Abstract
Heparan sulfate proteoglycans are highly glycosylated proteins in which heparan sulfate, a glycosaminoglycan sugar chain, is an acidic sugar chain consisting of a repeating disaccharide structure of glucuronic acid and N-acetylglucosamine is locally sulfated. Syndecan, one of the transmembrane HSPGs, functions as a receptor that transmits signals from the extracellular microenvironment to the inside of the cell. In the vascular system, heparan sulfate proteoglycans, a major component of the glycocalyx, enable the binding of various plasma-derived molecules due to their diversity, epimerization of glycosaminoglycans chains, long chains, and sulfation. Heparan sulfate proteoglycans present in the extracellular matrix serve as a reservoir for bioactive molecules such as chemokines, cytokines, and growth factors. Aberrant expression of heparan sulfate proteoglycans, heparanase, and sulfatase is observed in many pathological conditions. Therefore, it can be applied to therapeutic strategies for a wide range of fields including Alzheimer’s disease, heart failure, cancer, organ transplants, diabetes, chronic inflammation, aging, and autoimmune diseases.
1. Introduction
The heparan sulfate proteoglycans (HSPGs) are highly glycosylated proteins in which heparan sulfate (HS), a glycosaminoglycan sugar chain, is an acidic sugar chain consisting of a repeating disaccharide structure of glucuronic acid and N-acetylglucosamine is locally sulfated (Figure 1) [1,2]. Chondroitin sulfate (CS) and dermatan sulfate also belong to sulfated glycosaminoglycans (GAGs), but they differ from HS in that they contain galactosamine as an amino sugar. Furthermore, heparin localized within mast cells is homologous to HS, which exists on the cell surface and basement membrane as HSPG covalently bound to core protein and plays important physiological roles while interacting with various substances. The number of sugar chains of HSPGs on the cell surface varies from one to three or four [3].
In addition, in vivo, it is covalently bound to a protein, core protein, and expressed as HSPG, which are broadly classified into four types: Syndecan, glypican, perlecan, and agrin [4]. Among them, Syndecan and glypican are proteoglycans expressed on the cell surface. Three to five sugar chains and the core protein of glypican are bound to the cell membrane via glycated phosphatidylinositol (GPI) [5]. HSPGs with many sugar chains, including Syndecan, are recognized as a “full-time type”, in which the sugar chains are constantly modified [3]. Syndecan in mammals consists of four genetically distinct core proteins. The expression of Syndecan-1/Syndecan is primarily in the epithelium and some leukocytes, Syndecan-2/fibroglycan is expressed mostly in mesenchymal cells, Syndecan-3/N-syndecan is expressed in the nervous system, endothelium, and muscle tissue, and Syndecan-4/ryudocan is expressed almost ubiquitously [6,7]. Syndecan mRNAs encode a family of diverse extracellular domains consisting of an N-terminal signal peptide and an HS or CS addition site, a single transmembrane domain, and an intracellular domain of a short C-terminal consisting of conserved C1 and C2 domains separated by a diverse domain unique to each Syndecan [8]. Although the cytoplasmic domain of Syndecan is only a small portion of the molecules, this domain has important functions, such as regulating adhesion to the extracellular matrix, which contributes significantly to the progression of tumors. Syndecan-4 interacts with protein kinase C, alpha (PKCa), and Phosphatidylinositol 4,5-bisphosphate (PtdIns4,5P2) in fibroblasts cultured on fibronectin, resulting in enhanced activation of PKCa. This interaction is mediated by the LGKKPIYKK sequence, which is absent in other Syndecans and therefore cannot activate PKC, at the center of the short intracellular domain of Syndecan-4.
HSPGs, as a molecular group, have been involved in developmental and homeostatic processes but also many pathological conditions, particularly cancer [9,10,11]. There are six types of glypicans, including Glypican-1/Glypican, Glypican-2/Cerebroglycan, Glypican-3/OCI-5, Glypican-4/L-Glypican, Glypican-5, and Glypican-6 [12]. The glypican family has 14 cysteine residues necessary for forming a compact three-dimensional structure and a COOH-terminal structure that is covalently bounded to GPI and anchored to the cell membrane. The glypican family is mainly distributed on the cell membrane at the tip of the cell, but it is also expressed in a cell-type-specific manner, with only Glypican-1 present in vascular endothelial cells. On the other hand, perlecan and agrin are proteoglycans that are secreted outside the cell and form the extracellular matrix [13,14]. The core protein of perlecan has a molecular weight of 467 kDa in humans and consists of five functional domains like other extracellular matrix (ECM) molecules. The first domain at the N-terminus is a binding site for three HS chains, the second domain is a repeating structure like the low-density lipoprotein (LDL) receptor, the third domain is a repeating structure like a laminin short arm, the fourth domain contains an Ig-type NCAM (neural cell adhesion molecules)-like repeat, and the fifth domain contains a laminin A chain-like repeat and an epidermal growth factors (EGF) motif [14]. The core protein portion alone shows extreme multifunctionality [14,15]. There is also a hybrid type with added dermatan sulfate chains. In addition to HS chains, a total of about 20kDa of N-linked and O-linked oligosaccharide chains are added, suggesting their involvement in secretion. On the other hand, agrin was discovered as a protein that induces the aggregation of acetylcholine receptors and acetylcholinesterase in the postsynaptic membrane of the neuromuscular junction [16]. It is also present in basement membranes other than synapses and is more abundant than perlecan in renal glomeruli. The core protein consists of three globular domains, including a laminin-binding site at the head at N-terminus, a follistatin-like rod, and a laminin G-like globular domain at the tail at C-terminus. Four HS chains and four N-linked oligosaccharide chains are predicted to be modified [17]. Agrin exists in the basement membranes of nerve tissue and other tissues, but those that have undergone unique splicing are localized at synapses. In this review, we summarize the structure, biological activity, diversity, modification, and therapeutic applications of HSPGs.
