Machine Learning in Neuroimaging of Traumatic Brain Injury: Current Landscape, Research Gaps, and Future Directions
Abstract
1. Introduction
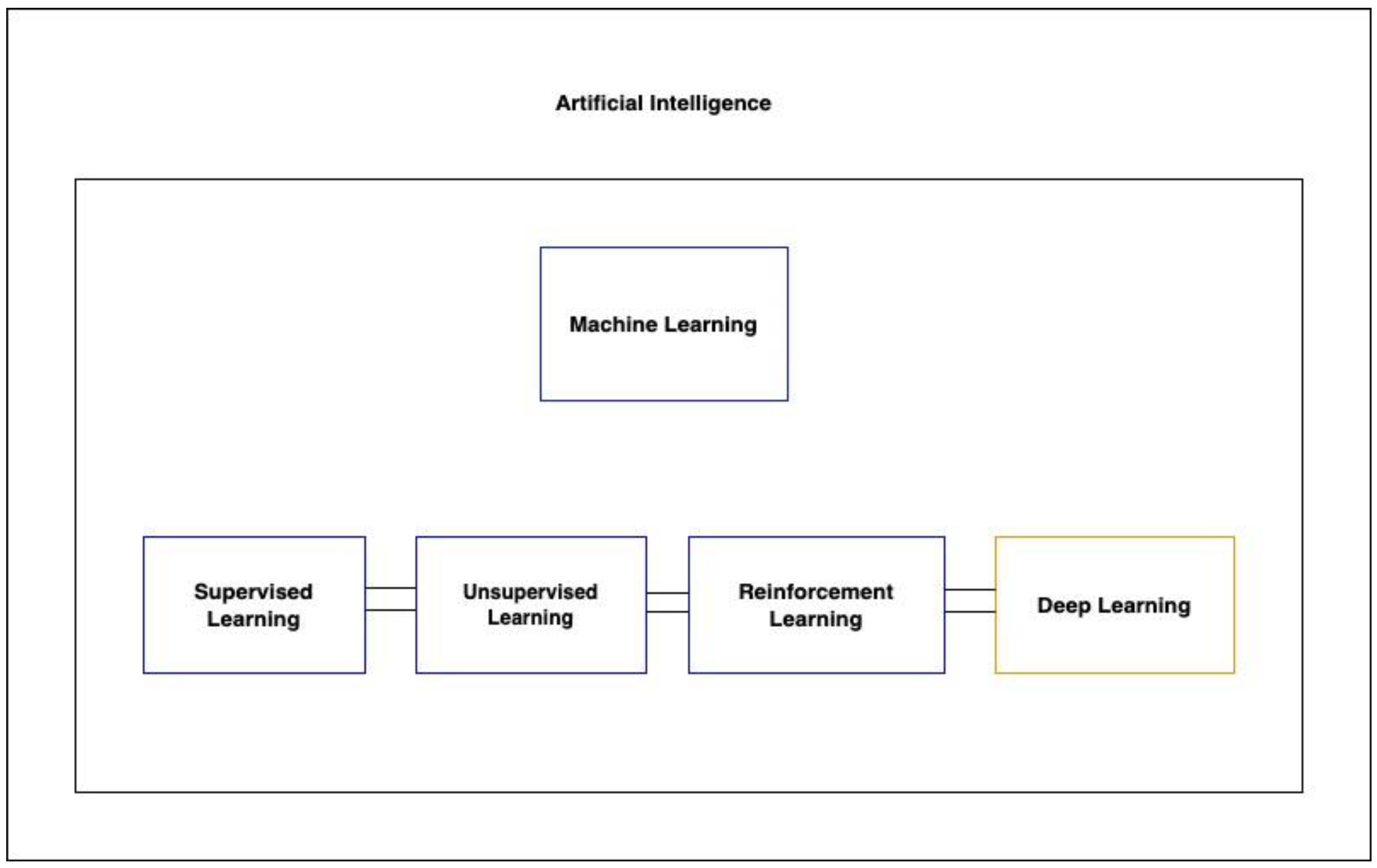
2. Machine Learning
3. Identifying mTBI Using Functional Brain Activity
4. Detecting Axonal Injury with Machine Learning
5. Predicting TBI with CT
6. Detecting and Quantifying Subdural Hematomas with Machine Learning
7. Clinical Applications of ML in Emergency Radiology: Achievements and Challenges
8. Research Frontiers: Expanding the Role of ML in TBI Diagnosis and Prognosis
9. Ethical and Legal Crossroads in AI-Powered TBI Diagnosis
10. Technical Hurdles
11. Traditional Methods and the Rise of AI
12. Cost Considerations
13. Global Perspective and Accessibility Issues
- (1).
- Collaborative International Research and Development: Promoting collaborative research efforts and technological exchanges between high-income countries and LMICs can aid in developing affordable and scalable ML solutions that are adaptable to various healthcare settings. Sharing data across nations will also build more robust and generalizable models.
- (2).
- Capacity Building: Investing in educational and training programs within LMICs is important for cultivating local expertise in ML. This initiative should focus on training healthcare professionals, data scientists, and technical staff to effectively manage and utilize ML systems.
- (3).
