Abstract
Catastrophic haemorrhage accounts for up to 40% of global trauma related mortality and is the leading cause of preventable deaths on the battlefield. Controlling abdominal and junctional haemorrhage is challenging, especially in the pre-hospital setting or ‘under fire’, yet there is no haemostatic agent which satisfies the seven characteristics of an ‘ideal haemostat’. We conducted a systematic search of Embase, Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Web of Science to evaluate the feasibility and efficacy of three types of haemostatic devices. Participants included any trauma patient in a pre-hospital setting, perfused human cadavers, or healthy human volunteer simulations. The haemostatic devices reviewed were REBOA, iTClampTM, and four junctional tourniquets: AAJT, CRoC, JETT, and SJT. The SJT had the best user survey performance of the junctional tourniquets, and the four junctional tourniquets had an overall efficacy of 26.6–100% and an application time of 10–203 s. The iTClampTM had an efficacy of 60–100% and an application time of 10–60 s. REBOA had an efficacy of 71–100% and an application time ranging from 5 min to >80 min. In civilian and military trauma patients the use of junctional tourniquets, iTClamp, or REBOA, mortality varied from 0–100%. All of these studies were deemed low to very low in quality, hence the reliability of data presented in each of the studies is called into question. We conclude that despite limited data for these devices, their use in the pre-hospital environment or ‘under fire’ is feasible with the correct training, portable imaging, and patient selection algorithms. However, higher quality studies are required to confirm the true efficacy of these devices.
1. Introduction
The leading cause of preventable death on the battlefield is catastrophic haemorrhage originating from abdominal or junctional regions of the body [1,2]. Gaining haemorrhage control is not always possible using manual pressure, particularly under fire, during transport, mass casualty events, or for extended periods of time. A US military study demonstrated that 90% of potentially survivable deaths were from massive haemorrhage, with 67% originating in the torso and 19% from junctional regions [1]. Similarly, a UK military study found that 75% of trauma patients died before reaching a medical treatment facility, 60% of which suffered from non-compressible torso haemorrhage [3].
Uncontrolled haemorrhage is the second leading cause of death worldwide after head injury, accounting for 40% of global trauma related mortality [4]. Although tourniquets have been used for centuries for extremity injuries, use was not commonplace until more recently. Through the Hartford Consensus, tourniquet use in pre-hospital civilian mass casualty events is promoted, such as the improvised versions used in the aftermath of the Boston marathon bombing of 2013 [2,5]. However, pre-hospital intrathoracic, intra-abdominal, and intra-pelvic haemorrhage control options are limited [2]. Therefore, there is a need for haemostatic agents or devices for these injuries that can be easily applied and maintained in both military and civilian pre-hospital settings.
There are seven criteria which have been cited as the ideal characteristics of a haemostat for pre-hospital use: haemorrhage cessation within two minutes of application; ready to use; simple application with minimal training; lightweight and durable; easily stored in a wide range of temperatures (−10 to 55 °C) for up to two years; no risk of injury or viral disease transmission; and inexpensive [6]. So far there is no haemostat available which satisfies all seven criteria, but the last decade has seen an increase in this research area.
The UK and US military currently employ the use of some haemostatic dressings, such as Celox gauze and QuickClot Combat Gauze [7,8]. However, both dressings rely on manual pressure application, making them less than ideal for austere or remote environments, or where transport time to a hospital is prolonged. The US military have developed an alternative device which does not require manual pressure known as XStat. It is a syringe filled with mini sponges, which are injected into the wound cavity to expand and fill it. However, X-ray imaging is required for removal, and they are contraindicated for use in the torso [9]. Although it was first approved solely for military use, it was later approved for civilian use in 2015 [9].
There are currently four junctional tourniquets (JTQ) which have been approved by the Food and Drug Administration (FDA): abdominal aortic junctional tourniquet (AAJT; Compression Works, Birmingham, AL, USA); junctional emergency treatment tool (JETT; Northern American Rescue, Greer, SC, USA); SAM® junctional tourniquet (SJT; SAM Medical Products, Portland, OR, USA); Combat Ready Clamp (CRoC; Combat Medical Systems, Fayetteville, NC, USA) [10]. These JTQs act similarly to a standard tourniquet, but their belt-like (AAJT, SJT, JETT) or C-clamp (CRoC) design allows control of more proximal wounds located in the groin or axilla for example.
The iTClampTM (Innovative Trauma Care Inc., Atlanta GA, USA) is a small clamp with needles on either side and controls haemorrhage by approximating the skin at either side of the wound, forming a haematoma under the skin to create a stable clot [11]. It was FDA approved for compressible injuries located in the extremities, groin, axilla, scalp, and neck [11].
Resuscitative endovascular occlusion of the aorta (REBOA) was first used in 1954 in combat casualty care and has recently demonstrated improved outcomes in the emergency department in comparison to resuscitative thoracotomy [12]. Whilst the use of REBOA in pre-hospital settings may be controversial (it may delay transport to a medical facility), alternative methods such as the iTClampTM and JTQs are contra-indicated for abdominal haemorrhage.
