Abstract
Background/Objectives: Obesity increases reactive oxygen species (ROS), thereby triggering oxidative stress. Coriander seeds contain polyphenolic compounds that act as natural antioxidants to reduce oxidative stress. Coriander seed ethanolic extract has been proven to decrease malondialdehyde and increase catalase activity in the liver of high-fat-diet-fed rats. Thus, coriander seeds are thought to protect against obesity-induced oxidative liver damage; however, their molecular mechanism has not been revealed. Nuclear factor erythroid 2-related factor 2 (Nrf2) and Forkhead Box O3 (FOXO3) are transcription factors involved in cellular antioxidant regulation (e.g., superoxide dismutase/SOD, glutathione peroxidase/GPx expression, and reduced glutathione/GSH) that are negatively regulated by Kelch-like ECH-associated Protein 1 (Keap1) and 14-3-3 protein to maintain cellular homeostasis. This study aimed to analyze the regulation of antioxidant expression through in silico and in vivo experiments. Methods: The in silico study assessed the potential of coriander seed ethanolic extract to inhibit Keap1 and 14-3-3 using molecular docking. Then, the drug-likeness, pharmacokinetics, and toxicity of the top three compounds were analyzed. Meanwhile, the in vivo study investigated how the coriander seed ethanolic extract impacted the level of Nrf2, FOXO3, and their downstream effectors (T-SOD, MnSOD, GPx, and GSH). The in vivo study involved five groups of rats with obesity induced by a high-fat diet that were fed with 100 mg/kgBW coriander seed ethanolic extract for 12 weeks. Results: The in silico tests revealed that shionoside b had the highest potential to inhibit Keap1 (ΔG = −8.90 kcal/mol; Ki = 298.01 nM) and 14-3-3 protein (ΔG = −6.85 kcal/mol; Ki = 9.46 µM). The in vivo tests showed that the Nrf2, FOXO3, MnSOD, and GPx mRNA expression was significantly different between the groups (p < 0.05). Meanwhile, T-SOD, MnSOD, GPx, and GSH activity were not significantly different between the groups (p > 0.05). Nrf2 was significantly correlated with FOXO3 as well as the T-SOD, MnSOD, and GPx activity, and FOXO3 was significantly correlated with the T-SOD, MnSOD, GPx, and GSH activity. Conclusions: In obese rats, coriander seeds tend to increase Nrf2 and FOXO3 expression, which is positively correlated with their downstream enzymatic and nonenzymatic antioxidant activity. This is possibly due to the interaction between the coriander seed phytoconstituents and protein inhibitors (Keap1 and 14-3-3), which contribute to the stability and nuclear mobilization of Nrf2 and FOXO3.
1. Introduction
Obesity is a condition that occurs due to the accumulation of adipocytes, which is a risk factor for degenerative diseases like type 2 diabetes mellitus and other metabolic diseases [1]. In 2022, the global prevalence of obesity surpassed one billion people, comprising 890 million adults and 160 million children and adolescents aged 5–19, or 1 in 8 individuals. Compared with 1990, the prevalence of obesity in adults more than doubled, while adolescent obesity increased fourfold [2]. Excessive intake of dietary fat is one of the risk factors for obesity [3]. The accumulation of free fatty acids in obese individuals leads to the buildup of reactive oxygen species (ROS), which may result in oxidative stress [1], caused by an imbalance between antioxidant capacity and ROS production [4]. Oxidative stress, together with hyperglycemia, hyperlipidemia, insulin resistance, and inflammation, comprise the pathological mechanisms that lead to non-alcoholic fatty liver disease (NAFLD) [5]. Obesity is linked to a higher risk of NAFLD, which is reflected by the elevated prevalence of NAFLD in obese people compared to those of a normal weight: the prevalence of NAFLD in the normal population is 15–30% while, in the obese population, it is 50–90% [5]. As a major detoxification organ, the liver possesses several defense mechanisms to combat oxidative stress [6]. This oxidative stress inhibition mechanism is inseparable from the role of nuclear factor erythroid 2-related factor 2 (Nrf2) and Forkhead Box O3 (FOXO3), which are two major transcription factors in the cellular process of ROS detoxification [7]. Nrf2 homeostasis is negatively regulated by Kelch-like ECH-associated Protein 1 (Keap1), which contributes to the ubiquitin–proteasomal breakdown of the Nrf2 protein within the cytoplasm [8]. Meanwhile, FOXO3 is regulated by 14-3-3 protein, which contributes to nuclear protein export and leads to the proteasomal degradation of FOXO3 in the cytoplasm [9,10].
In the nucleus, Nrf2 and FOXO3 act as transcription factors that enhance the expression of endogenous enzymatic and nonenzymatic antioxidants (such as Superoxide Dismutase/SOD, Glutathione Peroxidase/GPx, catalase (CAT), and reduced glutathione/GSH) [1,4]. Endogenous antioxidants can inhibit oxidative stress that occurs in the body. However, if Nrf2 is bound by its regulator (Keap1 protein) in the cytoplasm, it cannot migrate to the nucleus; therefore, it cannot perform its function [11]. Nrf2 bound by Keap1 will be tagged by ubiquitin and degraded through the ubiquitin–proteasome mechanism [12]. Meanwhile, FOXO3 is inactivated through Akt-mediated phosphorylation of serine 253 (S253) and is then bound by its regulator (14-3-3 protein) in the nucleus [13]. Phosphorylated FOXO3, which is bound by 14-3-3 protein, is exported from the nucleus to the cytoplasm to perform protein degradation through the ubiquitin–proteasome mechanism [9,14]. Therefore, the Keap1 and 14-3-3 proteins also contribute to the molecular mechanism of antioxidant regulation by inhibiting the cellular function of antioxidant-related transcription factors (Nrf2 and FOXO3) [9,15].
Even though the body is equipped with several endogenous antioxidants, a pathological condition such as obesity may result in excessive oxidative stress. This condition overwhelms the body’s ability to neutralize ROS through endogenous antioxidant defense mechanisms; therefore, exogenous antioxidants are needed to compensate for this. Previous studies have established that Nrf2 signaling has a positive role in obesity and insulin resistance [16]. Likewise, FOXO3 also contributes to reducing adipose tissue and improving insulin resistance and antioxidant activity in metabolic disease [17]. Besides Nrf2 and FOXO3, which act as transcription factors involved in antioxidant defense, Keap1 and 14-3-3 proteins, which are their regulatory proteins, also have essential roles related to antioxidant regulation. In addition, several studies have stated that obesity is associated with increased levels of Keap1 and 14-3-3 protein, which can interfere with the stability of antioxidant related transcription factors [6,18]. Additionally, several studies have also suggested that Keap1 and 14-3-3 protein inhibition serve as therapeutic targets for the treatment of some metabolic diseases, including obesity [11,14,15].
Natural antioxidant compounds, such as polyphenols, flavonoids, and terpenoids, are widely known as secondary plant metabolites [19]. These compounds are currently being investigated as treatments for the oxidative stress that occurs in obesity. The compounds provide cellular protection through an antioxidant mechanism. Coriander (Coriandrum sativum L.) seeds are not only a popular spice worldwide, but are also traditionally valued for their medicinal properties. Coriander seed ethanolic extract has been reported to prevent elevated hepatic oxidative stress markers (e.g., MDA) and increase the activity of CAT as a form of antioxidant protection in rats with obesity induced by a high-fat diet [20]. However, the molecular mechanism of how the extract inhibits liver oxidative stress has not been revealed. Therefore, this study aimed to explore the molecular mechanism of coriander seed ethanolic extract in the livers of high-fat-diet-fed rats by activating antioxidant-related transcription factors (Nrf2 and FOXO3). In addition, since the role of Keap1 and 14-3-3 proteins in activating these antioxidant-related transcription factors has already been proposed, an in silico study was also conducted to explore the potential therapeutic target of the compounds in coriander seed ethanolic extract in inhibiting regulatory proteins (Keap1 and 14-3-3), which contribute to the mobilization and stability of Nrf2 and FOXO3 in cells, therefore activating them. Furthermore, we also observed the effects of coriander seed ethanolic extract on the activity of downstream endogenous antioxidants, including T-SOD, MnSOD, GPx, and GSH.
2. Materials and Methods
2.1. Study Design
The in silico study was performed to examine the molecular mechanisms of Nrf2 and FOXO3 activation through the interaction between Coriandrum sativum L. ethanolic extract phytoconstituents and transcription factor inhibitors (Keap1 and 14-3-3 protein). Meanwhile, the in vivo study was a post-test-only experimental study to examine the mechanism of Coriandrum sativum L. ethanolic extract in cellular antioxidant regulation. The in vivo study measured the expression of Nrf2 and FOXO3 proteins, the expression of MnSOD and GPx mRNA, and the endogenous antioxidant activity (T-SOD, MnSOD, GPx, and GSH).
2.2. Ethanolic Extraction of Coriander Seeds (Coriandrum sativum L.)
The dried coriander seeds were collected from the Research Centre for Medicinal Plants and Spices, Ministry of Agriculture of Indonesia. The seeds were extracted by maceration using ethanol solvent for 24 h, and the evaporated extracts were freeze-dried and stored at 4 °C [20]. The coriander seed ethanolic extract contained flavonoids, terpenoids, and alkaloids, with a total flavonoid content of 178.62 mg EQ/g, as reported in our previous study [21]. Furthermore, the active compounds in the coriander seed ethanolic extract were identified using Gas Chromatography–Mass Spectrometry (GC-MS) and Liquid Chromatography–Mass Spectrometry (LC-MS), as described in our earlier work [22,23].
2.3. In Silico Study Procedures
2.3.1. Ligand Preparation
The coriander seed ethanolic extract’s phytoconstituents were identified using GC-MS and LC-MS [22,23]. The small molecule 3D structures of the identified constituents were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/ accessed on 5 November 2024) and ChemSpider (https://www.chemspider.com/ accessed on 5 November 2024), as mentioned in Table 1. The chemical structures were then translated into PDBQT format using AutoDockTools 1.5.7 for docking simulation.

Table 1.
Compound Identification.
2.3.2. Protein Preparation
The 3D protein crystal structures of Keap1 (PDB ID: 4L7B) and 14-3-3 (PDB ID: 6FN9) were obtained from the RCSB Protein Data Bank (https://www.rcsb.org/ accessed on 10 November 2024). The protein structure was initially prepared using AutoDockTools by removing the water molecules and native ligand; then, hydrogen atoms and Gasteiger charges were added to the protein. After that, it was translated into PDBQT format.
2.3.3. Molecular Docking
Molecular docking was carried out to predict the interaction between the coriander seed ethanolic extract’s compounds and the target proteins (Keap1 and 14-3-3), using Autodock4 to obtain the binding affinity (ΔG) and the predicted inhibition constant (Ki). ΔG refers to the difference in Gibbs free energy; spontaneous protein and ligand binding occur only when the ΔG of the system is negative. A lower ΔG value indicates a greater binding affinity between the ligand and the protein target. Meanwhile, Ki refers to the concentration needed to attain half of the maximum inhibition, which reflects how potent the substance is as an inhibitor. A smaller Ki means a smaller concentration of a substance is needed to inhibit its binding partner’s activity. Ki is calculated using the formula , where ΔG refers to free binding affinity, R refers to the gas constant (1.987 cal K−1 mol−1), and T refers to temperature (298.15 K) [24]. The target protein’s binding site was included in the dimensions of the grid box (40 × 40 × 40). The ligand–protein interactions (e.g., hydrogen bonds and hydrophobic interactions) were visualized using LigPlot (https://www.ebi.ac.uk/thornton-srv/software/LigPlus/, accessed on 10 November 2024). Redocking of each protein target with its native ligand was also performed to compare with the docking result.