- Development of Open-Source and Low-Cost Tools: Encouraging the creation of open-source ML platforms and economical diagnostic tools can increase accessibility in resource-limited settings.
- (4).
- Standardization of Data and Protocols: Implementing standardized protocols for data collection, sharing, and processing can improve the quality and accessibility of data worldwide, which are essential for the development and implementation of effective ML models.
- (5).
- Policy and Funding Support: The role of governments and international organizations is pivotal in providing policy and funding support for integrating ML into healthcare systems, particularly in LMICs. This support could include grants, subsidies, and incentives for adopting ML technologies.
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatuki, T.A.; Zvonarev, V.; Rodas, A.W. Prevention of Traumatic Brain Injury in the United States: Significance, New Findings, and Practical Applications. Cureus 2020, 12, e11225. [Google Scholar] [CrossRef]
- Rodríguez-Triviño, C.Y.; Torres Castro, I.; Dueñas, Z. Hypochloremia in Patients with Severe Traumatic Brain Injury: A Possible Risk Factor for Increased Mortality. World Neurosurg. 2019, 124, e783–e788. [Google Scholar] [CrossRef]
- McMahon, P.J.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T.; et al. Symptomatology and Functional Outcome in Mild Traumatic Brain Injury: Results from the Prospective TRACK-TBI Study. J. Neurotrauma 2014, 31, 26–33. [Google Scholar] [CrossRef]
- Keret, A.; Bennett-Back, O.; Rosenthal, G.; Gilboa, T.; Shweiki, M.; Shoshan, Y.; Benifla, M. Posttraumatic epilepsy: Long-term follow-up of children with mild traumatic brain injury. J. Neurosurg. Pediatr. PED 2017, 20, 64–70. [Google Scholar] [CrossRef]
- Pierre, K.; Dyson, K.; Dagra, A.; Williams, E.; Porche, K.; Lucke-Wold, B. Chronic Traumatic Encephalopathy: Update on Current Clinical Diagnosis and Management. Biomedicines 2021, 9, 415. [Google Scholar] [CrossRef]
- Gardner, R.C.; Yaffe, K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci. 2015, 66, 75–80. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert. Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Shenton, M.E.; Hamoda, H.M.; Schneiderman, J.S.; Bouix, S.; Pasternak, O.; Rathi, Y.; Vu, M.A.; Purohit, M.P.; Helmer, K.; Koerte, I.; et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012, 6, 137–192. [Google Scholar] [CrossRef]
- Wu, X.; Sun, Y.; Xu, X.; Steyerberg, E.W.; Helmrich, I.R.A.R.; Lecky, F.; Guo, J.; Li, X.; Feng, J.; Mao, Q.; et al. Mortality Prediction in Severe Traumatic Brain Injury Using Traditional and Machine Learning Algorithms. J. Neurotrauma 2023, 40, 1366–1375. [Google Scholar] [CrossRef]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mortality in patients on mechanical ventilation post traumatic brain injury: Machine learning approach. BMC Med. Inform. Decis. Mak. 2020, 20, 336. [Google Scholar] [CrossRef]
- Tunthanathip, T.; Oearsakul, T. Application of machine learning to predict the outcome of pediatric traumatic brain injury. Chin. J. Traumatol. 2021, 24, 350–355. [Google Scholar] [CrossRef]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mortality in patients with post traumatic brain injury using National Trauma Registry and Machine Learning Approach. Scand. J. Trauma. Resusc. Emerg. Med. 2020, 28, 44. [Google Scholar] [CrossRef]
- Hsu, S.-D.; Chao, E.; Chen, S.-J.; Hueng, D.-Y.; Lan, H.-Y.; Chiang, H.-H. Machine Learning Algorithms to Predict In-Hospital Mortality in Patients with Traumatic Brain Injury. J. Pers. Med. 2021, 11, 1144. [Google Scholar] [CrossRef]
- Folweiler, K.A.; Sandsmark, D.K.; Diaz-Arrastia, R.; Cohen, A.S.; Masino, A.J. Unsupervised Machine Learning Reveals Novel Traumatic Brain Injury Patient Phenotypes with Distinct Acute Injury Profiles and Long-Term Outcomes. J. Neurotrauma 2020, 37, 1431–1444. [Google Scholar] [CrossRef]
- Hale, A.T.; Stonko, D.P.; Brown, A.; Lim, J.; Voce, D.J.; Gannon, S.R.; Le, T.M.; Shannon, C.N. Machine-learning analysis outperforms conventional statistical models and CT classification systems in predicting 6-month outcomes in pediatric patients sustaining traumatic brain injury. Neurosurg. Focus FOC 2018, 45, E2. [Google Scholar] [CrossRef]