This systematic review aimed to evaluate the data related to the use of these haemostatic devices in the pre-hospital environment (civilian and military), as well as simulated environments that are translatable to the pre-hospital setting, such as healthy volunteers and in perfused human cadavers.
2. Materials and Methods
2.1. Preliminary Search
A scoping search was performed in May 2021 on PubMed using keywords such as ‘novel haemostatic agent’, ‘non-compressible haemorrhage’, and ‘pre-hospital’ to identify recent research. We aimed to address the research question: “what are the feasibility and efficacy of haemostatic devices in the pre-hospital setting for catastrophic haemorrhage originating from abdominal and junctional regions of the body?”. In our preliminary search it became clear that there was a paucity of evidence within the clinical domain (i.e., for pre-hospital trauma patients). Therefore, we also chose to include studies that used healthy volunteers and human cadavers to simulate a pre-hospital environment. This was so that we could assess the first part of our research question regarding feasibility. Our PICO statement is shown in Table 1.

Table 1.
Population, intervention, comparison, and outcome table.
2.2. Literature Search
A systematic literature search was conducted in July 2021, with data reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [13]. Four databases were used: Embase (OVID), Medline (OVID), CINAHL, and Web of Science, using both keywords and MeSH terms. The general terms used were ‘haemostatic device’, ‘REBOA’, ‘iTClamp’, ‘junctional tourniquet’, ‘pre-hospital’, ‘emergency health service’, and ‘out-of-hospital’ combined using Boolean operators. There were no publication date restrictions. Full details of the search strategies used for each database are found in Figures S1–S3 and Table S1. All searches were conducted by two authors (R.H. and Z.A.) and any discrepancies were resolved by discussion. This review was prospectively registered with PROSPERO (ID: CRD42021273077) before data extraction commenced.
2.3. Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met the following criteria: (1) observational studies, clinical, or pre-clinical trials, which investigated the use of haemostatic devices (REBOA, iTClampTM, or junctional tourniquets: AAJT, SJT, JETT, or CRoC) in a pre-hospital setting; (2) human participants of all ages as trauma patients, perfused human cadavers, or healthy human volunteers; (3) publications in English; (4) publications from any country or date of publication.
Studies were excluded if they exhibited any of the following characteristics: (1) any studies that investigated haemostatic sponges, dressings, bandages, and foams; (2) studies which only investigated device use in extremity injury; (3) any use of haemostatic devices within the hospital environment only (emergency department or operating theatre); (4) reviews (systematic or narrative), conference abstracts, guidelines, animal studies, or studies utilizing mannikin simulation models.
2.4. Data Collection and Extraction
After duplicate removal, titles and abstracts were screened against the eligibility criteria. The full texts of remaining papers were assessed for eligibility. Reference lists of included studies and review articles of interest were manually searched for any additional articles missed from the initial search. The following data was extracted from the eligible studies: (1) study characteristics and intervention assessed; (2) participant demographics; (3) outcomes reported such as mortality, device application time, overall success of the devices, and any associated adverse events.
2.5. Quality of Evidence and Risk of Bias
Each study included for review was assessed for the quality of the evidence it provided by two authors (R.H. and Z.A.) using the Grading of Recommendations, Assessment, Development, and Evaluation System (GRADE) [14].
A risk of bias assessment was conducted on each included study using the Risk of Bias in Non-Randomized Studies–of Interventions (ROBINS-I) tool [15]. Each of the seven domains: (1) confounding bias; (2) participant selection bias; (3) intervention classification bias; (4) intervention deviation bias; (5) missing data bias; (6) outcome measurement bias; (7) selective reporting bias were rated either as low, moderate, serious, or critical risk. Following the guidance of the tool, this produced an overall risk of bias for each study included for review [15].
Due to the heterogeneity of the data collection process, a meta-analysis was not possible and hence what follows is a narrative synthesis of the data.
3. Results
3.1. Literature Search
From the electronic databases searched, 458 studies were identified. Once duplicates were removed, 289 studies were screened by title and abstract. The full texts of 48 studies were sought for retrieval and assessed against the inclusion and exclusion criteria. This resulted in 18 studies included for review. The reference lists of eligible studies and review articles of interest were screened for any relevant publications which were not found in the initial search. Both manual searches identified eight studies eligible for inclusion, indicated as found through other sources. This resulted in 26 total studies included for review, with the process summarized in the PRISMA flow diagram (Figure 1).
