Microstructural and Magnetic Properties of Polyamide-Based Recycled Composites with Iron Oxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of IO Nanoparticles
2.3. Synthesis of RPA/IO Nanocomposites
3. Materials Characterization
4. Results and Discussion
4.1. Microstructural Properties
4.2. Magnetic Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koszewska, M. Circular Economy—Challenges for the Textile and Clothing Industry. Autex Res. J. 2018, 18, 337–347. [Google Scholar] [CrossRef]
- Roy Choudhury, A.K. Environmental Impacts of the Textile Industry and Its Assessment Through Life Cycle Assessment. In Roadmap to Sustainable Textiles and Clothing: Environmental and Social Aspects of Textiles and Clothing Supply Chain; Springer: Singapore, 2014; pp. 1–39. [Google Scholar] [CrossRef]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A Review on Textile Recycling Practices and Challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Wang, Y. Fiber and textile waste Utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Tang, K.H.D. State of the Art in Textile Waste Management: A Review. Textiles 2023, 3, 454–467. [Google Scholar] [CrossRef]
- Patwary, S. Clothing and Textile Sustainability: Current State of Environmental Challenges and the Ways Forward. Text. Leather Rev. 2020, 3, 158–173. [Google Scholar] [CrossRef]
- Markovičová, L.; Zatkalíková, V.; Kojnoková, T. Environmental impact on the life of a polymeric composite with polyamide matrix and glass fibres. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1178, 012043. [Google Scholar] [CrossRef]
- Hirschberg, V.; Rodrigue, D. Recycling of polyamides: Processes and conditions. J. Polym. Sci. 2023, 61, 1937–1958. [Google Scholar] [CrossRef]
- Al-Shawabkeh, A.F. Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer. E-Polymers 2022, 22, 858–869. [Google Scholar] [CrossRef]
- Hashemi Sanatgar, R.; Campagne, C.; Nierstrasz, V. Investigation of the adhesion properties of direct 3D printing of polymers and nanocomposites on textiles: Effect of FDM printing process parameters. Appl. Surf. Sci. 2017, 403, 551–563. [Google Scholar] [CrossRef]
- Baeg, K.-J.; Lee, J.; Baeg, J.K.; Lee, J. Flexible Electronic Systems on Plastic Substrates and Textiles for Smart Wearable Technologies. Adv. Mater. Technol. 2020, 5, 2000071. [Google Scholar] [CrossRef]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Mahalik, N.P.; Nambiar, A.N. Trends in food packaging and manufacturing systems and technology. Trends Food Sci. Technol. 2010, 21, 117–128. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Ranjan, N.; Penna, R.; Fraternali, F. On the recyclability of polyamide for sustainable composite structures in civil engineering. Compos. Struct. 2018, 184, 704–713. [Google Scholar] [CrossRef]