- Hernandes Rocha, T.A.; Elahi, C.; Cristina da Silva, N.; Sakita, F.M.; Fuller, A.; Mmbaga, B.T.; Green, E.P.; Haglund, M.M.; Staton, C.A.; Nickenig Vissoci, J.R. A traumatic brain injury prognostic model to support in-hospital triage in a low-income country: A machine learning–based approach. J. Neurosurg. JNS 2020, 132, 1961–1969. [Google Scholar] [CrossRef]
- Vishwanath, M.; Jafarlou, S.; Shin, I.; Lim, M.M.; Dutt, N.; Rahmani, A.M.; Cao, H. Investigation of Machine Learning Approaches for Traumatic Brain Injury Classification via EEG Assessment in Mice. Sensors 2020, 20, 2027. [Google Scholar] [CrossRef]
- Farzaneh, N.; Williamson, C.A.; Gryak, J.; Najarian, K. A hierarchical expert-guided machine learning framework for clinical decision support systems: An application to traumatic brain injury prognostication. NPJ Digit. Med. 2021, 4, 78. [Google Scholar] [CrossRef]
- Raj, R.; Wennervirta, J.M.; Tjerkaski, J.; Luoto, T.M.; Posti, J.P.; Nelson, D.W.; Takala, R.; Bendel, S.; Thelin, E.P.; Luostarinen, T.; et al. Dynamic prediction of mortality after traumatic brain injury using a machine learning algorithm. NPJ Digit. Med. 2022, 5, 96. [Google Scholar] [CrossRef]
- Warman, P.I.; Seas, A.; Satyadev, N.; Adil, S.M.; Kolls, B.J.; Haglund, M.M.; Dunn, T.W.; Fuller, A.T. Machine Learning for Predicting In-Hospital Mortality After Traumatic Brain Injury in Both High-Income and Low- and Middle-Income Countries. Neurosurgery 2022, 90, 605–612. [Google Scholar] [CrossRef]
- Bruschetta, R.; Tartarisco, G.; Lucca, L.F.; Leto, E.; Ursino, M.; Tonin, P.; Pioggia, G.; Cerasa, A. Predicting Outcome of Traumatic Brain Injury: Is Machine Learning the Best Way? Biomedicines 2022, 10, 686. [Google Scholar] [CrossRef]
- Satyadev, N.; Warman, P.I.; Seas, A.; Kolls, B.J.; Haglund, M.M.; Fuller, A.T.; Dunn, T.W. Machine Learning for Predicting Discharge Disposition After Traumatic Brain Injury. Neurosurgery 2022, 90, 768–774. [Google Scholar] [CrossRef]
- Lang, E.; Neuschwander, A.; Favé, G.; Abback, P.-S.; Esnault, P.; Geeraerts, T.; Harrois, A.; Hanouz, J.-L.; Kipnis, E.; Leone, M.; et al. Clinical decision support for severe trauma patients: Machine learning based definition of a bundle of care for hemorrhagic shock and traumatic brain injury. J. Trauma Acute Care Surg. 2022, 92, 135–143. [Google Scholar] [CrossRef]
- Mohd Noor, N.S.E.; Ibrahim, H. Predicting Outcomes in Patients with Traumatic Brain Injury Using Machine Learning Models. In Proceedings of the Intelligent Manufacturing and Mechatronics, Melaka, Malaysia, 8 July 2019; Springer: Singapore, 2020; pp. 12–20. [Google Scholar]
- Radabaugh, H.L.; Bonnell, J.; Dietrich, W.D.; Bramlett, H.M.; Schwartz, O.; Sarkar, D. Development and Evaluation of Machine Learning Models for Recovery Prediction after Treatment for Traumatic Brain Injury. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2416–2420. [Google Scholar]
- Lee, S.H.; Lee, C.H.; Hwang, S.H.; Kang, D.H. A Machine Learning–Based Prognostic Model for the Prediction of Early Death After Traumatic Brain Injury: Comparison with the Corticosteroid Randomization After Significant Head Injury (CRASH) Model. World Neurosurg. 2022, 166, e125–e134. [Google Scholar] [CrossRef]
- Guimarães, K.A.A.; de Amorim, R.L.O.; Costa, M.G.F.; Costa Filho, C.F.F. Predicting early traumatic brain injury mortality with 1D convolutional neural networks and conventional machine learning techniques. Inform. Med. Unlocked 2022, 31, 100984. [Google Scholar] [CrossRef]
- Amorim, R.L.; Oliveira, L.M.; Malbouisson, L.M.; Nagumo, M.M.; Simoes, M.; Miranda, L.; Bor-Seng-Shu, E.; Beer-Furlan, A.; De Andrade, A.F.; Rubiano, A.M.; et al. Prediction of Early TBI Mortality Using a Machine Learning Approach in a LMIC Population. Front. Neurol. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Signorini, D.F.; Andrews, P.J.; Jones, P.A.; Wardlaw, J.M.; Miller, J.D. Predicting survival using simple clinical variables: A case study in traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 1999, 66, 20–25. [Google Scholar] [CrossRef]
- Pang, B.C.; Kuralmani, V.; Joshi, R.; Hongli, Y.; Lee, K.K.; Ang, B.T.; Li, J.; Leong, T.Y.; Ng, I. Hybrid Outcome Prediction Model for Severe Traumatic Brain Injury. J. Neurotrauma 2007, 24, 136–146. [Google Scholar] [CrossRef]
