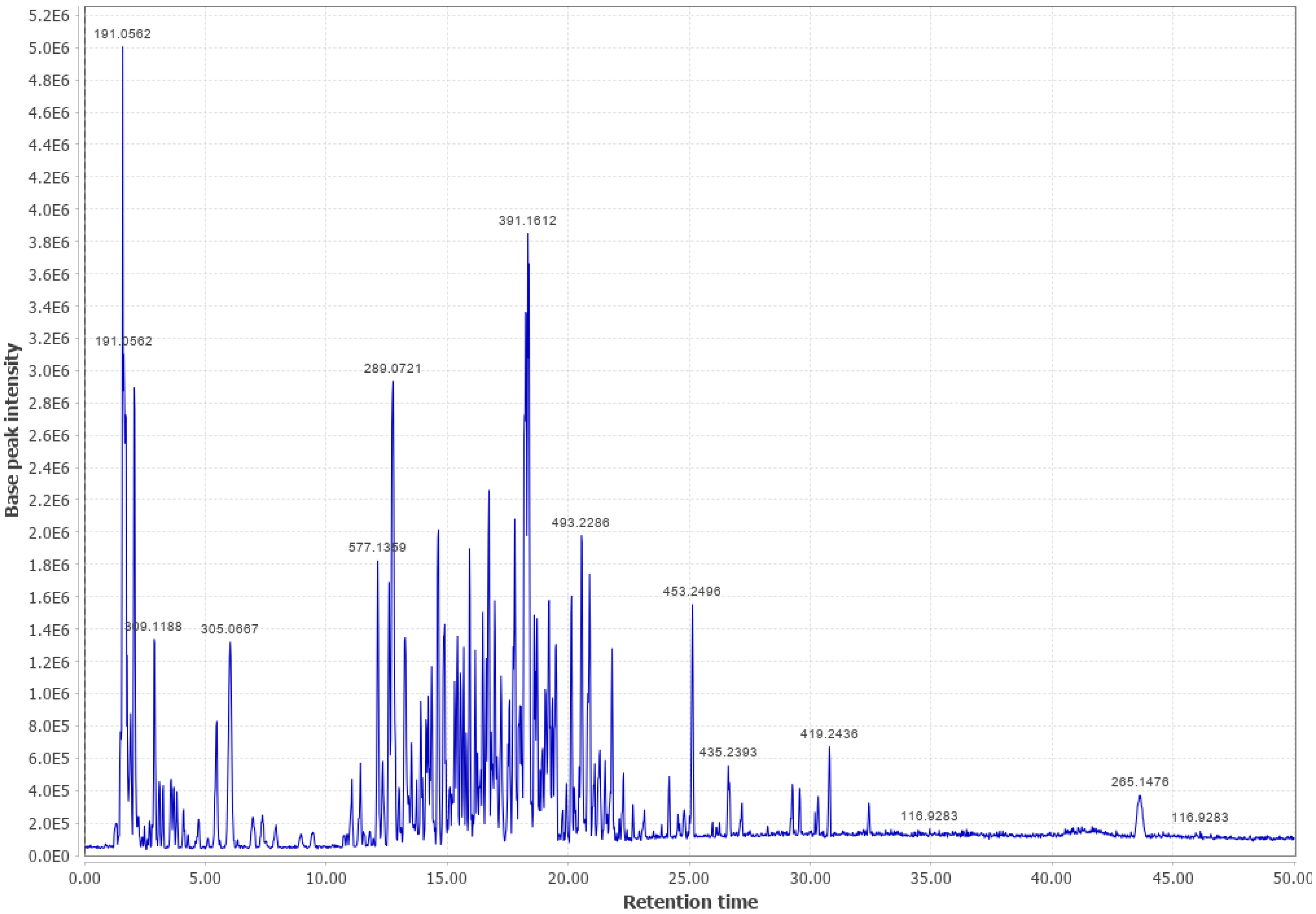
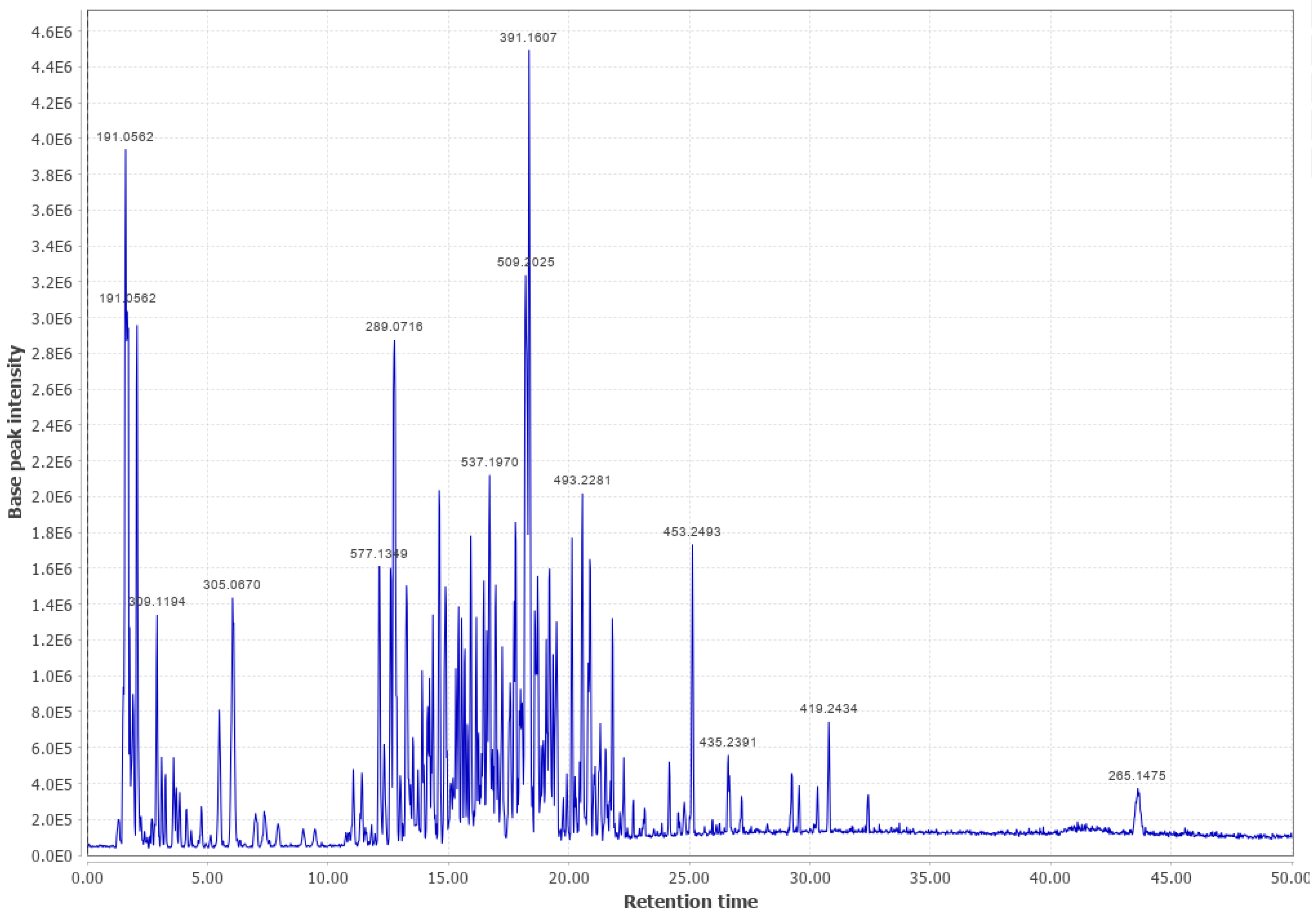

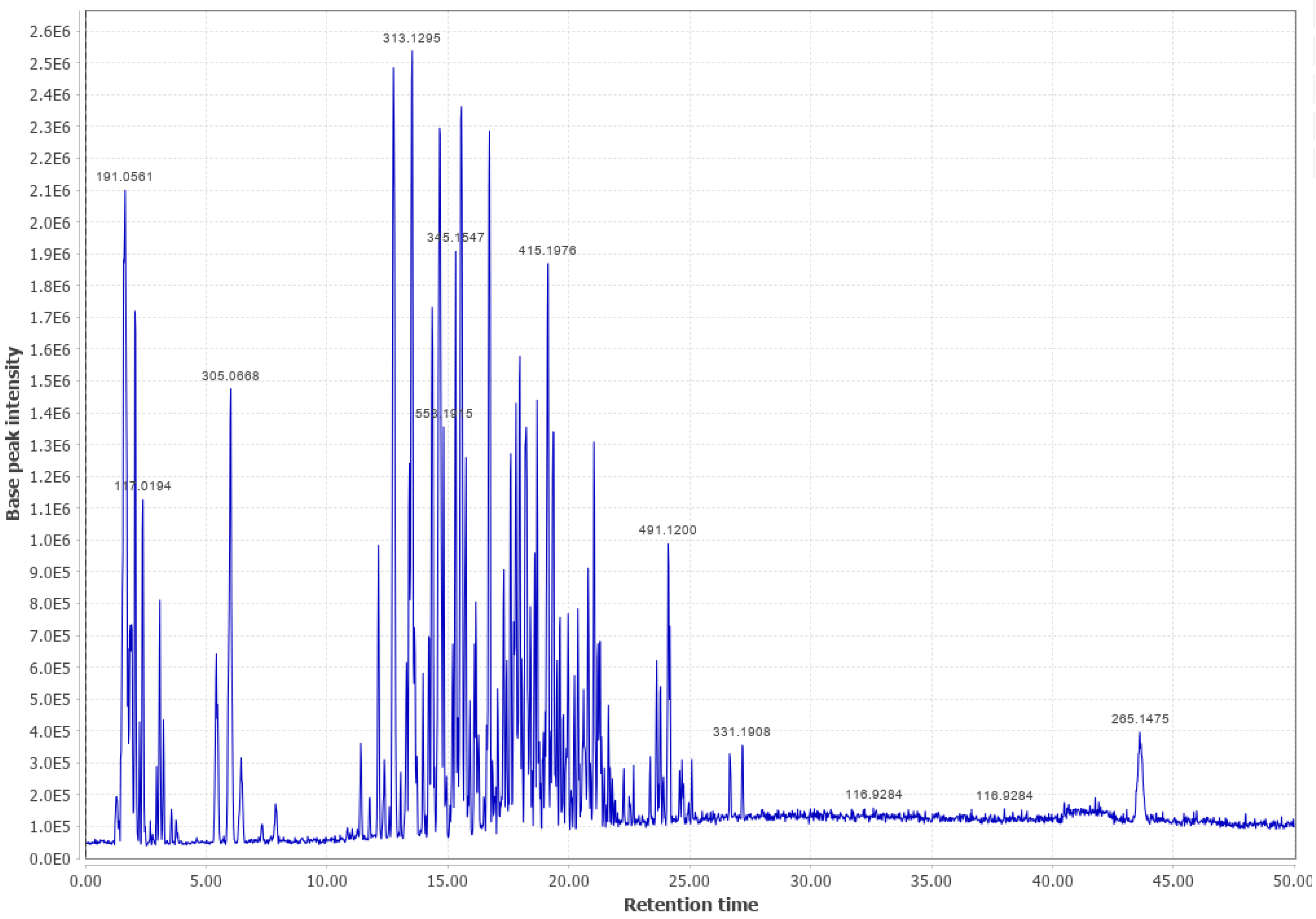
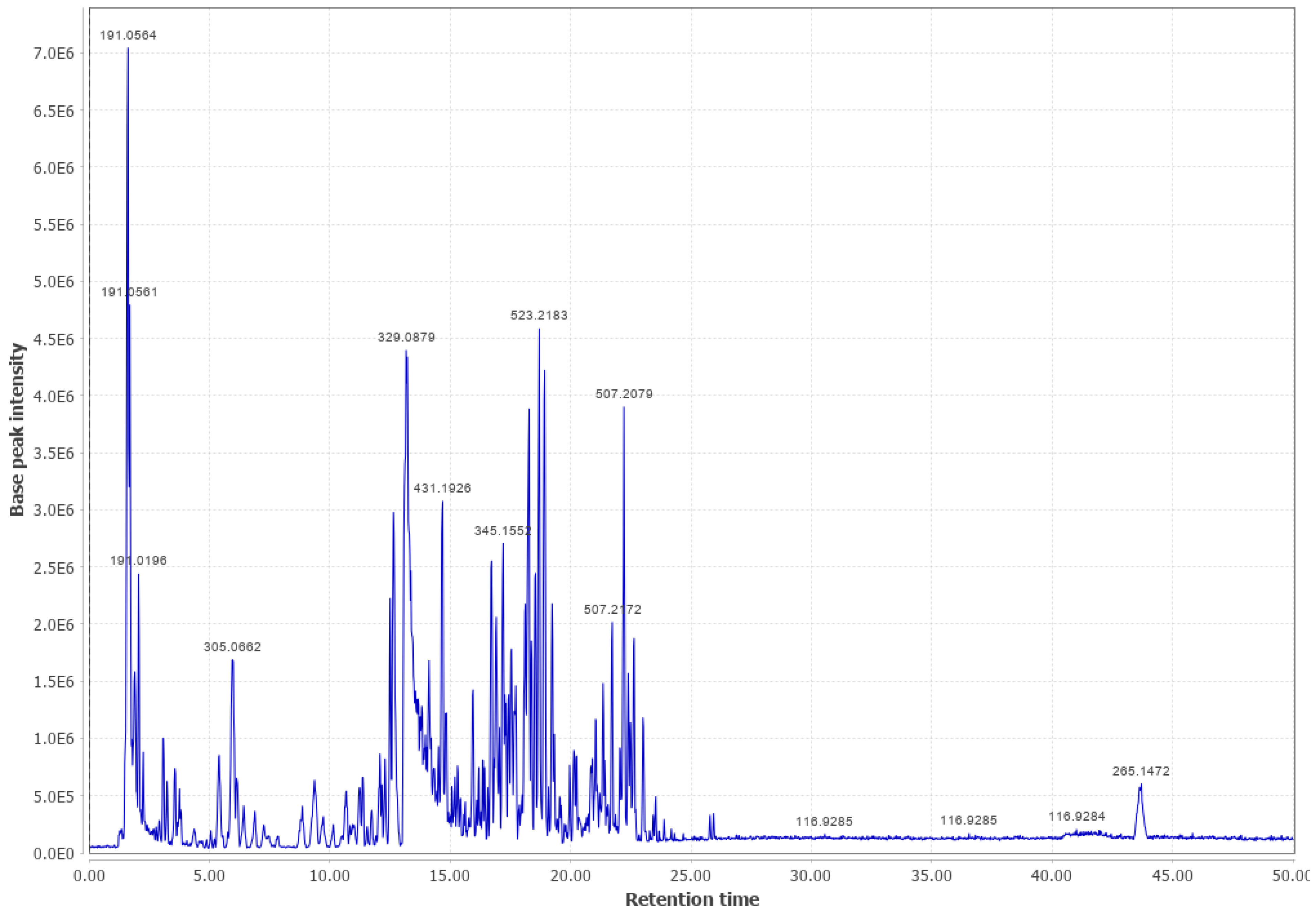
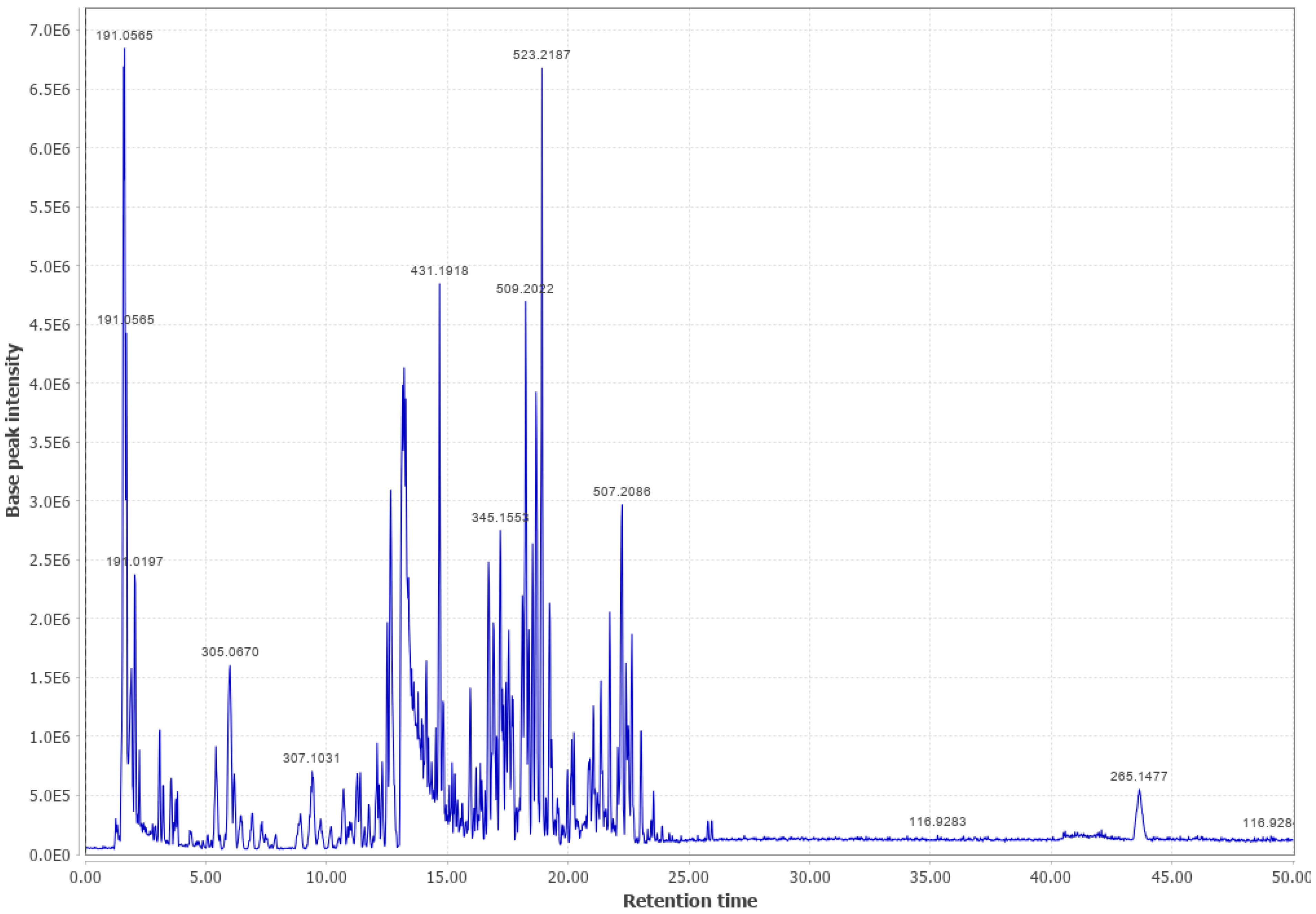
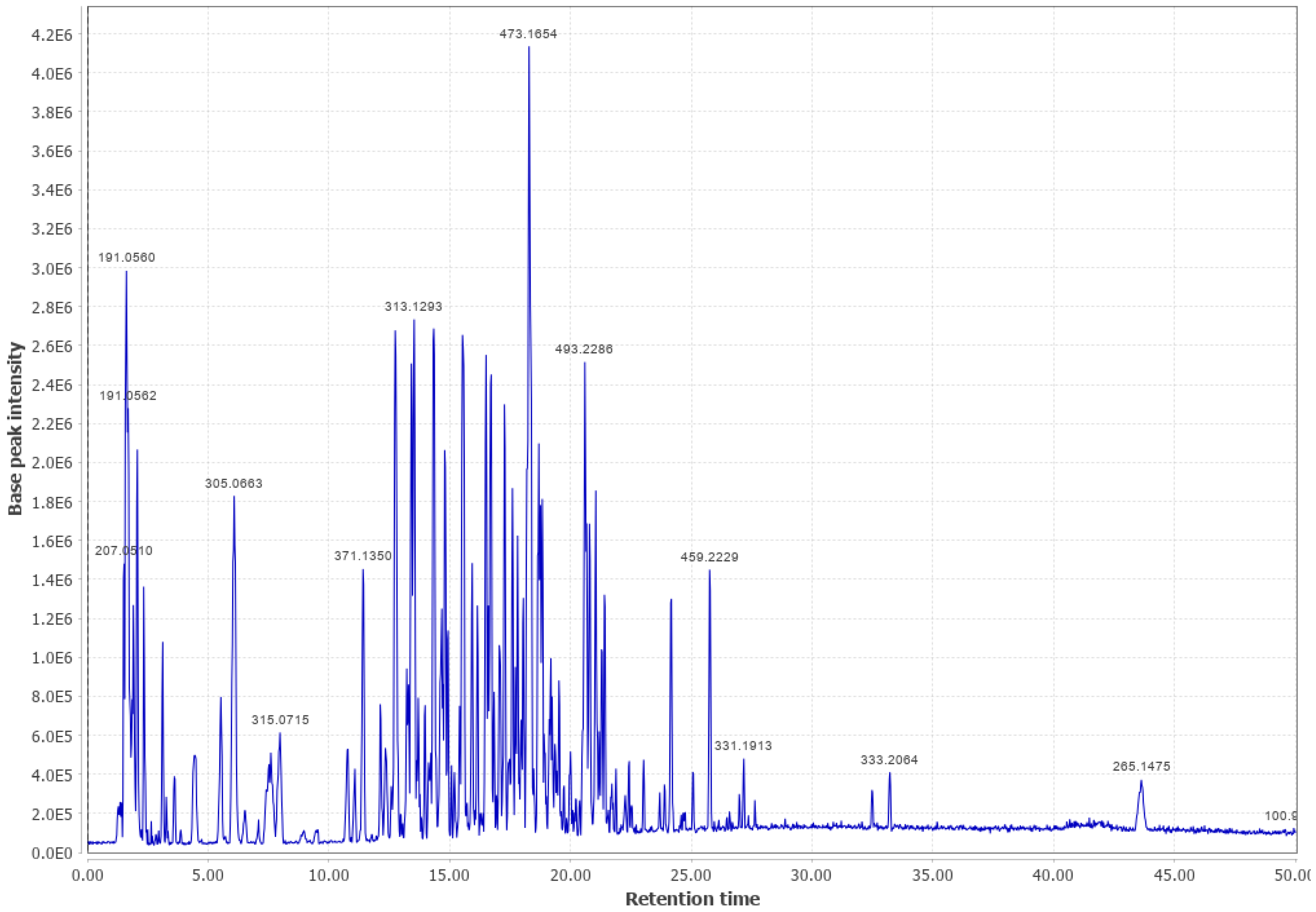
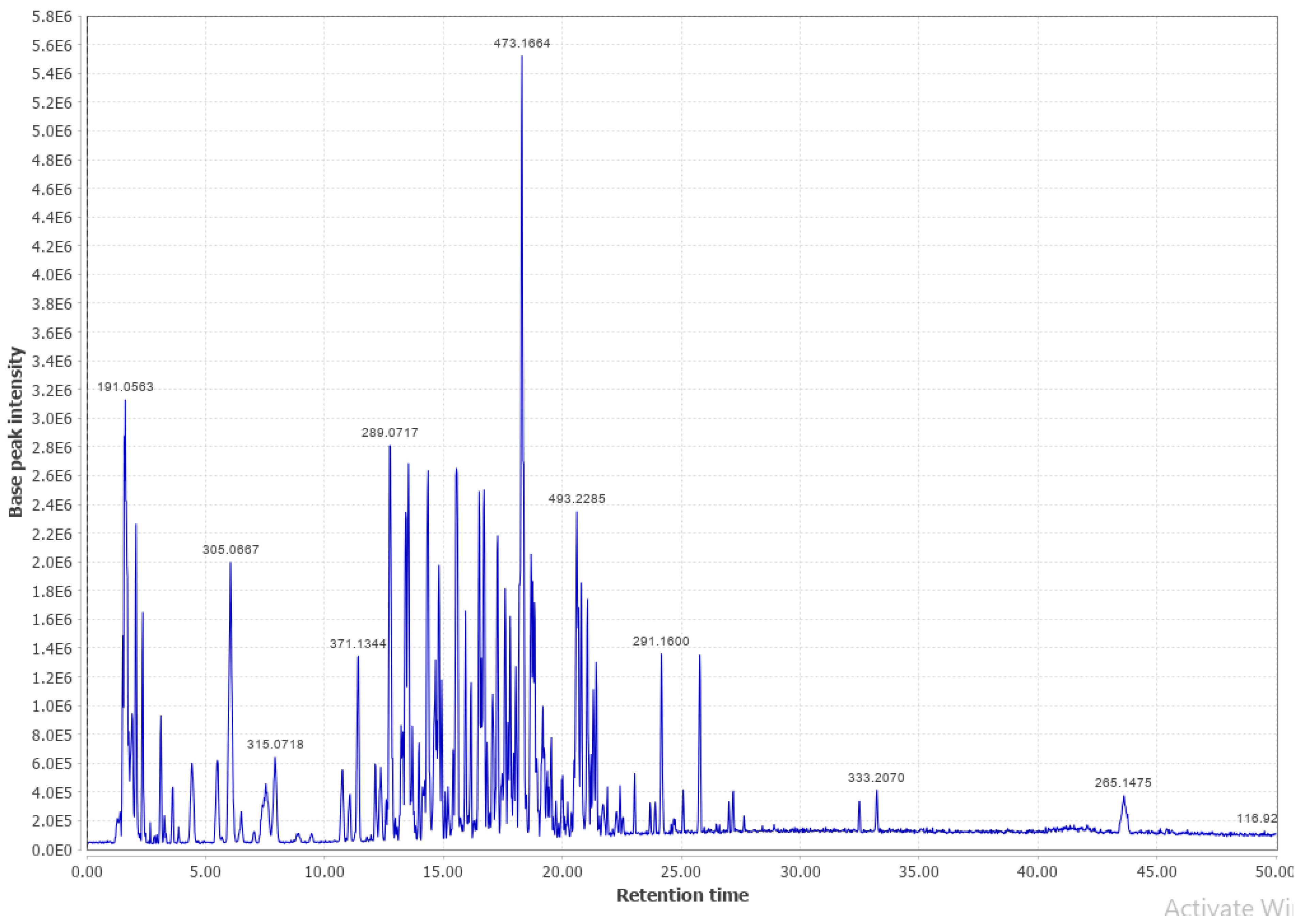
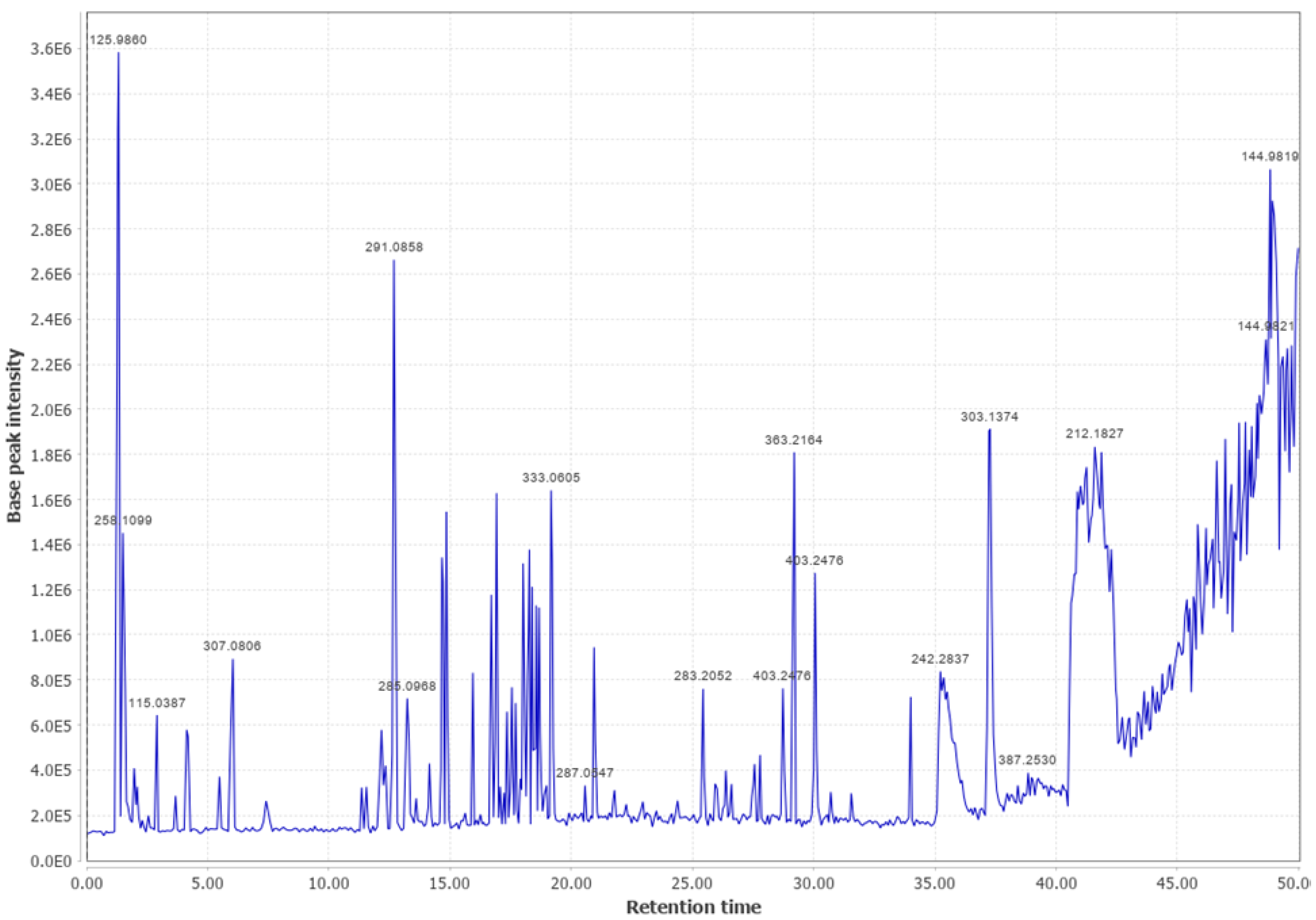
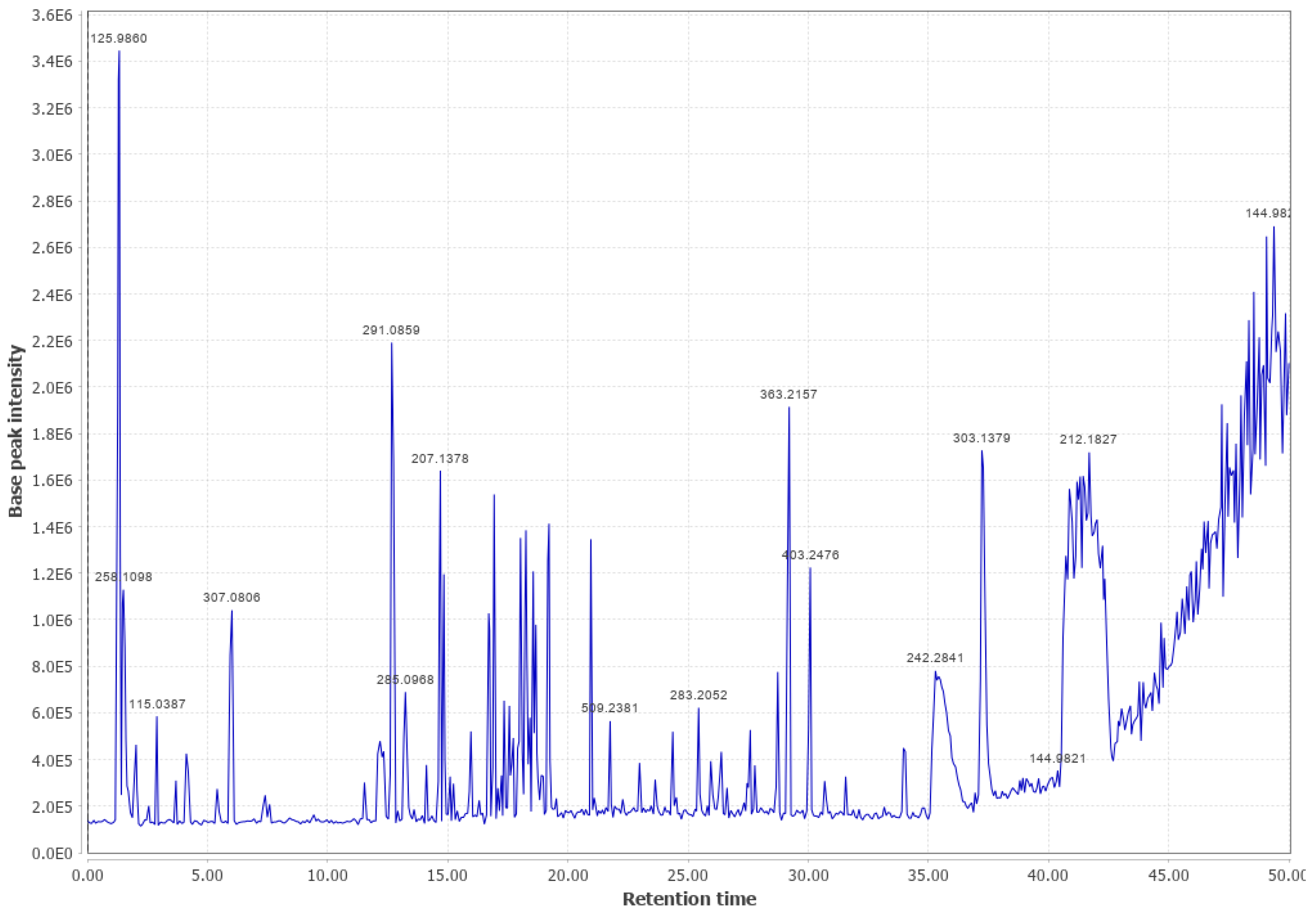
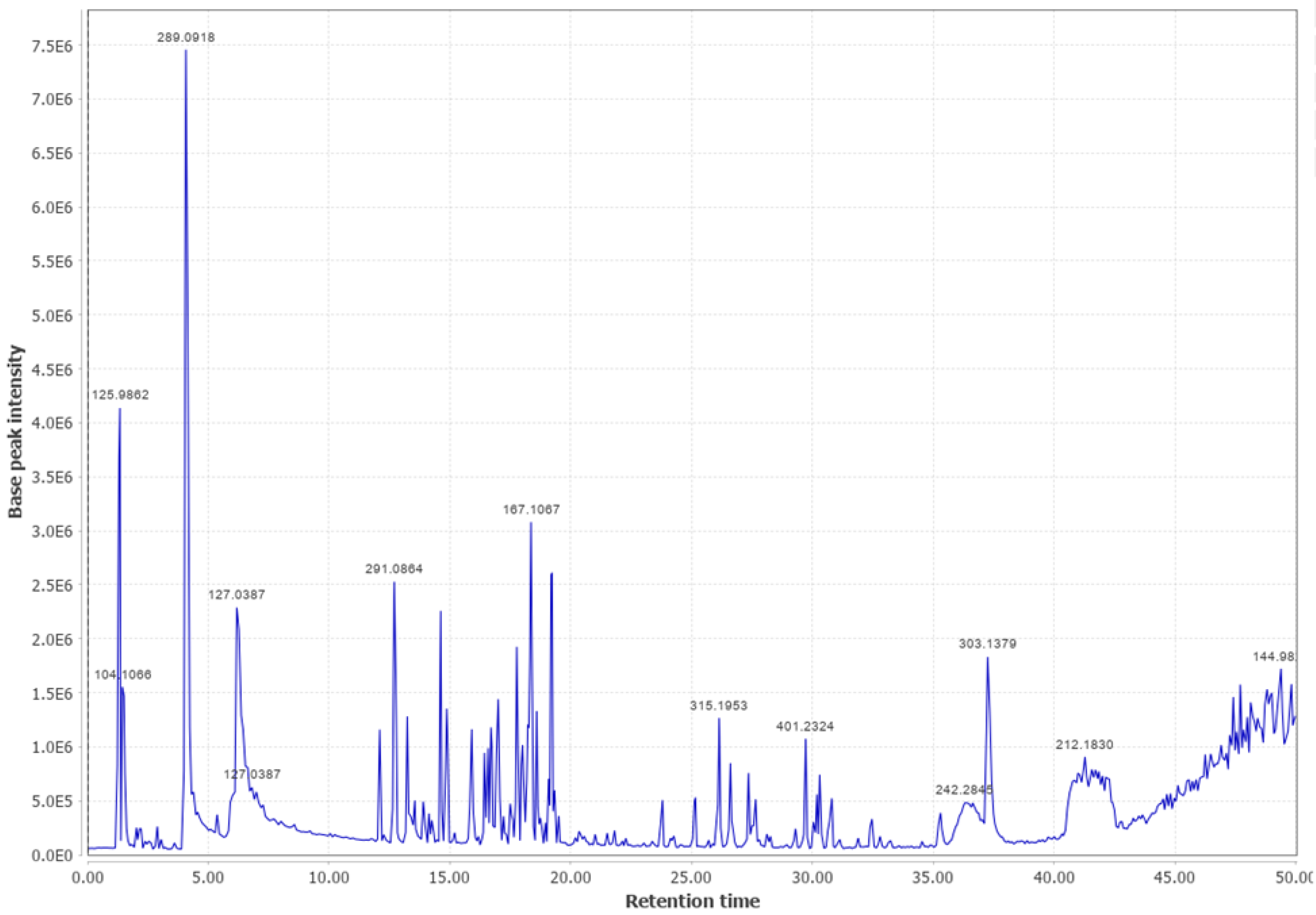
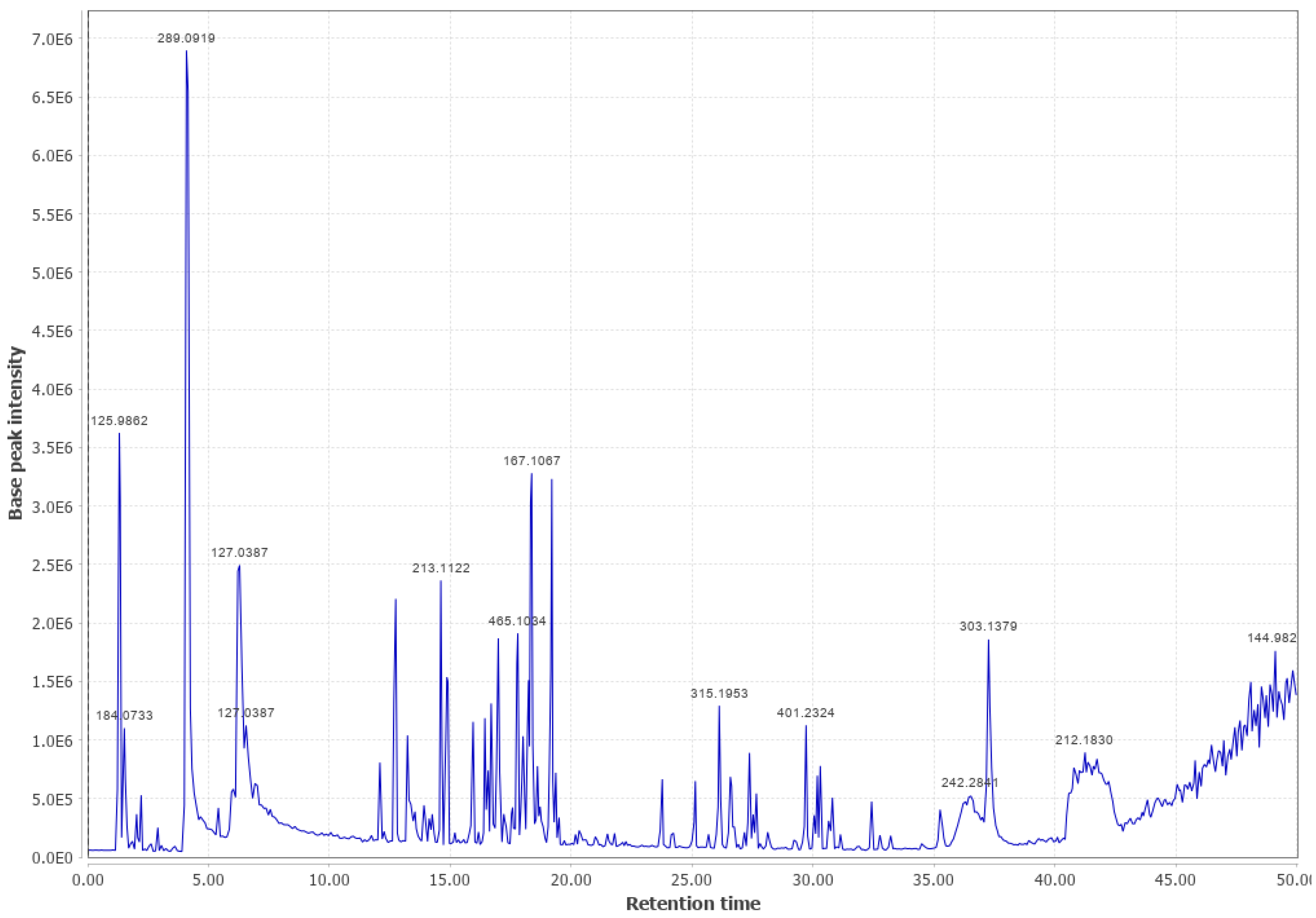
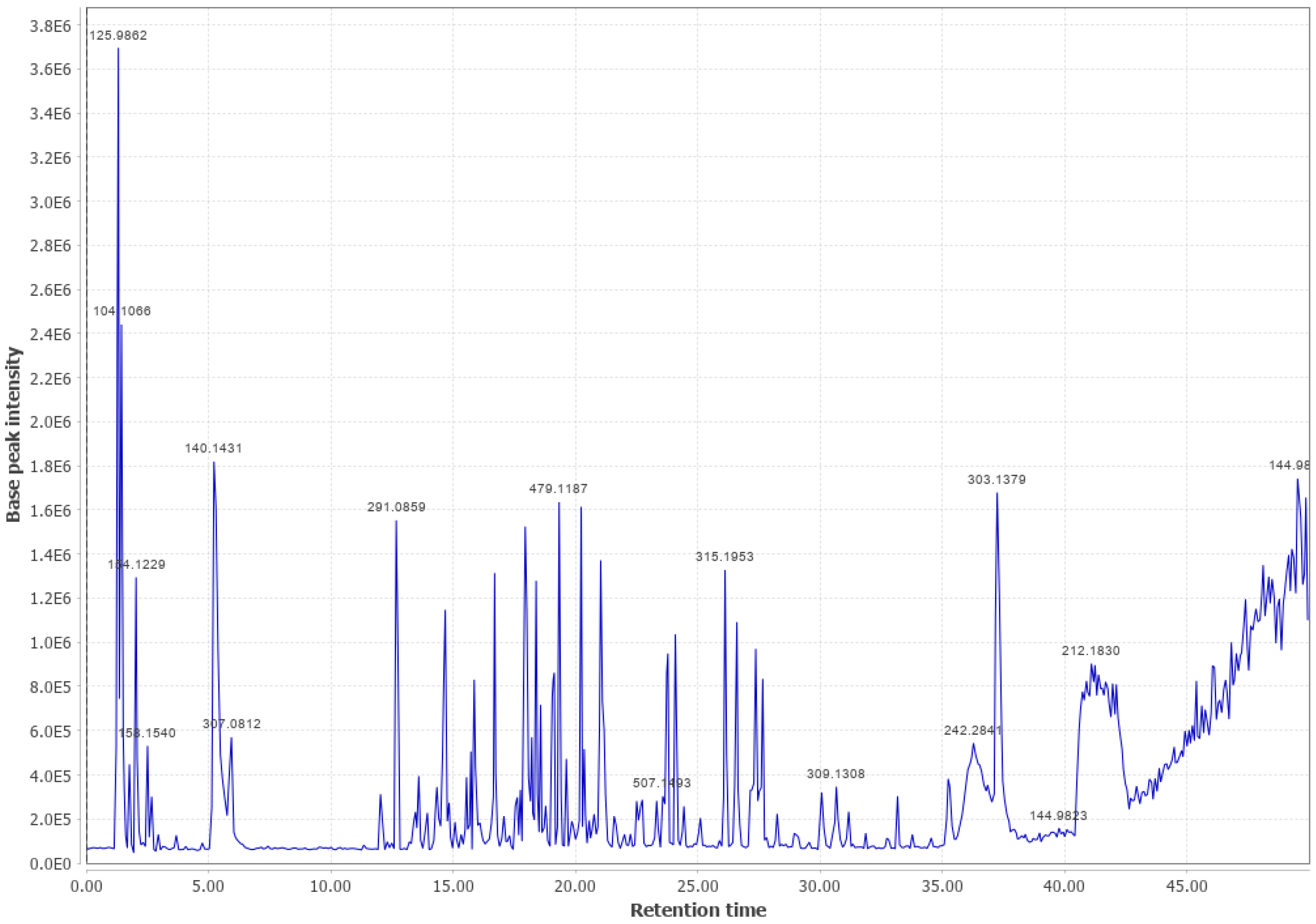
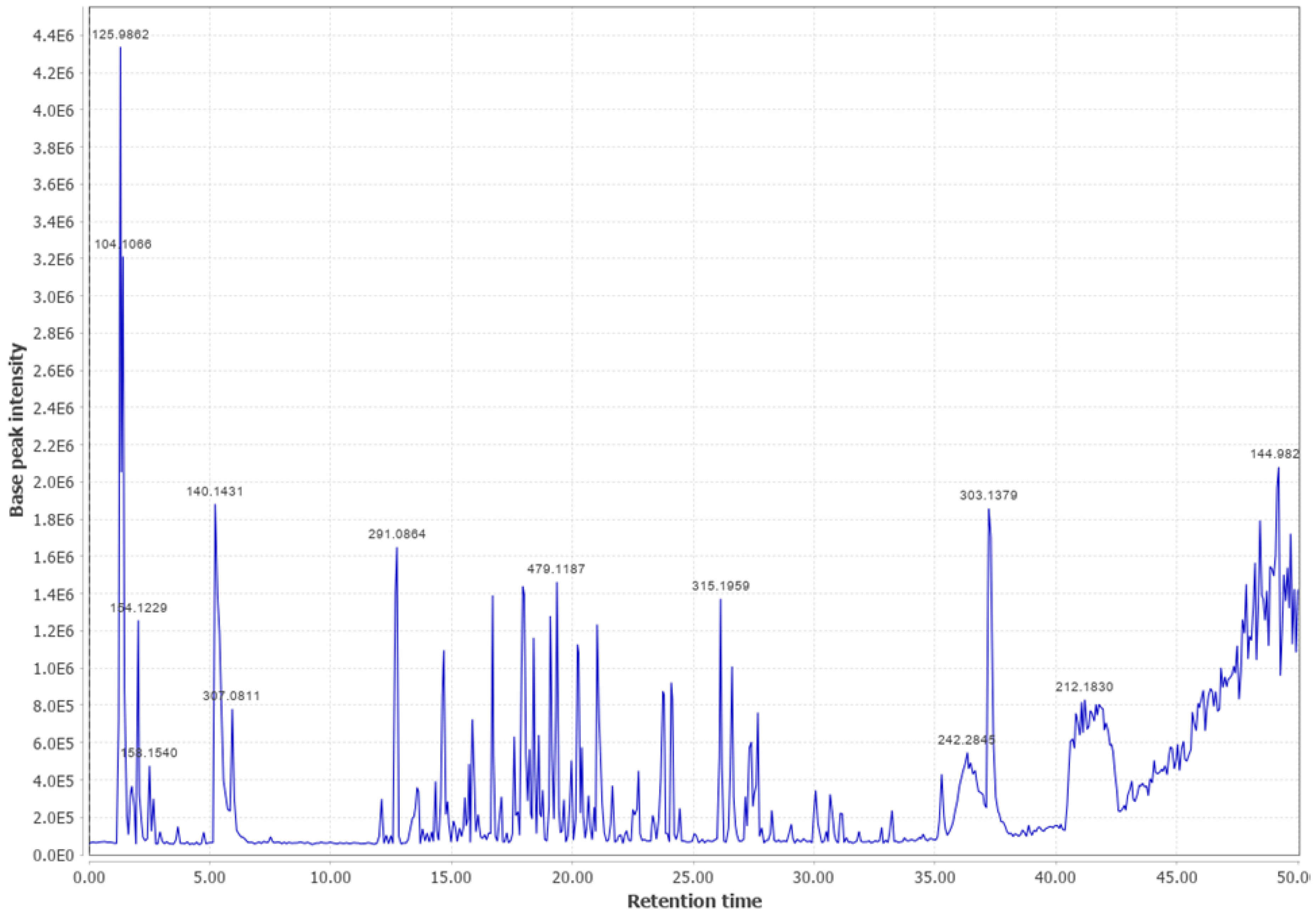
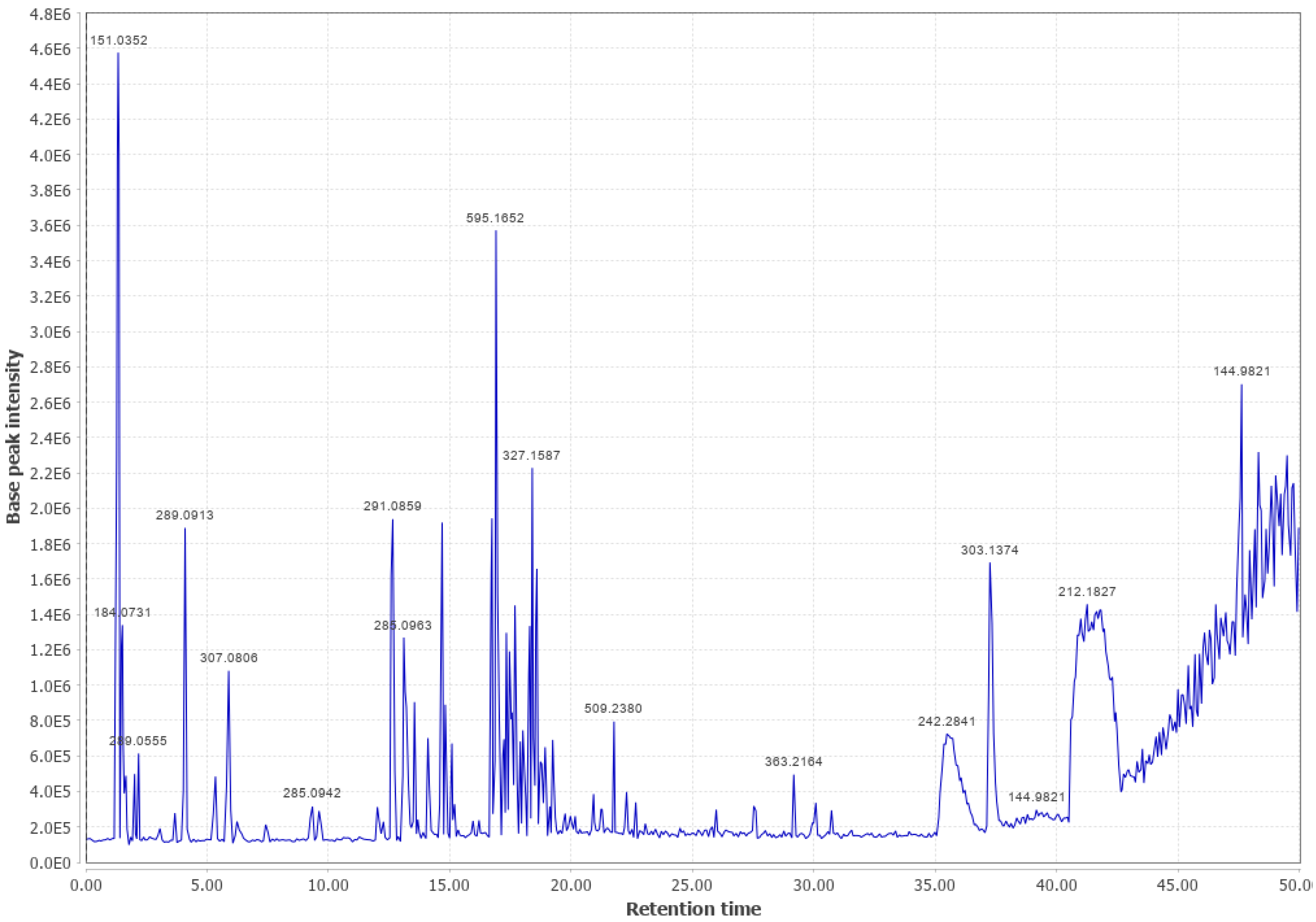
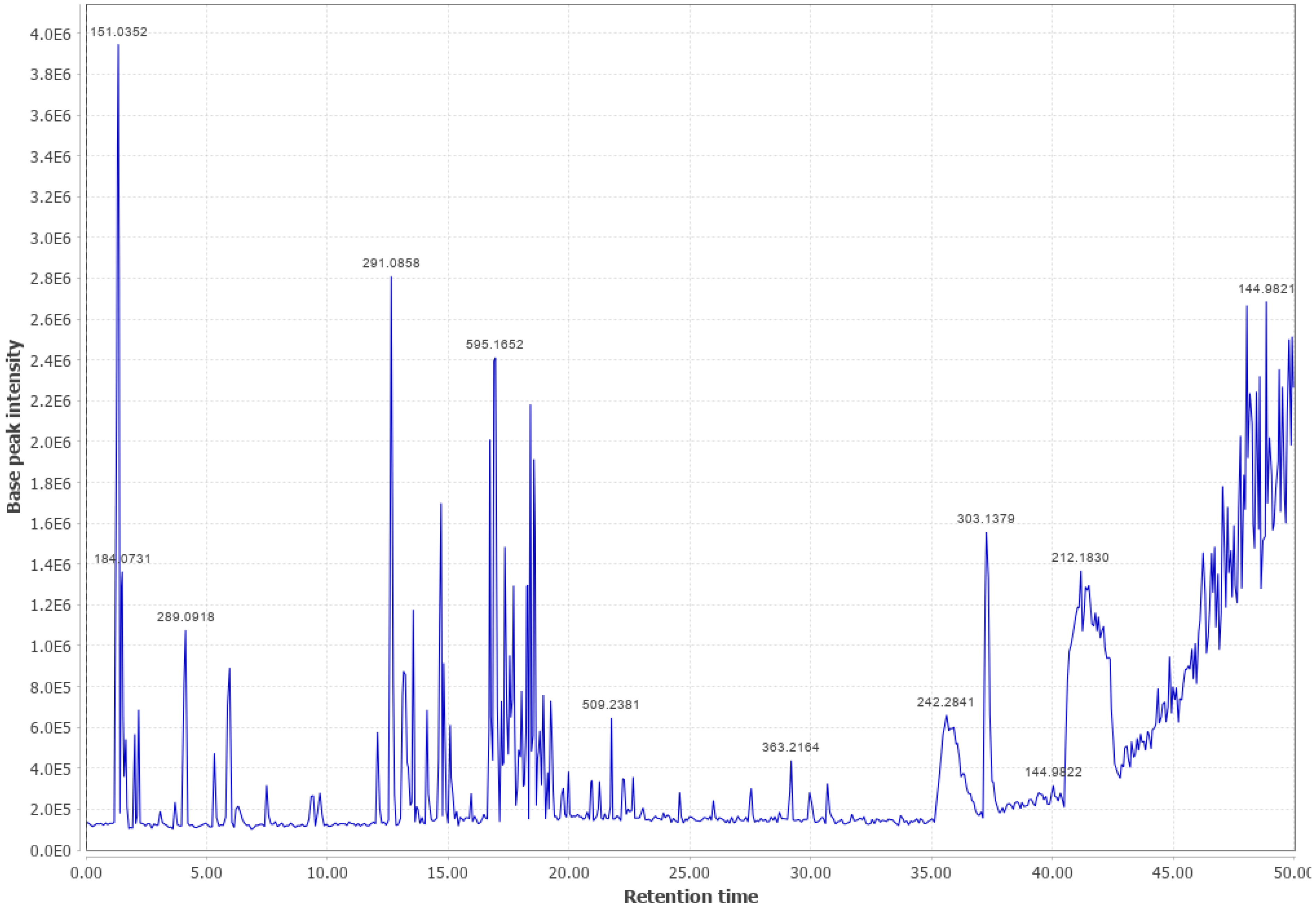
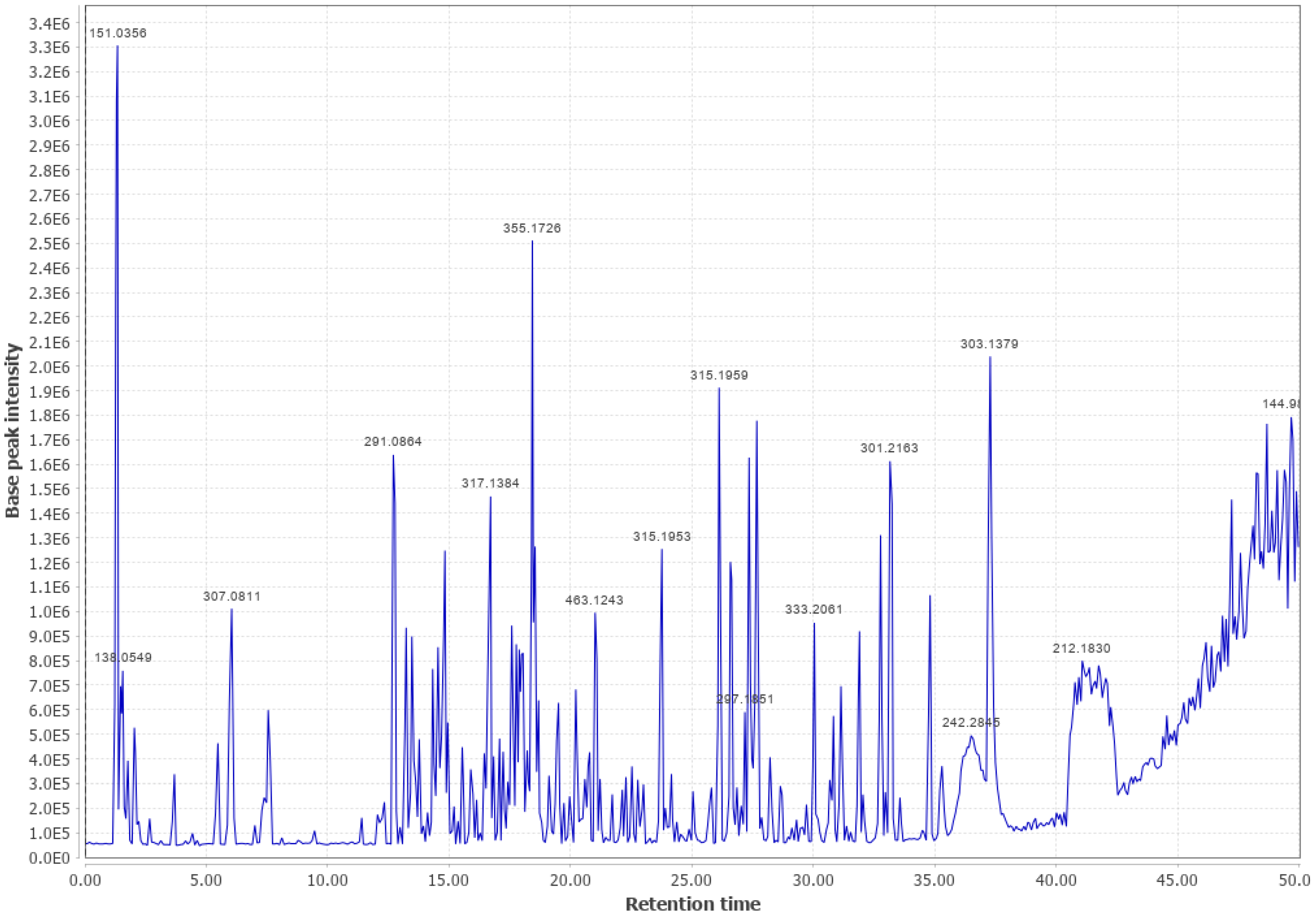
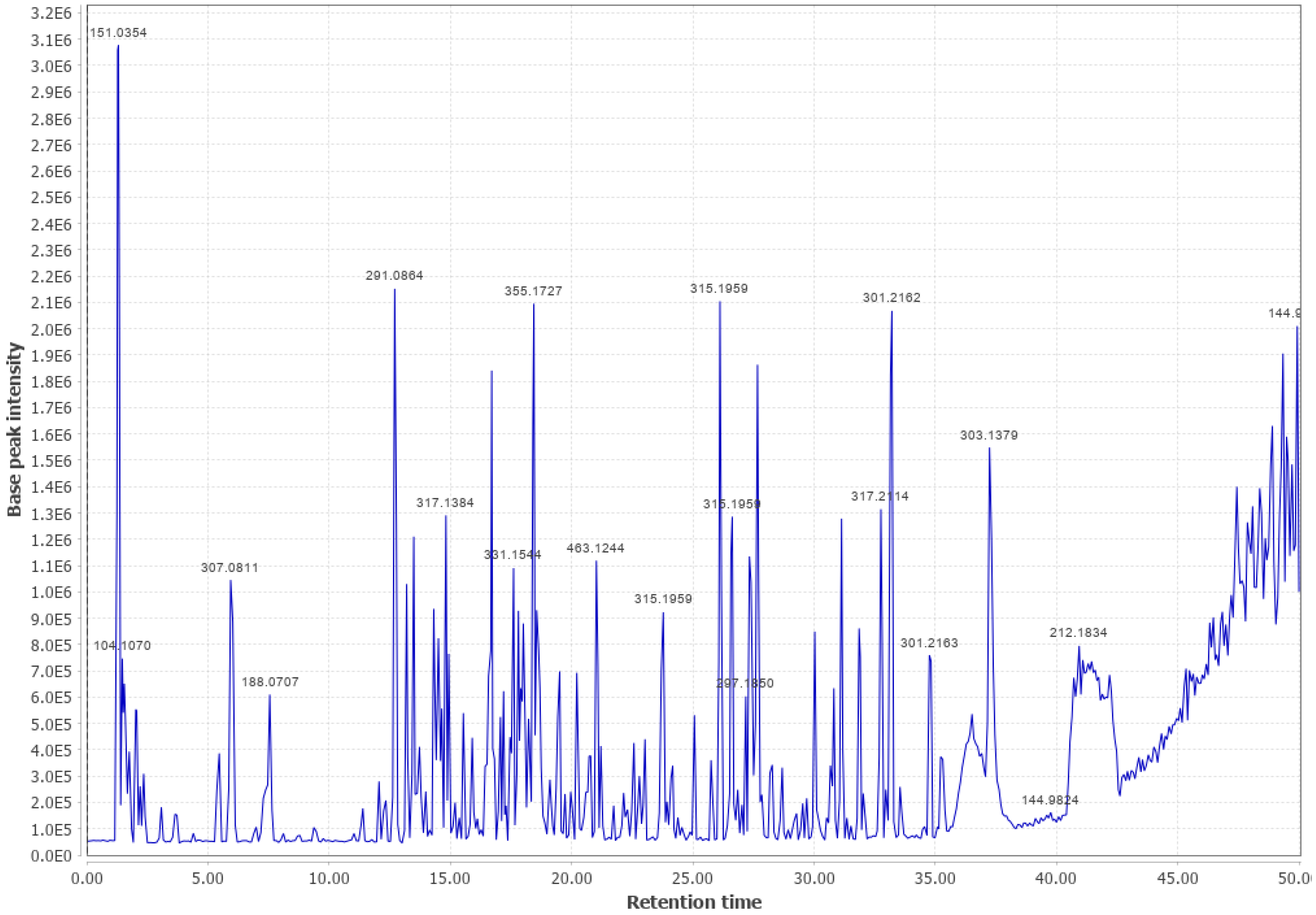
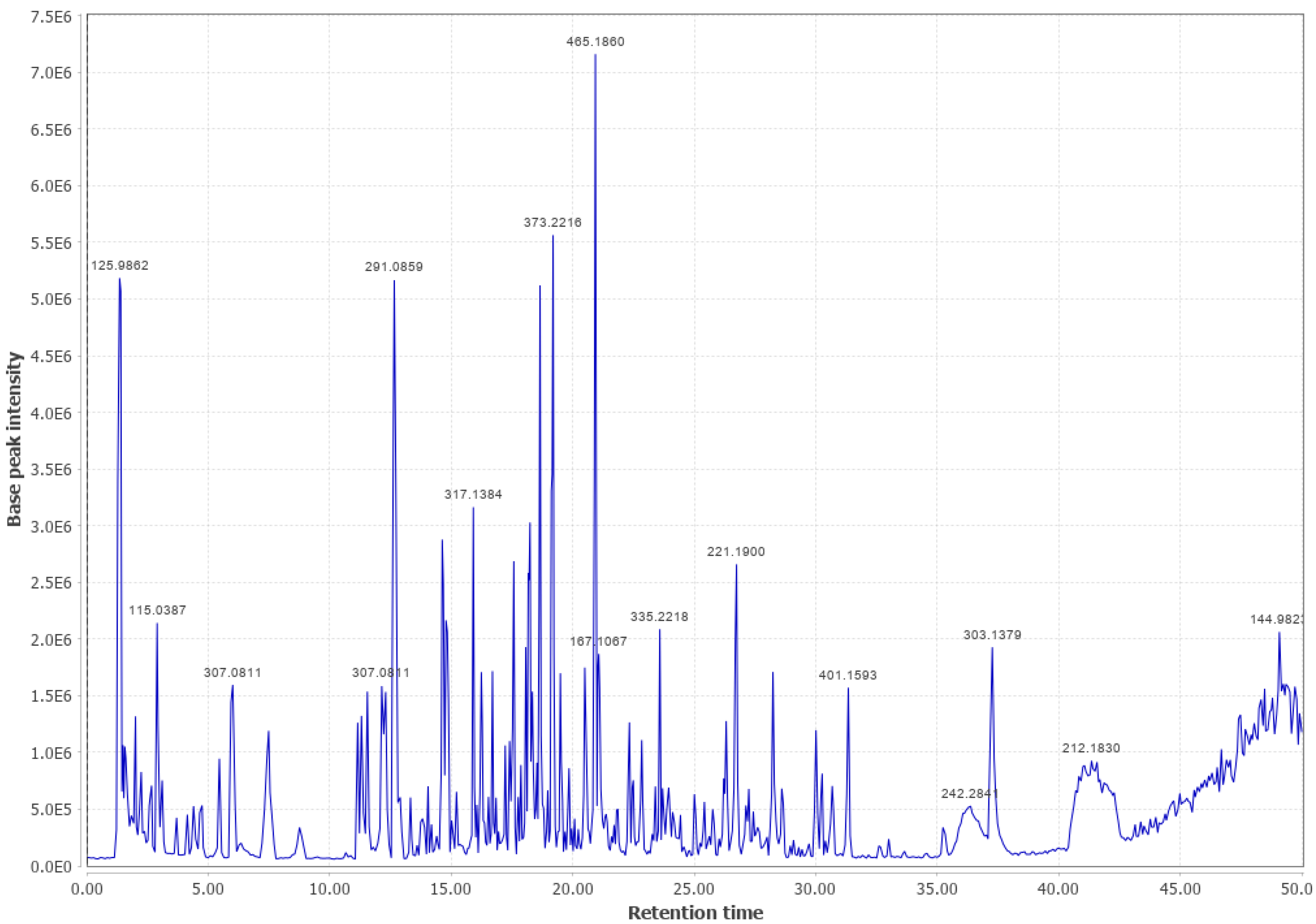
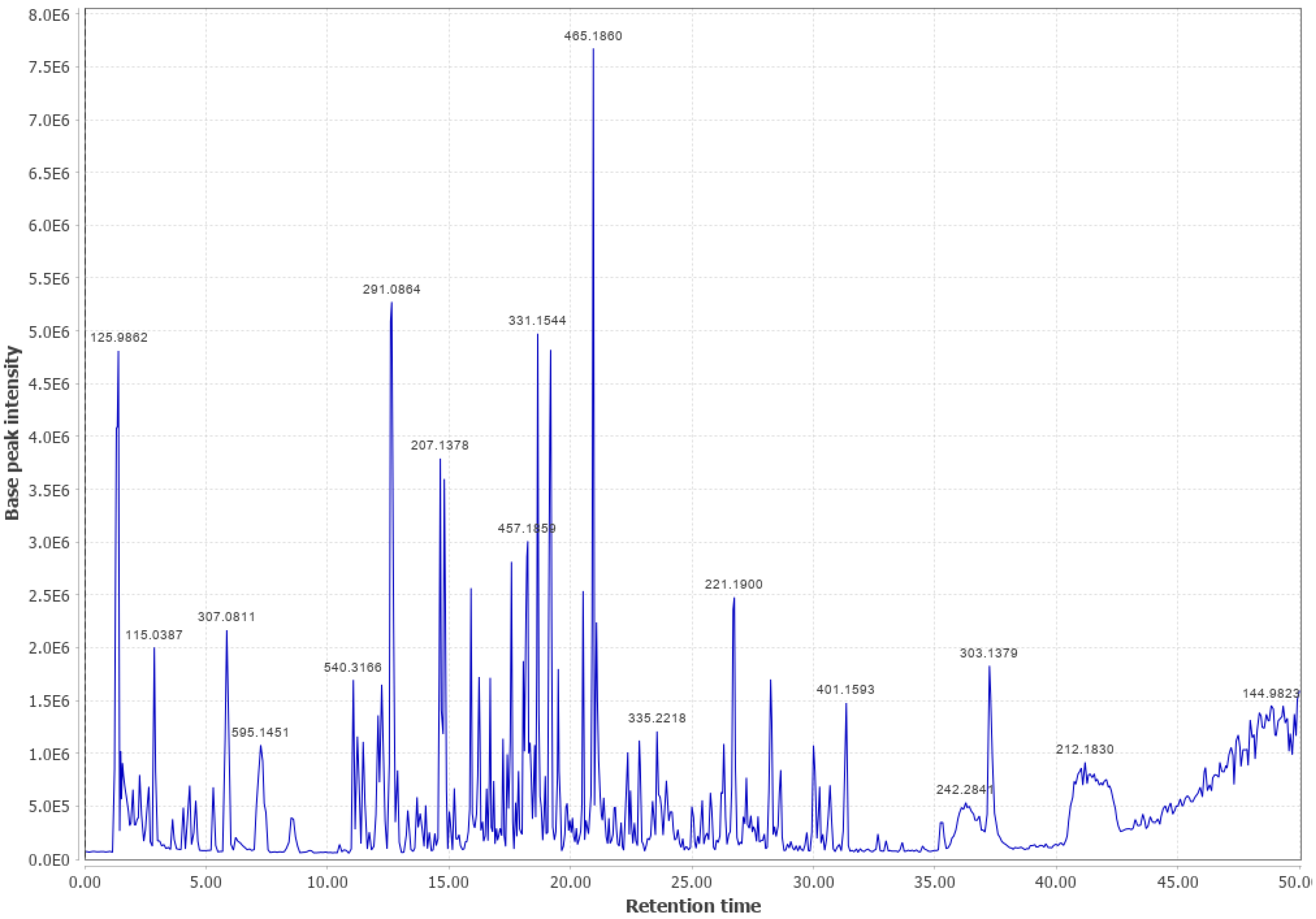
Appendix B
| Hazard Indicators | I | II | III | IV |
|---|---|---|---|---|
| Oral LD50 | Up to and including 50 mg/kg | >50 thru 500 mg/kg | >500 thru 5000 mg/kg | >5000 mg/kg |
| Dermal LD50 | Up to and including 200 mg/kg | >200 thru 2000 mg/kg | >2000 thru 20,000 mg/kg | >20,000 mg/kg |
| Inhalation LC50 | Up to and including 0.2 mg/L | >0.2 thru 2 mg/L | >2 thru 20 mg/L | >20 mg/L |
| Eye irritation | Corrosive; corneal opacity not reversible within 7 days | Corneal opacity reversible within 7 days; irritation persisting for 7 days | No corneal opacity; irritation reversible within 7 days | No irritation |
| Skin irritation | Corrosive | Severe irritation at 72 h | Moderate irritation at 72 h | Mild or slight irritation at 72 h |