- Yao, W.H. The preparation of modified polyamide clay nanocomposite/recycled maleic anhydride polyamide 6 and blending with low density polyethylene for film blowing application. Polym. Polym. Compos. 2021, 29, S631–S643. [Google Scholar] [CrossRef]
- Medeiros, D.G.; Jardim, P.M.; De Tatagiba, M.K.V.; D’Almeida, J.R.M. Composites of recycled nylon 11 and titanium based nanofillers. Polym. Test. 2015, 42, 108–114. [Google Scholar] [CrossRef]
- Benaducci, D.; de Oliveira, V.; Yin Tze, W.T.; Hafez, I.; Branciforti, M.C. Nanocomposites of recycled and of virgin polyamide 6.6 with cellulose nanofibers. Hybrid Adv. 2024, 6, 100261. [Google Scholar] [CrossRef]
- Korkees, F.; Aldrees, A.; Barsoum, I.; Alshammari, D. Functionalised graphene effect on the mechanical and thermal properties of recycled PA6/PA6,6 blends. J. Compos. Mater. 2021, 55, 2211–2224. [Google Scholar] [CrossRef]
- Bassyouni, D.; Mohamed, M.; El-Ashtoukhy, E.S.; El-Latif, M.A.; Zaatout, A.; Hamad, H. Fabrication and characterization of electrospun Fe3O4/o-MWCNTs/polyamide 6 hybrid nanofibrous membrane composite as an efficient and recoverable adsorbent for removal of Pb (II). Microchem. J. 2019, 149, 103998. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, X.; Luo, Y.; Jia, Z. Synthesis and performances of Fe2O3/PA-6 nanocomposite fiber. Mater. Lett. 2007, 61, 3269–3272. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; De Gruyter: Berlin, Germany, 2003. [Google Scholar]
- Gutierrez, F.V.; De Falco, A.; Yokoyama, E.; Mendoza, L.A.F.; Luz-Lima, C.; Perez, G.; Loreto, R.P.; Pottker, W.E.; La Porta, F.A.; Solorzano, G.; et al. Magnetic Characterization by Scanning Microscopy of Functionalized Iron Oxide Nanoparticles. Nanomaterials 2021, 11, 2197. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S. Synthesis and applications of nano-structured iron oxides/hydroxides—A review. Int. J. Eng. Sci. Technol. 2010, 2, 127–146. [Google Scholar] [CrossRef]
- Hamed, M.H.; Mueller, D.N.; Müller, M. Thermal phase design of ultrathin magnetic iron oxide films: From Fe3O4 to γ-Fe2O3 and FeO. J. Mater. Chem. C 2020, 8, 1335–1343. [Google Scholar] [CrossRef]
