Possibilities in Recycling Magnetic Materials in Applications of Polymer-Bonded Magnets
Abstract
:1. Introduction
1.1. Magnetic Properties
1.2. Urgent Requirement of Recycling Strategies for Polymer-Bonded Magnets
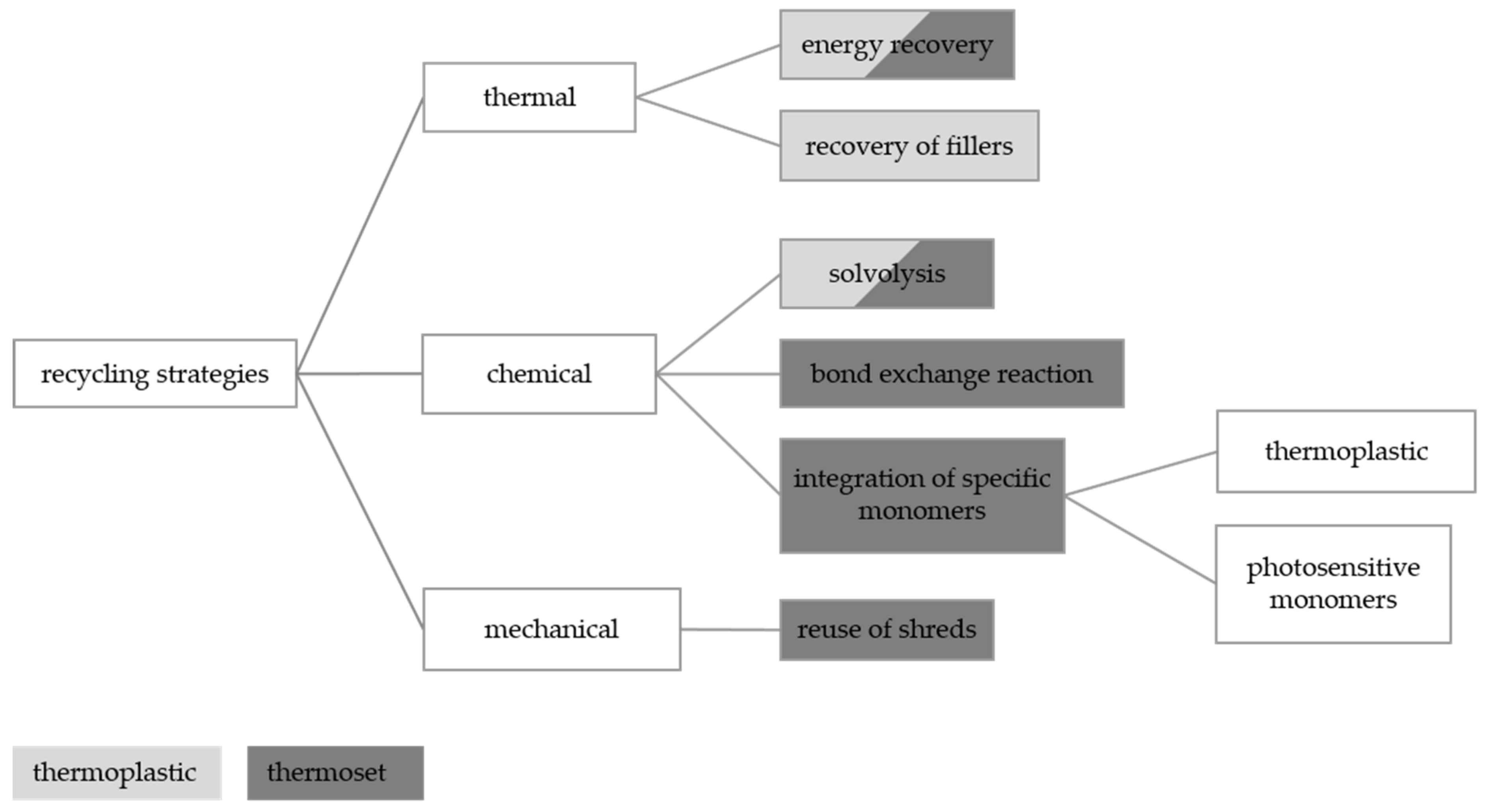
1.3. Reduction and Recycling Possibilities of Polymer-Bonded Magnets
2. Materials and Methods
2.1. Material
2.2. Fabrication of the Test Specimens
2.2.1. Thermoset-Based Test Specimens
2.2.2. Thermoplastic-Based Test Specimens
2.3. Realization of Recycling Strategy
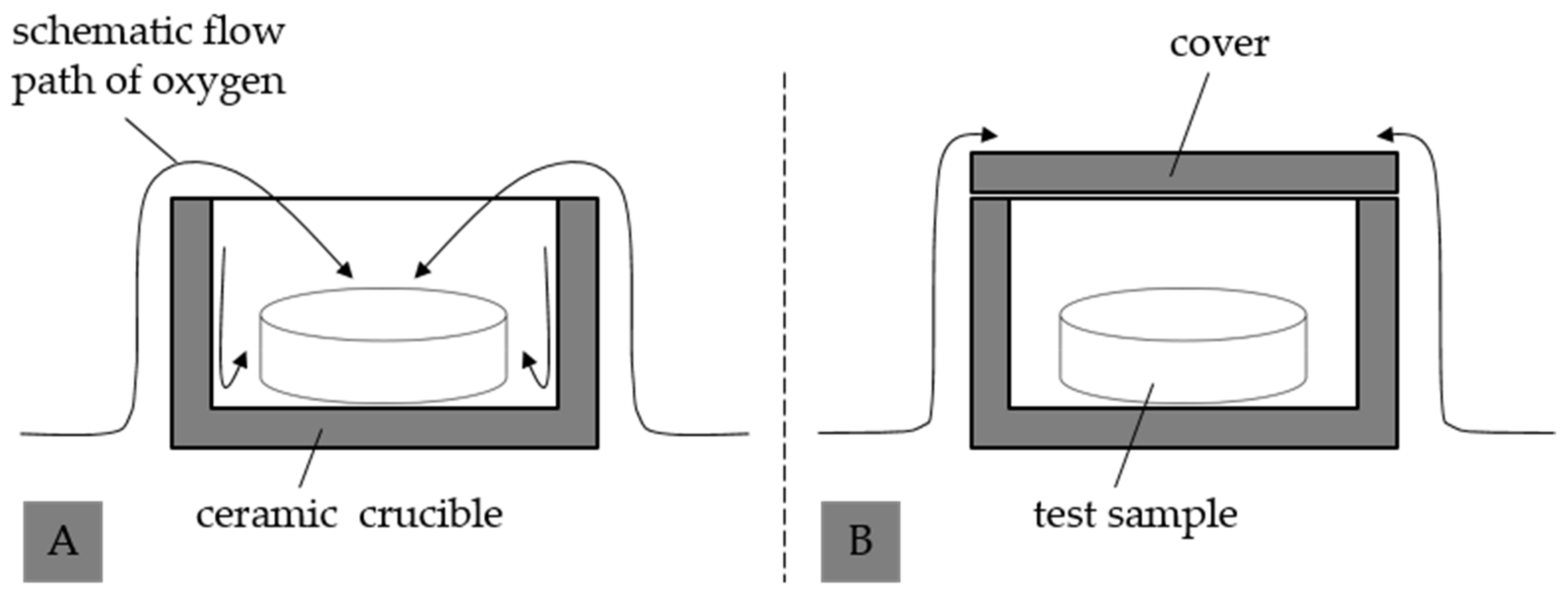
2.3.1. Thermal Recycling Strategy for Thermoset-Based Polymer-Bonded Magnets
2.3.2. Mechanical Reuse of Shreds for Thermoplastic-Based Polymer-Bonded Magnets
2.4. Characterization
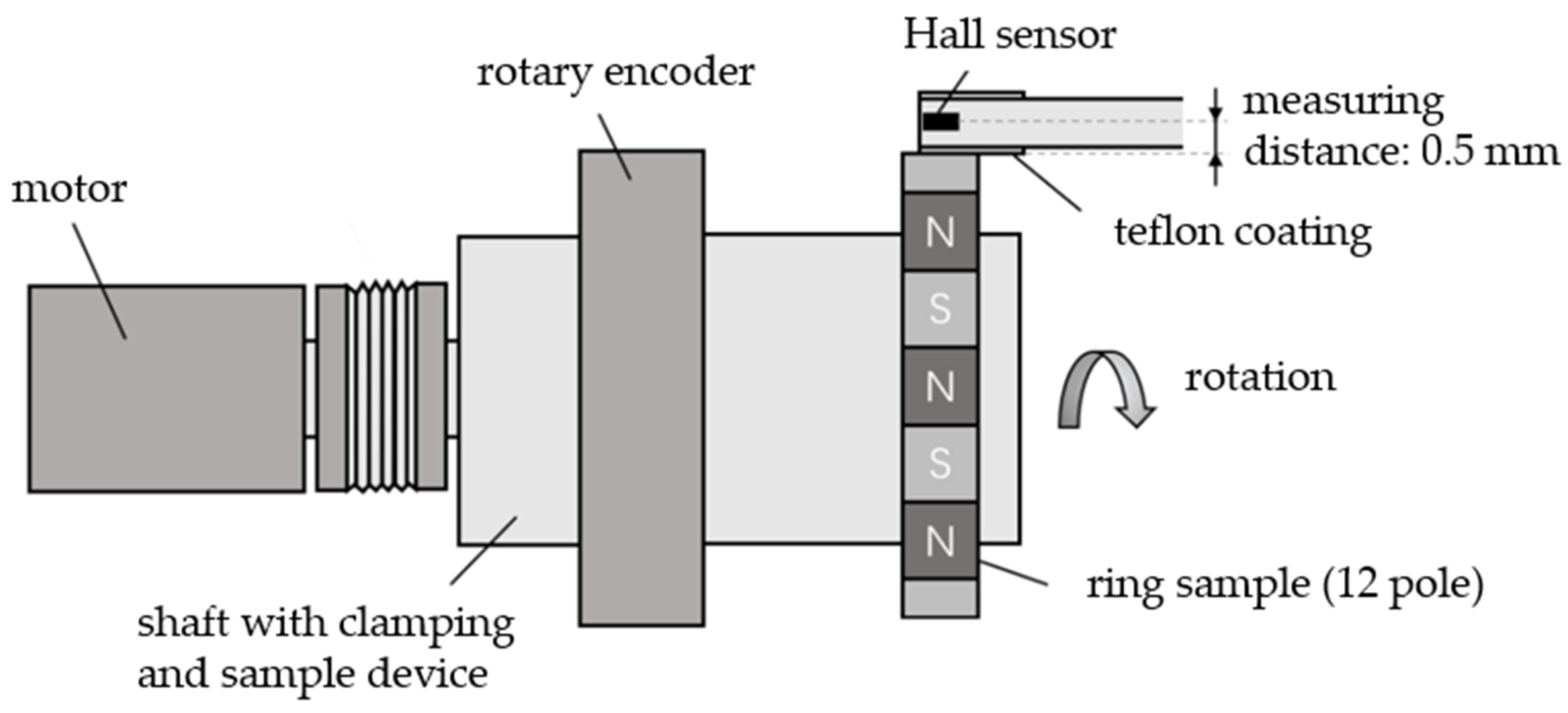
2.4.1. Magnetic Properties
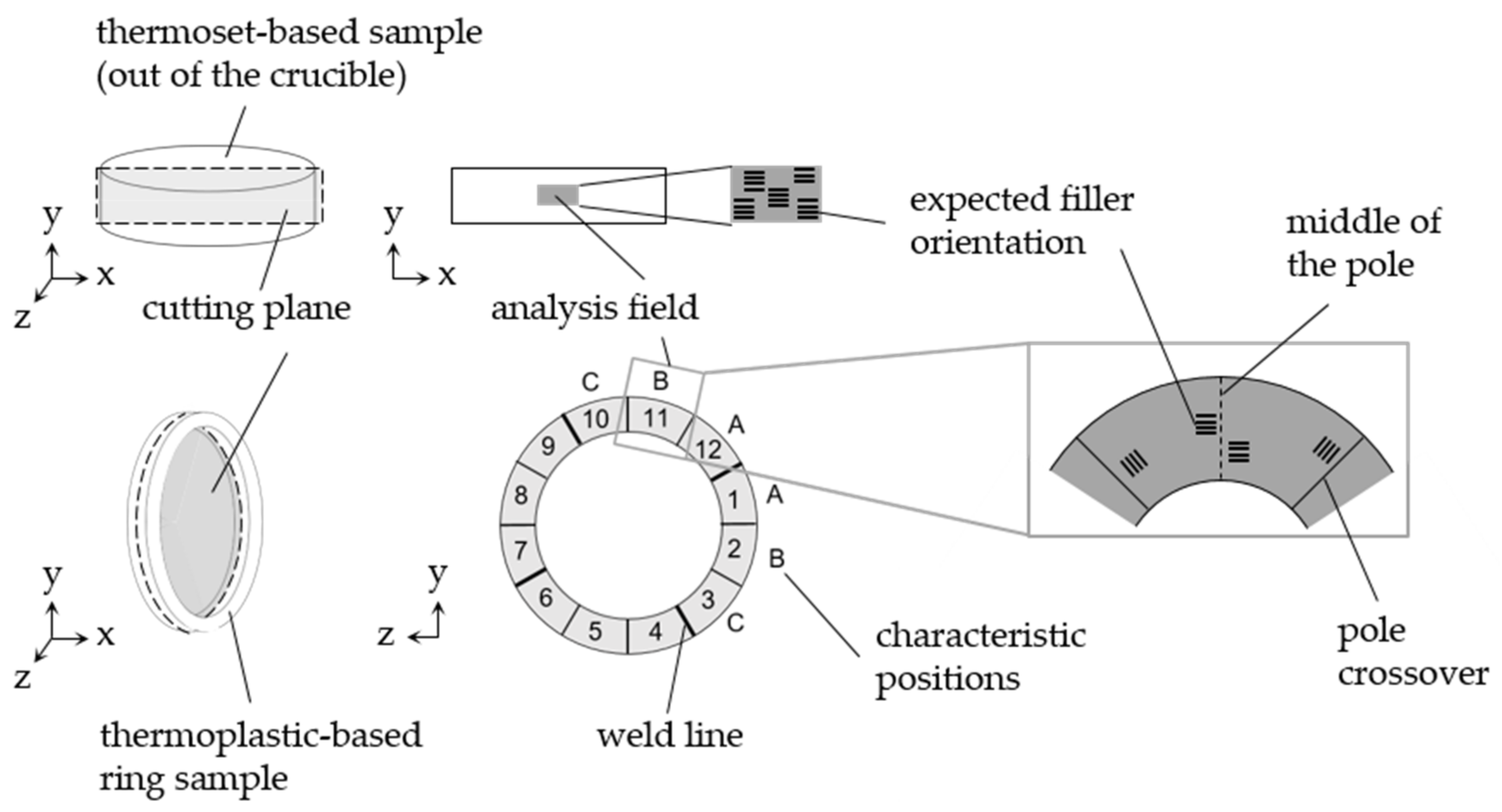
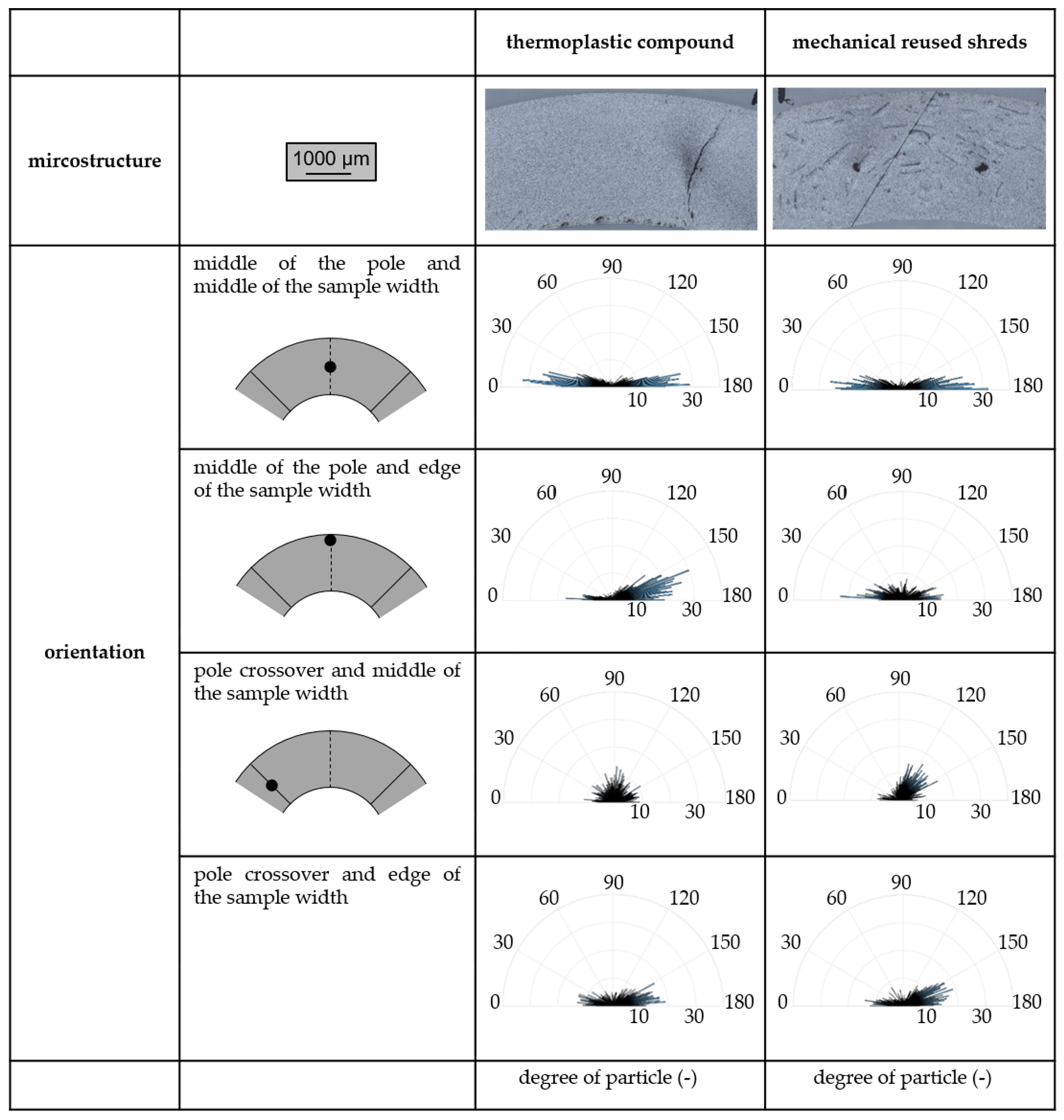
2.4.2. Filler Orientation
2.4.3. Thermogravimetric Analysis (TGA) Following DIN EN ISO 11358
2.4.4. Energy Dispersive X-ray Spectroscopy (EDX)
2.4.5. Differential Scanning Calorimetry (DSC) Following DIN EN ISO 11357
3. Results and Discussion
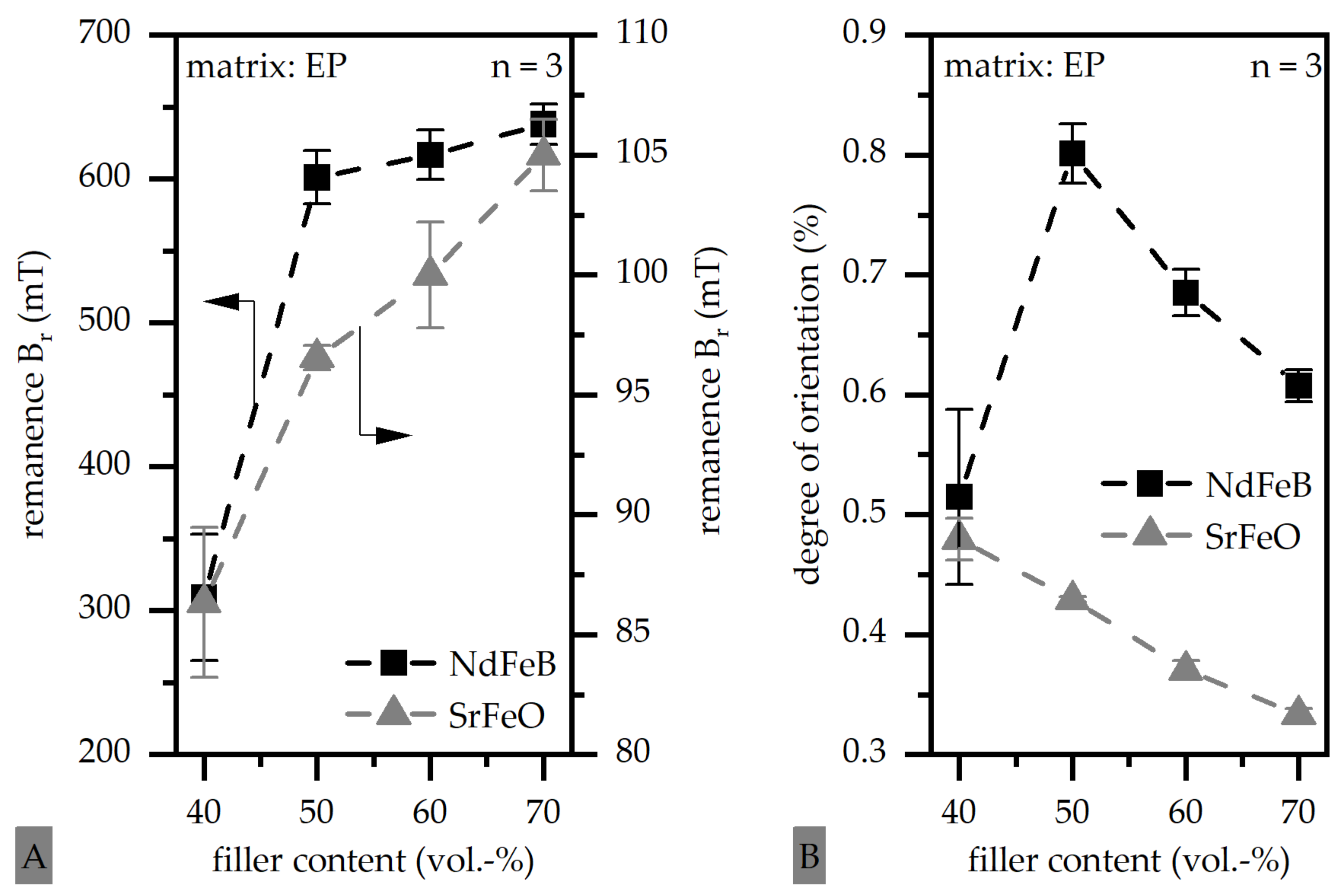
