Abstract
Background/Objectives: Sera from patients before and after organ transplantation were tested at two different temperatures, 21 °C and 37 °C. Currently, organs are transported under normothermic conditions (37 °C). This observational pilot study was conducted to define the effect of the incubation at 37 °C, comparing the results to the usual temperature of 21 °C for serum–bead incubation. Methods: We used the Luminex-based assay for the identification and characterization of HLA-specific antibodies. The assays were performed using single antigen beads for HLA class I and HLA class II. A total of 42 sera were assessed and tested, and 38 were analyzed on the Luminex 200 platform at both temperatures. Results: We noted varying outcomes: both an increase and a decrease in mean fluorescence intensity values. A shift from negative to positive values (n = 6) and vice versa (n = 1) was observed. Several sera (n = 4 for HLA class I and n = 5 for HLA class II) exhibited no alterations. In general, we observed an increase in the mean fluorescence intensity values by incubation at 37 °C. The analysis at the bead level revealed a significant deviation (37 °C vs. 21 °C) for the bead carrying HLA-A80 (p = 0.0006) and two HLA-DQ beads, DQA1*05:01-DQB1*02:01 (p = 0.0438) and DQA1*01:03-DQB1*06:03 (p = 0.0438). Conclusions: Mimicking physiological temperature conditions for the testing of HLA-specific antibodies will lead to the better and more accurate interpretation of the results. This method shows potential for use in the delisting strategy for highly sensitized patients as well, thus allowing a better and more reliable option for the patient awaiting a suitable crossmatch-negative organ.
1. Introduction
In the clinical setting of living or postmortal transplantation, it is crucial to delineate the donor-specific reactivity to the human leukocyte (HLA) antigens. This was previously achieved by utilizing the complement-dependent cytotoxicity assay (CDC), with various HLA-typed lymphocyte suspensions from blood donors as targets. The level of positivity was assessed by the ratio of positive responses to the panel. The Luminex-based assay for HLA-specific antibodies was introduced more than 20 years ago [1,2]. It is a bead-based multiplex immunoassay that facilitates the concurrent detection and definition of antibodies against HLA antigens in a single sample. It employs polystyrene microspheres referred to as beads internally infused with unique ratios of fluorophores. Each distinct HLA antigen is coupled to an individual bead, facilitating its simultaneous discrimination. During the assay, the serum sample of a proband/patient is incubated with a mixture of these beads; in the present report, 98 beads are coupled with HLA class I antigens and 100 with HLA class II antigens. After washing, a phycoerythrin (PE) reporter consisting of an anti-human Ig antibody is added to form a complex. The beads are analyzed by a LuminexTM instrument, which uses lasers to identify the bead region and define the PE signal leading to the mean fluorescence intensity (MFI) values. This technology transformed HLA antibody testing into an efficient and dependable approach for pre- and post-transplantation monitoring [3]. The assay is performed at ambient temperature, which is 21 °C. The incubation of the sera with EDTA eradicates possible prozone effects [4,5], hence enhancing the test’s reliability. However, there are still inconsistencies surrounding the assay. Conformational changes in the target HLA antigens, i.e., linearized molecules, make the decision difficult and lead to false positive or negative results [6]. In addition, sera contain IgM-specific antibodies, which might jeopardize the results as reported earlier [7]. Recently, we showed that day-to-day variations are significant in [8]. Similar results were observed by others [9].
Incubation at 37 °C typically enhances the efficacy of antibody testing [10]. Comparable outcomes were observed for clinical organ transplantation. An elevation in temperature substantially influences antibody binding [11]. In HLA immunogenetics, initially, incubation at 37 °C was employed for the detection of HLA-specific antibodies and the crossmatching technique; however, it was later abandoned, as most antibodies exhibit comparable efficacy at 30 °C or lower as they do at 37 °C [12]. Gel centrifugation assays for antibody identification demonstrated a strong correlation between room temperature incubation and incubation at 37 °C, indicating that room temperature incubation may be conducted concurrently with ABO blood group typing without compromising sensitivity or specificity [13]. Increased temperatures generally result in increased association and dissociation rates; however, the effect on equilibrium constants is not always clear-cut [14]. In clinical kidney transplantation, highly sensitized patients awaiting a suitable organ are penalized by the presence of denatured (linearized) HLA antigens detected in routine HLA antibody testing [15,16]. Cryoagglutinins, extensively described in red cell serology, are a kind of antibody species observed in human plasma that primarily react at lower temperatures and are ineffective at the physiological temperature of 37 °C. They are of the IgM class and may negatively influence any tests employed for screening and crossmatching [17]. This is consistent with the abovementioned reported results on the influence of IgM antibodies in the Luminex assay. Furthermore, the CDC crossmatch is performed for evaluating the risk of hyperacute rejection, particularly in the kidney transplantation [18]. However, it detects IgM-type anti-HLA antibodies, which are considered irrelevant for the outcome of grafting [19,20]. The prevailing agreement is that incubation at 37 °C is optimal for identifying clinically relevant IgG antibodies, which are implicated in hyperacute or acute accelerated rejection. IgM antibodies that typically demonstrate a response at lower temperatures (e.g., 4 °C) are regarded as clinically insignificant, thus not affecting graft survival. In contrast, all experiments for antibody characterization were performed at room temperature for practical reasons. From the findings outlined above, we can conclude that the definition of the HLA specificity of a given patient serum is of utmost importance to provide the patient with a suitable crossmatch-negative organ. In this context, only relevant antigens against which the patient produces antibodies should be set as unacceptable mismatches [21]. All the patient transplantation-irrelevant ones should be removed or delisted.