Glypicans consist of six members in mammals, are anchored in the cell membrane via a GPI anchor, and carry two to three HS chains [18]. Thus, multiple types of core proteins bind to HS, which is a sulfated sugar chain and has a structure, a sulfate group is added to a repeating disaccharide skeleton of uronic acid and glucosamine, and the local sulfation of their sugar chains caused by various sulfotransferases, such as N-deacetylase/N-sulfotransferase, C5 epi transferase, 6-O sulfotransferase, 2-O sulfotransferase, and 3-O sulfotransferase, etc., resulted in the construction of a diverse group of glycoproteins [19].
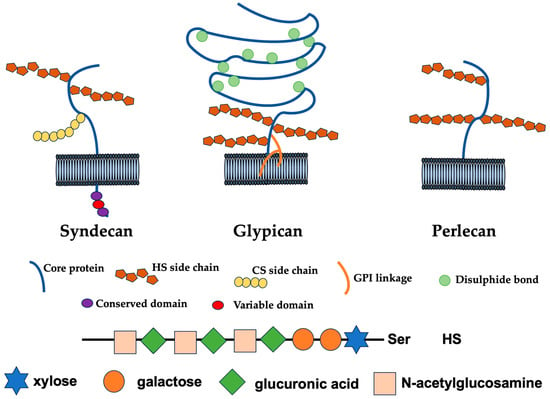
Figure 1.
Cell surface and extracellular HSPGs. Syndecan core proteins are transmembrane proteins that contain a highly conserved C-terminal cytoplasmic domain. HS chains attach to serine residues distal from the plasma membrane. Some Syndecans also contain a CS chain(s) that attaches to a serine residue(s) near the membrane. The glypican core proteins are disulfide-stabilized globular core proteins that are linked to the plasma membrane by a glycosylphosphatidylinositol (GPI) linkage. HS chains link to serine residues adjacent to the plasma membrane. Perlecans are secreted HSPGs that carry HS chains [20].
2. Modification and Biosynthesis of HS
2.1. Functions, Structures, and Biosynthesis of HS Chain
Due to its great structure diversity, HS chains of Syndecan bind with a wide range of ligands that strongly influence adhesion, migration, proliferation, and survival [21]. The HS chain is a strongly anionic, structurally heterogeneous, linear polysaccharide and retains a common overall pattern, characterized by highly, intermediately, and lightly sulfated regions. The expression of HS is in almost all cell types, and its synthesis occurs mainly in the Golgi apparatus [22,23]. Although HS chains are synthesized by the same set of enzymes, the length and amount of sulfation of HS chains on the same core protein differ in different tissues. For example, when calculating the ratio of saturated/unsaturated disaccharides, the average HS chain length in rats is found in muscle (to 60 disaccharides (dpc) per chain), liver (to 40 dpc), brain (to 50 dpc), spleen (to 62 dpc), lung (to 42 dpc), kidney (to 49 dpc), and heart (to 33 dpc). These differences are due to the expression of biosynthetic enzymes in the Golgi apparatus and differences in nucleotide sugar donors during synthesis.