- Li, Z.; Chanéac, C.; Berger, G.; Delaunay, S.; Graff, A.; Lefèvre, G. Mechanism and kinetics of magnetite oxidation under hydrothermal conditions. RSC Adv. 2019, 9, 33633–33642. [Google Scholar] [CrossRef] [PubMed]
- Winsett, J.; Moilanen, A.; Paudel, K.; Kamali, S.; Ding, K.; Cribb, W.; Seifu, D.; Neupane, S. Quantitative determination of magnetite and maghemite in iron oxide nanoparticles using Mössbauer spectroscopy. SN Appl. Sci. 2019, 1, 1636. [Google Scholar] [CrossRef]
- Buelvas, D.D.A.; Camargo, L.P.; Valezi, D.F.; Tupan, L.F.S.; Dall’Antonia, L.H.; Rocha, C.M.M.; Lopez, D.A.S.; Urbano, A.; Vicentin, B.L.S. Impact of Varying Magnetite Nanoparticle Concentrations on the Structural, Electrical, and Magnetic Properties of Polyaniline-Based Magnetic Nanocomposites. Synth. Met. 2024, 307, 117703. [Google Scholar] [CrossRef]
- Bragg, W.H. The Structure of Magnetite and the Spinels. Nature 1915, 95, 561. [Google Scholar] [CrossRef]
- Wareppam, B.; Kuzmann, E.; Garg, V.K.; Singh, L.H. Mössbauer spectroscopic investigations on iron oxides and modified nanostructures: A review. J. Mater. Res. 2022, 38, 937–957. [Google Scholar] [CrossRef] [PubMed]
- Petrychuk, M.; Kovalenko, V.; Pud, A.; Ogurtsov, N.; Gubin, A. Ternary magnetic nanocomposites based on core–shell Fe3O4/polyaniline nanoparticles distributed in PVDF matrix. Phys. Status Solidi 2010, 207, 442–447. [Google Scholar] [CrossRef]
- Buelvas, D.D.A.; Camargo, L.P.; Salgado, I.K.I.; Vicentin, B.L.S.; Valezi, D.F.; Dall’Antonia, L.H.; Tarley, C.R.T.; Mauro, E. Di: Study and optimization of the adsorption process of methylene blue dye in reusable polyaniline-magnetite composites. Synth. Met. 2023, 292, 117232. [Google Scholar] [CrossRef]
- Itoh, H.; Sugimoto, T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J. Colloid Interface Sci. 2003, 265, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.A.; Urquijo, J.P. Synthesis and characterization of magnetite-maghemite nanoparticles in presence of polyethylene glycol obtained by mechanical milling. Mater. Sci. Eng. B 2021, 263, 114873. [Google Scholar] [CrossRef]
- Andronenko, S.I.; Nikolaev, A.M.; Suharzhevsky, S.M.; Sinelnikov, A.A.; Kovalenko, A.S.; Ivanova, A.G.; Shilova, O.A. Phase Composition and Magnetic Properties of Nanoparticles with Magnetite–Maghemite Structure. Ceramics 2023, 6, 1623–1631. [Google Scholar] [CrossRef]