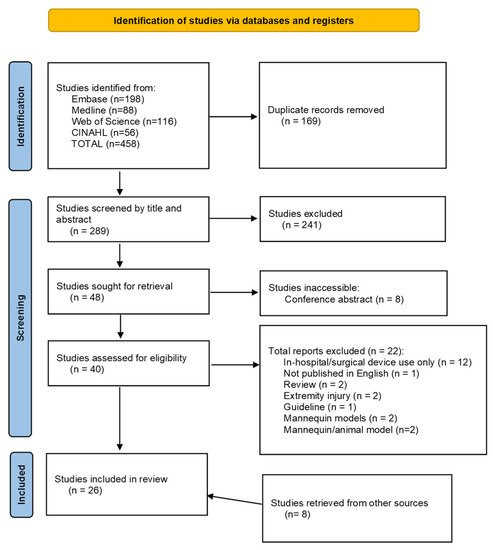
Figure 1.
PRISMA flow diagram.
3.2. Pre-Hospital Feasability Evidence
Of the 14 studies included for JTQ review, eight were conducted in 194 healthy human volunteers [16,17,18,19,20,21,22,23] (Table 2), 3 were conducted on 16 perfused human cadavers [24,25,26] (Table 3), and 2 case series [27,28] and 1 case report [29] on 21 trauma patients (Table 4). The total number of cadavers and volunteers was 210. Of these, 16 were perfused human cadavers and 194 were volunteers. The age range for these studies was 18–75. For the studies, which reported the proportion of males and females involved in their study, there were many more males involved (n = 155) compared to females (n = 25). Patient demographics and outcome measures (mortality, percentage efficacy, and application time) for civilian/military volunteer and cadaver studies are summarized in Table 2 and Table 3, respectively. Device efficacy was reported in all 11 studies involving cadavers or volunteers and ranged from 11–100% across all four JTQs. The efficacy of the CRoC ranged from 84–100% [18,19,21,22,23,24,25]; the AAJT ranged from 11–100% [16,17,18,19,20,21]; JETT had an efficacy range from 75–100% [18,19,21,23,25], and the SJT ranged from 82–100% [18,19,21,22,23,26]. Only one study assessed the efficacy of the SJT, JETT, and CRoC during the transport in volunteers, ranging from 24–48%. The SJT was reported to have a significantly higher transport efficacy (48%) than the JETT (24%; p = 0.03) [23]. Application time was recorded in 6 of the JTQ feasibility studies [16,19,21,22,23,25] and ranged from 10–203 s overall. Each JTQ had an application time of: CRoC 68–128 s [19,21,22,23,25], JETT 10–203 s [19,21,23,25], SJT 34–80 s [19,21,22,23], and AAJT 98–171 s [16,19,21]. Of the studies which assessed the safety of the JTQs (n = 7), none reported any incidences of adverse events [18,21,22]. User surveys reported the SJT to be the most preferred of the JTQs. It was perceived to be the most stable, reliable, easy to use, and fastest to apply when compared to the other three JTQs. JETT was consistently passed over for one of the other JTQs in each study recording user surveys. AAJT and CRoC had mixed reviews depending on whether the study was conducted in the field on trauma patients or in volunteer simulations, mostly due to higher pain ratings for the AAJT. Whilst CRoC was perceived to be more comfortable and scored lower on pain scales, it took the longest to assemble of the four JTQs. The AAJT was reported to be the most painful, although this was only recorded in one region of application.

Table 2.
Summary of junctional tourniquet study characteristics, patient demographics, and outcome measure results in civilian and military healthy volunteers.

Table 3.
Summary of junctional tourniquet study characteristics, patient demographics, and outcome measure results in perfused human cadaver studies.

Table 4.
Summary of junctional tourniquet study characteristics, patient demographics, and outcome measure results in civilian and military trauma patients.
There was one study conducted on human cadavers which investigated the use of the iTClampTM (Table 5). There were 3 cadavers, whose ages ranged from 49–81 [30] with injuries located on the thigh, inguinal region, neck, arm, and scalp. The device efficacy for these three participants was 100%, as measured by control or cessation of bleeding [30].

Table 5.
Summary of iTClampTM study characteristics, patient demographics, and outcome measure results.
3.3. Pre-Hospital Evidence
The three studies which used JTQs in pre-hospital trauma scenarios [27,28,29] had 13 military and 18 civilian patients (Table 4). Military patients presented with gun shot wounds (GSWs; n = 5) and blast injury (n = 8) whilst the civilian patients presented with blunt injury (n = 17) and penetrating injury (n = 1). The age range was 20–80. For the studies that reported the proportion of males and females involved in their study, there were many more males involved (n = 29) compared to females (n = 2). Patient demographics and outcome measures (mortality, percentage efficacy, and application time) military/civilian trauma patients are summarized in Table 4.
Mortality across the three trauma patient studies ranged from 0–100%, giving an overall mean of 87% mortality associated with AAJT, JETT, and SJT use. The CRoC tourniquet was not studied in pre-hospital trauma patients. Device efficacy was reported in the case report [29] and the case series [27], ranging from 70–100% for the SJT and JETT. Although the case report by Klotz et al. [29] did not specifically assess the efficacy of the SJT during transport, they reported that it remained in place throughout the MEDEVAC. Application was only recorded in the case report including 1 trauma patient where the application time was <180 s to apply the SJT [29].