2.3.4. Drug-Likeness, Pharmacokinetics, and Toxicity Prediction
The drug-likeness, pharmacokinetics, and toxicity were predicted for the three compounds that had the best molecular docking results. Drug-likeness prediction according to Lipinski’s rule of five was performed using SwissADME (http://www.swissadme.ch/index.php, accessed on 4 February 2025); pharmacokinetics prediction was performed using PKCSM (https://biosig.lab.uq.edu.au/pkcsm/prediction, accessed on 4 February 2025); and toxicity prediction was performed using PKCSM (https://biosig.lab.uq.edu.au/pkcsm/prediction, accessed on 4 February 2025) and ProTox-3.0 (https://tox.charite.de/protox3/index.php?site=compound_input, accessed on 4 February 2025). The compound’s canonical SMILES were obtained from the PubChem and ChemSpider databases, and their identification numbers (ID) are mentioned in Table 1.
2.4. In Vivo Study Procedures
The in vivo animal study was conducted on 25 adult male Rattus novergicus Wistar rats (8–10 weeks of age and ±200 g of initial weight) from Biofarma Ltd., Bandung, Indonesia. Sample size was determined using Federer’s formula, with five rats per group, which was adequate to detect significant effects. Before entering the experimental phase, all of the rats underwent acclimatization for one week, during which they were fed with a normal diet ad libitum. The experimental phase lasted 24 weeks, and coriander seed ethanolic extract was administered daily for the last 12 weeks (from the 13th week to the 24th week). The extract was administered orally to the rats in groups 2, 3, and 5 at a concentration of 100 mg/kg body weight/day in a volume of 1 mL/0.5 kg body weight [20,22,23]. The rats were allocated into five groups, as mentioned in Table 2.

Table 2.
Treatment group.
The Lee Index, Body Mass Index, and blood lipid profile were measured at the 12th week of treatment to determine the rats’ nutritional statuses. Necropsy was conducted at week 25 after treatment with ketamine 7.5 mg/kgBW and xylazine 0.4 mg/kgBW as anesthesia [20].
2.4.1. Tissue Homogenate Preparation and Total Protein Analysis
A mass of 100 mg of liver tissue was taken for each rat, which was then added to 1 mL RIPA Lysis Buffer (Cat No. 89900, Thermo Scientific™, Waltham, MA, USA) and 10 μL Protease Phosphatase Inhibitor Cocktail (Cat No. PPC1010, Sigma-Aldrich, St. Louis, MI, USA). The tissue was homogenized by a tissue homogenizer; then, it was centrifuged at 14,000× g for 15 min at 4 °C, with the supernatant separated into different microtubes. The total protein in the tissue supernatant was analyzed using the Bradford Microassay method using Bradford reagent (Biorad) with Bovine Serum Albumin (BSA) 1.4 mg/mL as the standard protein. The total protein was measured with a Varioskan Microplate Reader at λ 595 nm.
2.4.2. Nrf2 and FOXO3 Protein Expression Using Enzyme-Linked Immunosorbent Assay (ELISA)
The expression of Nrf2 and FOXO3 proteins was measured using a Sandwich ELISA Kit [FineTest®, Wuhan, China], and the absorbance was measured with a Varioskan Microplate Reader at λ 450 nm. After that, the protein expression was normalized through dividing by mg of total protein.
2.4.3. Nrf2 Protein Expression Using Western Blot
The expression of Nrf2 protein was measured using a Western blot. The protein was extracted using RIPA lysis buffer (Cat No. 89900, Thermo Scientific™), and Protease Phosphatase Inhibitor Cocktail (1:100, Cat No. PPC1010, Sigma-Aldrich) was added. The protein concentration of the tissue homogenate was determined by Bradford assay using Dual-Range™ Bradford Protein reagent (VisualProtein) with Bovine Serum Albumin (BSA) 1 mg/mL as the standard protein. The total protein absorbance was measured with a Varioskan Microplate Reader at λ 595 nm. The protein from the liver tissue homogenate was denatured at 97.5 °C for 5 min. An equal amount of loaded protein (35 μg per lane) was then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using SurePAGETM Precast Gel (Cat No. M00656, 4–20%, GenScript, Nanjing, China) at 100 volts or 500 mA for 120 min. The gel was then transferred onto a polyvinylidene difluoride (PVDF) membrane (pore size: 0.45 µm) with a voltage of 40 volts (400 mA) applied for 120 min, which was then increased to 60 volts (400 mA) for the next 30 min. The process of blocking the membrane involved incubating it with Tris-buffered saline tween 0.1% (TBST) containing 5% BSA for 1.5 h at room temperature, followed by washing using TBST for 4 × 5 min. Then, the membrane was incubated overnight at 4 °C using primary antibody anti-Nrf2 (dilution 1:1000, Cat No. PA5-68817, Invitrogen, Carlsbad, CA, USA) and anti-β-actin (dilution 1:1000, Cat No. E-AB-40517, Elabscience, Wuhan, China). The membrane was washed after primary antibody incubation using TBST for 4 × 5 min. After that, the membrane was incubated with goat anti-rabbit IgG HRP-labeled secondary antibody (dilution 1:20,000, Cat No. 31460, Invitrogen) for one hour at room temperature, then washed using TBST for 3 × 5 min. The blots were incubated with the visualization reagent PierceTM ECL Western Blotting Substrate (Cat No. 32209, Thermo Scientific™) for 5 min, and then, bands were detected with a chemiluminescence gel documentation system, with β-actin as a reference protein.
2.4.4. MnSOD and GPx Relative mRNA Expression Analysis
The total RNA from the liver tissue sample was extracted using Quick-RNA™ Miniprep Kit (Zymo Research®, Irvine, CA, USA). The purity index and total RNA concentration were examined at A230, A260, and A280 nm using a nanodrop. Using the SensiFAST SYBR No-ROX One-Step Kit (Bioline®, London, UK), the expression of MnSOD and GPx mRNA in liver tissue was measured through quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) with 100 ng of RNA. The thermocycling conditions are presented in Table 3. The forward and reverse primers were produced by Elabscience, and their sequences are presented in Table 4. The expression of MnSOD and GPx mRNA was adjusted using β-actin as the reference gene. The relative mRNA expression was measured using the Livak formula (2−ΔΔCq) relative to the mRNA expression of normal diet control rats (N group) to calibrate [25].

Table 3.
Thermocycling condition for qRT-PCR.

Table 4.
Primer for qRT-PCR.
2.4.5. Total SOD, MnSOD, and GPx Specific Enzymatic Activity Analysis
The T-SOD and MnSOD enzyme activity was analyzed using the T-SOD Activity Assay Kit (Elabscience). Since cyanide ions (CN-) react with Cu ions, which can inhibit the activity of CuZnSOD [28], to measure the MnSOD activity only, 5 mM of NaCN was added to the reaction and incubated for five minutes. This enables only the MnSOD enzyme to be identified in the reaction system. The Varioskan Microplate Reader was used to analyze the absorbance at λ 450 nm. Meanwhile, the GPx enzyme activity was analyzed by using the GPx Activity Assay Kit (Elabscience) and measured with a Varioskan Microplate Reader at λ 412 nm. The T-SOD, MnSOD, and GPx specific enzymatic activity (U/mg) was calculated by dividing the enzyme activity (units) by total protein (mg).
2.4.6. GSH Level Analysis
The GSH level in liver tissue homogenates was analyzed using the Ellman method with Ellman’s reagent (5,5-dithiobis-2-nitrobenzoic acid/DTNB), with the Varioskan Microplate Reader used to measure the absorbance at λ 412 nm. After that, the GSH level was normalized by dividing it by mg of total protein.
2.4.7. Statistical Analysis
The statistical analysis was conducted using the SPSS® Version 24 [IBM®] software. The data normality was analyzed using the Shapiro–Wilk test, and the homogeneity test was analyzed using the Levene test. The parametric data were analyzed using a One-way ANOVA followed by a Post hoc Tukey Test. Meanwhile, the nonparametric data were transformed (using the Log10 formula) to obtain parametric data. Then, the One-way ANOVA test followed by the Post hoc Tukey Test was performed to examine the statistical differences between groups. The data were interpreted as significantly different if the p-value < 0.05. The data are displayed as mean ± standard deviation (SD) in a graph created using the GraphPad Prism software version 9.5.1 (www.graphpad.com accessed on 4 February 2025). Data were also analyzed using the Pearson Correlation Test to observe the statistical correlations between the transcription factors (Nrf2 and FOXO3) and their downstream effectors (T-SOD, MnSOD, GPx, and GSH). A significant correlation is indicated if the p-value < 0.05, and a positive correlation is indicated if the Pearson correlation value is within the range of 0 to 1.
3. Results
3.1. In Silico Study
3.1.1. Molecular Docking
The interaction of the coriander seed ethanolic extract phytoconstituents with the Keap1 and 14-3-3 proteins was evaluated, with the ligand–protein interactions reflected by the binding affinity (ΔG) and inhibition constant (Ki). A more negative ΔG and a lower Ki indicate a stronger interaction between the phytoconstituent and the protein target.
The results in Table 5 indicate that the phytoconstituents from the coriander seed ethanolic extract are predicted to interact with the Keap1 protein. Specifically, it is shown that shionoside b (ΔG = −8.90 kcal/mol; Ki = 298.01 nM), arteannuin (ΔG = −7.44 kcal/mol; Ki = 3.50 µM), and aloeresin c (ΔG = −7.31 kcal/mol; Ki = 4.36 µM) are the three phytoconstituents that have the strongest interaction with the Keap1 protein. However, the ΔG values of these phytoconstituents were lower compared to that of the native ligand (ΔG = −9.37 kcal/mol). Shionoside b had the lowest Ki, which means that it is predicted to inhibit the Keap1 protein the most out of the other compounds.

Table 5.
Molecular docking result of coriander seeds ethanolic extract phytoconstituents with Keap1 and 14-3-3 protein.
The interaction between the coriander seed ethanolic extract’s phytoconstituents and 14-3-3 protein is explained by the values presented in Table 5. The result showed that shionoside b (ΔG = −6.85 kcal/mol; Ki = 9.46 µM), arteannuin (ΔG = −6.01 kcal/mol; Ki = 39.17 µM), and trinexapac-ethyl (ΔG = −5.85 kcal/mol; Ki = 51.36 µM) are the three phytoconstituents with the strongest interaction with 14-3-3 protein. The overall binding affinity between the coriander seed ethanolic extract’s phytoconstituents and Keap1 protein is still stronger than that between Keap1 and 14-3-3. This indicates that the interaction between the coriander seed ethanolic extract’s phytoconstituents and 14-3-3 protein was not as strong as the interaction with Keap1 protein. Shionoside b is also the compound with the lowest Ki, which means that it is predicted to inhibit 14-3-3 protein the most.