The objective of this study was not to investigate the assay’s utmost efficiency but to demonstrate the results within the physiological response of transplanted individuals. In this observational pilot study, we inquired as to whether incubating the patient’s serum with HLA antigen-coated polystyrene beads at 37 °C might yield significant results, thereby enhancing screening and crossmatching before and following transplantation monitoring by removing nonspecific reactivities. Our rationale is based on the fact that organs are currently transported and provided in normothermic circumstances and subsequently transplanted into a recipient under the same conditions. Consequently, antibodies demonstrate their efficacy at normothermic temperatures, specifically at 37 °C. Consequently, we examined the reactivity of sera, mainly from transplanted patients, at 37 °C and compared the findings with those at the standard 21 °C.
2. Materials and Methods
As part of the quarterly or the regular follow up HLA-antibody screening of transplantation patients, serum samples were taken and stored(−21 °C). No extra bleeding was performed for the study. Samples of thirty organ transplant patients were tested in parallel at 21 °C and 37 °C using the bead-based multiplexing assays for HLA antibody definition (LABScreenTM single antigen assay, provided by One Lambda, West Hills, CA, USA). Both preparations were performed using a shaker. We selected the sera because of the availability of the data with respect to antibody profile. The criteria were patients on the organ waiting list or follow-up list with known multiple or monospecific reactivity patterns and negative reactions. In total, 42 sera (n = 11 HLA class I only; n = 7 HLA class II only; n = 21 HLA class I and II and 10 controls) were tested on three different days. All three test runs were carried out by the same person. Negative control sera (NC lots 26 and 27 provided by One Lambda, West Hills, CA, USA) and positive control sera (for HLA class I IgG control from the ETRL Leiden, the Netherlands, and in-house HLA class II) were used. The testing of patient sera was not repeated on different days. In this observational study, we used the current LABScreen™ lot numbers: #15 for the HLA class I assay and lot #16 for the HLA class II assay. The sera were tested according to the manufacturer’s recommendations, except that for the first incubation (serum–bead incubation), and 37 °C was used where appropriate. After the initial incubation at 21 °C or 37 °C, we continued the assay at 21 °C. Finally, measurements were performed at ambient temperature using a Luminex 200 system, unless otherwise indicated in the Supplementary Materials (Tables S2 and S3). Since the study was performed using stored (−21 °C) and earlier-tested sera (in clinical diagnostics), no additional ethical approval was needed. In this study, patients on the waiting list or after organ transplantation were tested with the single-antigen solid-phase assay (One Lambda, West Hills, CA, USA). The characteristics of the cohort are shown in Table 1.

Table 1.
Characteristics of the cohort (status October 2024, age at serum collection).
The Luminex200TM or FLEXMAP 3D was used for HLA antibody testing, whereby the measurements were carried out with Xponent software version 4.3, and the antibody analysis was performed with HLA Fusion software version 4.7.0 (for further explanation, see Figure 1). In total, four patient sera in both cohorts were excluded from the analysis due to “low bead count” or because the positive and negative control beads were outside the valid measurement range. We scored the different groups as follows: red = 10,000 MFI or higher; orange = MFI between 3000 and 10,000; yellow = MFI between 1500 and 3000; blue = MFI between 500 and 1500; green = under 500 MFI. For better uniformity of the y-axis, we restricted the MFI values to a maximum of 10,000 in all figures. Sera with MFI < 1500 are regarded negative. We defined an MFI shift as the change in a category to any other, expressed by a higher or a lower MFI value. For this purpose, the sum of all MFI values of all beads (control beads excluded) was determined for each serum, and the change between bead incubation at 21 °C and 37 °C was determined as a % deviation (“change MFI in %”).
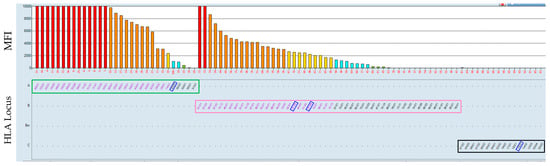
Figure 1.