There were four studies included for the iTClampTM, one of which was performed in perfused human cadavers [30] and three of which reported civilian trauma patients [11,31,32] (Table 5). There were 92 participants with an age range of 16–95. The lacerations treated varied in the location on the body: scalp, inguinal, head, neck, femur, chest, and craniomaxillofacial. Of the participants who had their sex recorded, there were more males (n = 55) than females (n = 22), with the sex of 15 participants unreported [32]. The mortality rate across all three of these studies was 0%, with a mean application time ranging from 10–60 s. Device efficacy ranged from 60–100% as recorded by cessation or control of bleeding. The studies also reported no adverse events associated with iTClampTM use. However, users reported failures in the device due to skin frailty. User surveys reported limited discomfort and demonstrated a positive satisfaction rate.
Of the eight studies that investigated the use of REBOA in a pre-hospital setting, four were case series and reports of military patients [12,33,34,35], and four were case series and reports of civilian patients [36,37,38,39] (Table 6). Studies reported whether REBOA was deployed in zone 1 (n = 3), zone 3 (n = 2), or a variation of zone 1 or 3 dependent on injury location (n = 3). There were 61 participants, one of which was the perfused human cadaver. The age range across all studies was 18–79 years old, with at least 25 male and 13 female participants across the studies that recorded this. The mechanism of injury amongst civilian patients was mostly blunt trauma, whereas the studies on military patients were a combination of blast and gunshot wounds.

Table 6.
Summary of REBOA study characteristics, patient demographics, and outcome measure results.
Mortality ranged from 0–100%. Of the studies which reported application time (n = 5), four studies had an overall range of 5 to 27 min, with one study reporting a median time of 80 min to inflate the balloon [32]. Device efficacy ranged from 71–100%. Extracted data for REBOA studies are summarized in Table 4. Regardless of the zone the balloon was inflated at, REBOA was associated with the formation of thromboembolic events, thus the total balloon inflation time was limited as much as was safe to prevent distal organ/limb ischemia [30,34]. No user surveys were recorded for these studies.
3.4. Quality of Evidence and Risk of Bias
All included studies were either of a low (n = 12) or very low (n = 14) quality of evidence, according to the GRADE system, due to their observational design (Table 7). The overall risk of bias for each study, measured by the ROBINS-I tool, varied between low (n = 10), moderate (n = 9), and serious (n = 4), with the three case reports not assessed due to a lack of comparators within the study. Any domain classed as ‘NI’ (no information), excluded an overall rating of ‘low’.

Table 7.
Quality of evidence (GRADE) and risk of bias assessment (ROBINS-I); Domain D1: Confounding bias; D2: Participant selection bias; D3: Intervention classification bias; D4: Intervention deviation bias; D5: Missing data bias; D6: Outcome measurement bias; D7: Selective reporting bias.
4. Discussion
The purpose of this review was to qualitatively assess the feasibility and efficacy of different haemostatic devices in a pre-hospital setting. Due to the lack of patient data, the feasibility of device use in a pre-hospital environment was also assessed by studies using a simulated pre-hospital environment. Only one study by Gaspary et al. [23] reported on the effectiveness during transport of JTQ application in a pre-hospital environment. Whilst other studies mentioned devices remaining in place during transport [29,30,31,37,38,39], it was not specifically assessed. An in-hospital case report by Croushorn et al. [40] reported that the AAJT remained in place during transport from the emergency department to the operating theatre with no blood loss seen throughout transfer.
4.1. Junctional Tourniquets
Mortality associated with all JTQ use was relatively high, although this can only be applied to the AAJT, JETT, and SJT. The AAJT case series had a mortality rate of 100%, whereas the SJT and JETT case series had mortality rates of <25%. However, Balian et al. [28] aimed to assess the effects of the AAJT on physiological parameters associated with traumatic cardiac arrest rather than reducing blood loss from a junctional or abdominal wound.
Device efficacy was reported by the following methods: elimination of blood flow as measured by doppler ultrasound in healthy volunteers; the visual cessation of blood flow from a wound or changes in physiological parameters for trauma patients; or pressure sensors or transducers for cadaver studies. The AAJT recorded the largest range in device efficacy, and this was largely due to the amount of pain experienced by the healthy participants, which led to early termination of the studies. Although interestingly, in a case report on AAJT use in the emergency department, the patient reported less pain associated with the AAJT compared to the Combat-Application-Tourniquet [41], indicating that other injuries or painkillers may reduce the confounding bias of pain associated with the AAJT. Whilst the CRoC appears to be the most efficacious and time efficient overall, this does not account for the device assembly time, as the volunteer studies had the devices pre-assembled before timing began.