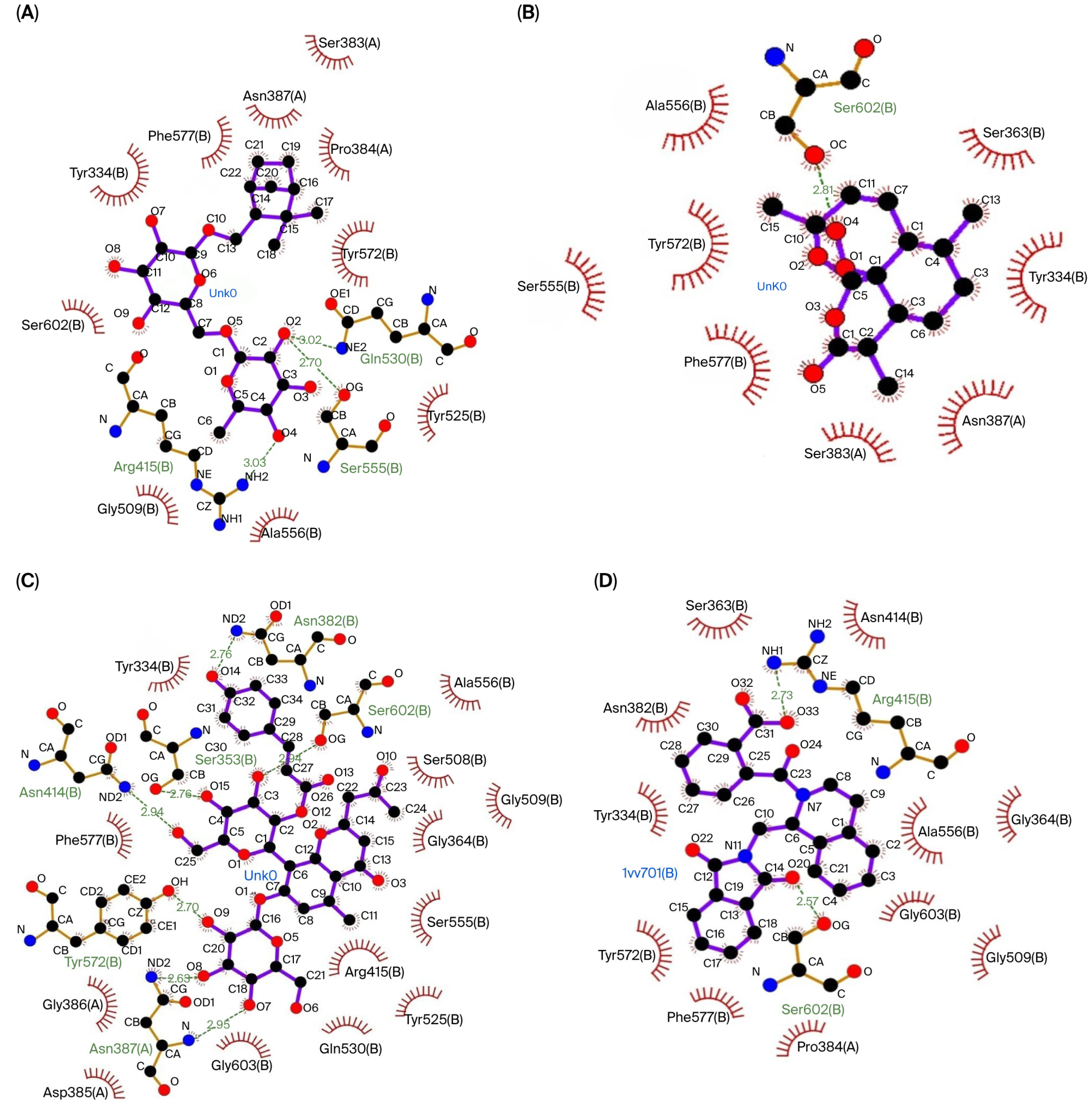
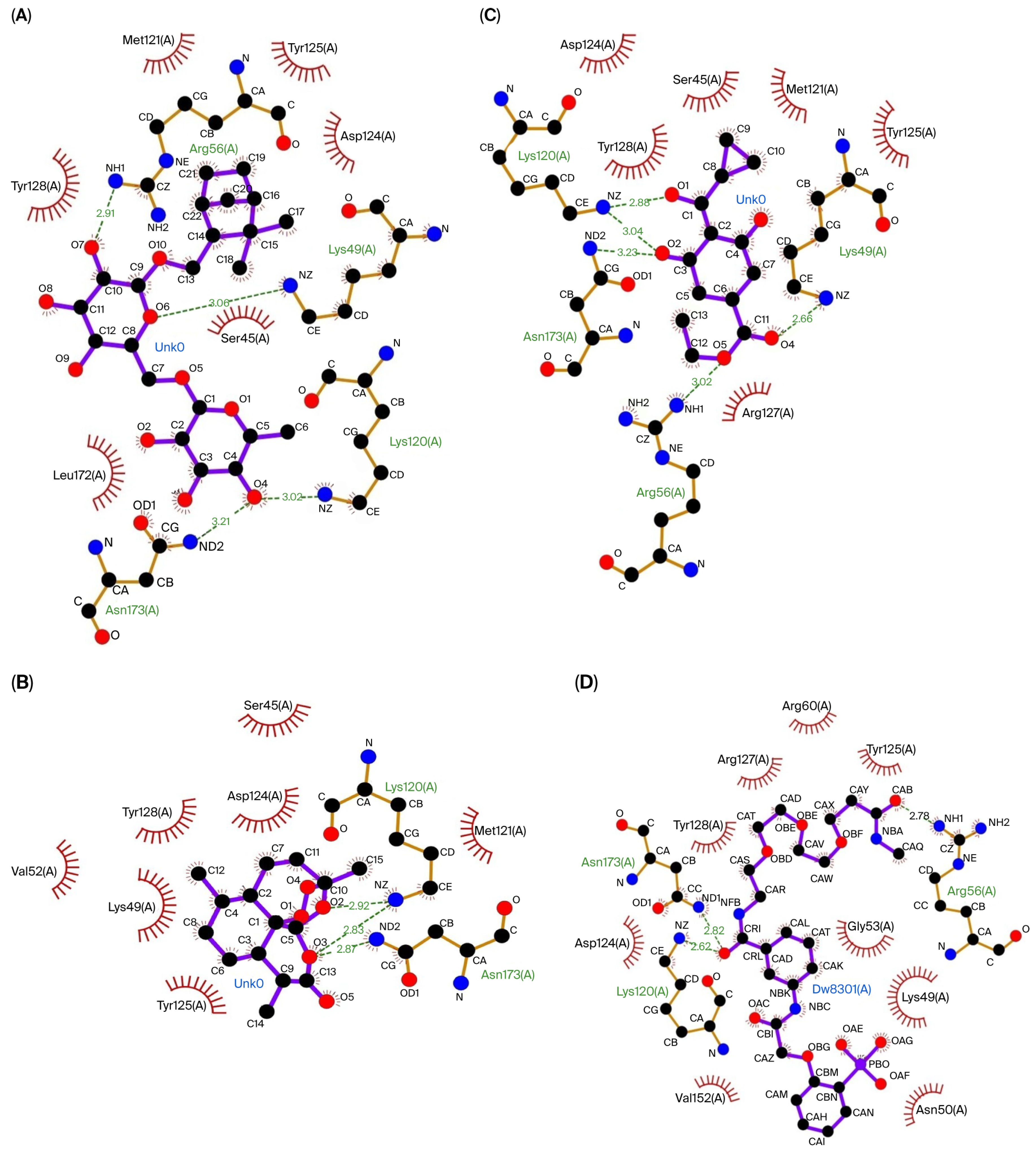
The three compounds that showed the best molecular docking results were visualized using LigPlot to predict their interactions with the amino acid residues in each protein. Figure 1 visualizes the predicted interactions between the three compounds showing the best molecular docking results and the amino acid residues in the Keap1 protein. Figure 2 visualizes the predicted interactions between the three compounds, showing the best molecular docking results and the amino acid residues in the 14-3-3 protein.
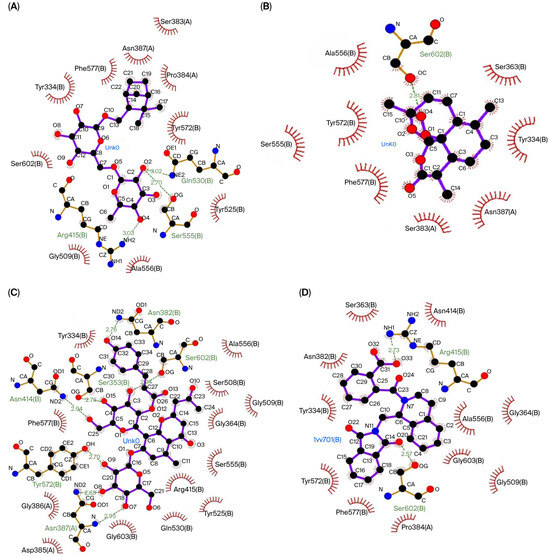
Figure 1.
The interactions between the top three compounds in coriander seed ethanolic extract and amino acid residues in Keap1 protein. (A) Shionoside b, (B) arteannuin, (C) aloeresin c, and (D) native ligand.
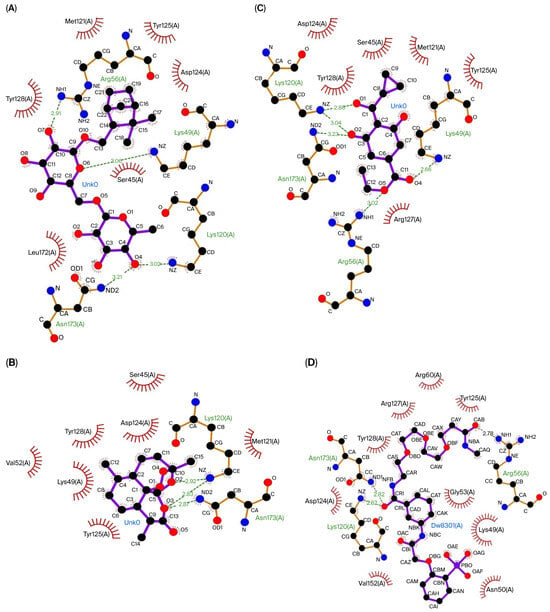
Figure 2.
The interactions between the top three compounds in coriander seed ethanolic extract and amino acid residues in 14-3-3 protein. (A) Shionoside b, (B) arteannuin, (C) trinexapac-ethyl, and (D) native ligand.
The amino acid–ligand interactions are presented in Table 6, and these indicate that Arg415 and Ser602 are the key amino acids assumed to play an essential role in the interaction between the coriander seed ethanolic extract’s phytoconstituents and Keap1 protein. Table 6 indicates that Arg56, Lys120, and Asn173 are predicted to have essential roles in the interaction between the coriander seed ethanolic extract’s phytoconstituents and the 14-3-3 protein.

Table 6.
Interaction between compound and amino acid residues from docking result.
3.1.2. Drug-Likeness, Pharmacokinetics, and Toxicity Prediction
Shionoside b and arteannuin were two compounds in the coriander seed ethanolic extract that had the best molecular docking results with both the Keap1 and 14-3-3 proteins. The third-best molecular docking result with the Keap1 protein was found for aloeresin C. Meanwhile, the third-best molecular docking result with the 14-3-3 protein was found for trinexapac-ethyl. Therefore, these four compounds were further analyzed to predict their drug-likeness, pharmacokinetics, and toxicity.
Table 7 presents the drug-likeness predictions of the four phytoconstituents according to Lipinski’s rule of five criteria, which reflect how good the drug absorption and permeability are. If the compound meets all of the criteria (which means none of the criteria are violated), it is predicted to have physicochemical properties making it orally bioavailable. A compound with fewer than five hydrogen bond donors, fewer than ten hydrogen bond acceptors, a molecular weight under 500 g/mol, and a LogP value below five is regarded as having favorable absorption and permeability [29]. LogP is a crucial parameter that indicates the lipophilicity of a compound, predicting the compound’s ability to cross biological membranes. The drug-likeness result demonstrated that arteannuin and trinexapac-ethyl were the two compounds that met all of the drug-likeness criteria (Table 7). It can be assumed that these two compounds could potentially be orally administered with high absorption and permeability.