- Meftah, S.; Ngo, A.T.; Bouteiller, L.; Russier, V.; Hrabovsky, D.; Konaté, A.; Kondo, D.; Bedoui, F.; Lisiecki, I. Synthesis and Magnetic Properties of Spherical Maghemite Nanoparticles with Tunable Size and Surface Chemistry. Langmuir 2024, 40, 22673–22683. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Pancholi, K.; De Sa, R.; Murray, D.; Huo, D.; Droubi, G.; White, M.; Njuguna, J. Effect of Oleic Acid Coating of Iron Oxide Nanoparticles on Properties of Magnetic Polyamide-6 Nanocomposite. JOM 2019, 71, 3119–3128. [Google Scholar] [CrossRef]
- Muhazeli, N.S.; Nordin, N.A.; Mazlan, S.A.; Abdul Aziz, S.A.; Ubaidillah Nazmi, N. Mini review: An insight on the fabrication methods of smart magnetic polymer foam. J. Magn. Magn. Mater. 2021, 534, 168038. [Google Scholar] [CrossRef]
- Nordin, A.H.; Ahmad, Z.; Husna, S.M.N.; Ilyas, R.A.; Azemi, A.K.; Ismail, N.; Nordin, M.L.; Ngadi, N.; Siti, N.H.; Nabgan, W.; et al. The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review. Gels 2023, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Shen, J.; Zheng, Z.; Liu, J. Role of a nanoparticle network in polymer mechanical reinforcement: Insights from molecular dynamics simulations. Phys. Chem. Chem. Phys. 2021, 23, 21797–21807. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, B.; Liu, J.; Hu, X.; Zheng, Z.J. Revealing the reinforcing effect of a nanorod network on a polymer matrix through molecular dynamics simulations. Phys. Chem. Chem. Phys. 2023, 25, 18757–18765. [Google Scholar] [CrossRef] [PubMed]
- Wenlei, X.; Ning, M. Immobilized lipase on Fe3O4 nanoparticles as biocatalyst for biodiesel production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Guinier, A. X-Ray Diffraction: In Crystals, Imperfect Crystals, and Amorphous Bodies; W. H. Freeman & Company: San Francisco, CA, USA, 1963. [Google Scholar]
- Buelvas, D.D.A.; Vicentin, B.L.S.; Urbano, A.; Besegato, J.F.; Hoeppner, M.G.; Galvão, T.D.; Parreira, P.S.; Di Mauro, E. Crystalline properties and morphology of bulk-fill dental resin composites as function of light-cure protocol and composition. Polym. Bull. 2023, 80, 2349–2366. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Nica, S.L.; Nica, V.; Grigoras, V.C.; Varganici, C.D.; Popovici, D.; Hulubei, C.; Ioan, S. Influence of two structural phases of Fe3O4 and γ-Fe2O3 on the properties of polyimide/iron oxide composites. Polym. Int. 2015, 64, 1172–1181. [Google Scholar] [CrossRef]
- Stern, P.; Polymer, E.S. On the structure of polypropylene fibres. Polymer 1968, 9, 471–477. [Google Scholar] [CrossRef]