User surveys recorded an overall perception of decreased pain scores associated with increased efficacy. Overall, the SJT was preferred by users when considering application time, ease of use, stability, and maintenance of vessel occlusion during transport. The AAJT, JETT, and SJT all have the ability to act as a pelvic binder if necessary and have a bilateral effect, whereas the CRoC acts unilaterally. However, the AAJT was the only JTQ to break during volunteer testing [21]. Overall, the JTQs are relatively transportable, easy, quick, and safe to use by any member of the pre-hospital team.
4.2. iTClampTM
The iTClampTM pre-hospital studies showed no mortality, no morbidity, and high rates of efficacy. However, the lack of mortality in this group of patients may be due reduced severity of the patients compared to other treatments. Nonetheless, the device has been successfully used in conjunction with a haemostatic gauze, Hemcom Chitogauze [11], and also on injuries across many regions of the body. Barnung et al. [31] reported an off-licence use of the iTClampTM to successfully secure a chest drain in challenging conditions, indicating potential versatility of the device. They are easily transported and can be applied to multiple different areas of the body. However, they are limited by the width of the presenting wound as approximation of the skin on either side of the wound is required for haematoma formation to stop blood flow.
4.3. REBOA
A recent systematic review by Nunez et al. [42] suggested that REBOA had a positive effect on mortality in comparison to resuscitative thoracotomy performed in hospital and this low mortality rate was also confirmed in a pre-hospital setting by the results from this review, alongside a high efficacy rate. Device efficacy was found to be higher when the balloon catheter was inflated in zone 1 compared to zone 3. However, it is unclear whether this is significant, as this was not an aspect of the studies which was investigated.
There is a much shorter time limit for the safe inflation of the balloon (<40 min) compared to the application of the iTClampTM or JTQs, which are safe to use for up to 3 or 4 h, respectively. Whilst the application time was longer than for the iTClampTM or JTQs, it was deemed necessary by the attending physician for patient survival to a medical facility. However, patient selection for these studies was limited to patients with sub-diaphragmatic injuries, with access to the femoral artery. The 2018 study by de Schouthee et al. [36] used the mechanism, injury, signs, and treatment (MIST) acronym to facilitate correct patient selection for the procedure as in-hospital algorithms were not applicable for pre-hospital diagnostic purposes. Eligible patients had experienced high energy sub-diaphragmatic trauma associated with shock. This study used a dynamic approach, by partially occluding the vessel (partial REBOA) in a stepwise manner according to the response seen in blood pressure. This approach could reduce the risk of distal limb ischemia by allowing some perfusion and increase the length of device use [35].
Cannulation can be challenging, especially in patients with a weak or immeasurable pulse. REBOA performed in-hospital has historically been performed under fluoroscopy to ensure the correct positioning of the catheter and prevent over-inflation of the balloon. Some studies have investigated the use of REBOA with ultrasound guidance and blood pressure monitoring, which could increase the feasibility of translation into the pre-hospital setting [35,37,43,44].
So far there is no one ‘ideal’ haemostatic device to be universally applied to all injury types. There is a need to develop a rapid and simple algorithm to identify those patients who would benefit most from each device. The pre-hospital data for each of these devices is poor and limited, particularly when assessing the maintenance of efficacy during transport. However, the data available so far indicates the feasibility of using these devices in a pre-hospital setting with the correct training and patient selection. Methods which can supplement the translation into pre-hospital care, such as portable ultrasound imaging for REBOA and partial REBOA, should also be investigated further in higher quality human studies. Ideally, randomized control trials for these devices would be the best way to assess application and efficacy of all these devices for different wound types, during transportation, long term follow-up, and any other physiological changes associated with the devices. However, we are aware that this is extremely challenging and probably unlikely due to constraints in study design, industry involvement, patient recruitment, and ethical considerations.
4.4. Limitations
The biggest limitation of this systematic review is the poor quality of evidence in all of the included studies, ranging from low to very low in quality. In addition, there is significant heterogeneity in outcome measures reported. Due to lack of patient data, we were compelled to include data from perfused cadaver and healthy volunteer studies that were deemed to be translatable to the pre-hospital environment. These were used to address our research question regarding the feasibility and efficacy of these devices within the pre-hospital setting.
Between the studies, there was limited consistency with which outcome measures were reported and how they were recorded. This is in part due to the inclusion of perfused cadaver and human simulation studies, as neither of these models would exhibit the physiological properties of a trauma patient following massive haemorrhage, and mortality cannot be reported with these models. The cadaver studies did not have any coagulation, so whilst this does prove the mechanical haemorrhage cessation to be due to the device application, it does not account for the physiological effects of massive haemorrhage and shock. Outcome measures for the AAJT were particularly skewed due to the presence of pain in volunteers, leading to early termination of the studies. Although applicable to junctional or abdominal injuries, the appropriate pattern of injury to which the device can be applied varies, resulting in limited comparisons between the devices themselves.