Table 7.
Drug-likeness prediction.
The pharmacokinetics predictions of the four compounds are presented in Table 8. According to the absorption parameters, Arteannuin and trinexapac-ethyl are predicted to be absorbed well and can more readily cross the human intestinal epithelial cell barrier compared to other compounds. P-glycoprotein (P-gp) is a transporter located on the apical intestinal membrane that actively pumps a substance back out into the intestinal lumen, which significantly reduces the substance’s entry into the systemic circulation. Arteannuin and Trinexapac-Ethyl are predicted not to be P-gp substrates, which means that these two compounds have better intestinal permeability and can enter the systemic circulation. Meanwhile, according to the Caco-2 permeability, only arteannuin is predicted to have high permeability in the human intestinal mucosa (Caco-2 permeability is considered high if the predicted value is above 0.90) [30]. The distribution can be predicted by the steady-state volume of distribution (VDss) value, which is measured when the concentration of the drug in the plasma is equal to the concentration of the drug in tissues. VDss is considered low if it is below 0.71 L/kg (log VDss < −0.15) and high if it is above 2.81 L/kg (log VDss > 0.45) [30]. Therefore, arteannuin, which has the highest VdSS, is predicted to be highly distributed in the tissue, rather than the plasma, compared to the other top three compounds. Arteannuin is predicted to be a CYP3A4 substrate, which means it is likely to be metabolized by CYP3A4; however, arteannuin is also predicted to be a CYP1A2 enzyme inhibitor, which means it could possibly slow down the enzyme’s ability to metabolize this substance.

Table 8.
Pharmacokinetics prediction.
Table 9 presents the toxicity predictions for the four compounds: shionoside b is predicted to have a narrower therapeutic window and more organ toxicity compared to the other three compounds; however, four of the compounds are predicted not to be hepatotoxic.

Table 9.
Toxicity prediction.
These in silico findings suggest inhibitory potential against Keap1 and 14-3-3, which may facilitate Nrf2 and FOXO3 activation; this hypothesis was evaluated in vivo through protein quantification and mRNA expression analysis in rat liver tissues.
3.2. In Vivo Study
The in vivo study was conducted to evaluate the efficacy of the coriander seed ethanolic extract on the cellular antioxidant regulation of high-fat-diet-fed rats by evaluating the level of transcription factor proteins (Nrf2 and FOXO3) and their downstream antioxidant enzyme mRNA expression and enzymatic specific activity.
3.2.1. The Efficacy of Coriander Seed Ethanolic Extract on Hepatic Nrf2 and FOXO3 Protein Expression
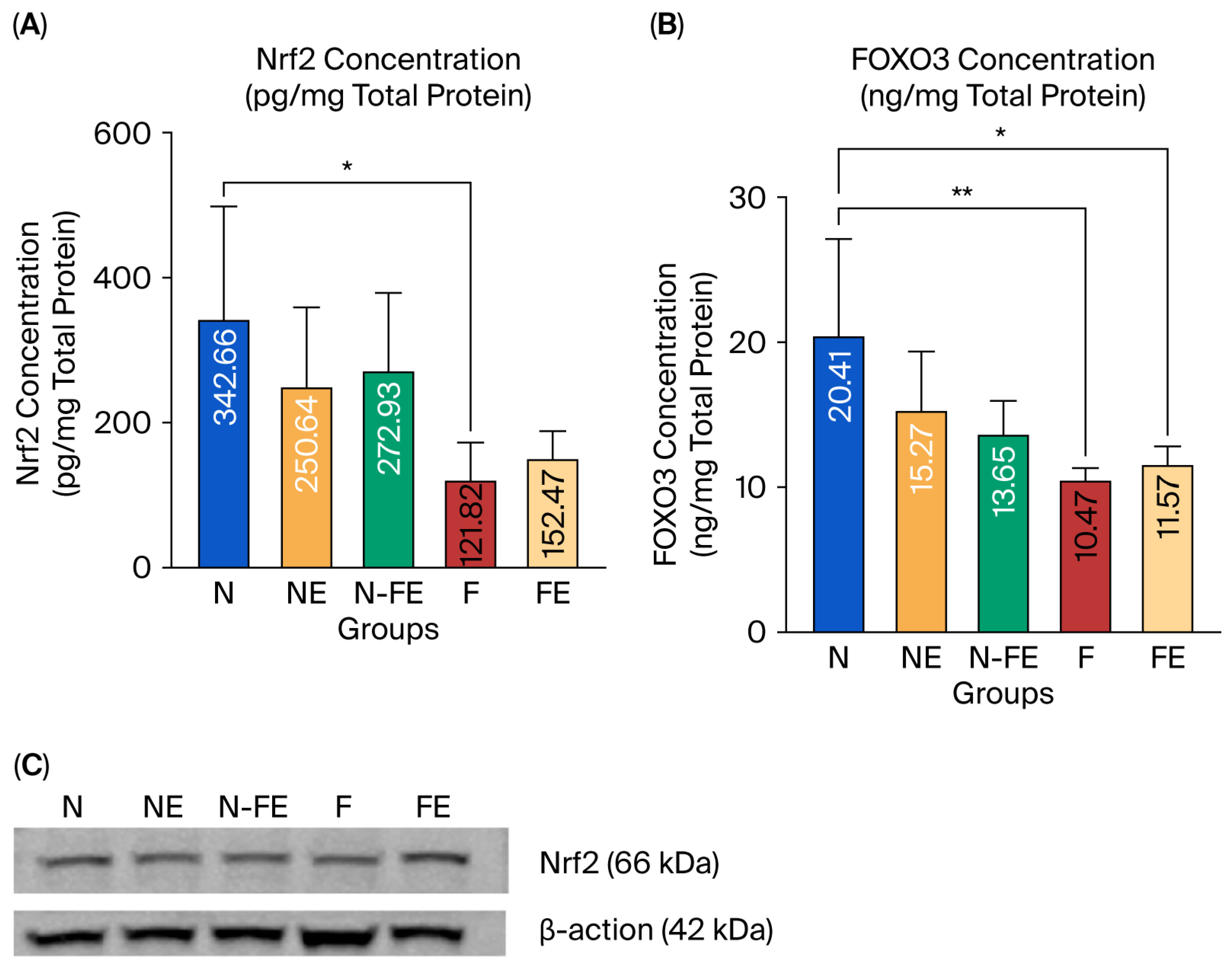
The expression of Nrf2 and FOXO3 protein in liver tissue was observed to analyze the role of ethanolic coriander seed extract in preventing liver damage caused by a high-fat diet. Based on the statistical results, Nrf2 expression was significantly different between the groups (p = 0.016, Figure 3A). Similarly, FOXO3 expression was also significantly different between the groups (p = 0.004, Figure 3B). There was a significant decrease in Nrf2 expression (p = 0.020, Figure 3A), along with a significant decrease in FOXO3 expression (p = 0.004, Figure 3B), in the obese control group (F) compared to the normal diet group (N). It can be concluded that a high-fat diet decreases the expression of Nrf2 and FOXO3 proteins in liver tissue. Ethanolic coriander seed extract given for 12 weeks to obese mice in the preventive group (N-FE) and curative group (FE) tended to increase the mean Nrf2 levels compared to those in the obese control group (F), although neither was significantly different (p = 0.171 and p = 0.989, respectively). A similar result was found for the expression of FOXO3 protein in that when the ethanolic coriander seed extract was given for 12 weeks to obese mice in the preventive group (N-FE) and curative group (FE), the FOXO3 protein expression tended to be higher compared to the mean in the obese control group (F), although both were not significantly different (p = 0.677 and p = 0.990, respectively). The result for Nrf2 protein expression from the ELISA method (Figure 3A) was confirmed by the Western blot, which also showed elevated Nrf2 protein expression in the obese rats given the coriander seed ethanolic extract for 12 weeks (Figure 3C).
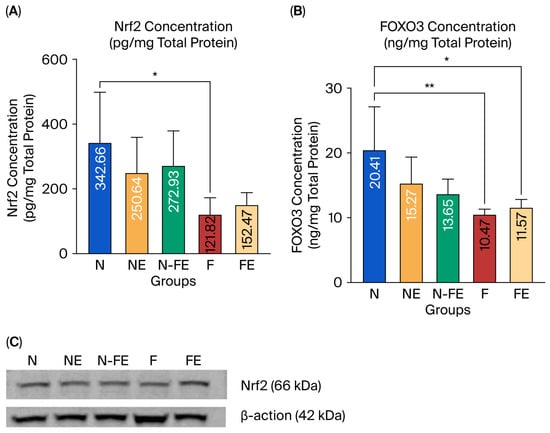
Figure 3.
Efficacy of coriander seed ethanolic extract on the antioxidant-related transcription factors in the liver. (A) Comparison of Nrf2 protein expression between groups (by the ELISA method). (B) Comparison of Nrf2 protein expression between groups confirmed via Western blot. (C) Comparison of FOXO3 protein expression between groups (by the ELISA method). Data from the ELISA results were transformed using the Log10 formula and analyzed using One-way ANOVA followed by Post hoc Tukey Test (* p < 0.05, ** p < 0.01). N: normal diet control; NE: normal diet and coriander seed ethanolic extract; N-FE: normal diet, high-fat diet, and coriander seed ethanolic extract; F: high-fat diet control; FE: high-fat diet and coriander seed ethanolic extract.