- Chong, S.L.; Liu, N.; Barbier, S.; Ong, M.E. Predictive modeling in pediatric traumatic brain injury using machine learning. BMC Med. Res. Methodol. 2015, 15, 22. [Google Scholar] [CrossRef]
- Adil, S.M.; Elahi, C.; Gramer, R.; Spears, C.A.; Fuller, A.T.; Haglund, M.M.; Dunn, T.W. Predicting the Individual Treatment Effect of Neurosurgery for Patients with Traumatic Brain Injury in the Low-Resource Setting: A Machine Learning Approach in Uganda. J. Neurotrauma 2020, 38, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Daley, M.; Cameron, S.; Ganesan, S.L.; Patel, M.A.; Stewart, T.C.; Miller, M.R.; Alharfi, I.; Fraser, D.D. Pediatric severe traumatic brain injury mortality prediction determined with machine learning-based modeling. Injury 2022, 53, 992–998. [Google Scholar] [CrossRef]
- Nourelahi, M.; Dadboud, F.; Khalili, H.; Niakan, A.; Parsaei, H. A machine learning model for predicting favorable outcome in severe traumatic brain injury patients after 6 months. Acute Crit. Care 2022, 37, 45–52. [Google Scholar] [CrossRef]
- Matsuo, K.; Aihara, H.; Nakai, T.; Morishita, A.; Tohma, Y.; Kohmura, E. Machine Learning to Predict In-Hospital Morbidity and Mortality after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 202–210. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Y.; Peng, J.; Sun, M.; Zeng, J.; Jiang, H. Comparison between logistic regression and machine learning algorithms on survival prediction of traumatic brain injuries. J. Crit. Care 2019, 54, 110–116. [Google Scholar] [CrossRef]
- Noor, N.S.E.M.; Ibrahim, H. Machine Learning Algorithms and Quantitative Electroencephalography Predictors for Outcome Prediction in Traumatic Brain Injury: A Systematic Review. IEEE Access 2020, 8, 102075–102092. [Google Scholar] [CrossRef]
- Palacios, E.M.; Owen, J.P.; Yuh, E.L.; Wang, M.B.; Vassar, M.J.; Ferguson, A.R.; Diaz-Arrastia, R.; Giacino, J.T.; Okonkwo, D.O.; Robertson, C.S.; et al. The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci. Adv. 2020, 6, eaaz6892. [Google Scholar] [CrossRef]
- Tjerkaski, J.; Nyström, H.; Raj, R.; Lindblad, C.; Bellander, B.-M.; Nelson, D.W.; Thelin, E.P. Extended Analysis of Axonal Injuries Detected Using Magnetic Resonance Imaging in Critically Ill Traumatic Brain Injury Patients. J. Neurotrauma 2022, 39, 58–66. [Google Scholar] [CrossRef]
- Chen, W.; Cockrell, C.; Ward, K.R.; Najarian, K. Intracranial pressure level prediction in traumatic brain injury by extracting features from multiple sources and using machine learning methods. In Proceedings of the 2010 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Hong Kong, China, 18–21 December 2010; pp. 510–515. [Google Scholar]
- Ye, G.; Balasubramanian, V.; Li, J.K.J.; Kaya, M. Machine Learning-Based Continuous Intracranial Pressure Prediction for Traumatic Injury Patients. IEEE J. Transl. Eng. Health Med. 2022, 10, 4901008. [Google Scholar] [CrossRef]
- Chen, W.; Cockrell, C.H.; Ward, K.; Najarian, K. Predictability of intracranial pressure level in traumatic brain injury: Features extraction, statistical analysis and machine learning-based evaluation. Int. J. Data Min. Bioinform. 2013, 8, 480–494. [Google Scholar] [CrossRef]
- Tunthanathip, T.; Duangsuwan, J.; Wattanakitrungroj, N.; Tongman, S.; Phuenpathom, N. Comparison of intracranial injury predictability between machine learning algorithms and the nomogram in pediatric traumatic brain injury. Neurosurg. Focus 2021, 51, E7. [Google Scholar] [CrossRef]
- Molaei, S.; Korley, F.K.; Soroushmehr, S.M.R.; Falk, H.; Sair, H.; Ward, K.; Najarían, K. A machine learning based approach for identifying traumatic brain injury patients for whom a head CT scan can be avoided. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2258–2261. [Google Scholar]
- Li, Y.-C.; Liu, L.; Chiu, W.-T.; Jian, W.-S. Neural network modeling for surgical decisions on traumatic brain injury patients. Int. J. Med. Inform. 2000, 57, 1–9. [Google Scholar] [CrossRef]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Using trauma registry data to predict prolonged mechanical ventilation in patients with traumatic brain injury: Machine learning approach. PLoS ONE 2020, 15, e0235231. [Google Scholar] [CrossRef]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Al-Thani, H.; El-Menyar, A. Machine Learning Model to Predict Ventilator Associated Pneumonia in patients with Traumatic Brain Injury: The C.5 Decision Tree Approach. Brain Inj. 2021, 35, 1095–1102. [Google Scholar] [CrossRef]