- Tsukimura, K.; Sasaki, S.; Kimizuka, N. Cation distributions in nickel ferrites. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 1997, 36, 3609–3612. [Google Scholar] [CrossRef]
- Solano, E.; Frontera, C.; Puig, T.; Obradors, X.; Ricart, S.; Ros, J. Neutron and X-ray diffraction study of ferrite nanocrystals obtained by microwave-assisted growth. A structural comparison with the thermal synthetic route. J. Appl. Crystallogr. 2014, 47, 414–420. [Google Scholar] [CrossRef]
- Rebodos, R.L.; Vikesland, P.J. Effects of oxidation on the magnetization of nanoparticulate magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhao, X.; Yang, G. Double in situ synthesis of Fe3O4/polyamide 6 magnetic nanocomposite. Mater. Lett. 2013, 98, 90–93. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, B.; Dou, W.; Wu, Y.; Luo, L. Preparation of Nano-Fe3O4/Nylon Composite Fabric with Magnetic Properties by Post Finishing Method. Fibers Polym. 2019, 20, 1396–1403. [Google Scholar] [CrossRef]
- Ranganathan, P.; Mutharani, B.; Tseng, C.-H.; Tsai, P.-S. The Isothermal and Nonisothermal Crystallization Kinetics and Morphology of Solvent-Precipitated Nylon 66. Polymers 2022, 14, 442. [Google Scholar] [CrossRef]
- Qianhui, X.; Hongmei, H.; Ruishu, Z.; Lina, S.; Jianyong, Y.; Xueli, W. A non-destructive, environment-friendly method for separating and recycling polyamide 6 from waste and scrap polyamide 6 blended textiles. Text. Res. J. 2023, 93, 3327–3340. [Google Scholar] [CrossRef]
- Colucci, G.; Ostrovskaya, O.; Frache, A.; Martorana, B.; Badini, C. The effect of mechanical recycling on the microstructure and properties of PA66 composites reinforced with carbon fibers. J. Appl. Polym. Sci. 2015, 132, 42275. [Google Scholar] [CrossRef]
- Morales-Luckie, R.A.; Sánchez-Mendieta, V.; Olea-Mejia, O.; Vilchis-Nestor, A.R.; López-Téllez, G.; Varela-Guerrero, V.; Huerta, L.; Arenas-Alatorre, J. Facile Solventless Synthesis of a Nylon-6,6/Silver Nanoparticles Composite and Its XPS Study. Int. J. Polym. Sci. 2013, 2013, 235850. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Safapour, S.; Mirnezhad, S. Thermodynamic and kinetic studies of the adsorption behaviour of the natural dye cochineal on polyamide 66. Color. Technol. 2018, 134, 308–314. [Google Scholar] [CrossRef]
- Cheval, N.; Gindy, N.; Flowkes, C.; Fahmi, A. Polyamide 66 microspheres metallised with in situ synthesised gold nanoparticles for a catalytic application. Nanoscale Res. Lett. 2012, 7, 182. [Google Scholar] [CrossRef]