3.1. Magnetic Properties of Thermoset-Based Polymer-Bonded Magnets with Respect to the Thermal Recycling Strategy
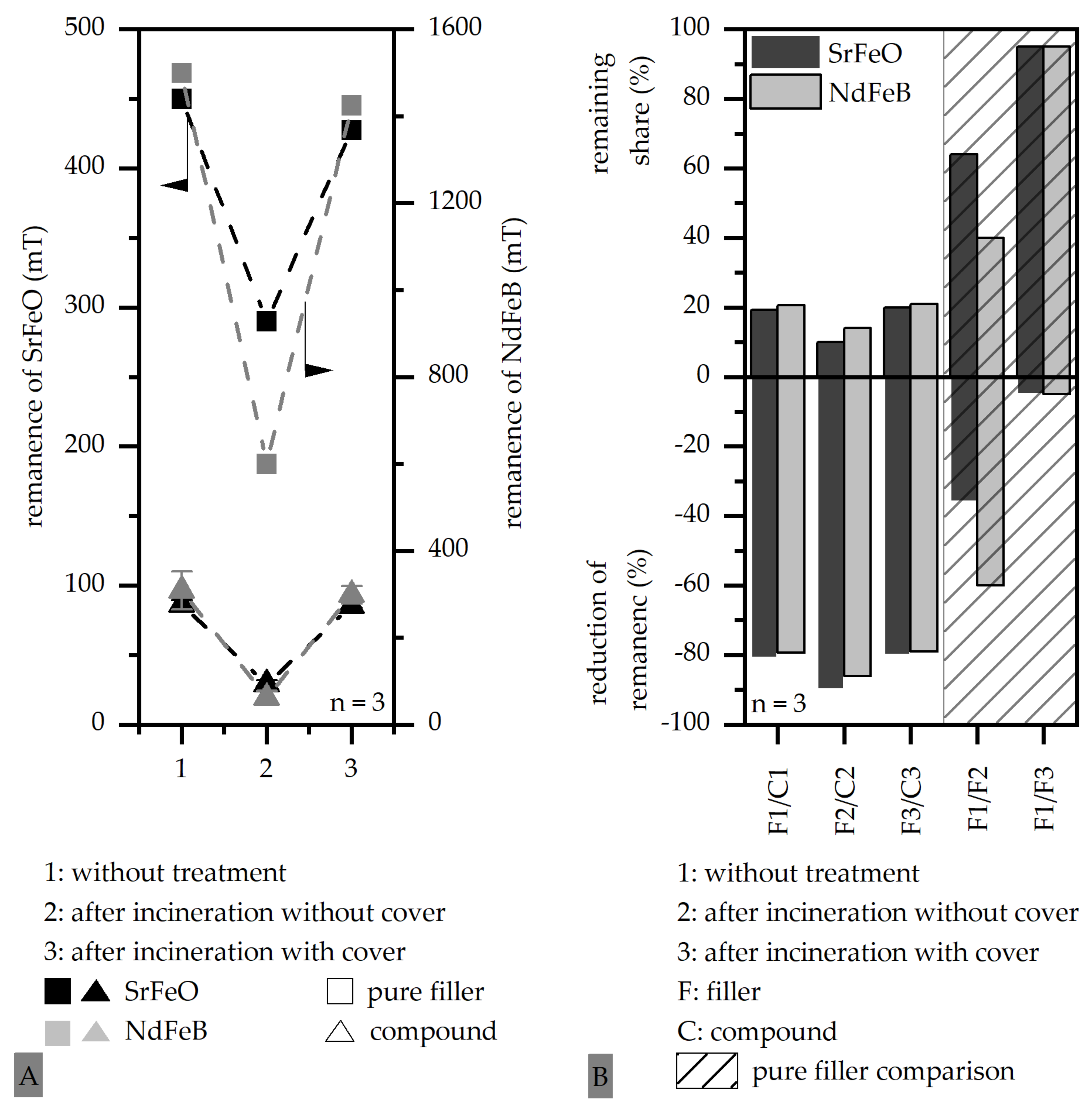
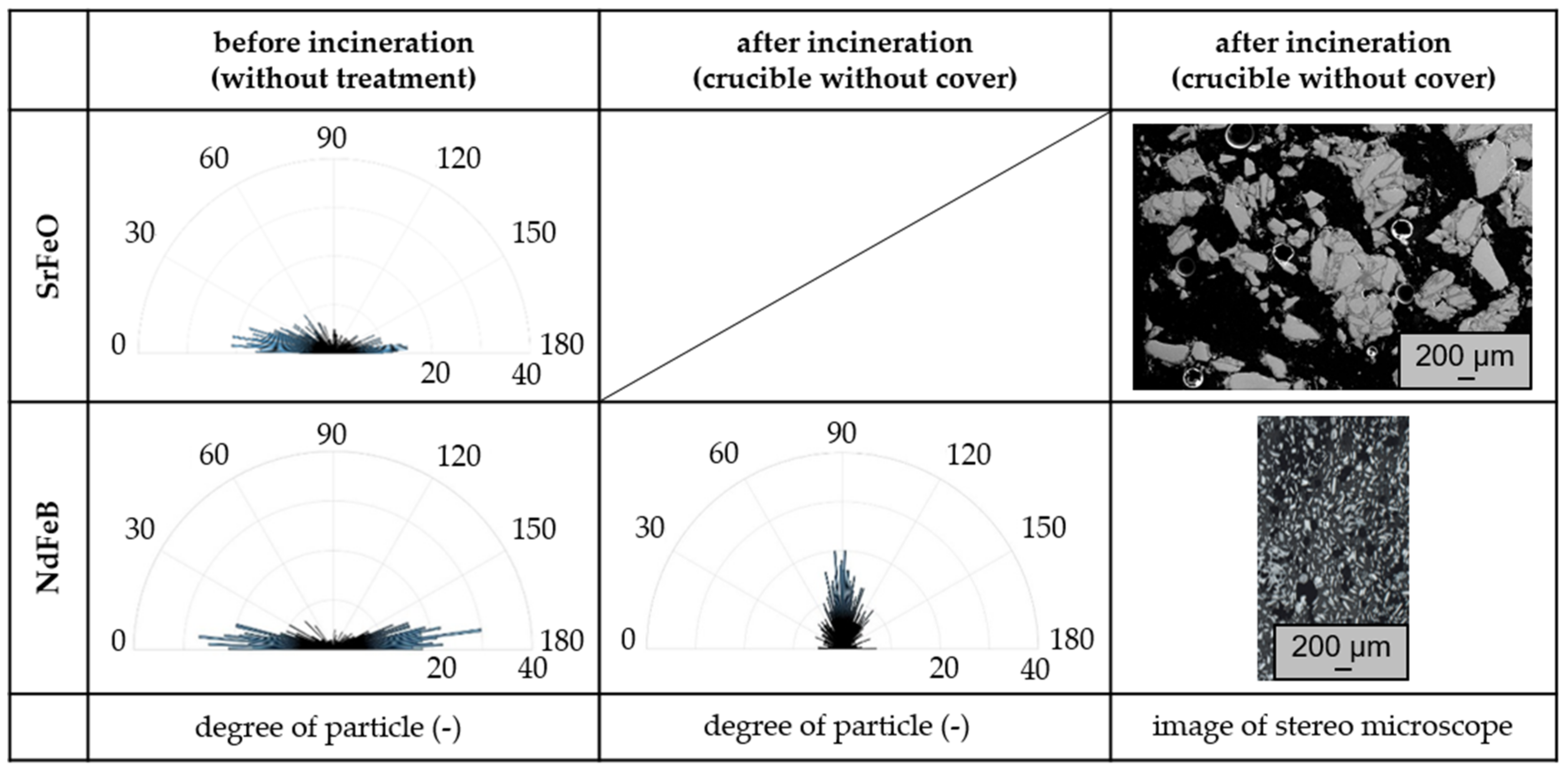
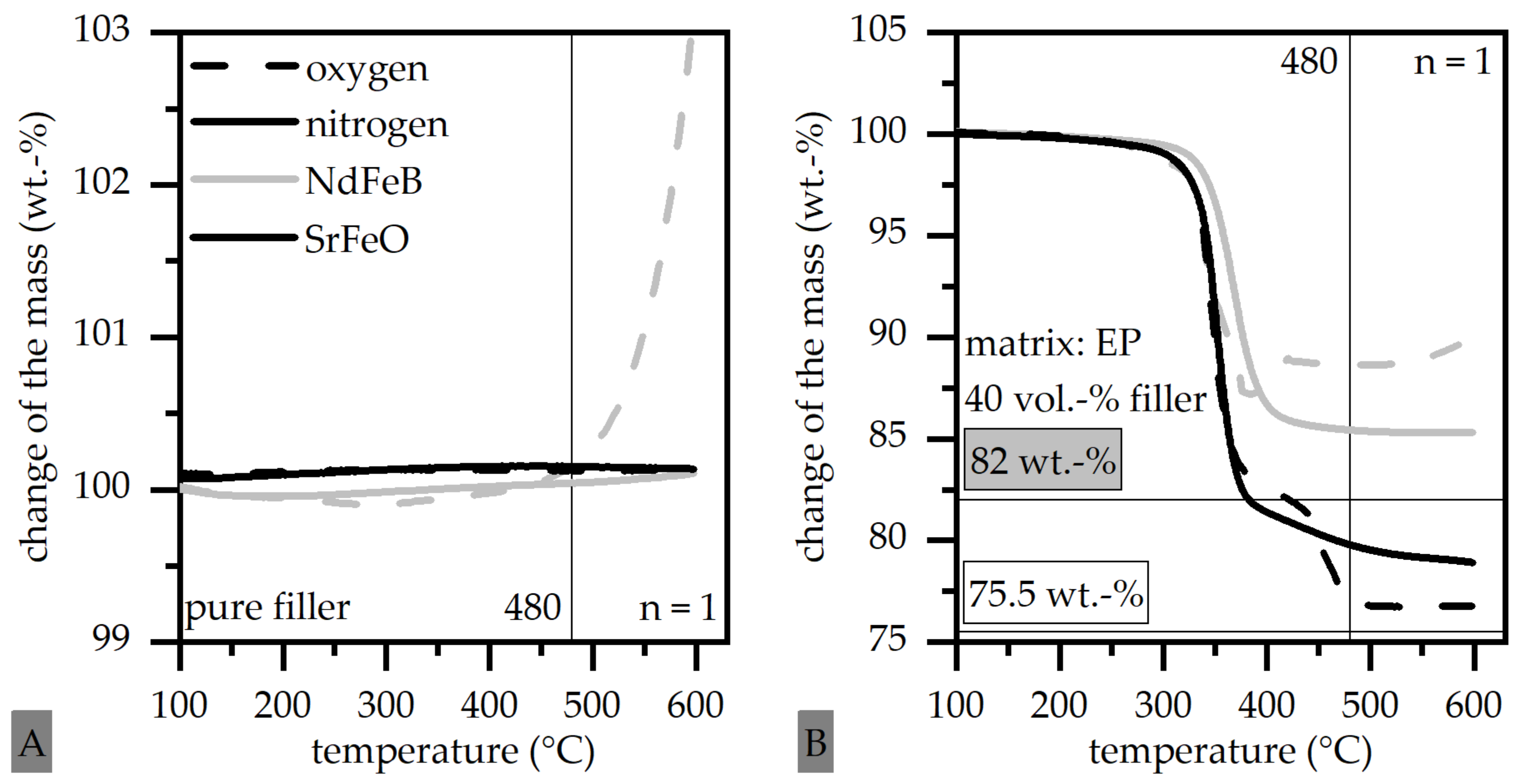
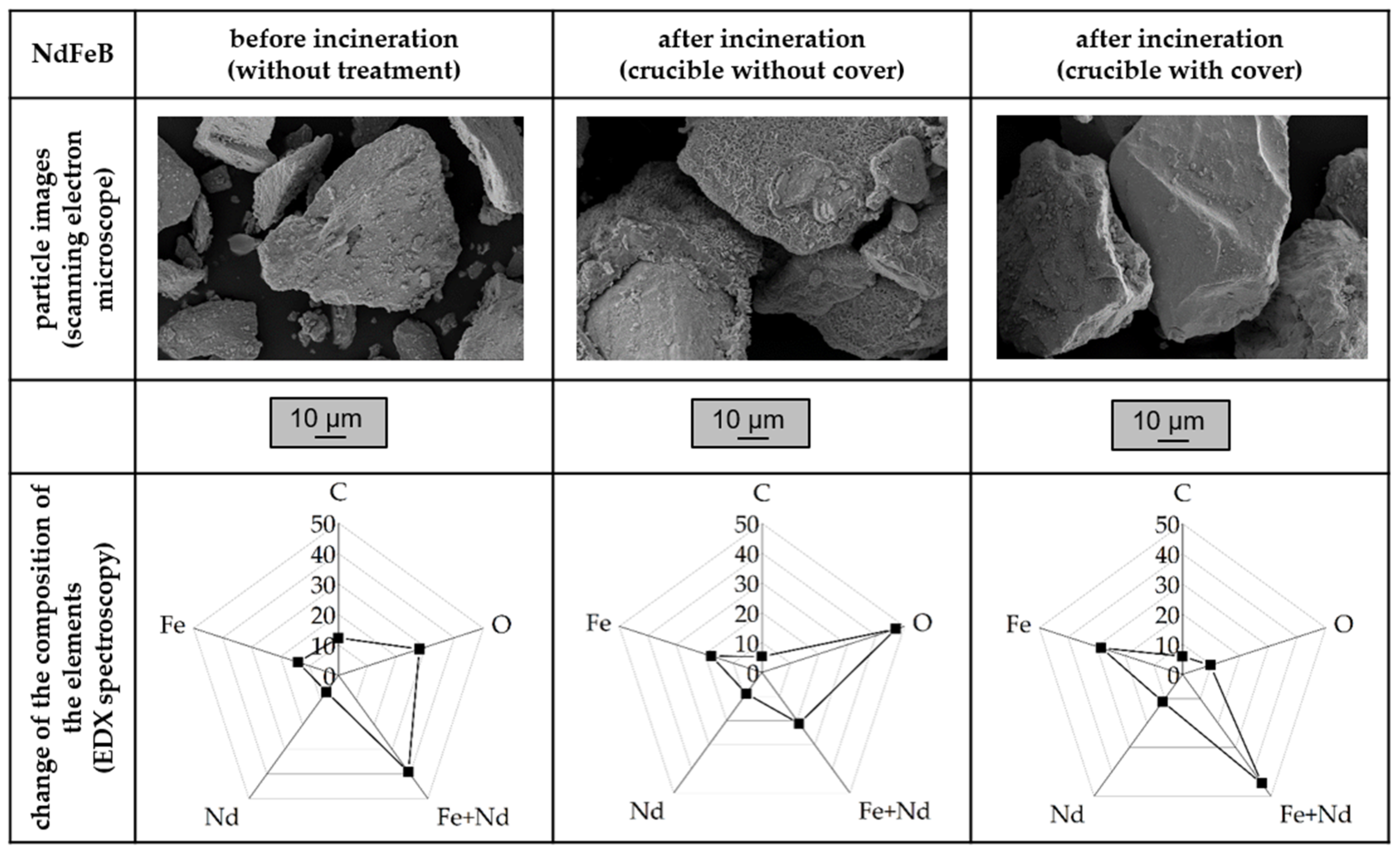
3.2. Influence of the Atmosphere and the Oxidation of Thermoset-Based Polymer-Bonded Magnets with Respect to the Thermal Recycling Strategy
3.3. Magnetic Properties of Thermoplastic-Based Polymer-Bonded Magnets with Respect to the Mechanical Reuse of Shreds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Drummer, D. Prozessführung beim Spritzgießen kunststoffgebundener Dauermagnete. In Kunststoffgebundene Dauermagnete; Springer: Düsseldorf, Germany, 2004. [Google Scholar]
- Hagemann, B. Kunststoffgebundene Magnete in der Antriebstechnik—Möglichkeiten und Grenzen. In Kunststoffgebundene Dauermagnete; Springer: Düsseldorf, Germany, 2004. [Google Scholar]
- Schliesch, T. Sensormagnete: Vielfältig Gestaltbare Spritzgussteile: Magnete mit Virtuoser Polverteilung; Industrieanzeiger: Leinfelden-Echterdingen, Germany, 2010. [Google Scholar]