References
- Bostrom, N.; Cirkovic, M.M. (Eds.) Global Catastrophic Risks; Oxford University Press: Oxford, UK; New York, NY, USA, 2011. [Google Scholar]
- Robock, A.; Oman, L.; Stenchikov, G.L. Nuclear winter revisited with a modern climate model and current nuclear arsenals: Still catastrophic consequences. J. Geophys. Res. 2007, 112, D13. [Google Scholar] [CrossRef]
- Xia, L.; Robock, A.; Scherrer, K.; Harrison, C.S.; Bodirsky, B.L.; Weindl, I.; Jägermeyr, J.; Bardeen, C.G.; Toon, O.B.; Heneghan, R. Global food insecurity and famine from reduced crop, marine fishery and livestock production due to climate disruption from nuclear war soot injection. Nat. Food 2022, 3, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Denkenberger, D.C.; Pearce, J.M. Feeding everyone: Solving the food crisis in event of global catastrophes that kill crops or obscure the sun. Futures 2015, 72, 57–68. [Google Scholar] [CrossRef]
- Denkenberger, D.; Sandberg, A.; Tieman, R.J.; Pearce, J.M. Long term cost-effectiveness of resilient foods for global catastrophes compared to artificial general intelligence safety. Int. J. Disaster Risk Reduct. 2022, 73, 102798. [Google Scholar] [CrossRef]
- Denkenberger, D.; Pearce, J. Feeding Everyone No Matter What: Managing Food Security after Global Catastrophe; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- García Martínez, J.B.; Pearce, J.M.; Throup, J.; Cates, J.; Lackner, M.; Denkenberger, D.C. Methane Single Cell Protein: Potential to Secure a Global Protein Supply Against Catastrophic Food Shocks. Front. Bioeng. Biotechnol. 2022, 10, 1125. [Google Scholar] [CrossRef]