5. Conclusions
It is likely from available evidence that each of the devices examined in our review could be used in the pre-hospital setting (civilian or military) with the appropriate training, injury pattern, and patient selection, and may be useful in scenarios where manual pressure cannot be maintained. However, the poor quality of evidence precludes any definitive conclusions regarding clinical efficacy. Of the JTQs, SJT was preferred by users in comparison to the AAJT, JETT, and CRoC. The iTClampTM is the easiest to use, as well as the most portable, but there is a size limit to the wounds to which it can be applied. REBOA may also be useful and can be quickly deployed, minimising transport delays to the hospital, but has a much shorter time frame for which it can safely be used in comparison to the JTQs or iTClampTM.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/traumacare2010003/s1, Figure S1. Embase search strategy, Figure S2. Medline search strategy, Figure S3. CINAHL search strategy Table S1. Web of Science search strategy.
Author Contributions
Conceptualization: R.H., D.N.N. and Z.A.; methodology: R.H. and Z.A.; formal analysis: R.H. and Z.A.; investigation: R.H.; data curation: R.H. and Z.A.; writing—original draft preparation: R.H.; writing—review and editing: R.H., D.N.N. and Z.A.; supervision: D.N.N. and Z.A.; project administration: R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as per advice from the NHS Health Re-search Authority (UK) decision tool, since it is a systematic review of published literature.
Informed Consent Statement
Any patient consent was waived since no patients or members of the public were involved in the design, conduct of this study, or reporting of this research.
Data Availability Statement
All data generated as part of this study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eastridge, B.J.; Mabry, R.L.; Seguin, P.; Cantrell, J.; Tops, T.; Uribe, P.; Mallett, O.; Zubko, T.; Oetjen-Gerdes, L.; Rasmussen, T.E.; et al. Death on the battlefield (2001–2011): Implications for the future of combat casualty care. J. Trauma Acute Care Surg. 2012, 73, S431–S437. [Google Scholar] [CrossRef] [PubMed]
- van Oostendorp, S.E.; Tan, E.C.T.H.; Geeraedts, L.M.G. Pre-hospital control of life-threatening truncal and junctional haemorrhage is the ultimate challenge in optimizing trauma care; a review of treatment options and their applicability in the civilian trauma setting. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 110. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.J.; Stannard, A.; Rasmussen, T.E.; Jansen, J.O.; Tai, N.R.M.; Midwinter, M.J. Injury pattern and mortality of noncompressible torso hemorrhage in UK combat casualties. J. Trauma Acute Care Surg. 2013, 75 (Suppl. 2), S263–S268. [Google Scholar] [CrossRef]
- Chaudery, M.; Clark, J.; Wilson, M.H.; Bew, D.; Yang, G.Z.; Darzi, A. Traumatic intra-abdominal hemorrhage control: Has current technology tipped the balance toward a role for pre-hospital intervention? J. Trauma Acute Care Surg. 2015, 78, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kue, R.C.; Temin, E.S.; Weiner, S.G.; Gates, J.; Coleman, M.H.; Fisher, J.; Dyer, S. Tourniquet use in a civilian emergency medical services setting: A descriptive analysis of the Boston EMS experience. Prehospital Emerg. Care 2015, 19, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Brooks, A. Use of local pro-coagulant haemostatic agents for intra-cavity control of haemorrhage after trauma. Eur. J. Trauma Emerg. Surg. 2014, 41, 493–500. [Google Scholar] [CrossRef]
- Granville-Chapman, J.; Jacobs, N.; Midwinter, M.J. Pre-hospital haemostatic dressings: A systematic review. Injury 2011, 42, 447–459. [Google Scholar] [CrossRef]
- UK MoD selects Celox Rapid Haemostatic Gauze for all branches of the UK Military Celox Hemostats. Available online: https://www.celoxmedical.com/uk-mod-selects-celox-rapid/ (accessed on 18 August 2021).
- XStat–RevMedx. Available online: https://www.revmedx.com/xstat/ (accessed on 18 August 2021).