- Ormerod, J.; Constantinides, S. Bonded permanent magnets: Current status and future opportunities (invited). J. Appl. Phys. 1997, 81, 4816–4820. [Google Scholar] [CrossRef] [Green Version]
- Reppel, G.W. Duroplastgepresste Magnete—Werkstoffe, Verfahren und Eigenschaften. In Kunststoffgebundene Dauermagnete: Werkstoffe, Fertigungsverfahren und Eigenschaften; Ehrenstein, G.W., Drummer, D., Eds.; Springer: Düsseldorf, Germany, 2004; pp. 14–36. [Google Scholar]
- Baran, W. Magnetische Werkstoffe für Energiewandler und statistische Systeme. In Magnetische Werkstoffe; Verlag TÜV: Rheinland, Germany, 1990. [Google Scholar]
- Wellmann, P. Materialien der Elektronik und Energietechnik: Halbleiter, Graphen, Funktionale Materialien; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2017. [Google Scholar]
- Cassing, W.; Kuntze, K.; Ross, G. Dauermagnete: Mess- und Magnetisierungstechnik, 3rd ed.; Expert-Verlag: Renningen, Germany, 2018. [Google Scholar]
- Hering, E.; Martin, R.; Stohrer, M. Physik für Ingenieure, 12th ed.; Springer Vieweg: Berlin, Germany, 2016. [Google Scholar]
- Koch, J. Dauermagentische Werkstoffe und ihre Anwendungen. Phys. Blätter 1975, 31, 439–455. [Google Scholar] [CrossRef]