3.2.2. The Efficacy of Coriander Seed Ethanolic Extract on Hepatic MnSOD and GPx mRNA Expression
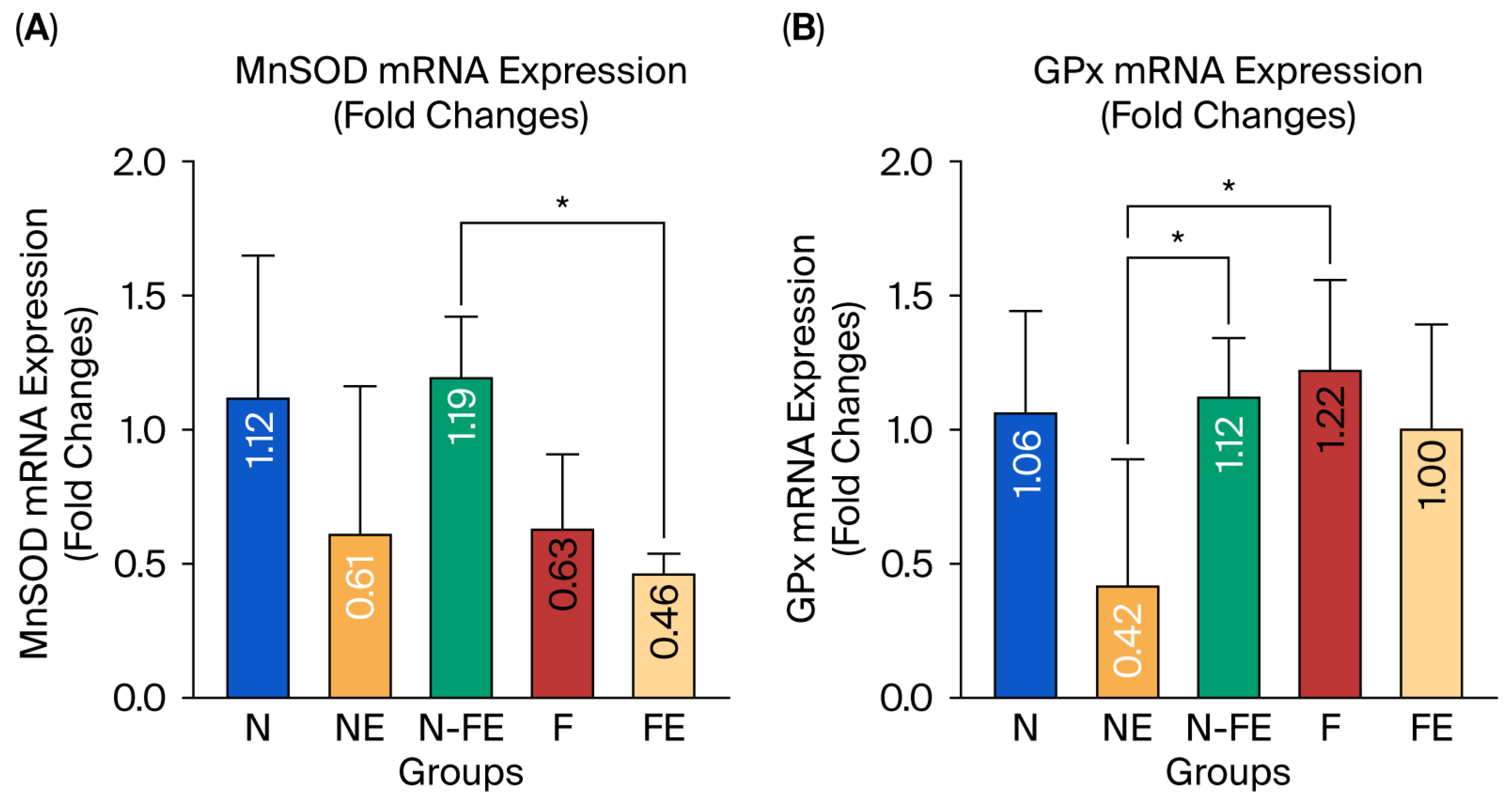
The hepatic MnSOD and GPx mRNA expression was measured to evaluate the role of the coriander seed ethanolic extract in preventing liver damage induced by a high-fat diet. Figure 4A,B reveal that the relative expression of MnSOD and GPx mRNA was significantly different between the groups (p = 0.019 and p = 0.020, respectively). The coriander seed ethanolic extract administered for 12 weeks to the high-fat-diet-fed rats in the preventive group (N-FE) tended to increase the relative expression of MnSOD mRNA compared to the high-fat diet group (F) (p = 0.174). On the other hand, the relative expression of GPx mRNA in the preventive group (N-FE) and curative group (FE) tended to be lower compared to that in the high-fat diet control group (F), but this was not statistically significant (p = 0.993 and p = 0.876, respectively). The relative expression of GPx mRNA in the preventive group (N-FE) and obese control group (F) was significantly different compared to that in the normal group given coriander seed ethanolic extract (NE) (p = 0.045 and p = 0.019, respectively).
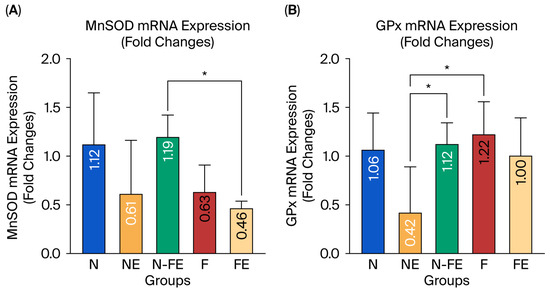
Figure 4.
Efficacy of coriander seed ethanolic extract on hepatic MnSOD and GPx mRNA expression. (A) Comparison of Mn-SOD mRNA expression between groups. (B) Comparison of GPx mRNA expression between groups. Data were analyzed using One-way ANOVA followed by Post hoc Tukey Test (* p < 0.05). N: normal diet control; NE: normal diet and coriander seed ethanolic extract; N-FE: normal diet, high-fat diet, and coriander seed ethanolic extract; F: high-fat diet control; FE: high-fat diet and coriander seed ethanolic extract.
3.2.3. The Efficacy of Coriander Seed Ethanolic Extract on Hepatic Antioxidant Activity
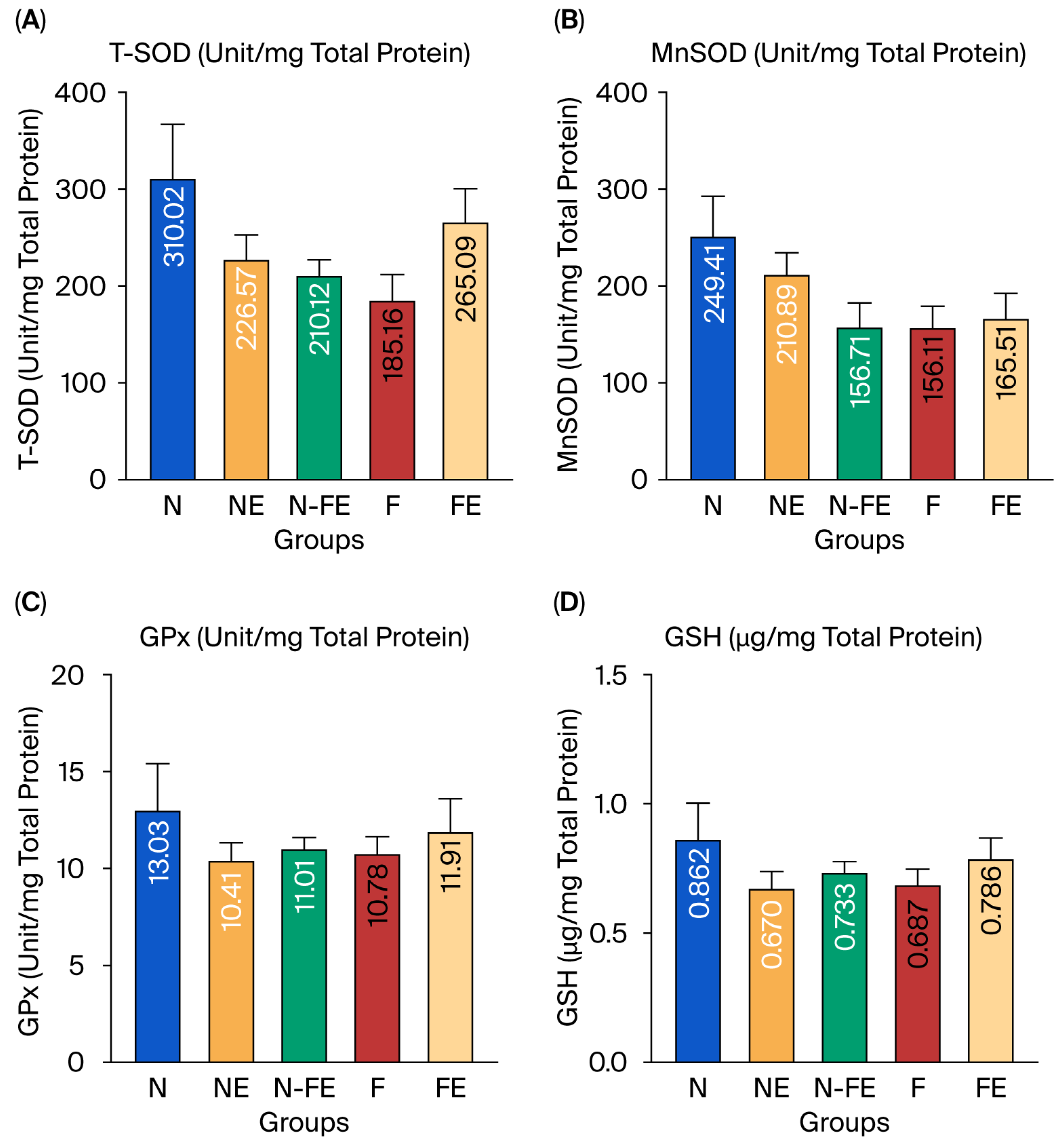
In addition to measuring the endogenous antioxidant conditions at the mRNA level, the hepatic endogenous antioxidant activity was also observed by measuring the specific activity of antioxidant enzymes (T-SOD, MnSOD, and GPx) and nonenzymatic antioxidant levels (GSH). Despite the upregulation of transcription factors, no significant functional increase in antioxidant activity was observed. Figure 5 shows that T-SOD, MnSOD, GPx, and GSH levels did not differ significantly between the groups (p = 0.135, p = 0.115, p = 0.736, and p = 0.529, respectively). However, Figure 5 shows a tendency for decreased T-SOD, MnSOD, GPx, and GSH levels in the obesity control group (F) compared to those in the normal diet group (N). It can be assumed that a high-fat diet tended to decrease hepatic antioxidants (T-SOD, MnSOD, GPx, and GSH), although this decrease was not significant. The ethanolic coriander seed extract given for 12 weeks to rats fed a high-fat diet (FE) tended to increase the enzymatic activity of T-SOD, especially in the curative group (FE). Meanwhile, the MnSOD, GPx, and GSH levels were only slightly higher compared to those in the high-fat diet group (F).
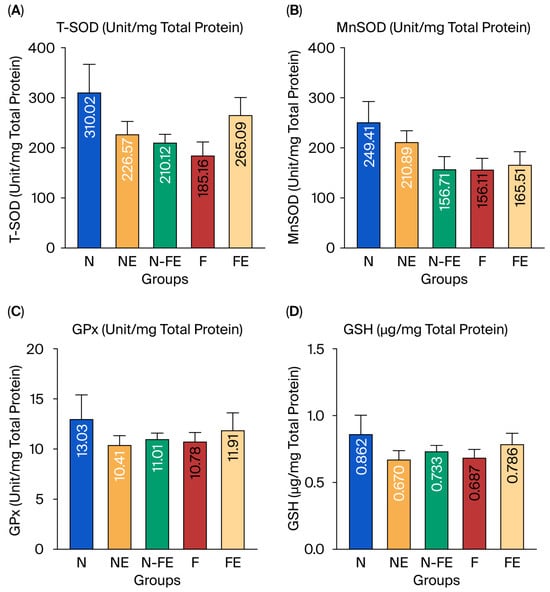
Figure 5.
The efficacy of coriander seed ethanolic extract on hepatic antioxidant activity. (A) Comparison of T-SOD specific enzyme activity between groups. (B) Comparison of Mn-SOD specific enzyme activity between groups. (C) Comparison of GPx specific enzyme activity between groups. (D) Comparison of GSH level between groups. The data were analyzed using one-way ANOVA followed by post hoc Tukey Test. N: normal diet control; NE: normal diet and coriander seed ethanolic extract; N-FE: normal diet, high-fat diet, and coriander seed ethanolic extract; F: high-fat diet control; FE: high-fat diet and coriander seed ethanolic extract.
3.2.4. Correlation Between the Transcription Factors (Nrf2 and FOXO3) and Their Downstream Effectors
A correlation analysis was performed on the transcription factors and their downstream effectors. The results in Table 10 show that Nrf2 has a significant positive correlation with FOXO3; furthermore, there is a significant positive correlation between the transcription factors (Nrf2 and FOXO3) and their downstream effectors (T-SOD, MnSOD, GPx, and GSH).

Table 10.
Correlation between the transcription factors and their downstream.