- Kotwal, R.S.; Butler, F.K. Junctional Hemorrhage Control for Tactical Combat Casualty Care. Wilderness Environ. Med. 2017, 28, S33–S38. [Google Scholar] [CrossRef]
- Tan, E.C.T.H.; Peters, J.H.; Edwards, M.J.R.; McKee, J.L. The iTClamp in the management of pre-hospital haemorrhage. Injury 2016, 47, 1012–1015. [Google Scholar] [CrossRef]
- Northern, D.M.; Manley, J.D.; Lyon, R.; Farber, D.; Mitchell, B.J.; Filak, K.J.; Lundy, J.; DuBose, J.J.; Rasmussen, T.E.; Holcomb, J.B. Recent advances in austere combat surgery: Use of aortic balloon occlusion as well as blood challenges by special operations medical forces in recent combat operations. J. Trauma Acute Care Surg. 2018, 85 (Suppl. 2), S98–S103. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. Rating Quality of Evidence and Strength of Recommendations: GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Shiver, S.A.; Greenfield, E.M.; Reynolds, B.Z.; Lerner, E.B.; Wedmore, I.S.; Richard, B. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J. Trauma Acute Care Surg. 2012, 73 (Suppl. 1), S103–S105. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Coleman, M.; Parker, P.J. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil. Med. 2013, 178, 1196–1201. [Google Scholar] [CrossRef]
- Kragh, J.F.J.; Parsons, D.L.; Kotwal, R.S.; Kheirabadi, B.S.; Aden, J.K., 3rd; Gerhardt, R.T.; Baer, D.G.; Dubick, M.A. Testing of junctional tourniquets by military medics to control simulated groin hemorrhage. J. Spec. Oper. Med. 2014, 14, 58–63. [Google Scholar] [PubMed]
- Kragh, J.F.; Kotwal, R.S.; Cap, A.P.; Aden, J.K.; Walters, T.J.; Kheirabadi, B.S.; Gerhardt, R.T.; De Lorenzo, R.A.; Pidcoke, H.F.; Cancio, L.C. Performance of Junctional Tourniquets in Normal Human Volunteers. Preshop. Emerg. Care 2015, 19, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Johnson, D.; Gordon, R. Use of a Novel Abdominal Aortic and Junctional Tourniquet to Reduce or Eliminate Flow in the Brachial and Popliteal Arteries in Human Subjects. Preshop. Emerg. Care 2015, 19, 405–408. [Google Scholar] [CrossRef]
- Chen, J.; Benov, A.; Nadler, R.; Landau, G.; Sorkin, A.; Aden, J.K., 3rd; Kragh, J.F., Jr.; Glassberg, F. Testing of Junctional Tourniquets by Medics of the Israeli Defense Force in Control of Simulated Groin Hemorrhage. J. Spec. Oper. Med. 2016, 16, 36–42. [Google Scholar]
- Meusnier, J.-G.; Dewar, C.; Mavrovi, E.; Caremil, F.; Wey, P.-F.; Martinez, J.-Y. Evaluation of Two Junctional Tourniquets Used on the Battlefield: Combat Ready Clamp® versus SAM® Junctional Tourniquet. J. Spec. Oper. Med. 2016, 16, 41–46. [Google Scholar]
- Gaspary, M.J.; Zarow, G.J.; Barry, M.J.; Walchak, A.C.; Conley, S.P.; Roszko Micah, J. Comparison of Three Junctional Tourniquets Using a Randomized Trial Design. Prehosp. Emerg. Care 2019, 23, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kragh, J.F., Jr.; Murphy, C.; Steinbaugh, J.; Dubick, M.A.; Baer, D.G.; Johnson, J.; Henkel, C.K.; Blackbourne, L.H. Pre-hospital emergency inguinal clamp controls hemorrhage in cadaver model. Mil. Med. 2013, 178, 799–805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gates, K.S.; Baer, L.; Holcomb, J.B. Pre-hospital emergency care: Evaluation of the junctional emergency tourniquet tool with a perfused cadaver model. J. Spec. Oper. Med. 2014, 14, 40–44. [Google Scholar] [PubMed]
- Johnson, J.E.; Sims, R.K.; Hamilton, D.J.; Kragh, J.F., Jr. Safety and Effectiveness Evidence of SAM® Junctional Tourniquet to Control Inguinal Hemorrhage in a Perfused Cadaver Model. J. Spec. Oper. Med. 2014, 14, 21–25. [Google Scholar]
- Schauer, S.G.; April, M.D.; Fisher, A.D.; Cunningham, C.W.; Gurney, J. Junctional Tourniquet Use During Combat Operations in Afghanistan: The Pre-hospital Trauma Registry Experience. J. Spec. Oper. Med. 2018, 18, 71–74. [Google Scholar]
- Balian, F.; Garner, A.A.; Weatherall, A.; Lee, A. First experience with the abdominal aortic and junctional tourniquet in pre-hospital traumatic cardiac arrest. Resuscitation 2020, 156, 210–214. [Google Scholar] [CrossRef]
- Klotz, J.K.; Leo, M.; Andersen, B.L.; Nkodo, A.A.; Garcia, G.; Wichern, A.M.; Chambers, M.J.; Gonzalez, O.N.; Pahle, M.U.; Wagner, J.A.; et al. First case report of SAM® Junctional tourniquet use in Afghanistan to control inguinal hemorrhage on the battlefield. J. Spec. Oper. Med. 2014, 14, 1–5. [Google Scholar]