- Schliesch, T. Kunststoffgebundene Dauermagnete: Werkstoffe, Auslegung und Prüftechnik. In Hochgefüllte Kunststoffe mit Definierten Magnetischen, Thermischen und Elektrischen Eigenschaften; Ehrenstein, G.W., Drummer, D., Eds.; VDI Fachmedien GmbH & Co. KG: Düsseldorf, Germany, 2002; pp. 179–210. [Google Scholar]
- Johannaber, F.; Michaeli, W. Handbuch Spritzgießen, 2nd ed.; Carl Hanser Verlag: München, Germany, 2004. [Google Scholar]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 9506–9513. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, B.; Kleijn, R.; Kramer, G.J. Recycling potential of neodymium: The case of computer hard disk drives. Environ. Sci. Technol. 2014, 48, 9506–9513. [Google Scholar] [CrossRef] [PubMed]
- Glöser-Chahoud, S.; Kühn, A.; Tercero Espinoza, L.A. Globale Verwendungsstrukturen der Magnetwerkstoffe Neodym und Dysprosium: Eine szenariobasierte Analyse der Auswirkung der Diffusion der Elektromobilität auf den Bedarf an Seltenen Erden. In Working Paper Sustainability and Innovation; Fraunhofer-Institut für System- und Innovationsforschung ISI: Karlsruhe, Germany, 2016. [Google Scholar]