Evaluation in the Fusion software using the example of HLA class I beads. On the y-axis in the upper part of the figure, the MFI values are indicated by color codes; red indicates MFI values over 10,000, orange indicates MFI between 3000 and 10,000, yellow indicates MFI between 1500 and 3000, blue indicates MFI between 500 and 1500, and green is under 500 MFI. On the x-axis, red numbers are shown, which indicate bead IDs with specific HLA antigens, according to the manufacturer’s instructions. In this figure, the reactions/bars have been sorted according to HLA loci: HLA-A locus (green box), HLA-B locus (pink box), and HLA-C locus (black box) in the lower part of the figure. Blue rectangles indicate the HLA mismatches the patient received.
3. Results
The study includes transplant recipients (n = 30) from whom pretested sera were used (see Table 1). Most of the patients were males, and the mean age at the date of bleeding was 47.8 years. Most of the transplants were kidneys. An overview of all tested sera and a comparison of serum–bead incubation at 21 °C and 37 °C is shown in Figure 2a,b. Figure S1 shows the change in MFI (%) for HLA class I. In the cohort studied, differences in the effect of 37 °C incubation of the HLA-A (Figure S1a), B (Figure S1b), and C loci (Figure S1c) are evident. The largest change is observed in the HLA locus C (up to >400% increase in MFI), followed by the HLA-B locus and HLA-A locus, with up to 200% increase in MFI. HLA class II shows in few sera an extreme increase in MFI values at 37 °C. This seems to be serum/patient specific (Figure S2). The comparison between the 21 °C incubation and the 37 °C incubation is depicted in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8.
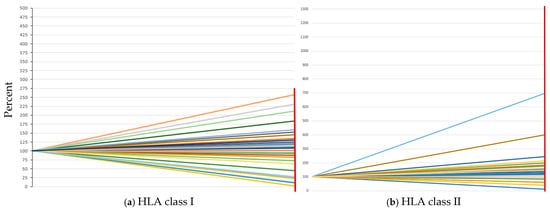
Figure 2.
Comparison of bead–serum incubation at 21 °C (100% on the y-axis) and 37 °C (red vertical line on the right side of the diagram). (a) Change in the sum of all MFI values in percent at 21 °C and 37 °C for HLA class I. (b) Change in the sum of all MFI values in percent at 21 °C and 37 °C for HLA class II. Each colored line indicates an individual serum.
In our study, we observed changes both in the HLA class I and HLA class II antigens. As shown in Table 2, in the category of no changes, it was equally distributed between the HLA class I and HLA class II antigens. Mainly, the change to more negative results was seen. In general, the results obtained after an incubation at the higher temperature resulted in clearer results, in our opinion. Table 3 depicts the qualitative changes in the results (positive to negative or negative to positive) in the different HLA loci A, B, C, DR, DQ, and DP with respect to the number of sera and to the MFI values observed. The respective percentage values are also shown. A number of sera showed no changes for HLA-A n = 12 (41.4%), HLA-B n = 15 (51.7%), and HLA-C n = 21 (72.4%). While n = 17 (58.6% HLA-A), n = 14 (48.3% HLA-B), and n = 8 (27.6% HLA-C), respectively, show changes. Regarding the HLA class II loci, DR, DQ, and DP, the results were as follows. No qualitative changes in the results: n = 19 (67.9% HLA-DR), n = 15 (53.6% HLA-DQ), and n = 21 (75.0% HLA-DP), respectively, with changes in n = 9 (32.1% HLA-DR), n = 13 (46.3% HLA-DQ), and n = 7 (25.0% HLA-DP), respectively. Analyzing the individual beads separately in a 2 × 2 formation of beads revealed a statistically significant deviation, as seen in Table 3 (change MFI %): bead ID33 (HLA-A*80) p = 0.0006, ID 42 (DQA1*05:01-DQB1*02:01) p = 0.0438, and bead ID53 (DQA1*01:03-DQB1*06:03) p = 0.0438.

Table 2.
Categories of changes observed in comparison with 21 °C and 37 °C bead incubation.

Table 3.
Qualitative changes in the serum results for the different HLA-loci (from 21 °C to 37 °C).
In the following section, we present screenshots of the results of patients’ sera incubated at the two different temperatures. NB: The sera were pretreated with EDTA, as mentioned earlier [4], before the incubation reported here.
3.1. No Qualitative Change in Result by Incubation at 37 °C
Figure 3 shows the results of patients transplanted with a heart in 10/2023. The incubation of the serum obtained in 10/2024 and tested towards HLA class I beads at 37 °C resulted in the following data. For a previously defined HLA-negative serum, it showed, as expected, no altered results. Both HLA class I (a) and HLA class II (b) beads also remained negative one-year post-transplantation (Figure 3).