- Marincowitz, C.; Paton, L.; Lecky, F.; Tiffin, P. Predicting need for hospital admission in patients with traumatic brain injury or skull fractures identified on CT imaging: A machine learning approach. Emerg. Med. J. 2022, 39, 394–401. [Google Scholar] [CrossRef]
- Abujaber, A.; Fadlalla, A.; Nashwan, A.; El-Menyar, A.; Al-Thani, H. Predicting prolonged length of stay in patients with traumatic brain injury: A machine learning approach. Intell.-Based Med. 2022, 6, 100052. [Google Scholar] [CrossRef]
- Prichep, L.S.; Jacquin, A.; Filipenko, J.; Dastidar, S.G.; Zabele, S.; Vodencarević, A.; Rothman, N.S. Classification of traumatic brain injury severity using informed data reduction in a series of binary classifier algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 806–822. [Google Scholar] [CrossRef]
- Yadav, K.; Sarioglu, E.; Choi, H.A.; Cartwright Iv, W.B.; Hinds, P.S.; Chamberlain, J.M. Automated Outcome Classification of Computed Tomography Imaging Reports for Pediatric Traumatic Brain Injury. Acad. Emerg. Med. 2016, 23, 171–178. [Google Scholar] [CrossRef]
- Ellethy, H.; Chandra, S.S.; Nasrallah, F.A. The detection of mild traumatic brain injury in paediatrics using artificial neural networks. Comput. Biol. Med. 2021, 135, 104614. [Google Scholar] [CrossRef]
- Dhillon, N.S.; Sutandi, A.; Vishwanath, M.; Lim, M.M.; Cao, H.; Si, D. A Raspberry Pi-Based Traumatic Brain Injury Detection System for Single-Channel Electroencephalogram. Sensors 2021, 21, 2779. [Google Scholar] [CrossRef]
- Peacock, W.F.; Van Meter, T.E.; Mirshahi, N.; Ferber, K.; Gerwien, R.; Rao, V.; Sair, H.I.; Diaz-Arrastia, R.; Korley, F.K. Derivation of a Three Biomarker Panel to Improve Diagnosis in Patients with Mild Traumatic Brain Injury. Front. Neurol. 2017, 8, 641. [Google Scholar] [CrossRef]
- Rau, C.S.; Kuo, P.J.; Chien, P.C.; Huang, C.Y.; Hsieh, H.Y.; Hsieh, C.H. Mortality prediction in patients with isolated moderate and severe traumatic brain injury using machine learning models. PLoS ONE 2018, 13, e0207192. [Google Scholar] [CrossRef]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine Learning for Medical Imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef]
- Wagner, M.W.; Namdar, K.; Biswas, A.; Monah, S.; Khalvati, F.; Ertl-Wagner, B.B. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology 2021, 63, 1957–1967. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Mushkudiani, N.; Perel, P.; Butcher, I.; Lu, J.; McHugh, G.S.; Murray, G.D.; Marmarou, A.; Roberts, I.; Habbema, J.D.F.; et al. Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. PLoS Med. 2008, 5, e165. [Google Scholar] [CrossRef]
- Perel, P.; Arango, M.; Clayton, T.; Edwards, P.; Komolafe, E.; Poccock, S.; Roberts, I.; Shakur, H.; Steyerberg, E.; Yutthakasemsunt, S. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ 2008, 336, 425–429. [Google Scholar] [CrossRef]
- Safar, K.; Zhang, J.; Emami, Z.; Gharehgazlou, A.; Ibrahim, G.; Dunkley, B.T. Mild traumatic brain injury is associated with dysregulated neural network functioning in children and adolescents. Brain Commun. 2021, 3, fcab044. [Google Scholar] [CrossRef]
- Vergara, V.M.; Mayer, A.R.; Kiehl, K.A.; Calhoun, V.D. Dynamic functional network connectivity discriminates mild traumatic brain injury through machine learning. NeuroImage Clin. 2018, 19, 30–37. [Google Scholar] [CrossRef]
- Vergara, V.M.; Mayer, A.R.; Damaraju, E.; Kiehl, K.A.; Calhoun, V. Detection of Mild Traumatic Brain Injury by Machine Learning Classification Using Resting State Functional Network Connectivity and Fractional Anisotropy. J. Neurotrauma 2017, 34, 1045–1053. [Google Scholar] [CrossRef]
- Rangaprakash, D.; Dretsch, M.N.; Venkataraman, A.; Katz, J.S.; Denney, T.S., Jr.; Deshpande, G. Identifying disease foci from static and dynamic effective connectivity networks: Illustration in soldiers with trauma. Hum. Brain Mapp. 2018, 39, 264–287. [Google Scholar] [CrossRef]
- Luo, X.; Lin, D.; Xia, S.; Wang, D.; Weng, X.; Huang, W.; Ye, H. Machine Learning Classification of Mild Traumatic Brain Injury Using Whole-Brain Functional Activity: A Radiomics Analysis. Dis. Markers 2021, 2021, 3015238. [Google Scholar] [CrossRef]