- Throup, J.; García Martínez, J.B.; Bals, B.; Cates, J.; Pearce, J.M.; Denkenberger, D.C. Rapid repurposing of pulp and paper mills, biorefineries, and breweries for lignocellulosic sugar production in global food catastrophes. Food Bioprod. Process. 2022, 131, 22–39. [Google Scholar] [CrossRef]
- Alvarado, K.A.; Mill, A.; Pearce, J.M.; Vocaet, A.; Denkenberger, D. Scaling of greenhouse crop production in low sunlight scenarios. Sci. Total Environ. 2020, 707, 136012. [Google Scholar] [CrossRef]
- Stewart, H. Cedar: Tree of Life to the Northwest Coast Indians; Douglas & McIntyre: Vancouver, BC, Canada, 1984. [Google Scholar]
- Winstead, D.J.; Jacobson, M.G. Food resilience in a dark catastrophe: A new way of looking at tropical wild edible plants. Ambio 2022, 51, 1949–1962. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Egbejimba, J.; Throup, J.; Matassa, S.; Pearce, J.M.; Denkenberger, D.C. Potential of microbial protein from hydrogen for preventing mass starvation in catastrophic scenarios. Sustain. Prod. Consum. 2021, 25, 234–247. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Brown, M.M.; Christodoulou, X.; Alvarado, K.A.; Denkenberger, D.C. Potential of microbial electrosynthesis for contributing to food production using CO2 during global agriculture-inhibiting disasters. Clean. Eng. Technol. 2021, 4, 100139. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Alvarado, K.A.; Christodoulou, X.; Denkenberger, D.C. Chemical synthesis of food from CO2 for space missions and food resilience. J. CO2 Util. 2021, 53, 101726. [Google Scholar] [CrossRef]