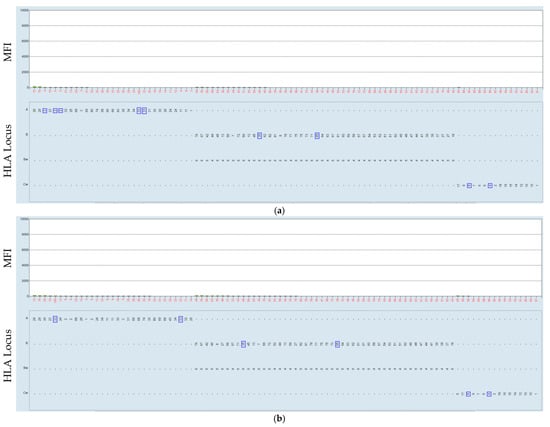

Figure 3.
Negative serum serum–bead incubation of the HLA class I beads was performed at 21 °C (a) and at 37 °C (b). The HLA was mismatched with that which the patient received in consecutive transplantations, marked by the blue rectangle.
The patient of this serum received their first transplant in 1985, followed by a combined transplant in 06/2009. For the serum of 08/2024, shown in Figure 4, the incubation of the HLA class I beads at 37 °C resulted in no change in the results. However, we observed a shift to a higher category for the MFI values (from 6846 MFI at 21 °C to 12,682 at 37 °C) for the mismatch HLA-A1 the patient received.
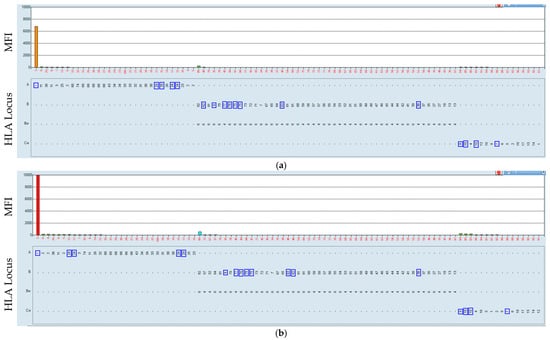

Figure 4.
Positive serum reacting with the HLA-A1 antigen-coated bead. Serum–bead incubation of the HLA class I beads was performed at 21 °C (a) and at 37 °C (b). The HLA was mismatched with that which the patient received in consecutive transplantations, marked by the blue rectangle.
3.2. Elimination of Presumably Irrelevant Positive Reactions
The serum was obtained on 07/2024 from a kidney transplantation patient; the transplant was performed on 06/2000. The incubation at 37 °C of the HLA class I beads (Figure 5) resulted in a shift from positive to negative results with regard to the MFI values (16,636 at 21 °C to 1349 at 37 °C). The remaining HLA class I (a) and HLA class II (b) beads were unaffected.
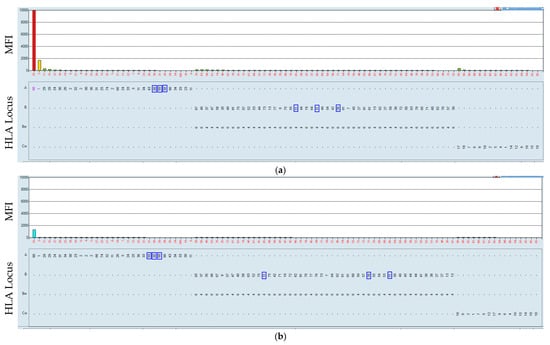

Figure 5.
Positive serum reacting with the HLA-A80 antigen-coated bead (a). The incubation of the HLA class I beads at 37 °C resulted in a negative result (b). Serum–bead incubation at 21 °C (a) and at 37 °C (b). The HLA was mismatched with that which the patient received, marked by blue rectangles. In this case, we observed that all but one of the beads were negative. The HLA-A80-coated bead was positive at 21 °C and negative, as expected, at 37 °C.
3.3. Negative to Positive Reactions
Serum from 04/2024 of a mother who underwent a kidney transplant was tested using the HLA class II beads as targets. The first reported immunizing event, i.e., the delivery of a child, was in 2014, and the patient received their transplant in 12/2024. The incubation of the serum using the HLA class II beads at 37 °C resulted in a shift from negative (lower MFI) to positive (higher MFI) results, as shown in Figure 6. Some beads (bead ID 59 (from 3856 to 17,705), ID 60 (from 3703 to 18,224), ID 63 (from 3681 to 15,796), ID 58 (from 3379 to 16,905), ID 66 (from 3245 to 16,277), ID 65 (from 2397 to 12,326), and ID 56 (from 1994 to 12,834) were observed as having an increased MFI value at 37 °C. The beads are coated with the HLA-DQB1*03 (DQ7) HLA antigen, which was the immunizing antigen during the pregnancy. Regarding the HLA mismatches to the transplant, the organ donor-specific HLA antibody HLA-DRB4*01:03 was negative at 21 °C but positive at 37 °C (MFI 173 to 3000). The testing was performed ca. 8 months prior to the transplantation. In this case, testing at a higher temperature would have prevented possible complications post-transplantation. The patient suffered rejection episodes, treated through several rounds of plasmapheresis and the administration of daratumumab and eculizumab, drugs used to treat humoral rejections. The patient is back on dialysis while being treated with drugs for humoral rejection. All the remaining HLA class I (a) and HLA class II (b) beads remained unaffected.