- Fan, L.; Xu, H.; Su, J.; Qin, J.; Gao, K.; Ou, M.; Peng, S.; Shen, H.; Li, N. Discriminating mild traumatic brain injury using sparse dictionary learning of functional network dynamics. Brain Behav. 2021, 11, e2414. [Google Scholar] [CrossRef]
- Rangaprakash, D.; Deshpande, G.; Daniel, T.A.; Goodman, A.M.; Robinson, J.L.; Salibi, N.; Katz, J.S.; Denney, T.S., Jr.; Dretsch, M.N. Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and posttraumatic stress disorder. Hum. Brain Mapp. 2017, 38, 2843–2864. [Google Scholar] [CrossRef]
- Vedaei, F.; Mashhadi, N.; Zabrecky, G.; Monti, D.; Navarreto, E.; Hriso, C.; Wintering, N.; Newberg, A.B.; Mohamed, F.B. Identification of chronic mild traumatic brain injury using resting state functional MRI and machine learning techniques. Front. Neurosci. 2022, 16, 1099560. [Google Scholar] [CrossRef]
- Hellyer, P.J.; Leech, R.; Ham, T.E.; Bonnelle, V.; Sharp, D.J. Individual prediction of white matter injury following traumatic brain injury. Ann. Neurol. 2013, 73, 489–499. [Google Scholar] [CrossRef]
- Fagerholm, E.D.; Hellyer, P.J.; Scott, G.; Leech, R.; Sharp, D.J. Disconnection of network hubs and cognitive impairment after traumatic brain injury. Brain 2015, 138 Pt 6, 1696–1709. [Google Scholar] [CrossRef]
- Mitra, J.; Shen, K.-k.; Ghose, S.; Bourgeat, P.; Fripp, J.; Salvado, O.; Pannek, K.; Taylor, D.J.; Mathias, J.L.; Rose, S. Statistical machine learning to identify traumatic brain injury (TBI) from structural disconnections of white matter networks. NeuroImage 2016, 129, 247–259. [Google Scholar] [CrossRef]
- Stone, J.R.; Wilde, E.A.; Taylor, B.A.; Tate, D.F.; Levin, H.; Bigler, E.D.; Scheibel, R.S.; Newsome, M.R.; Mayer, A.R.; Abildskov, T.; et al. Supervised learning technique for the automated identification of white matter hyperintensities in traumatic brain injury. Brain Inj. 2016, 30, 1458–1468. [Google Scholar] [CrossRef]
- Bai, L.; Bai, G.; Wang, S.; Yang, X.; Gan, S.; Jia, X.; Yin, B.; Yan, Z. Strategic white matter injury associated with long-term information processing speed deficits in mild traumatic brain injury. Hum. Brain Mapp. 2020, 41, 4431–4441. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, S.; Zhao, W.; Li, Z.; Wu, Z.; Ji, S. Concussion classification via deep learning using whole-brain white matter fiber strains. PLoS ONE 2018, 13, e0197992. [Google Scholar] [CrossRef] [PubMed]
- Minaee, S.; Wang, Y.; Chung, S.; Wang, X.H.; Fieremans, E.; Flanagan, S.; Rath, J.F.; Lui, Y.W. A Machine Learning Approach For Identifying Patients with Mild Traumatic Brain Injury Using Diffusion MRI Modeling. arXiv 2017, arXiv:1708.09000. [Google Scholar]
- Senyukova, O.; Galanine, V.; Krylov, A.; Petraikin, A.; Akhadov, T.; Sidorin, S. Diffuse Axonal Injury Lesion Segmentation Using Contouring Algorithm. In Proceedings of the 21st International Conference on Computer Graphics and Vision, GraphiCon’2011-Conference Proceedings, Moscow, Russia, 26–30 September 2011. [Google Scholar]
- Abdelrahman, H.A.F.; Ubukata, S.; Ueda, K.; Fujimoto, G.; Oishi, N.; Aso, T.; Murai, T. Combining Multiple Indices of Diffusion Tensor Imaging Can Better Differentiate Patients with Traumatic Brain Injury from Healthy Subjects. Neuropsychiatr. Dis. Treat. 2022, 18, 1801–1814. [Google Scholar] [CrossRef]
- Mohamed, M.; Alamri, A.; Mohamed, M.; Khalid, N.; O’Halloran, P.; Staartjes, V.; Uff, C. Prognosticating outcome using magnetic resonance imaging in patients with moderate to severe traumatic brain injury: A machine learning approach. Brain Inj. 2022, 36, 353–358. [Google Scholar] [CrossRef]
- Bohyn, C.; Vyvere, T.V.; Keyzer, F.; Sima, D.M.; Demaerel, P. Morphometric evaluation of traumatic axonal injury and the correlation with post-traumatic cerebral atrophy and functional outcome. Neuroradiol. J. 2022, 35, 468–476. [Google Scholar] [CrossRef]
- Monteiro, M.; Newcombe, V.F.J.; Mathieu, F.; Adatia, K.; Kamnitsas, K.; Ferrante, E.; Das, T.; Whitehouse, D.; Rueckert, D.; Menon, D.K.; et al. Multiclass semantic segmentation and quantification of traumatic brain injury lesions on head CT using deep learning: An algorithm development and multicentre validation study. Lancet Digit. Health 2020, 2, e314–e322. [Google Scholar] [CrossRef]