- Zakaria, Z.; Izzah, Z.; Tarmizi, A.; Jawaid, M.; Hassan, A. Effect of degree of deacetylation of chitosan on thermal stability and compatibility of chitosan-polyamide blend. BioResources 2012, 7, 5568–5580. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; El-Said, W.A.; Sayed, E.M.; Abdel-Wahab, A.M.A. Synthesis, Characterization of Some Conductive Aromatic Polyamides/Fe3O4 NPs/ITO, and Their Utilization for Methotrexate Sensing. Surfaces 2023, 6, 83–96. [Google Scholar] [CrossRef]
- Yu, D.; Ni, H.; Wang, L.; Wu, M.; Yang, X. Nanoscale-confined precursor of CuFe2O4 mediated by hyperbranched polyamide as an unusual heterogeneous Fenton catalyst for efficient dye degradation. J. Clean. Prod. 2018, 186, 146–154. [Google Scholar] [CrossRef]
- Mohan, A.; Singhal, R.; Ramanan, S.R. A study on the effect of the collector properties on the fabrication of magnetic polystyrene nanocomposite fibers using the electrospinning technique. J. Appl. Polym. Sci. 2023, 140, e53461. [Google Scholar] [CrossRef]
- O’Handley, R.C. Modern Magnetic Materials: Principles and Applications; IEEE Electrical Insulation Magazine: Hoboken, NJ, USA, 1999; Volume 13, p. 768. [Google Scholar]
- Hu, P.; Zhang, S.; Wang, H.; Pan, D.; Tian, J.; Tang, Z.; Volinsky, A.A. Heat treatment effects on Fe3O4 nanoparticles structure and magnetic properties prepared by carbothermal reduction. J. Alloys Compd. 2011, 509, 2316–2319. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm. 2017, 19, 246–255. [Google Scholar] [CrossRef]
- Faaliyan, K.; Abdoos, H.; Borhani, E.; Afghahi, S.S.S. Magnetite-silica nanoparticles with core-shell structure: Single-step synthesis, characterization and magnetic behavior. J. Sol-Gel Sci. Technol. 2018, 88, 609–617. [Google Scholar] [CrossRef]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Chirita, M.; Bezergheanu, A.; Bazil Cizmas, C.; Ercuta, A. Superparamagnetic-like Micrometric Single Crystalline Magnetite for Biomedical Application Synthesis and Characterization. Magnetochemistry 2022, 9, 5. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G. One-step hydrothermal synthesis of magnetic Fe3O4 nanoparticles immobilized on polyamide fabric. Appl. Surf. Sci. 2012, 258, 4952–4959. [Google Scholar] [CrossRef]
- Gupta, R.; Pancholi, P.V.; Yu, X.; Gupta, L.; Stenning, G.B.G.; Bucknall, D.; Flynn, D.; Pancholi, K. Role of interface in optimisation of polyamide-6/Fe3O4 nanocomposite properties suitable for induction heating. Nano-Struct. Nano-Objects 2023, 34, 100973. [Google Scholar] [CrossRef]