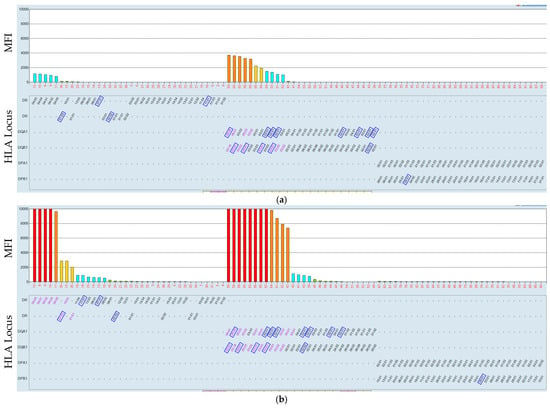

Figure 6.
Low-HLA class II MFI values increased after incubation at the higher temperature (37 °C). Serum–bead incubation of the HLA class II beads was at 21 °C (a) and at 37 °C (b). HLA was mismatched with the antigens the patient received, marked by a blue rectangle. The position of the rectangle might differ between (a,b) due to the sorting procedure of the software offered by the provider.
3.4. Shift in MFI Values
The serum of the patient transplanted in 08/1996 and 02/2007 was collected in 04/2024. The testing was performed using the HLA class I beads. The comparison of the incubation temperature towards the beads resulted in more negative results, as shown in Figure 7. Markedly, a higher number of beads show a negative result with, MFI values < 1500 at 37 °C compared to 21 °C, specifically for HLA-A bead ID 8 (from 19,070 to 1171), ID 9 (from 19,855 to 359), ID 10 (from 20,067 to 152), ID 11 (from 19,067 to 881), ID 12 (from 20,358 to 125), ID 14 (from 19,204 to 856), ID 15 (from 20,047 to 58), ID 17 (19,483 to 34), ID 18 (from 6048 to 1076), ID 22 (from 2281 to 931), ID 26 (from 19,641 to 470), and ID 32 (from 3720 to 618). The other HLA antigens of the B and C loci behave similarly.
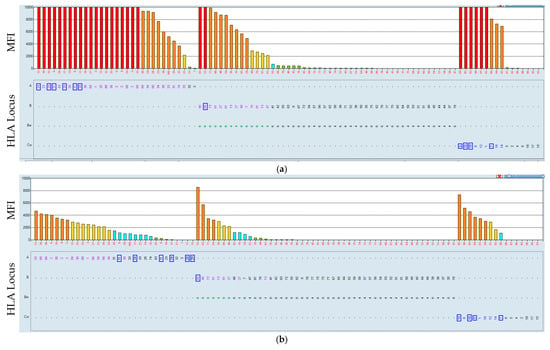

Figure 7.
Incubation of serum of a highly sensitized patient with HLA class I beads at 21 °C and 37 °C. Serum–bead incubation at 21 °C (a) and at 37 °C (b). The HLA is mismatched with that which the patient received in his transplantation history, marked by a blue rectangle.
In the same category as reported above, the serum, the same as in Figure 5, of the patient using the HLA class II beads as targets. The incubation of the serum of the patient showed (Figure 8), at 37 °C, a shift to more negative results, together with lower MFI values. The respective MFI values are ID 40 (from 3064 to 487), ID 49 (from 4099 to 591), ID 52 (from 2083 to 895), ID 54 (from 1613 to 270). These results point to a possible factor in the serum of the patient that influences the reactivity.
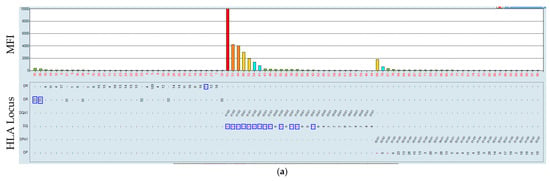
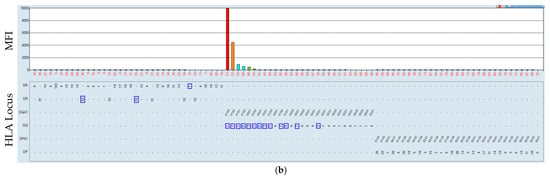


Figure 8.
Incubation of the serum of a sensitized patient at 21 and 37 °C. Serum–bead incubation for the HLA class II beads at 21 °C (a) and at 37 °C (b). The HLA is mismatched to that which the patient received, marked by a blue rectangle.