4. Discussion
The in silico results revealed a possible mechanism of Nrf2 and FOXO3 activation through the coriander seed ethanolic extract’s phytoconstituents, inhibiting their regulatory proteins (Keap1 and 14-3-3) by interacting with key amino acid residues. The coriander seed ethanolic extract’s phytoconstituents can interact with the Keap1 protein, which is the regulatory protein for Nrf2. Shionoside b, a monoterpene glycoside, is the compound that exhibited the strongest binding affinity with Keap1, primarily through hydrogen and hydrophobic bonds. The key amino acid residues that play a role in ligand and protein binding have been identified as Gln530, Ser555, Arg415, Ser383, Pro384, Tyr572, Tyr525, Ala556, Gly509, Ser602, Tyr334, Phe577, and Asn387. Overall, the interactions between the top three compounds in the coriander seed ethanolic extract and Keap1 primarily occurred at Arg415 and Ser602, located in the Nrf2 binding domain. These are key amino acids in the Keap1 protein–protein interaction domain that are involved in the interaction with Nrf2 through influencing the stability and activity of Nrf2. Arg415 is often referred to as the main key amino acid residue located within the binding pocket that interacts with the Nrf2 protein, specifically on its “ETGE” motif. This is because it facilitates a strong ionic interaction which is necessary for the proper binding and regulation of the Keap1–Nrf2 complex. Arg415 acts as a critical anchor point for Nrf2 binding, allowing Keap1 to control the cellular antioxidant response by modulating Nrf2′s stability and activity within cells [31]. The interaction between coriander seeds’ phytoconstituents and these key amino acids could potentially interfere with the interaction between Keap1 and Nrf2. The consequence of this phytoconstituent–Keap1 interaction is that Nrf2 will be free to escape proteasomal degradation regulated by Keap1, leading to Nrf2 stabilization [32].
Besides the Keap1–Nrf2 mechanism, a similar mechanism also occurs in the interaction between the compounds in the coriander seed ethanolic extract and 14-3-3 protein, which is the regulatory protein for FOXO3. It is known that FOXO3 also has an important role in antioxidant mechanisms [33]. The nuclear export of phosphorylated FOXO3 is strongly regulated by the 14-3-3 protein [9]; therefore, inhibiting the 14-3-3 protein will potentially affect the stability of FOXO3 in the nucleus. Shionoside b was the compound with the strongest binding affinity for 14-3-3. The in silico study also revealed that the interactions between the coriander seed ethanolic extract and 14-3-3 protein primarily occurred at the Arg56, Lys49, Lys120, and Asn173 amino acid residues, which are located within the 14-3-3 protein–protein interaction pocket. This finding is in line with earlier studies showing that the area in the 14-3-3 protein where phosphoproteins bind is composed of positively charged residues such as Lys49, Arg56, Arg60, Arg127, and Tyr128 [34]. Small molecules can disrupt the 14-3-3 binding domain, which modulates its regulatory function. The interactions between small molecules and 14-3-3 protein potentially prevent FOXO3 nuclear exclusion and cytoplasmic ubiquitin–proteasome degradation, depending on 14-3-3 binding regulation, resulting in FOXO3 nuclear stabilization.
Shionoside b, arteannuin, aloeresin c, and trinexapac-ethyl have been reported to be beneficial for several therapeutic purposes. Shionoside b is a monoterpene glycoside. Monoterpenes are a class of terpenoids that contribute to the aroma of many plants. Monoterpenes act as antioxidants, are anti-inflammatory, and have been reported as antidiabetic agents [35]. Arteannuin is a sesquiterpene lactone, while Aloeresin c is a phenolic compound. Both of them also demonstrate antioxidant and anti-inflammatory effects on metabolic disorders [36].
Even though shionoside b has the strongest interaction with Keap1 and 14-3-3 protein, its drug-likeness, pharmacokinetics, and toxicity results were not as promising as those for arteannuin, aloeresin c, and trinexapac-ethyl. Shionoside b has low absorption and permeability in the gastrointestinal tract and has a narrower therapeutic window compared to other compounds. Therefore, shionoside b should be explored using various strategies (e.g., formulation optimization and structural modification) to enhance its absorption and minimize its toxicity. Meanwhile, arteannuin and trinexapac-ethyl, the compounds with the second strongest binding affinities for Nrf2 and FOXO3, showed better results for drug-likeness and pharmacokinetics, and also had a wider therapeutic window. Arteannuin and trinexapac-ethyl could also be isolated from coriander seed ethanolic extract for further research to elucidate their potential in combating oxidative stress or other pathological conditions as a single compound. Moreover, the four compounds were predicted not to have hepatotoxic properties; therefore, it can be assumed that the four compounds are relatively safe for the liver. Even though arteannuin and trinexapac-ethyl are absorbed well by the intestine, only arteannuin is well-distributed in the tissue. This might reflect that a higher dosage of the coriander seed ethanolic extract is needed to achieve beneficial effects on the targeted tissue. However, in vivo studies are needed to confirm the in silico results regarding the predicted pharmacokinetics and toxicity.
Oxidative stress in obesity is the result of a high-fat diet [3]. Oxidative stress occurs when elevated ROS cannot be neutralized by the antioxidants existing in the body [4]. Increased ROS are generated due to the accumulation of free fatty acids that occurs in obesity. A high-fat diet induces elevated circulating FFA, which mediates ROS production. The liver plays an essential role in metabolism and detoxification; in high-fat-diet-induced obesity, the liver is also affected by this pathological condition. The present in vivo study revealed that a high-fat diet tends to decrease the hepatic Nrf2 and FOXO3 transcription factors, which are essential for regulating antioxidant expression in cells. Nrf2 and FOXO3 are transcription factors that contribute to the expression of both enzymatic and nonenzymatic antioxidants, such as MnSOD, CuZnSOD, GPx, catalase, and GSH [4,37]. Hence, decreases in Nrf2 and FOXO3 lead to increased oxidative stress in the obese. This result aligned with our previous study that revealed the MDA concentration is elevated by a high-fat diet [20].
Consistent with the in silico results, the in vivo study revealed that the coriander seed ethanolic extract given for 12 weeks was able to increase Nrf2 and FOXO3 levels. This effect may be due to the interaction of coriander seeds’ components with the Keap1 and 14-3-3 proteins, as demonstrated in our in silico study. Coriander seed ethanolic extract contains a variety of phytoconstituents (including phenolic compounds and terpenes) that have antioxidant activity. These compounds are able to act as direct antioxidants via a free radical scavenging mechanism [38,39]. These compounds not only act as direct antioxidants, but also act as indirect antioxidants by increasing endogenous enzymatic and nonenzymatic antioxidants through regulating gene expression involving antioxidant-related transcription factors such as Nrf2 and FOXO3 [40]. This is in line with our T-SOD and GPx specific activity and GSH level results, which tended to increase with elevated Nrf2 and FOXO3. Meanwhile, the MnSOD specific activity did not increase, even though T-SOD increased. It is assumed that the coriander seed ethanolic extract might have affected the CuZnSOD isoform located in the cytoplasm instead of the MnSOD isoform located in the mitochondria. However, the expression of MnSOD mRNA in the preventive group (N-FE) was significantly elevated. This might indicate that the coriander seed ethanolic extract was more beneficial for the preventive group than the curative group in terms of MnSOD’s ability to protect against superoxide anion radicals at the mRNA level, although the enzyme activity level did not increase. The discrepancy between the MnSOD mRNA levels and enzyme activity may result from inhibition at the post-transcriptional stages that regulate protein synthesis, folding, and activation, thereby preventing changes in enzymatic activity [41]. Another possible explanation is the presence of inhibitory compounds that impair proper enzyme function [41]. The expression of GPx mRNA in the obese rats given coriander seed ethanolic extract was lower than that in the high-fat diet control group, whereas the GPx specific activity showed an opposite result. This might be due to the negative feedback effect on mRNA expression from its protein product. This negative feedback mechanism reduces the effect of mRNA expression when the protein is not needed, helping to maintain stable and balanced gene expression. One possible mechanism is transcriptional autoregulation, where a protein product can bind to the cis-regulatory element associated with the promoter region of its own gene, repressing its transcription [42].
The coriander seed ethanolic extract did not affect the hepatic GPx specific activity; however, our previous study showed that coriander seed ethanolic extract significantly increased the CAT activity [20]. Both GPx and CAT are regulated by Nrf2 and FOXO3, and these enzymes also catalyze the same reaction, which converts H2O2 into H2O and O2 [43]; however, their enzymatic mechanisms are slightly different. CAT can directly convert two molecules of H2O2 into two molecules of H2O and one molecule of O2 without the need for any reducing agents [43]. Meanwhile, GPx enzymatic activity relies on the availability of reduced glutathione (GSH), which acts as a reducing agent, to convert H2O2 into H2O and O2 [43]. The present study also revealed that GSH was not significantly increased. GSH can exist in a saturated state within cells, meaning that the cell’s capacity to store or utilize GSH might be reached, and further intake or production of GSH will not lead to a significant increase in its concentration and activity. Moreover, producing GSH is a long process involving synthesizing GSH synthase (the key enzyme for GSH synthesis) and GSH reductase (the key enzyme for maintaining the reduced state of glutathione by converting glutathione disulfide/GSSG into GSH using NADPH as its hydrogen donor) [44]. The redox balance between GSH and GSSG also influences the GPx activity. Both GSH synthetase and GSH reductase rely on Nrf2 and FOXO3 as their transcription factors [1].
Catalase is the most abundant endogenous antioxidant in the liver, which contains numerous amounts of peroxisomes; instead, GPx is mostly found in the kidney and digestive tract epithelium [45,46]. This might indicate that each tissue expresses different tissue-specific antioxidant responses. In addition, CAT has the greatest turnover number out of all the enzymes, including GPx [47]. The turnover number of an enzyme (kcat) reflects the amount of substrate molecules that can be converted by a single enzyme molecule into a product per unit of time (typically per second) when the enzyme is saturated with substrate. More than 2.8 million H2O2 molecules can be converted into oxygen and water every second by one molecule of CAT. In contrast, GPx’s turnover numbers can range from 4.7 to 727.8 molecules per second [48]. Due to its high turnover number, CAT serves as a primary enzymatic antioxidant for dissociating H2O2 [49].
This study also aligns with other research describing in detail the degradation of H2O2 by CAT and GPx. CAT catalytic activity exhibits a linear relationship with the level of H2O2; therefore, it can be assumed that an increased H2O2 level will be directly followed by elevated CAT activity as it breaks down this H2O2. As mentioned above, CAT is the primary enzyme that eliminates H2O2, especially when the cellular H2O2 quantity is over 10−6 mol/L. On the other hand, when the H2O2 concentration exceeds 10−6 mol/L, GPx will be saturated. It can be assumed that the maximum detoxifying activity of GPx is reached at a higher level of H2O2, and it does not increase further with additional H2O2 past this point. At 10−6 mol/L of H2O2, the rate of CAT activity is 12.5 times more rapid than that of GPx. Nevertheless, the rate dramatically increased to 100 times that of GPx when the H2O2 level was raised to 10−4 mol/L. As a result, CAT almost entirely contributes to the total turnover of H2O2 at concentrations above 10−6 mol/L [49]. Even though the present study has not analyzed the H2O2 level, our previous study revealed that the MDA level was elevated to double that in the high-fat diet group, which may indicate overall increased ROS in obese rats [20]. Therefore, it could be assumed that in some cases, CAT’s ability to compensate for elevated H2O2 is much higher compared to that of GPx. It could also be assumed that Nrf2 and FOXO3 are employed in the synthesis of CAT rather than GPx to respond more rapidly to elevated H2O2 in the liver caused by a high-fat diet. This may serve as an adaptive response to counteract H2O2 elevation when the GPx activity is not elevated significantly, which otherwise would result in an inability to compensate for the elevated H2O2. It is possible that due to a higher H2O2 concentration, which may be linked to the GPx activity remaining unchanged, CAT plays a major role in neutralizing this ROS [50].