- Yoon, S. Determination of the Temperature Dependence of the Magnetic Anisotropy Constant in Magnetite Nanoparticles. J. Korean Phys. Soc. 2011, 59, 3069–3073. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, J.M.; Yoon, H.Y.; Lee, J.K.; Kim, Y.K. Magnetic multi-granule nanoclusters: A model system that exhibits universal size effect of magnetic coercivity. Sci. Rep. 2015, 5, 12135. [Google Scholar] [CrossRef]
- Lima, E.; Brandl, A.L.; Arelaro, A.D.; Goya, G.F. Spin disorder and magnetic anisotropy in Fe3O4 nanoparticles. J. Appl. Phys. 2006, 99, 083908. [Google Scholar] [CrossRef]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature. J. Phys. Chem. Solids. 2021, 151, 109928. [Google Scholar] [CrossRef]
- Rondinone, A.J.; Samia, A.C.S.; Zhang, Z.J. Characterizing the magnetic anisotropy constant of spinel cobalt ferrite nanoparticles. Appl. Phys. Lett. 2000, 76, 3624–3626. [Google Scholar] [CrossRef]
- Chikazumi, S. Physics of Ferromagnetism; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Muscas, G.; Peddis, D.; Cobianchi, M.; Lascialfari, A.; Cannas, C.; Musinu, A.; Omelyanchik, A.; Rodionova, V.; Fiorani, D.; Mameli, V. Magnetic Interactions Versus Magnetic Anisotropy in Spinel Ferrite Nanoparticles. IEEE Magn. Lett. 2019, 10, 6110305. [Google Scholar] [CrossRef]
- Knobel, M.; Nunes, W.C.; Socolovsky, L.M.; De Biasi, E.; Vargas, J.M.; Denardin, J.C. Superparamagnetism and Other Magnetic Features in Granular Materials: A Review on Ideal and Real Systems. J. Nanosci. Nanotechnol. 2008, 8, 2836–2857. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, K.; Granitzer, P.; Morales, P.M.; Poelt, P.; Reissner, M. Variable blocking temperature of a porous silicon/Fe3O4 composite due to different interactions of the magnetic nanoparticles. Nanoscale Res. Lett. 2012, 7, 445. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Melo, W.W.M.; Granada, M.; Troiani, H.E.; MacEdo, W.A.A.; Ardison, J.D.; Vaz, M.G.F.; Novak, M.A. Magnetic properties of nanoscale crystalline maghemite obtained by a new synthetic route. J. Magn. Magn. Mater. 2012, 324, 3029–3033. [Google Scholar] [CrossRef]
- Gogoi, B.; Das, U. Enhanced Study of Magnetic Properties of Polyvinyl Alcohol-Coated Superparamagnetic Iron Oxide Nanoparticles Below Blocking Temperatures. Powder Metall. Met. Ceram. 2023, 62, 41–57. [Google Scholar] [CrossRef]