- Keshavamurthy, K.N.; Leary, O.P.; Merck, L.H.; Kimia, B.B.; Collins, S.; Wright, D.W.; Allen, J.W.; Brock, J.F.; Merck, D. Machine learning algorithm for automatic detection of CT-identifiable hyperdense lesions associated with traumatic brain injury. In Proceedings of the Medical Imaging 2017: Computer-Aided Diagnosis, Orlando, FL, USA, 13–16 February 2017. [Google Scholar]
- Salehinejad, H.; Kitamura, J.; Ditkofsky, N.; Lin, A.; Bharatha, A.; Suthiphosuwan, S.; Lin, H.M.; Wilson, J.R.; Mamdani, M.; Colak, E. A real-world demonstration of machine learning generalizability in the detection of intracranial hemorrhage on head computerized tomography. Sci. Rep. 2021, 11, 17051. [Google Scholar] [CrossRef]
- Puffer, R.C.; Yue, J.K.; Mesley, M.; Billigen, J.B.; Sharpless, J.; Fetzick, A.L.; Puccio, A.; Diaz-Arrastia, R.; Okonkwo, D.O. Long-term outcome in traumatic brain injury patients with midline shift: A secondary analysis of the Phase 3 COBRIT clinical trial. J. Neurosurg. 2018, 131, 596–603. [Google Scholar] [CrossRef]
- Nag, M.K.; Gupta, A.; Hariharasudhan, A.S.; Sadhu, A.K.; Das, A.; Ghosh, N. Quantitative analysis of brain herniation from non-contrast CT images using deep learning. J. Neurosci. Methods 2021, 349, 109033. [Google Scholar] [CrossRef]
- Yan, J.L.; Chen, Y.L.; Chen, M.Y.; Chen, B.A.; Chang, J.X.; Kao, C.C.; Hsieh, M.C.; Peng, Y.T.; Huang, K.C.; Chen, P.Y. A Robust, Fully Automatic Detection Method and Calculation Technique of Midline Shift in Intracranial Hemorrhage and Its Clinical Application. Diagnostics 2022, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Pease, M.; Arefan, D.; Barber, J.; Yuh, E.; Puccio, A.; Hochberger, K.; Nwachuku, E.; Roy, S.; Casillo, S.; Temkin, N.; et al. Outcome Prediction in Patients with Severe Traumatic Brain Injury Using Deep Learning from Head CT Scans. Radiology 2022, 304, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Stanišić, M.; Hald, J.; Rasmussen, I.A.; Pripp, A.H.; Ivanović, J.; Kolstad, F.; Sundseth, J.; Züchner, M.; Lindegaard, K.F. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: A prospective study of 107 operated patients. Acta Neurochir. 2013, 155, 323–333; discussion 333. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.R.; Chesnut, R.; Ghajar, J.; Gordon, D.; Hartl, R.; Newell, D.W.; Servadei, F.; Walters, B.C.; Wilberger, J.E. Surgical management of acute subdural hematomas. Neurosurgery 2006, 58, S16–S24; discussion Si-iv. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, N.; Williamson, C.A.; Jiang, C.; Srinivasan, A.; Bapuraj, J.R.; Gryak, J.; Najarian, K.; Soroushmehr, S.M.R. Automated Segmentation and Severity Analysis of Subdural Hematoma for Patients with Traumatic Brain Injuries. Diagnostics 2020, 10, 773. [Google Scholar] [CrossRef]
- Chen, D.; Bian, L.; He, H.Y.; Li, Y.D.; Ma, C.; Mao, L.G. Evaluation of Traumatic Subdural Hematoma Volume by Using Image Segmentation Assessment Based on Deep Learning. Comput. Math. Methods Med. 2022, 2022, 3830245. [Google Scholar] [CrossRef]
- Colasurdo, M.; Leibushor, N.; Robledo, A.; Vasandani, V.; Luna, Z.A.; Rao, A.S.; Garcia, R.; Srinivasan, V.M.; Sheth, S.A.; Avni, N.; et al. Automated detection and analysis of subdural hematomas using a machine learning algorithm. J. Neurosurg. 2023, 138, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Rismani, M.; Nematollahi, M.A.; Masoudi, M.S.; Asadollahi, A.; Taheri, R.; Pourmontaseri, H.; Valibeygi, A.; Roshanzamir, M.; Alizadehsani, R.; et al. Prognosis prediction in traumatic brain injury patients using machine learning algorithms. Sci. Rep. 2023, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, R.T.; Vargas, J.; Barros, G.; Sen, R.; Bass, D.; Mason, J.R.; Levitt, M. Segmentation of Chronic Subdural Hematomas Using 3D Convolutional Neural Networks. World Neurosurg. 2021, 148, e58–e65. [Google Scholar] [CrossRef]
- Kung, W.M.; Lin, M.S. CT-Based Quantitative Analysis for Pathological Features Associated With Postoperative Recurrence and Potential Application Upon Artificial Intelligence: A Narrative Review With a Focus on Chronic Subdural Hematomas. Mol. Imaging 2020, 19, 1536012120914773. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Cè, M.; Irmici, G.; Ascenti, V.; Caloro, E.; Bianchi, L.; Pellegrino, G.; D’Amico, N.; Papa, S.; Carrafiello, G. Artificial Intelligence in Emergency Radiology: Where Are We Going? Diagnostics 2022, 12, 3223. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Vyvere, T.V.; Terzopoulos, V.; Sima, D.M.; Roura, E.; Maas, A.; Wilms, G.; Verheyden, J. Automatic Quantification of Computed Tomography Features in Acute Traumatic Brain Injury. J. Neurotrauma 2019, 36, 1794–1803. [Google Scholar] [CrossRef]