- Mottet, K.; Filips, D.; Logsetty, S.; Atkinson, I. Evaluation of the iTClamp 50 in a human cadaver model of severe compressible bleeding. J. Trauma Acute Care Surg. 2014, 76, 791–797. [Google Scholar] [CrossRef]
- Barnung, S.; Steinmetz, J. A pre-hospital use of ITClamp for haemostatic control and fixation of a chest tube. Acta Anaesthesiol. Scand. 2014, 58, 251–253. [Google Scholar] [CrossRef]
- Mckee, J.L.; Mckee, I.A.; Ball, C.G.; Tan, E.; Moloff, A.; McBeth, P.; LaPorta, A.; Bennett, B.; Filips, D.; Teicher, C.; et al. The iTClamp in the treatment of pre-hospital craniomaxillofacial injury: A case series study. J. Inj. Violence Res. 2019, 11, 29–34. [Google Scholar]
- Knipp, B.S.; Needham, K.E.; Nguyen, P.T.; Keville, M.P.; Brzuchalski, J.T.; Srivilasa, C.; Lewis, C.J.; Betzold, R.D.; DuBose, J.J. Leaning forward: Early arterial access promotes resuscitative endovascular balloon occlusion of the aorta utilization in battlefield casualties. J. Trauma Acute Care Surg. 2020, 89 (Suppl. 2), S88–S92. [Google Scholar] [CrossRef] [PubMed]
- Manley, J.D.; Mitchell, B.J.; DuBose, J.J.; Rasmussen, T.E. A Modern Case Series of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in an Out-of-Hospital, Combat Casualty Care Setting. J. Spec. Oper. Med. 2017, 17, 1–8. [Google Scholar]
- de Schoutheete, J.C.; Fourneau, I.; Waroquier, F.; De Cupere, L.; O’Connor, M.; Van Cleynenbreugel, K.; Ceccaldi, J.C.; Nijs, S. Three cases of resuscitative endovascular balloon occlusion of the aorta (REBOA) in austere pre-hospital environmenttechnical and methodological aspects. World J. Emerg. Surg. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Lendrum, R.; Perkins, Z.; Chana, M.; Marsden, M.; Davenport, R.; Grier, G.; Sadek, S.; Davies, G. Pre-hospital Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for exsanguinating pelvic haemorrhage. Resuscitation 2019, 135, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Sadek, S.; Lockey, D.J.; Lendrum, R.A.; Perkins, Z.; Price, J.; Davies, G.E. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the pre-hospital setting: An additional resuscitation option for uncontrolled catastrophic haemorrhage. Resuscitation 2016, 107, 135–138. [Google Scholar] [CrossRef]
- Lamhaut, L.; Qasim, Z.; Hutin, A.; Dagron, C.; Orsini, J.-P.; Haegel, A.; Perkins, Z.; Pirracchio, C.P. First description of successful use of zone 1 resuscitative endovascular balloon occlusion of the aorta in the pre-hospital setting. Resuscitation 2018, 133, e1–e2. [Google Scholar] [CrossRef]
- Gamberini, L.; Coniglio, C.; Lupi, C.; Tartaglione, M.; Mazzoli, C.A.; Baldazzi, M.; Cecchi, A.; Ferri, E.; Chiarini, V.; Semeraro, F.; et al. Resuscitative endovascular occlusion of the aorta (REBOA) for refractory out of hospital cardiac arrest. An Utstein-based case series. Resuscitation 2021, 165, 161–169. [Google Scholar] [CrossRef]
- Croushorn, J.; Thomas, G.; McCord, S.R. Abdominal aortic tourniquet controls junctional hemorrhage from a gunshot wound of the axilla. J. Spec. Oper. Med. 2013, 13, 1–4. [Google Scholar]
- Croushorn, J. Abdominal aortic and junctional tourniquet controls hemorrhage from a gunshot wound of the left groin. J. Spec. Oper. Med. 2014, 14, 6–8. [Google Scholar]
- Nunez, M.R.; Naranjo, M.P.; Foianini, E.; Ferrada, P.; Rincon, E.; García-Perdomo, H.A.; Burbano, P.; Herrera, J.P.; García, A.F.; Ordonez, C.A. A meta-analysis of resuscitative endovascular balloon occlusion of the aorta (REBOA) or open aortic cross-clamping by resuscitative thoracotomy in non-compressible torso hemorrhage patients. World J. Emerg. Surg. 2017, 12, 1–9. [Google Scholar]
- Reva, V.A.; Perevedentcev, A.V.; Pochtarnik, A.A.; Khupov, M.T.; Kalinina, A.A.; Samokhvalov, I.M.; Khan, M.A. Ultrasound-guided versus blind vascular access followed by REBOA on board of a medical helicopter in a hemorrhagic ovine model. Injury 2021, 52, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Lefor, A.K.; Nakamura, M.; Fujizuka, K.; Shiroto, K.; Nakano, M. Ultrasound-Guided Resuscitative Endovascular Balloon Occlusion of the Aorta in the Resuscitation Area. J. Emerg. Med. 2017, 52, 715–722. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).