- Rösel, U.; Drummer, D. Injection Moulding of Multipolar Polymer-Bonded Magnets into Soft Magnetic Inserts for Rotors in Reluctance Motors. Magnetism 2021, 1, 3–21. [Google Scholar] [CrossRef]
- Buchert, M.; Manhart, A.; Suttler, J. Untersuchung zu Seltenen Erden: Permanentmagnete im Industriellen Einsatz in Baden-Württemberg; Öko-Institut eV: Breisgau, Germany, 2014. [Google Scholar]
- Dolgirev, J.; Kalter, M.; Urschel, S.; Funck, R.; Jung, J.; Schimmelpfennig, V. Resource-saving Cirulating Pump on basis of an Integrated Synchronous-Reluctance Drive System. In Proceedings of the IEEE 4th International Future Energy Electronics Conference (IFEEC), Singapore, 25–28 November 2019; pp. 1–7. [Google Scholar]
- Michalowsky, L.; Schneider, J. Magnettechnik: Grundlagen, Werkstoffe, Anwendungen, 3rd ed.; Vulkan Verlag: Essen, Germany, 2006. [Google Scholar]
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical recycling of glass fiber reinforced epoxy resin cured with amine using nitric acid. Polymer 2005, 46, 1905–1912. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent adapted networks: A unique paradigm in Cross-linked polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Yu, K.; Mu, X.; Shi, X.; Wei, Y.; Guo, Y.; Qi, H.J. A molecular dynamics study of bond exchange reactions in covalent adaptable networks. Soft Matter 2015, 11, 6305–6317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–986. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M. Neues Leben für alte Kunststoffe: So Lassen Sich Duroplasten Recyclen. Available online: https://www.ingenieur.de/technik/fachbereiche/chemie/so-lassen-sich-duroplaste-recyceln/ (accessed on 11 November 2021).
- Davidson, T.A.; Wagener, K.B. The polymerization of dicyclopentadiene: An investigation of mechanism. J. Mol. Catal. A Chem. 1998, 133, 67–74. [Google Scholar] [CrossRef]
- Rast, U.; Blank, R.; Buchert, M.; Elwert, T.; Finsterwalder, F.; Hörnig, G.; Klier, T.; Langkau, S.; Mrscheider-Weidermann, F.; Müller, J.-O.; et al. Recycling von Komponenten und Strategische Metallen aus Elektrischen Fahrantrieben: Kennwort: MORE (Motor Recycling); TK Verlag Karl Thomé-Kozmiensky: Nietwerder, Germany, 2014. [Google Scholar]
- Meyer, F. Recycling von Neodym aus NdFeB-Magneten in Elektroaltgeräten: Potential und Mögliche Recyclingverfahren; Bachelorarbeit: Hamburg, Germany, 2021. [Google Scholar]
- Terada, T.; Onishi, H.; Yamagata, Y.; Yamashita, F. Method of Producing Recycled Raw Material Powder for Use in Bonded Magnet and Method of Recycling Bonded Magnet. U.S. Patent 6,599,450 B1, 29 July 2003. [Google Scholar]
- Ziegmann, G.; Kapustka, K. Recycling von Magnetischen Materialien aus Generatoren von Windkraftanlagen, Elektromotoren und Elektronikschrott: Kurztitel: IrmagMat; Abschlussbericht DBU, AZ: 34799/01; Projektträger; Technische Universität Clausthal Insitut für Polymerwerkstoffe und Kunststofftechnik: Clausthal-Zellerfeld, Germany, 2020. [Google Scholar]
- Drummer, D. Verarbeitung und Eigenschaften Kunststoffgebundener Dauermagnete. Ph.D. Dissertation, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2004. [Google Scholar]
- Frick, A.; Stern, C. Einführung in Die Kunststoffprüfung: Prüfmethoden und Anwendungen; Carl Hanser Verlag: München, Germany, 2017. [Google Scholar]
- Kurth, K. Zum Spritzgießen Polorientierter Kunststoffgebundener Dauermagnete. Ph.D. Dissertation, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2019. [Google Scholar]





| Filler Material (-) | Type in (-) | Mean Particle Size in µm | Density in g/cm3 | Thermal Conductivity λ in W/(mK) | Heat Capacity c in J/(gK) |
|---|---|---|---|---|---|