- van Eijck, M.M.; Schoonman, G.G.; van der Naalt, J.; de Vries, J.; Roks, G. Diffuse axonal injury after traumatic brain injury is a prognostic factor for functional outcome: A systematic review and meta-analysis. Brain Inj. 2018, 32, 395–402. [Google Scholar] [CrossRef]
- Yu, H.; Ande, S.R.; Batoo, D.; Linton, J.; Shankar, J. Prognostic Value of Initial Diagnostic Imaging Findings for Patient Outcomes in Adult Patients with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Tomography 2023, 9, 509–528. [Google Scholar] [CrossRef]
- Shafie, M.; Mahmoodkhani, M.; Salehi, I.; Dehghan, A. Clinical predictors of abnormal brain computed tomography findings in mild traumatic brain injury: A cross-sectional study. Medicine 2023, 102, e34167. [Google Scholar] [CrossRef]
- Bigler, E.D.; Abildskov, T.J.; Goodrich-Hunsaker, N.J.; Black, G.; Christensen, Z.P.; Huff, T.; Wood, D.M.; Hesselink, J.R.; Wilde, E.A.; Max, J.E. Structural Neuroimaging Findings in Mild Traumatic Brain Injury. Sports Med. Arthrosc. Rev. 2016, 24, e42–e52. [Google Scholar] [CrossRef] [PubMed]
- Pierre, K.; Haneberg, A.G.; Kwak, S.; Peters, K.R.; Hochhegger, B.; Sananmuang, T.; Tunlayadechanont, P.; Tighe, P.J.; Mancuso, A.; Forghani, R. Applications of Artificial Intelligence in the Radiology Roundtrip: Process Streamlining, Workflow Optimization, and Beyond. Semin. Roentgenol. 2023, 58, 158–169. [Google Scholar] [CrossRef]
- Rossiter, N.D. Trauma-the forgotten pandemic? Int. Orthop. 2022, 46, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Purkayastha, S.; Padmanaban, G.P.; Krupinski, E.; Trivedi, H.; Banerjee, I.; Gichoya, J.W. Current Clinical Applications of Artificial Intelligence in Radiology and Their Best Supporting Evidence. J. Am. Coll. Radiol. 2020, 17, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.S.; Davidson, E. Governance of artificial intelligence and personal health information. Digit. Policy Regul. Gov. 2019, 21, 280–290. [Google Scholar] [CrossRef]
- Jungmann, F.; Jorg, T.; Hahn, F.; Pinto Dos Santos, D.; Jungmann, S.M.; Düber, C.; Mildenberger, P.; Kloeckner, R. Attitudes Toward Artificial Intelligence Among Radiologists, IT Specialists, and Industry. Acad. Radiol. 2021, 28, 834–840. [Google Scholar] [CrossRef]
- Kals, M.; Kunzmann, K.; Parodi, L.; Radmanesh, F.; Wilson, L.; Izzy, S.; Anderson, C.D.; Puccio, A.M.; Okonkwo, D.O.; Temkin, N.; et al. A genome-wide association study of outcome from traumatic brain injury. EBioMedicine 2022, 77, 103933. [Google Scholar] [CrossRef]
- Wilde, E.A.; Wanner, I.B.; Kenney, K.; Gill, J.; Stone, J.R.; Disner, S.; Schnakers, C.; Meyer, R.; Prager, E.M.; Haas, M.; et al. A Framework to Advance Biomarker Development in the Diagnosis, Outcome Prediction, and Treatment of Traumatic Brain Injury. J. Neurotrauma 2022, 39, 436–457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierre, K.; Turetsky, J.; Raviprasad, A.; Sadat Razavi, S.M.; Mathelier, M.; Patel, A.; Lucke-Wold, B. Machine Learning in Neuroimaging of Traumatic Brain Injury: Current Landscape, Research Gaps, and Future Directions. Trauma Care 2024, 4, 31-43. https://doi.org/10.3390/traumacare4010004
Pierre K, Turetsky J, Raviprasad A, Sadat Razavi SM, Mathelier M, Patel A, Lucke-Wold B. Machine Learning in Neuroimaging of Traumatic Brain Injury: Current Landscape, Research Gaps, and Future Directions. Trauma Care. 2024; 4(1):31-43. https://doi.org/10.3390/traumacare4010004
Chicago/Turabian StylePierre, Kevin, Jordan Turetsky, Abheek Raviprasad, Seyedeh Mehrsa Sadat Razavi, Michael Mathelier, Anjali Patel, and Brandon Lucke-Wold. 2024. "Machine Learning in Neuroimaging of Traumatic Brain Injury: Current Landscape, Research Gaps, and Future Directions" Trauma Care 4, no. 1: 31-43. https://doi.org/10.3390/traumacare4010004
APA StylePierre, K., Turetsky, J., Raviprasad, A., Sadat Razavi, S. M., Mathelier, M., Patel, A., & Lucke-Wold, B. (2024). Machine Learning in Neuroimaging of Traumatic Brain Injury: Current Landscape, Research Gaps, and Future Directions. Trauma Care, 4(1), 31-43. https://doi.org/10.3390/traumacare4010004