4. Discussion
HLA antibody screening is a crucial and integral step before accepting an organ for transplantation as well as the longitudinal monitoring after grafting. Usually, a panel of HLA-type cells is used. The panels generally contain between 30 and 50 different cell preparations. The percentage of positivity was reported as vPRA [22] or cPRA [23] for virtual or calculated panel reactive antibodies. This value was used and is used for allocation purposes, following the idea that the higher the percentage, the more difficult it will be to obtain a suitable crossmatch-negative organ for the patient. Therefore, the patients receive additional points for allocation. The definition, and especially the specification, of HLA antibodies became easier and more reliable. Recently, we reported that the assay is hampered by several unforeseen problems, such as the reproducibility of the day-to-day testing or the results obtained when different people perform the assay [8]. Therefore, to avoid this type of inconsistency, the same performed all of the comparison experiments in parallel on the same day. Our intention was not to establish a procedure to obtain the best results by optimizing the conditions of the assay but to analyze the role of the physiological conditions in which the organ is transplanted.
In the present report, we attempted to analyze the serum reactivity of patients, most of whom had undergone transplantation, by reproducing the assays of the early days of HLA antibody screening using the now outdated CDC methodology. We introduced an incubation at 37 °C and compared the results with the data obtained at 21 °C. The rationale behind our testing is to (i) mimic the physiological conditions of the transplanted patients and organ; (ii) adhere to the now widely used normothermic transport of the organs, i.e., at 37 °C, in normothermic perfusion incubators; and (iii) avoid as much as possible unwanted interferences with the many molecules in the serum of patients as well.
It is widely accepted that the increase in the testing temperature results in diminishing the effectiveness of so-called cryoglobulin, primarily observed in the setting of the red cell serology. Cryoagglutinins usually have no clinical relevance but might add additional information for diagnostic purposes. In the physiological context, antibodies are well adapted to function and persist at 37 °C due to inherent stability and biological recycling mechanisms. It is also well accepted that an increase in the testing temperature from 21 °C to 37 °C mainly influences the on-and-off rate of the antibodies from their target. Many factors can influence the binding. According to the preliminary results shown here, the reason for a change is probably mainly due to serum itself, a fact that should be substantiated with more data. Our cohort was not tested for the occurrence of conformational changes in the HLA antigens coupled to the beads, and therefore no information is available on the possible reactivity of the antigens with the sera of the patients at 37 °C. The clinical relevance of that is still disputed [24].
We observed expected and unexpected results as well. In the first category lies the data of negative sera, which remained negative after incubation at 37 °C. Furthermore, sera recognizing specific HLA antigens showed no alteration by an incubation at 37 °C (Table 3 and Table 4).

Table 4.
Qualitative changes, positive to negative result or negative to positive result (from 21 °C to 37 °C), based on MFI values of individual beads.
It is also immunologically logical that in some instances, an increase in the MFI value was observed for specific HLA antigens. This increase in the serum reactivity, meaning an increase in the mean fluorescence intensity, is probably due to inactivation of factors interfering with the primary binding of the antibodies to the target coupled onto the polystyrene beads [25]. Variations in the MFI values can be ascribed to a combination of the aforementioned factors. The removal of reactivities targeting individual HLA specificities, such as HLA-A80, shown here, is warranted, as they are considered false positives due to the absence of a specific epitope exclusively associated with this specificity. This specificity was also found to be significant in terms of deviations, together with antigens of the HLA-DQ locus. Extensive repeated testing is required to confirm the results. Similar results are expected with other HLA antigens occurring at a very low incidence in the population analyzed. The reactivity can then be attributed as nonspecific or not affecting a possible transplant. Under these conditions, a prospective organ donor with no deleterious consequences can express the HLA antigen. It would be premature, given the current state of the data, to offer a list of possible serum reactivities towards specific HLA antigens, such as HLA-A80. However, such data are collected and can be made available in due time. It is worth noting that a collaboration of many centers will help to identify such HLA antigens.
An intriguing observation was that a positive serum at 21 °C was seen to be less reactive at 37 °C. This unexpected observation calls for an explanation. We can eliminate the possibility of a mix-up of the samples because the incubation was performed on plates together with other samples shown above. Possibly, we are dealing with antibodies of lower avidity/affinity at 37 °C, which may be used for a delisting strategy in the case of patients with many different HLA-specific antibodies, termed as highly sensitized. A number of such lower-reacting antibodies, or antibodies with a lower avidity/affinity, can delisted from the list of unacceptable HLA antigens for transplantation. This will open up new pathways for the treatment of such difficult patients, e.g., using Imlifidase or CD38-specific antibodies to eliminate plasma cells [26,27].
For the patients, if tested with the increase in temperature, as proposed here, the path to the clear detection of HLA-specific antibodies will offer additional possibilities in terms of offering a crossmatch-negative graft. Following the reasoning of the virtual crossmatches, as used in many organ procurement organizations, data that are more accurate can enter the procedure. Especially for highly sensitized patients, the procedure can and will offer possibilities to safely delist antigens from the unacceptable antigens list, as shown above in Figure 5, Figure 7 and Figure 8. There are several possibilities that explain the results: (i) inactivation of blocking factors in the serum of the patients at 37 °C, as observed here; (ii) an additive effect of HLA antigen and serum as seen for the HLA-A80 and DQ antigens. Surely, additional possibilities are possible and will be discussed when more data are available. We propose that to avoid extensive unproductive discussions, confirmation of the immunizing event should be reported. For plausibility reasons, HLA typing should be performed at the highest resolution possible [28].