The endogenous antioxidant activity in the liver is also inseparable from the SOD activity, although the present study revealed that SOD was not significantly increased. This might be linked to the longer half-life of H2O2 compared to O2●− (superoxide anion radicals), which is SOD’s substrate [50]. The longer half-life of H2O2 might indicate its superior stability compared to other ROS [51]. Moreover, unlike superoxide, H2O2 can easily traverse lipid membranes or be transported through channels. This trait might explain the noticeable changes in the CAT or GPx activity, while no significant changes were observed in terms of the SOD activity [50].
The transcriptional activity of Nrf2 and FOXO3 for endogenous antioxidant synthesis is also influenced by several mechanisms that could contribute to inhibiting their nuclear activity as transcription factors in endogenous antioxidant mRNA synthesis. miRNA could possibly interfere with the downstream target of mRNA synthesis. For example, miR-212 is highly expressed in the liver of high-fat-diet-induced obese rats [52] and can target the expression of MnSOD mRNA, resulting in its degradation [53]. When the miRNA binds to its complementary sequences in the target mRNA’s 3′ untranslated region (3′ UTR), this can modify gene expression by causing translation suppression or mRNA degradation [54,55]. Furthermore, at the transcriptional level, the NF-κB p65 subunit that mediates inflammatory mechanisms can directly block the Nrf2 pathway. The Nrf2 pathway may become inactive as a result of competition between the Nuclear factor-κB/NF-κB p65 subunit and Nrf2 to bind the CH1-KIX domain of the transcriptional co-activator CBP (CREB-binding protein). The proportion of translocated Nrf2 and NF-κB determines whether either transcription factor binds to CBP. In addition, NF-κB also encourages histone deacetylase 3 (HDAC3) to engage with CBP or MafK, resulting in local hypoacetylation and blocking Nrf2 signaling. HDAC3 recruitment to the ARE region leads to histone hypoacetylation and hinders Nrf2 signaling [56].
The present study also revealed that Nrf2 has a strong positive correlation with FOXO3, which is assumed to occur through an SIRT1-regulated mechanism. SIRT1 acts as the upstream protein regulator of Nrf2 and FOXO3 [57] and can also be activated by natural compounds (e.g., polyphenolic and monoterpene products) [58,59]. The crosstalk ensuring strict control of SIRT1, Nrf2, and FOXO3 has been revealed. SIRT1 deacetylates Nrf2 and FOXO3, which enhances Nrf2 and FOXO3 upregulation [57]. Therefore, Nrf2 and FOXO3 upregulation occur simultaneously once natural compound consumption activates SIRT1. A correlation also exists between the two transcription factors (Nrf2 and FOXO3) and their downstream effectors (SOD, GPx, and GSH). Nrf2 and FOXO3 act as transcription factors to actively enhance SOD, GPx, and GSH synthesis when antioxidant-containing natural compounds are consumed [1,4].
Nrf2 and FOXO3 act as transcription factors of endogenous antioxidants, such as SOD, GPx, and GSH, which comprise the frontline defense against ROS to maintain intracellular redox homeostasis. A decline in the activity of these transcription factors can lead to oxidative damage [43]. Elevated Nrf2 and FOXO3 might contribute to ameliorating the oxidative stress that occurs in obesity induced by a high-fat diet. There are some limitations to this study; for example, it does not rule out other possible mechanisms of coriander seed ethanolic extract’s strong effect on ameliorating oxidative stress in obesity (e.g., the inflammation mechanism). In addition, in this study, we only used a 100 mg/BW dosage for 12 weeks, which is assumed to be suboptimal for significantly enhancing Nrf2 and FOXO3 expression as well as their downstream endogenous antioxidants. According to the pharmacokinetics predictions, only arteannuin exhibits high intestinal absorption and tissue distribution out of the top four phytoconstituents in coriander seed ethanolic extract. This might indicate that a higher dosage and longer duration of coriander seed ethanolic extract administration need to be explored to achieve the desired plasma concentration and be well-distributed in the targeted tissue. In addition, establishing the most appropriate drug delivery system (e.g., using a nanocarrier) is also essential for significantly improving the overall absorption and enhancing the distribution of natural extracts within the body. Nanocarriers not only improve medicinal herbs’ efficiency, including increasing their solubility, bioavailability, and tissue distribution, but can also be beneficial for reducing the medicinal dosage and its side effects [60]. The present study did not analyze the enzymatic activity of glutathione synthetase, glutathione reductase, GSSG, or H2O2 to gain an in-depth understanding of CAT/GPx/GSH regulation. Furthermore, the in silico study performed using molecular docking was limited for predicting the ligand–protein interaction under static conditions. The stability and dynamic interaction between coriander seeds’ phytoconstituents and the target protein in a complex system over a certain duration have not been revealed.
5. Conclusions
In this study, it was concluded that a high-fat diet tends to decrease Nrf2 and FOXO3, which are essential transcription factors for endogenous antioxidant protection. This is also followed by the repression of their downstream endogenous antioxidant activity (e.g., SOD, GPx, and GSH). Coriander seed ethanolic extract activates Nrf2 and FOXO3, which act as transcription factors that prevent hepatic oxidative stress. This is also followed by elevated T-SOD, GPx, and GSH activity. A possible mechanism for this is the interaction between the transcription factor’s regulatory proteins (Keap1 and 14-3-3) and compounds in the coriander seed ethanolic extract that can potentially inhibit the binding between the transcription factors and their regulatory protein. Therefore, Nrf2 and FOXO3 can escape proteasomal degradation, and their stability increases in hepatic cells. However, there might also be another mechanism that involves direct free radical scavenging by compounds in the coriander seed ethanolic extract, other than antioxidant-related transcription factors activation. In addition, inflammatory pathways affect antioxidant-related transcription factor activation; therefore, further research needs to be performed to identify other mechanisms through which coriander seed ethanolic extract can strongly inhibit the oxidative stress induced by obesity. In addition, further research employing a higher dosage and longer intervention duration should be performed to establish the optimal dosage. It might also be important to explore possible drug delivery systems to ensure coriander seed ethanolic extract easily reaches the target tissue and significantly enhances Nrf2 and FOXO3, as well as their downstream endogenous antioxidants for protection against obesity-related oxidative stress with minimum side effects. Further studies should also analyze the enzymatic activity of glutathione synthetase, glutathione reductase, and GSSG, as well as the H2O2 level, in order to explain CAT/GPx/GSH regulation in detail. In addition, molecular dynamics analyses need to be performed to evaluate the protein–ligand interaction stability in a complex system more similar to the human body. This could enable prediction of the interaction stability of coriander seed phytoconstituents in inhibiting Keap1/Nrf2 and FOXO3/14-3-3 binding under conditions mimicking the environment in the human body.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biologics5040032/s1, Table S1: Raw Data.
Author Contributions
K.D.P. conducted the experiment, performed data analysis, and wrote the manuscript. N.S.H. and S.D. participated in the conceptualization, research design development, and supervised the research conduct, data analysis, data interpretation, and manuscript writing. B.A.T. advised regarding the in silico study and manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from Directorate of Research Funding & Ecosystem Universitas Indonesia (HIBAH PUTI 2024, Grant Number: NKB-285/UN2.RST/HKP.05.00/2024).
Institutional Review Board Statement
This study complied with the animal research ethics requirements of the Health Research Ethics Committee Faculty of Medicine Universitas Indonesia with approval number KET-661/UN2.F1/ETIK/PPM.00.02/2024 dated 10 May 2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data in this study are available in the Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
All authors thank to Directorate of Research Funding & Ecosystem Universitas Indonesia for funding this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3′ UTR | 3′ untranslated region |
| BSA | Bovine serum albumin |
| CAT | Catalase |
| CBP | CREB-binding protein |
| FOXO3 | Forkhead Box O3 |
| GC-MS | Gas Chromatography–Mass Spectrophotometry |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| GSSG | Glutathione disulfide |
| H2O2 | Hydrogen peroxide |
| HDAC3 | histone deacetylase 3 |
| Keap1 | Kelch-like ECH-associated Protein 1 |
| Ki | Inhibition constant |
| LC-MS | Liquid Chromatography–Mass Spectrophotometry |
| MafK | MAF bZIP transcription factor K |
| MDA | Malondialdehyde |
| MnSOD | Manganese superoxide dismutase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NAFLD | Non-alcoholic fatty liver disease |
| NFκB | Nuclear factor-κB |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PVDF | Polyvinylidene difluoride |
| ROS | Radical oxygen species |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SIRT | Sirtuin |
| TBST | Tris-buffered saline tween 0.1% |
| T-SOD | Total superoxide dismutase |
References
- Xia, Y.; Zhai, X.; Qiu, Y.; Lu, X.; Jiao, Y. The Nrf2 in Obesity: A Friend or Foe? Antioxidants 2022, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 8 April 2025).