- Meyer, T.K.; Pascaris, A.; Denkenberger, D.; Pearce, J.M.U.S. Potential of Sustainable Backyard Distributed Animal and Plant Protein Production during and after Pandemics. Sustainability 2021, 13, 5067. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Alvarado, K.A.; Denkenberger, D.C. Synthetic fat from petroleum as a resilient food for global catastrophes: Preliminary techno-economic assessment and technology roadmap. Chem. Eng. Res. Des. 2022, 177, 255–272. [Google Scholar] [CrossRef]
- Denkenberger, D.; Pearce, J. Micronutrient Availability in Alternative Foods During Agricultural Catastrophes. Agriculture 2018, 8, 169. [Google Scholar] [CrossRef]
- Denkenberger, D.C.; Cole, D.D.; Abdelkhaliq, M.; Griswold, M.; Hundley, A.B.; Pearce, J.M. Feeding everyone if the sun is obscured and industry is disabled. Int. J. Disaster Risk Reduct. 2017, 21, 284–290. [Google Scholar] [CrossRef]
- Fist, T.; Adesanya, A.A.; Denkenberger, D.; Pearce, J.M. Global distribution of forest classes and leaf biomass for use as alternative foods to minimize malnutrition. World Food Policy 2021, 7, 128–146. [Google Scholar] [CrossRef]
- Addo-Danso, S.D. Responses of Western Hemlock, Western Redcedar, and Amabilis Fir to Fertilization: A Synthesis; Province of British Columbia: Victoria, BC, Canada, 2019.