5. Conclusions
Obviously, the number of patients as well as the number of sera tested in the present pilot study is rather low. We would like, however, to emphasize that in view of the transplantation procedure, a normothermic testing might provide better results both for those patients showing lower activity and those with higher activity. Our objective was not to optimize any test conditions but to enhance the physiological potential of the assay by selecting conditions that could most benefit the patients to obtain a suitable organ. These results, however, present the possibility of a larger and possibly multicenter study to confirm the findings.
Normothermic conditions when testing patients’ sera for HLA-specific antibodies prior to and post-transplantation as a form of monitoring might help in the decision-making process by increasing the understanding of the results and will lead to better and more accurate results for the benefit of the patients.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biologics5040031/s1: Table S1: HLA Typing of recipients and immunizer; Table S2: Comparison of the effect of different temperatures on HLA class I in different Luminex devices; Table S3: Comparison of the effect of different temperatures on HLA class II in different Luminex devices; Figures S1: Comparison of bead–serum incubation with HLA class I; Figures S2: Comparison of bead–serum incubation with HLA class II.
Author Contributions
Conceptualization, C.L. and I.D.; methodology, C.L. and R.L.; software, C.L.; formal analysis, C.L.; investigation, C.L.; resources, C.L. and R.L.; data curation, C.L.; writing—original draft preparation, C.L. and I.D.; writing—review and editing, R.L.; visualization, C.L.; supervision, I.D.; project administration, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of University Leipzig, Medical Faculty; ethics vote is 336/23-ek dated 24 October 2023).
Informed Consent Statement
Informed consent was obtained from all subjects as part of routine diagnostics.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tait, B.D. Detection of HLA Antibodies in Organ Transplant Recipients—Triumphs and Challenges of the Solid Phase Bead Assay. Front. Immunol. 2016, 7, 570. [Google Scholar] [CrossRef]
- Slavcev, A. Prediction of organ transplant rejection by HLA-specific and non-HLA antibodies--brief literature review. Int. J. Immunogenet. 2013, 40, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Lee, J.; Chen, T.; Rojo, S.; Terasaki, P.I. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Hum. Immunol. 1999, 60, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Schnaidt, M.; Weinstock, C.; Jurisic, M.; Schmid-Horch, B.; Ender, A.; Wernet, D. HLA antibody specification using single-antigen beads—A technical solution for the prozone effect. Transplantation 2011, 92, 510–515. [Google Scholar] [CrossRef]
- Battle, R.K.; Henderson, L.; Phelan, P.J.; Latham, K.; Turner, D.M. A case report-Two manufacturers SAB testing kits can reveal different HLA antibody profiles-Identifying prozone and denatured antigen. HLA 2020, 96, 76–82. [Google Scholar] [CrossRef]
- Jucaud, V.; Ravindranath, M.H.; Terasaki, P.I. Conformational Variants of the Individual HLA-I Antigens on Luminex Single Antigen Beads Used in Monitoring HLA Antibodies: Problems and Solutions. Transplantation 2017, 101, 764–777. [Google Scholar] [CrossRef]
- Kosmoliaptsis, V.; Bradley, J.A.; Peacock, S.; Chaudhry, A.N.; Taylor, C.J. Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigen-beads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies. Transplantation 2009, 87, 813–820. [Google Scholar] [CrossRef]
- Schacker, T.; Landgraf, R.; Doxiadis, I.; Loeffler-Wirth, H.; Lehmann, C. Luminex MFI—Efforts from a Qualitative to a Quantitative Analysis. Biology 2025, 14, 686. [Google Scholar] [CrossRef] [PubMed]
- Middleton, D.; Jones, J.; Lowe, D. Nothing’s perfect: The art of defining HLA-specific antibodies. Transpl. Immunol. 2014, 30, 115–121. [Google Scholar] [CrossRef]
- AuBuchon, J.P.; de Wildt-Eggen, J.; Dumont, L.J. Reducing the variation in performance of antibody titrations. Arch. Pathol. Lab. Med. 2008, 132, 1194–1201. [Google Scholar] [CrossRef]
- Das, A.; Taner, T.; Kim, J.; Emamaullee, J. Crossmatch, Donor-specific Antibody Testing, and Immunosuppression in Simultaneous Liver and Kidney Transplantation: A Review. Transplantation 2021, 105, e285–e291. [Google Scholar] [CrossRef] [PubMed]