| SrFeO | OP 71 | 1.25 | 5.382 | 2.30 | 0.639 |
| NdFeB | MQA 38-14 | 105 | 7.501 | 6.10 | 0.423 |
| EP | Epofix | - | 1.100 | 0.20 | 1.384 |
| PA12 | Vestamid BS 1636 | - | 1.009 | 0.33 | 1.840 |
| Mass temperature | 280 °C |
| Mold temperature | 80 °C |
| Injection speed | 80 mm/s |
| Changeover point | 1000 bar |
| Holding pressure | 500 bar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rösel, U.; Drummer, D. Possibilities in Recycling Magnetic Materials in Applications of Polymer-Bonded Magnets. Magnetism 2022, 2, 251-270. https://doi.org/10.3390/magnetism2030019
Rösel U, Drummer D. Possibilities in Recycling Magnetic Materials in Applications of Polymer-Bonded Magnets. Magnetism. 2022; 2(3):251-270. https://doi.org/10.3390/magnetism2030019
Chicago/Turabian StyleRösel, Uta, and Dietmar Drummer. 2022. "Possibilities in Recycling Magnetic Materials in Applications of Polymer-Bonded Magnets" Magnetism 2, no. 3: 251-270. https://doi.org/10.3390/magnetism2030019
APA StyleRösel, U., & Drummer, D. (2022). Possibilities in Recycling Magnetic Materials in Applications of Polymer-Bonded Magnets. Magnetism, 2(3), 251-270. https://doi.org/10.3390/magnetism2030019