- BC Market Outreach Network. BC Forest Information. Managing B.C. Cedar for the Future; BC Market Outreach Network: Vancouver, BC, Canada, 2003. [Google Scholar]
- McArdle, R.E.; Meyer, W.H.; Bruce, D. The Yield of Douglas Fir in the Pacific Northwest; IDEAS Working Paper Series from RePEc; US Department of Agriculture: Washington, DC, USA, 1930.
- Gazol, A.; Valeriano, C.; Cantero, A.; Vergarechea, M.; Camarero, J.J. Douglas Fir Growth Is Constrained by Drought: Delineating the Climatic Limits of Timber Species under Seasonally Dry Conditions. Forests 2022, 13, 1796. [Google Scholar] [CrossRef]
- Schmid, M.; Pautasso, M.; Holdenrieder, O. Ecological consequences of Douglas fir (Pseudotsuga menziesii) cultivation in Europe. Eur. J. For. Res. 2014, 133, 13–29. [Google Scholar] [CrossRef]
- Nuszdorfer, F.C. Old and Large Douglas-Fir and Western Redcedar in the Squamish Forest District, British Columbia, Canada [Microform]; Vancouver Forest Region: Nanaimo, BC, Canada, 2000. [Google Scholar]
- Alldritt, M.J. The Ecology of the Ponderosa Pine Zone; British Columbia Ministry of Forests: Govt Victoria, BC, Canada, 1998.
- Safford, H.D.; Stevens, J.T. Natural Range of Variation for Yellow Pine and Mixed-Conifer Forests in the Sierra Nevada, Southern Cascades, and Modoc and Inyo National Forests, California, USA; General Technical Report; Pacific Southwest Research Station, USDA Forest Service: Washington, DC, USA, 2017.
- Arno, S. Northwest Trees: Identifying and Understanding the Region’s Native Trees; Mountaineers Books: Seattle, WA, USA, 2020. [Google Scholar]