- Arndt, P.; Garratty, G. Evaluation of the optimal incubation temperature for detecting certain IgG antibodies with potential clinical significance. Transfusion 1988, 28, 210–213. [Google Scholar] [CrossRef]
- Hitzler, W.E.; Ulrich, M.; Däumer, C.; Beeser, H. Der Gelzentrifugationstest: Vergleich der Antikörpersuche bei 37 degrees C mit der Antikörpersuche bei Raumtemperatur. Transfus. Med. Hemotherapy 1993, 20 (Suppl. S2), 61–63. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Andrew, S.M.; Hogarth, M.P.; Pietersz, G.A.; McKenzie, I.F. The effect of temperature on the binding kinetics and equilibrium constants of monoclonal antibodies to cell surface antigens. Mol. Immunol. 1990, 27, 327–333. [Google Scholar] [CrossRef]
- Cai, J.; Terasaki, P.I.; Anderson, N.; Lachmann, N.; Schönemann, C. Intact HLA not beta2m-free heavy chain-specific HLA class I antibodies are predictive of graft failure. Transplantation 2009, 88, 226–230. [Google Scholar] [CrossRef]
- Battle, R.K.; Rennie, T.J.W.; Phelan, P.J.; Abel, A.A.; McConnell, S.; Turner, D.M. Highly sensitised patients awaiting deceased donor renal transplants are disadvantaged by the presence of denatured HLA antibody detected in routine HLA antibody testing. HLA 2022, 100, 24–36. [Google Scholar] [CrossRef]
- Williams, R.C.; Emmons, J.D.; Yunis, E.J. Studies of human sera with cytotoxic activity. J. Clin. Investig. 1971, 50, 1514–1524. [Google Scholar] [CrossRef][Green Version]
- Doxiadis, I.I.N.; Roelen, D.; Claas, F.H.J. Mature wines are better: CDC as the leading method to define highly sensitized patients. Curr. Opin. Organ Transplant. 2010, 15, 716–719. [Google Scholar] [CrossRef]
- Suzuki, M.; Ishida, H.; Komatsu, T.; Kennoki, T.; Ishizuka, T.; Tanabe, K. Kidney transplantation in a recipient with anti-HLA antibody IgM positive. Transpl. Immunol. 2009, 21, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Viglietti, D.; Lefaucheur, C.; Glotz, D. Evidence for an important role of both complement-binding and noncomplement-binding donor-specific antibodies in renal transplantation. Curr. Opin. Organ Transplant. 2016, 21, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Claas, F.H.J.; Rahmel, A.; Doxiadis, I.I.N. Enhanced kidney allocation to highly sensitized patients by the acceptable mismatch program. Transplantation 2009, 88, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Doxiadis, I.; Haasnoot, G.; Witvliet, M.; Claas, F. 10-P: The virtual PRA: An excellent tool to predict the chance to find a crossmatch negative donor. Hum. Immunol. 2007, 68, S20. [Google Scholar] [CrossRef]
- Gebel, H.M.; Kasiske, B.L.; Gustafson, S.K.; Pyke, J.; Shteyn, E.; Israni, A.K.; Bray, R.A.; Snyder, J.J.; Friedewald, J.J.; Segev, D.L. Allocating Deceased Donor Kidneys to Candidates with High Panel-Reactive Antibodies. Clin. J. Am. Soc. Nephrol. 2016, 11, 505–511. [Google Scholar] [CrossRef]
- Visentin, J.; Guidicelli, G.; Moreau, J.-F.; Lee, J.-H.; Taupin, J.-L. Deciphering allogeneic antibody response against native and denatured HLA epitopes in organ transplantation. Eur. J. Immunol. 2015, 45, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Zachary, A.A.; Lucas, D.P.; Detrick, B.; Leffell, M.S. Naturally occurring interference in Luminex assays for HLA-specific antibodies: Characteristics and resolution. Hum. Immunol. 2009, 70, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Kwun, J.; Matignon, M.; Manook, M.; Guendouz, S.; Audard, V.; Kheav, D.; Poullot, E.; Gautreau, C.; Ezekian, B.; Bodez, D.; et al. Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use. J. Am. Soc. Nephrol. 2019, 30, 1206–1219. [Google Scholar] [CrossRef]
- Jordan, S.C.; Legendre, C.; Desai, N.M.; Lorant, T.; Bengtsson, M.; Lonze, B.E.; Vo, A.A.; Runström, A.; Laxmyr, L.; Sjöholm, K.; et al. Imlifidase Desensitization in Crossmatch-positive, Highly Sensitized Kidney Transplant Recipients: Results of an International Phase 2 Trial (Highdes). Transplantation 2021, 105, 1808–1817. [Google Scholar] [CrossRef]
- Lehmann, C.; Pehnke, S.; Weimann, A.; Bachmann, A.; Dittrich, K.; Petzold, F.; Fürst, D.; de Fallois, J.; Landgraf, R.; Henschler, R.; et al. Extended genomic HLA typing identifies previously unrecognized mismatches in living kidney transplantation. Front. Immunol. 2023, 14, 1094862. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).