- Shabrina, E.; Briawan, D.; Ekayanti, I.; Riyadina, W. Changes in sugar, salt, and fat intake among obese adults: Cohort study. J. Gizi Diet. (Indones. Indones. J. Nutr. Diet.) 2022, 10, 109–118. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, L.; He, J.; Wu, M.; Jia, L.; Guo, J. Role of FOXO3a Transcription Factor in the Regulation of Liver Oxidative Injury. Antioxidants 2022, 11, 2478. [Google Scholar] [CrossRef]
- Divella, R.; Mazzocca, A.; Daniele, A.; Sabbà, C.; Paradiso, A. Obesity, Nonalcoholic Fatty Liver Disease and Adipocytokines Network in Promotion of Cancer. Int. J. Biol. Sci. 2019, 15, 610–616. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Lucano-Landeros, S.; Monroy-Ramirez, H.C.; Silva-Gomez, J.; Gutierrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Roles of Nrf2 in Liver Diseases: Molecular, Pharmacological, and Epigenetic Aspects. Antioxidants 2020, 9, 980. [Google Scholar] [CrossRef]
- Li, L.; Fu, J.; Sun, J.; Liu, D.; Chen, C.; Wang, H.; Hou, Y.; Xu, Y.; Pi, J. Is Nrf2-ARE a potential target in NAFLD mitigation? Curr. Opin. Toxicol. 2019, 13, 35–44. [Google Scholar] [CrossRef]
- Song, M.Y.; Lee, D.Y.; Chun, K.S.; Kim, E.-H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef]
- Dobson, M.; Ramakrishnan, G.; Ma, S.; Kaplun, L.; Balan, V.; Fridman, R.; Tzivion, G. Bimodal regulation of FoxO3 by AKT and 14-3-3. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011, 1813, 1453–1464. [Google Scholar] [CrossRef]
- Obsilova, V.; Vecer, J.; Herman, P.; Pabianova, A.; Sulc, M.; Teisinger, J.; Boura, E.; Obsil, T. 14-3-3 Protein Interacts with Nuclear Localization Sequence of Forkhead Transcription Factor FoxO4. Biochemistry 2005, 44, 11608–11617. [Google Scholar] [CrossRef]
- Khalili, F.; Vaisi-Raygani, A.; Shakiba, E.; Kohsari, M.; Dehbani, M.; Naseri, R.; Asadi, S.; Rahimi, Z.; Rahimi, M.; Rahimi, Z. Oxidative stress parameters and keap 1 variants in T2DM: Association with T2DM, diabetic neuropathy, diabetic retinopathy, and obesity. J. Clin. Lab. Anal. 2022, 36, e24163. [Google Scholar] [CrossRef]
- Yahya, M.A.; Alshammari, G.M.; Osman, M.A.; Al-Harbi, L.N.; Yagoub, A.E.A.; AlSedairy, S.A. Isoliquiritigenin attenuates high-fat diet-induced intestinal damage by suppressing inflammation and oxidative stress and through activating Nrf2. J. Funct. Foods 2022, 92, 105058. [Google Scholar] [CrossRef]
- Mathivanan, S.; Chunchagatta Lakshman, P.K.; Singh, M.; Giridharan, S.; Sathish, K.; Hurakadli, M.A.; Bharatham, K.; Kamariah, N. Structure of a 14-3-3ε:FOXO3apS253 Phosphopeptide Complex Reveals 14-3-3 Isoform-Specific Binding of Forkhead Box Class O Transcription Factor (FOXO) Phosphoproteins. ACS Omega 2022, 7, 24344–24352. [Google Scholar] [CrossRef]
- Diallo, K.; Oppong, A.K.; Lim, G.E. Can 14-3-3 proteins serve as therapeutic targets for the treatment of metabolic diseases? Pharmacol. Res. 2019, 139, 199–206. [Google Scholar] [CrossRef]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1–Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Rahimi, Z.; Sadijam, M.; Shabab, N.; Goodarzi, M.T. Effects of Resveratrol on FOXO1 and FOXO3 a Genes Expression in Adipose Tissue, Serum Insulin, Insulin Resistance and Serum SOD Activity in Type 2 Diabetic Rats. Int. J. Mol. Cell. Med. (IJMCM) 2018, 7, 176–184. [Google Scholar]
- Lim, G.E.; Albrecht, T.; Piske, M.; Sarai, K.; Lee, J.T.C.; Ramshaw, H.S.; Sinha, S.; Guthridge, M.A.; Acker-Palmer, A.; Lopez, A.F.; et al. 14-3-3ζ coordinates adipogenesis of visceral fat. Nat. Commun. 2015, 6, 7671. [Google Scholar] [CrossRef]
- Hayat, J.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon 2020, 6, e05609. [Google Scholar] [CrossRef] [PubMed]
- de Lima, F.V.I.; Hardiany, N.S.; Dewi, S. Role of Coriander Seed Ethanolic Extract (Coriandrum sativum L.) on the Liver Tissue of High Fat Diet-Induced Rat: Focused on the Oxidative Stress and Cellular Senescence. Master’s Thesis, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia, 2022. [Google Scholar]
- Hardiany, N.S.; Fadilah, F.; de Lima, F.V.I.; Dewi, S.; Namirah, I. Flavonoid content and total antioxidant capacity of coriander (Coriandrum sativum L.) seed extract in different level polarity of solvent. AIP Conf. Proc. 2025, 3186, 20063. [Google Scholar]
- Namirah, I.; Hardiany, N.; Arozal, W. The Effects of Ethanolic Extract of C. sativum (Coriandrum sativum L.) Seed on Cellular Senescence in the Cardiovascular Organs of High-Fat Diet-Induced Rats. Ph.D. Dissertation, Faculty of Medicine, University of Indonesia, Jakarta, 2024. [Google Scholar]
- Namirah, I.; Wimbanu, K.S.; Rompies, A.M.E.; Prayogo, Y.S.; Arozal, W.; Fadilah, F.; Hanafi, M.; Hardiany, N.S. The effect of ethanol-based coriander (Coriandrum sativum L.) seed extract on oxidative stress, antioxidant level and cellular senescence in the heart of obese rat. J. Pharm. Pharmacogn. Res. 2024, 12, 1111–1120. [Google Scholar] [CrossRef]
- Pandey, A.K.; Verma, S. An in-silico evaluation of dietary components for structural inhibition of SARS-CoV-2 main protease. J. Biomol. Struct. Dyn. 2022, 40, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wanandi, S.I.; Dewi, S.; Jusman, S.W.A.; Sadikin, M. Expression of manganese superoxide dismutase in rat blood, heart and brain during induced systemic hypoxia. Med. J. Indones. 2011, 20, 27. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Popova, T.N.; Agarkov, A.A.; de Carvalho, M.Â.A.P.; Rjevskiy, S.G. Expression of Glutathione Peroxidase and Glutathione Reductase and Level of Free Radical Processes under Toxic Hepatitis in Rats. J. Toxicol. 2013, 2013, 870628. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Douglas, E.V.; Pires, T.L.B.; David, B.A. pkCSM: Predicting Small-Molecule Pharmacokinetic Properties Using Graph-Based Signatures: 1–4. Available online: https://biosig.lab.uq.edu.au/pkcsm/theory (accessed on 4 June 2025).
- Crisman, E.; Duarte, P.; Dauden, E.; Cuadrado, A.; Rodríguez-Franco, M.I.; López, M.G.; León, R. KEAP1-NRF2 protein–protein interaction inhibitors: Design, pharmacological properties and therapeutic potential. Med. Res. Rev. 2023, 43, 237–287. [Google Scholar] [CrossRef]
- Oishi, T.; Matsumaru, D.; Ota, N.; Kitamura, H.; Zhang, T.; Honkura, Y.; Katori, Y.; Motohashi, H. Activation of the NRF2 pathway in Keap1-knockdown mice attenuates progression of age-related hearing loss. NPJ Aging Mech. Dis. 2020, 6, 14. [Google Scholar] [CrossRef]
- McIntyre, R.L.; Liu, Y.J.; Hu, M.; Morris, B.J.; Willcox, B.J.; Donlon, T.A.; Houtkooper, R.H.; Janssens, G.E. Pharmaceutical and nutraceutical activation of FOXO3 for healthy longevity. Ageing Res. Rev. 2022, 78, 101621. [Google Scholar] [CrossRef]
- Pair, F.S.; Yacoubian, T.A. 14-3-3 Proteins: Novel Pharmacological Targets in Neurodegenerative Diseases. Trends Pharmacol. Sci. 2021, 42, 226–238. [Google Scholar] [CrossRef] [PubMed]
- da Sousa, L.R.; Viana, N.R.; Coêlho, A.G.; Barbosa, C.d.O.; Barros, D.S.L.; Martins, M.D.C.d.C.e.; Ramos, R.M.; Arcanjo, D.D.R. Use of Monoterpenes as Potential Therapeutics in Diabetes Mellitus: A Prospective Review. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1512974. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.-S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharmacol. 2015, 19, 21–27. [Google Scholar]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Dinkova-Kostova, A.T.; Georgiev, M.I. Obesity and NRF2-mediated cytoprotection: Where is the missing link? Pharmacol. Res. 2020, 156, 104760. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar]
- Scandar, S.; Zadra, C.; Marcotullio, M.C. Coriander (Coriandrum sativum) Polyphenols and Their Nutraceutical Value against Obesity and Metabolic Syndrome. Molecules 2023, 28, 4187. [Google Scholar] [CrossRef]
- Ji, L.L.; Sheng, Y.C.; Zheng, Z.Y.; Shi, L.; Wang, Z.-T. The involvement of p62–Keap1–Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic. Biol. Med. 2015, 85, 12–23. [Google Scholar] [CrossRef]
- Grujicic, J.; Allen, A.R. Manganese Superoxide Dismutase: Structure, Function, and Implications in Human Disease. Antioxidants 2025, 14, 848. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Kreiman, G. Multiple transcription auto regulatory loops can act as robust oscillators and decision-making motifs. Comput. Struct. Biotechnol. J. 2022, 20, 5115–5135. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Esworthy, R.S.; Doroshow, J.H.; Chu, F.F. The beginning of GPX2 and 30 years later. Free Radic. Biol. Med. 2022, 188, 419–433. [Google Scholar] [CrossRef]
- Takio, N.; Yadav, M.; Yadav, H.S. Catalase-mediated remediation of environmental pollutants and potential application–a review. Biocatal. Biotransform. 2021, 39, 389–407. [Google Scholar] [CrossRef]
- Smejkal, G.B.; Kakumanu, S. Enzymes and their turnover numbers. Expert Rev. Proteom. 2019, 16, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Hadwan, M.H.; Hussein, M.J.; Mohammed, R.M.; Hadwan, A.M.; Al-Kawaz, H.S.; Al-Obaidy, S.S.M.; Al Talebi, Z.A. An improved method for measuring catalase activity in biological samples. Biol. Methods Protoc. 2024, 9, bpae015. [Google Scholar] [CrossRef] [PubMed]
- Bizoń, A.; Chojdak-Łukasiewicz, J.; Budrewicz, S.; Pokryszko-Dragan, A.; Piwowar, A. Exploring the Relationship between Antioxidant Enzymes, Oxidative Stress Markers, and Clinical Profile in Relapsing-Remitting Multiple Sclerosis. Antioxidants 2023, 12, 1638. [Google Scholar] [CrossRef] [PubMed]
- Meilhoc, E.; Boscari, A.; Pauly, N.; Lepetit, M.; Frendo, P.; Bruand, C.; Puppo, A.; Brouquisse, R. Oxygen and derived reactive species in legume–rhizobia interactions: Paradoxes and dual roles. J. Exp. Bot. 2025, 76, 3758–3773. [Google Scholar] [CrossRef]
- Xiao, J.; Bei, Y.; Liu, J.; Dimitrova-Shumkovska, J.; Kuang, D.; Zhou, Q.; Li, J.; Yang, Y.; Xiang, Y.; Wang, F.; et al. miR-212 downregulation contributes to the protective effect of exercise against non-alcoholic fatty liver via targeting FGF-21. J. Cell Mol. Med. 2016, 20, 204–216. [Google Scholar] [CrossRef]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the regulation of cellular redox status and its implications in myocardial ischemia-reperfusion injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef]
- Guo, J.; Yang, P.; Li, Y.F.; Tang, J.-F.; He, Z.-X.; Yu, S.-G.; Yin, H.-Y. MicroRNA: Crucial modulator in purinergic signalling involved diseases. Purin. Signal. 2023, 19, 329–341. [Google Scholar] [CrossRef]
- Ruiz-Manriquez, L.M.; Carrasco-Morales, O.; Sanchez, Z.E.A.; Osorio-Perez, S.M.; Estrada-Meza, C.; Pathak, S.; Banerjee, A.; Bandyopadhyay, A.; Duttaroy, A.K.; Paul, S. MicroRNA-mediated regulation of key signaling pathways in hepatocellular carcinoma: A mechanistic insight. Front. Genet. 2022, 13, 910733. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, M.M.; Hassanein, E.H.M.; Sayed, A.M.; Alsufyani, S.E.; El-Sheikh, A.A.K.; Arab, H.H.; Mohamed, W.R. Targeting SIRT1/FoxO3a/Nrf2 and PI3K/AKT Pathways with Rebamipide Attenuates Acetic Acid-Induced Colitis in Rats. Pharmaceuticals 2023, 16, 533. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Sousa, C.; Mendes, A.F. Monoterpenes as Sirtuin-1 Activators: Therapeutic Potential in Aging and Related Diseases. Biomolecules 2022, 12, 921. [Google Scholar] [CrossRef]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel Drug Delivery Systems for Loading of Natural Plant Extracts and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).