- Schuler, J.L. National Wildlife Federation Field Guide to Trees of North America. J. Environ. Qual. 2009, 38, 1330. [Google Scholar] [CrossRef]
- Final Report of the Lodgepole Pine Seed Set Task Group, Interior Technical Advisory Committee, Forest Genetics Council of British Columbia; Forest Genetics Council of British Columbia: Victoria, BC, Canada, 2002.
- Lodgepole Pine 2022. Available online: https://www.for.gov.bc.ca/hfd/library/documents/treebook/lodgepolepine.htm (accessed on 18 November 2022).
- Hubbard, B.R.; Putman, L.I.; Techtmann, S.; Pearce, J.M. Open Source Vacuum Oven Design for Low-Temperature Drying: Performance Evaluation for Recycled PET and Biomass. J. Manuf. Mater. Process. 2021, 5, 52. [Google Scholar] [CrossRef]
- Pearce, J.M.; Khaksari, M.; Denkenberger, D. Preliminary Automated Determination of Edibility of Alternative Foods: Non-Targeted Screening for Toxins in Red Maple Leaf Concentrate. Plants 2019, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Breuer, S.W.; Toppen, L.; Schum, S.K.; Pearce, J.M. Open source software toolchain for automated non-targeted screening for toxins in alternative foods. MethodsX 2021, 8, 101551. [Google Scholar] [CrossRef] [PubMed]
- Dorne, J.L.C.M.; Richardson, J.; Livaniou, A.; Carnesecchi, E.; Ceriani, L.; Baldin, R.; Kovarich, S.; Pavan, M.; Saouter, E.; Biganzoli, F.; et al. EFSA’s OpenFoodTox: An open source toxicological database on chemicals in food and feed and its future developments. Environ. Int. 2021, 146, 106293. [Google Scholar] [CrossRef]
- Code of Federal Regulation 2022. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-E/part-156/subpart-D/section-156.62 (accessed on 18 November 2022).
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Case Definition: Trichothecene Mycotoxin 2018. Available online: https://emergency.cdc.gov/agent/trichothecene/casedef.asp (accessed on 25 November 2022).
- Dionisio, K.L.; Phillips, K.; Price, P.S.; Grulke, C.M.; Williams, A.; Biryol, D.; Hong, T.; Isaacs, K.K. The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Sci. Data 2018, 5, 180125. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Hiratsuka, A.; Aizawa, T.; Sawahata, T.; Ozawa, N.; Isobe, M.; Takabatake, E. Studies on metabolism and toxicity of styrene IV. 1-Vinylbenzene 3,4-oxide, a potent mutagen formed as a possible intermediate in the metabolism in vivo of styrene to 4-vinylphenol. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1982, 93, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Haschek, W.M.; Voss, K.A. Mycotoxins. Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1187–1258. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.-P.F.L.; Liaubet, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.D.; et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-d-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Dimethyl Dicarbonate 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3086 (accessed on 25 November 2022).