- Correa, J.R.; Bordallo, E.; Canetti, D.; León, V.; Otero-Díaz, L.C.; Negro, C.; Gómez, A.; Sáez-Puche, R. Structure and superparamagnetic behaviour of magnetite nanoparticles in cellulose beads. Mater. Res. Bull. 2010, 45, 946–953. [Google Scholar] [CrossRef]
- Zheng, R.K.; Gu, H.; Xu, B.; Zhang, X.X. The origin of the non-monotonic field dependence of the blocking temperature in magneticnanoparticles. J. Phys. Condens. Matter. 2006, 18, 5905. [Google Scholar] [CrossRef]
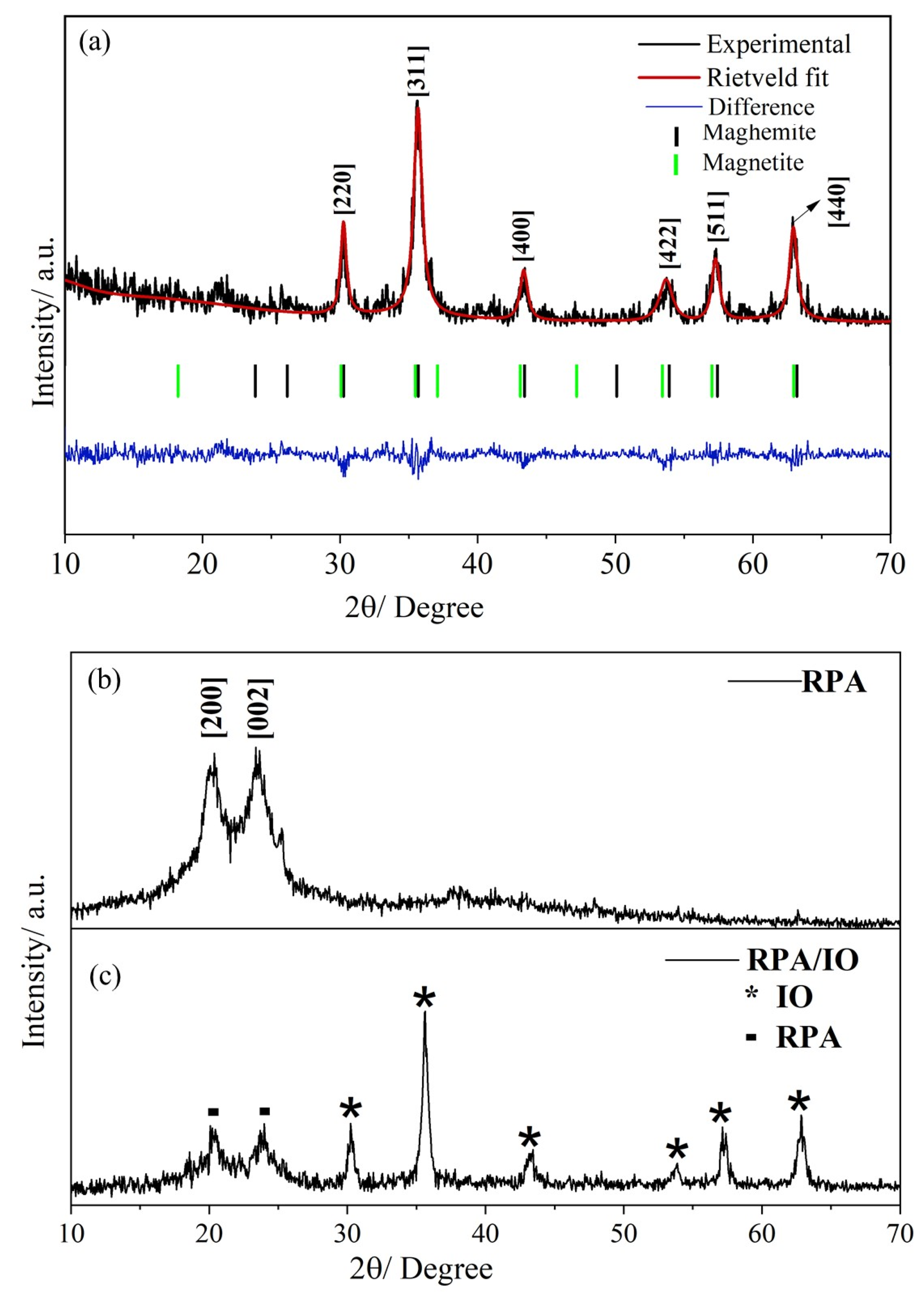

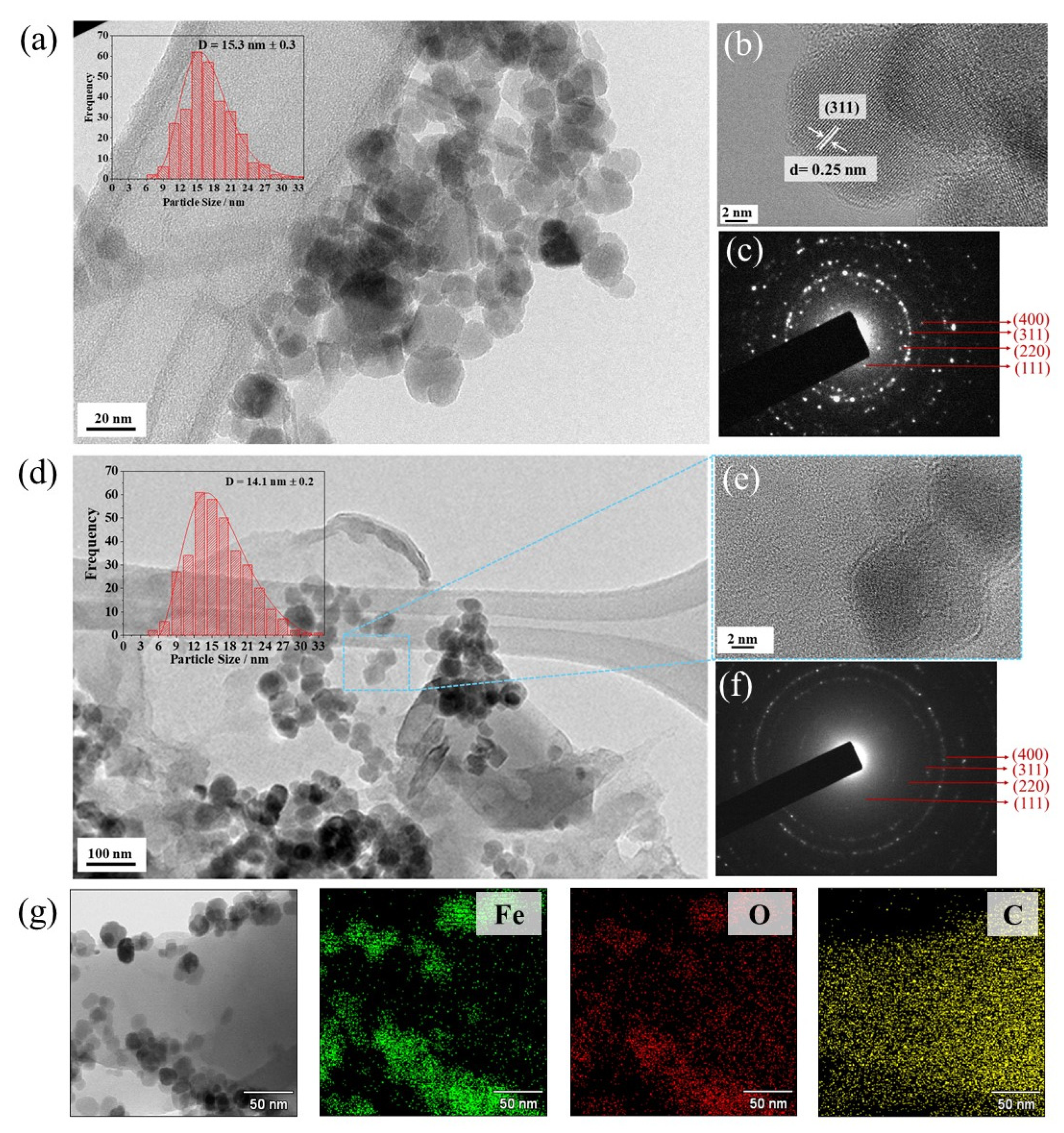

| Sample | Planes/h k l | 2θ° | * β | ** S/nm | *** %DOC |
|---|---|---|---|---|---|
| IO | 311 | 35.6 | 0.8341 | 10.0 | 69.2 |
| RPA | 200 | 20.1 | 2.6408 | 3.2 | 35.8 |
| 002 | 23.6 | 3.0403 | 2.9 | ||
| RPA/IO | 200 | 20.3 | 1.9383 | 4.4 | 11.5 |
| 002 | 23.9 | 1.8330 | 4.6 |
| Sample | Temperature/K | Ms/emu g–1 | Mr/emu g–1 | Mr/Ms | Hc/kOe | K1/erg cm–3 |
|---|---|---|---|---|---|---|
| IO | 5 | 48.83 ± 0.59 | 13.21 ± 0.01 | 0.271 ± 0.003 | 0.4 × 10−3 ± 0.1 × 10−3 | 5.44 × 105 ± 0.05 |
| 300 | 43.42 ± 0.12 | 0.75 ± 0.02 | 0.017 ± 0.001 | 1.9 × 10−4 ± 5.7 × 10−5 | 3.34 × 105 ± 0.01 | |
| RPA/IO | 5 | 21.81 ± 0.05 | 5.55 ± 0.01 | 0.255 ± 0.001 | 0.22 ± 0.01 | 4.28 × 105 ± 0.01 |
| 300 | 18.84 ± 0.01 | 0.83 ± 0.01 | 0.044 ± 0.001 | 0.02 ± 0.001 | 1.53 × 105 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.G.D.; Buelvas, D.D.A.; Valezi, D.F.; Vicentin, B.L.S.; Rocha, C.M.M.; Mauro, E.D.; Porta, F.d.A.L. Microstructural and Magnetic Properties of Polyamide-Based Recycled Composites with Iron Oxide Nanoparticles. Magnetism 2025, 5, 5. https://doi.org/10.3390/magnetism5010005
Santos LGD, Buelvas DDA, Valezi DF, Vicentin BLS, Rocha CMM, Mauro ED, Porta FdAL. Microstructural and Magnetic Properties of Polyamide-Based Recycled Composites with Iron Oxide Nanoparticles. Magnetism. 2025; 5(1):5. https://doi.org/10.3390/magnetism5010005
Chicago/Turabian StyleSantos, Lucas G. Dos, Daina D. A. Buelvas, Daniel F. Valezi, Bruno L. S. Vicentin, Christian M. M. Rocha, Eduardo Di Mauro, and Felipe de A. La Porta. 2025. "Microstructural and Magnetic Properties of Polyamide-Based Recycled Composites with Iron Oxide Nanoparticles" Magnetism 5, no. 1: 5. https://doi.org/10.3390/magnetism5010005
APA StyleSantos, L. G. D., Buelvas, D. D. A., Valezi, D. F., Vicentin, B. L. S., Rocha, C. M. M., Mauro, E. D., & Porta, F. d. A. L. (2025). Microstructural and Magnetic Properties of Polyamide-Based Recycled Composites with Iron Oxide Nanoparticles. Magnetism, 5(1), 5. https://doi.org/10.3390/magnetism5010005