- Yu, J.; Proctor, R.H.; Brown, D.W.; Abe, K.; Gomi, K.; Machida, M.; Hasegawa, F.; Nierman, W.C.; Bhatnagar, D.; Cleveland, T.E. Genomics of Economically Significant Aspergillus and Fusarium Species. Appl. Mycol. Biotechnol. 2004, 4, 249–283. [Google Scholar] [CrossRef]
- Boonen, J.; Malysheva, S.V.; Taevernier, L.; Di Mavungu, J.D.; De Saeger, S.; De Spiegeleer, B. Human skin penetration of selected model mycotoxins. Toxicology 2012, 301, 21–32. [Google Scholar] [CrossRef]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef] [PubMed]
- Substance Information—ECHA 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.013.416 (accessed on 18 November 2022).
- Meyar, T.K.; Tieman, R.J.; Breuer, S.W.; Denkenberger, D.; Joshua, M.P. Yield and Toxic Analysis of Leaf Protein Concentrate of Common Agricultural Residues. submitted.
- Kennedy, D. Leaf for Life. Leaf Concentrate: A Field Guide for Small Scale Programs. Available online: https://www.leafforlife.org/gen/leaf_concentrate.html (accessed on 9 January 2023).
- Can You Eat Pine Needles? 2022. Available online: https://www.primalsurvivor.net/can-you-eat-pine-needles/ (accessed on 25 November 2022).
- Amazon.ca: Pine Needle Tea 2022. Available online: https://www.amazon.ca/s (accessed on 25 November 2022).
- Denkenberger, D.; Pearce, J.; Taylor, A.R.; Black, R. Food without sun: Price and life-saving potential. Foresight 2019, 21, 118–129. [Google Scholar] [CrossRef]
- Denkenberger, D.C.; Pearce, J.M. Cost-effectiveness of interventions for alternate food in the United States to address agricultural catastrophes. Int. J. Disaster Risk Reduct. 2018, 27, 278–289. [Google Scholar] [CrossRef]
- Denkenberger, D.C.; Pearce, J.M. Cost-Effectiveness of Interventions for Alternate Food to Address Agricultural Catastrophes Globally. Int. J. Disaster Risk Sci. 2016, 7, 205–215. [Google Scholar] [CrossRef]
| Sample | WC #1 |
|---|---|
| Wet weight of leaf (g wet biomass) | 50.01 |
| LPC drying paper weight (g) | 2.33 |
| Fiber Mass drying paper weight (g) | 1.79 |
| Drying time (hours) | 16 |
| Heating time (minutes) | 3.0 |
| Blending time (minutes) | 3.0 |
| Paper + Fiber Mass (g dry) | 23.59 |
| Fiber Mass (g dry) | 21.8 |
| Fiber Mass yield (% dry fiber mass to dry leaf weight) | 81.89% |
| Paper + LPC (g dry) | 2.88 |
| LPC (g dry) | 0.55 |
| LPC yield % (dry LPC to dry leaf weight) | 2.07% |
| Material | Class | Toxin (In ESI+ or ESI−) | Run 1 | Run 2 |
|---|---|---|---|---|
| WC #1 | Toxic Class 1 | Alternariol monomethyl ether (+,−) | ✓ | ✓ |
| 4-Vinylphenol (−) | ✓ | ✓ | ||
| HT-2 toxin (−) | ✓ | ✓ | ||
| T-2 Toxin tetraol (−) | ✓ | - | ||
| Toxic Class 2 | Deoxynivalenol 3-glucoside (−) | ✓ | ✓ |
| Material | Class | Toxin (In ESI+ or ESI−) | Run 1 | Run 2 |
|---|---|---|---|---|
| WC #2 LPC (+) | Toxic Class 1 | 4-Vinylphenol (+,−) | ✓ | ✓ |
| Alternariol monomethyl ether (+,−) | ✓ | ✓ | ||
| Aflatoxin M1 (−) | ✓ | ✓ | ||
| HT-2 toxin (+) | ✓ | ✓ | ||
| T-2 Toxin tetraol (−) | ✓ | - | ||
| Altenuene (+) | ✓ | - | ||
| Neosolaniol (+) | - | ✓ | ||
| Toxic Class 2 | Deoxynivalenol 3-glucoside (+,−) | ✓ | ✓ | |
| Aflatoxin G1 (−) | ✓ | ✓ | ||
| 15-Acetyldeoxynivalenol (−) | ✓ | ✓ | ||
| Glutaraldehyde (+) | ✓ | ✓ | ||
| Carbofuran (+) | ✓ | ✓ | ||
| 2-Acetylfuran (+) | ✓ | - | ||
| Anguidine (−) | - | ✓ | ||
| Nivalenol (+) | - | ✓ |
| Sample | DF |
|---|---|
| Wet weight of leaf (g wet biomass) | 50.2 |
| LPC drying paper weight (g) | 1.27 |
| Fiber Mass drying paper weight (g) | 1.55 |
| Drying time (hours) | 16 |
| Heating time (minutes) | 3.0 |
| Blending time (minutes) | 3.0 |
| Paper + Fiber Mass (g dry) | 19.6 |
| Fiber Mass (g dry) | 18.05 |
| Fiber Mass yield (% dry fiber mass to dry leaf weight) | 61.65% |
| Paper + LPC (g dry) | 1.58 |
| LPC (g dry) | 0.31 |
| LPC yield % (dry LPC to dry leaf weight) | 1.06% |
| Material | Class | Toxin (In ESI+ or ESI−) | Run 1 | Run 2 |
|---|---|---|---|---|
| DF | Toxic Class 1 | Alternariol monomethyl ether (+,−) | ✓ | ✓ |
| 4-Vinylphenol (+,−) | ✓ | ✓ | ||
| Toxic Class 2 | T-2 Toxin tetraol (−) | - | ✓ | |
| 15-Acetyldeoxynivalenol (−) | ✓ | ✓ | ||
| Dimethyl dicarbonate (−) | ✓ | - |
| Sample | #1 | #2 | Average |
|---|---|---|---|
| Wet weight of leaf (g wet biomass) | 50 | 50 | 50 |
| LPC drying paper weight (g) | 2.1 | 1.9 | 2.0 |
| Fiber Mass drying paper weight (g) | 2.12 | 2.60 | 2.36 |
| Drying time (hours) | 14 | 14 | 14 |
| Heating time (minutes) | 3.0 | 3.0 | 3.0 |
| Blending time (minutes) | 3.0 | 3.0 | 3.0 |
| Fiber Mass (g dry) | 20.58 | 20.58 | 20.58 |
| Fiber Mass yield (% dry fiber mass to dry leaf weight) | 78.12% | 78.12% | 78.12% |
| Paper + LPC (g dry) | 3.01 | 2.59 | 2.85 |
| LPC (g dry) | 0.91 | 0.7 | 0.80 |
| LPC yield % (dry LPC to dry leaf weight) | 3.45% | 2.66% | 3.05% |
| Material | Class | Toxin (In ESI+ or ESI−) | Run 1 | Run 2 |
|---|---|---|---|---|
| PP | Toxic Class 1 | 4-Vinylphenol (+,−) | ✓ | - |
| Alternariol monomethyl ether (−) | ✓ | ✓ | ||
| Altenuene (−) | - | ✓ | ||
| Toxic Class 2 | T-2 Toxin tetraol (+) | - | ✓ | |
| Deoxynivalenol 3-glucoside (−) | ✓ | ✓ | ||
| Dimethyl dicarbonate (−) | - | ✓ |
| Sample | WHL |
|---|---|
| Wet weight of leaf (g wet biomass) | 42.7 |
| LPC drying paper weight (g) | 0.97 |
| Fiber Mass drying paper weight (g) | 2.52 |
| Drying time (hours) | 16 |
| Heating time (minutes) | 3.0 |
| Blending time (minutes) | 3.0 |
| Paper + Fiber Mass (g dry) | 18.4 |
| Fiber Mass (g dry) | 15.88 |
| Fiber Mass yield (% dry fiber mass to dry leaf weight) | 74.71% |
| Paper + LPC (g dry) | 2.52 |
| LPC (g dry) | 1.55 |
| LPC yield % (dry LPC to dry leaf weight) | 7.29% |
| Material | Class | Toxin (In ESI+ or ESI−) | Run 1 | Run 2 |
|---|---|---|---|---|
| Western Hemlock | Toxic Class 1 | Alternariol monomethyl ether (+,−) | ✓ | ✓ |
| 4-Vinylphenol (+,−) | ✓ | ✓ | ||
| Toxic Class 2 | Carbofuran (+) | ✓ | ||
| Aflatoxin B2 (−) | - | ✓ | ||
| Deoxynivalenol 3-glucoside (−) | - | ✓ | ||
| Dimethyl dicarbonate (−) | - | ✓ |
| Sample | LPP |
|---|---|
| Wet weight of leaf (g wet biomass) | 50.27 |
| LPC drying paper weight (g) | 1.26 |
| Fiber Mass drying paper weight (g) | 2.07 |
| Drying time (hours) | 16 |
| Heating time (minutes) | 3.0 |
| Blending time (minutes) | 3.0 |
| Paper + Fiber Mass (g dry) | 22.1 |
| Fiber Mass (g dry) | 20.03 |
| Fiber Mass yield (% dry fiber mass to dry leaf weight) | 23% |
| Paper + LPC (g dry) | 2 |
| LPC (g dry) | 0.74 |
| LPC yield % (dry LPC to dry leaf weight) | 2.55% |
| Material | Class | Toxin (In ESI+ or ESI−) | Run 1 | Run 2 |
|---|---|---|---|---|
| LPP | Toxic Class 1 | Alternariol monomethyl ether (+,−) | ✓ | ✓ |
| 4-Vinylphenol (−) | ✓ | ✓ | ||
| Aflatoxin M1 (−) | - | ✓ | ||
| Toxic Class 2 | Dimethyl dicarbonate (−) | ✓ | ✓ | |
| Deoxynivalenol 3-glucoside (+) | ✓ | ✓ | ||
| Aflatoxin G1 (−) | - | ✓ | ||
| 2-Acetylfuran (+) | ✓ | - |
| Tree | Yield % (Dry LPC/Dry Leaf Weight) |
|---|---|
| Western Cedar | 2.07 |
| Douglas Fir | 1.06 |
| Ponderosa Pine | 3.05 (2.66–3.45) |
| Western Hemlock | 7.29 |
| Lodgepole Pine | 2.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottaghi, M.; Meyer, T.K.; Tieman, R.J.; Denkenberger, D.; Pearce, J.M. Yield and Toxin Analysis of Leaf Protein Concentrate from Common North American Coniferous Trees. Biomass 2023, 3, 163-187. https://doi.org/10.3390/biomass3020011
Mottaghi M, Meyer TK, Tieman RJ, Denkenberger D, Pearce JM. Yield and Toxin Analysis of Leaf Protein Concentrate from Common North American Coniferous Trees. Biomass. 2023; 3(2):163-187. https://doi.org/10.3390/biomass3020011
Chicago/Turabian StyleMottaghi, Maryam, Theresa K. Meyer, Ross John Tieman, David Denkenberger, and Joshua M. Pearce. 2023. "Yield and Toxin Analysis of Leaf Protein Concentrate from Common North American Coniferous Trees" Biomass 3, no. 2: 163-187. https://doi.org/10.3390/biomass3020011
APA StyleMottaghi, M., Meyer, T. K., Tieman, R. J., Denkenberger, D., & Pearce, J. M. (2023). Yield and Toxin Analysis of Leaf Protein Concentrate from Common North American Coniferous Trees. Biomass, 3(2), 163-187. https://doi.org/10.3390/biomass3020011