Biosynthesis of the HS chain initiates with the generation of the core tetrasaccharide, in which stepwise addition of a tetrasaccharide chain (GlcA-Gal-Gal-Xyl-O-Ser) consisting of xylose (Xyl), galactose (Gal), and glucuronic acid (GlcA) to serine residues of core proteins [23]. This occurs through the translocation of xylose to a specific serine residue in the HSPG core protein by xylose transferase, followed by the addition of two galactose residues by galactosyltransferases I and II and glucuronic acid (GlucA) by glucuronidyltransferase. After the core tetrasaccharide is formed, elongation of the disaccharide repeating structure (GlcA-GlcNAc) of acetylglucosamine (GlcNAc) and GlcA. Elongation of the HS chain begins with the addition of N-acetylglucosamine (GlcNAc) by N-acetylglucosamine transferase I (GlcNAcT-I), followed by alternating addition of GlcA and GlcNAc by exostosis family enzymes [24]. Subsequently, sulfation and epimerization modify the extending [GlcA-GlcNAc]n polymer. The first modification that occurs and is required in advance of all other modifications is GlcNAc N-deacetylation/N-sulfation of GlcNAc residues by N-deacetylases/N-sulfotransferases (NDSTs). This reaction is followed by the isomerization of GlcA and the resulting 2-O-sulfation of the iduronic acid (IdoA) residue. Finally, O-sulfation at position 6 and/or position 3 of the glucosamine residue occurs. After the initial N-deacetylation/N-sulfation, all modification reactions are incompletely accomplished, resulting in various sulfation states of HS, in which HS chain composition determines HSPG-mediated cellular responses [23]. Due to the exostosis glycosyltransferase 1 (Ext1) gene mutation, primary fibroblasts express a smaller amount and shorter chain of HS than the wild-type (Wt) fibroblasts and are unable to form vinculin-containing focal adhesions compared to the Wt, which is consistent with the known role of Syndecan-4 in promoting focal adhesion formation through interaction with the fibronectin Hep II domain [25]. N-sulfation is essential for focal adhesion formation in primary rat fibroblasts plated on fibronectin in the presence of competitive heparin oligomers or variably desulfated heparin oligomers or in the absence of oligomers, and 6-O-sulfate is the next most important.
2.2. Functions of the Syndecan Family
The sulfation pattern of HS also influences the mode of ligand binding to HSPGs [23]. Syndecan-1, which are expressed at approximately the same level on the surface of myeloma cells, share a common core protein and have HS chain sugars of approximately similar size. However, only Syndecan-1 on MPC-11 cells, which is a B lymphocyte cell line of a mouse with plasmacytoma, mediates cell adhesion by binding to type I collagen. Syndecan-1 HS from MPC-11 exhibits high N-sulfation 2-0-sulfation and low 6-O sulfation. This indicates that HS sulfation and the specific sulfation pattern, rather than the true charge, are important for differences in binding to collagen. Each of these HSPGs has a cell-type-specific distribution, and in vascular endothelial cells, expression of Syndecan-1, -2, and -4 is mainly observed between cells and on the basement membrane side [1]. The Syndecan family belongs to type I membrane proteins and forms a family with homology to each other in their transmembrane and cytoplasmic domains. The cytoplasmic amino acid sequences of Syndecan proteins show high homology within the family. In particular, the submembrane and C-terminal regions are highly conserved and are therefore responsible for triggering signal transduction cascades common to the family. Also, since the central arrangement is unique to each member, it triggers a cascade unique to each member. In addition, this transmembrane–intracellular domain contains four tyrosine residues with conserved positions, and phosphorylation of any of the tyrosine residues participates in intracellular signal transduction [26]. These intracellular domain ends are involved in association with intracellular microfilaments in Syndecan-1 and focal adhesion in Syndecan-4.
Furthermore, in Syndecan-1, which was cloned from mouse mammary epithelial cells, the sugar chain contains CS in addition to heparan sulfate, and the ratio and size of these differ depending on the tissue in which they are expressed. Additionally, Syndecan-1 is expressed as an HSPG with anticoagulant activity in rat microvascular endothelial cells. It was isolated from golden hamsters as a fibroblast growth factor 2 (FGF-2) receptor, and the sugar chain has been modified to have different functions depending on the cell in which it is expressed [27,28].
2.3. Sugar Skeleton of HS
The sugar skeleton of HS is a repeat of GlcA/IdoA-GlcNAc, but there are theoretically 1036 different molecules depending on the position and number of sulfate groups attached. Therefore, it is currently not possible to determine whether the “ligand binding redundancy” of HS is truly redundant or binding via each specific sugar chain structure. Signal pathways mediated by cell surface receptors usually involve a dimerization process upon ligand binding. Syndecan can self-associate through a transmembrane domain containing a specific conserved motif GXXXG [26]. The delicate balance between anabolism and catabolism is critical to tissue homeostasis, structure, and function. For example, matrix metalloproteinases (MMPs) cleave many extracellular matrix proteins, shedding Syndecan from the cell membrane and affecting signaling [29,30].
Furthermore, in some cancer types, HSPG expression is different from healthy tissue. Thus, enzymes that modify, cleave, shed, and degrade HSPG are important factors in catabolic reactions, while glycosaminoglycan biosynthetic enzymes control anabolic reactions [22,30,31,32]. Syndecan functions as a receptor that transmits signals from the extracellular microenvironment to the inside of the cell [8,33]. Fibroblast growth factor-2 (FGF-2) bound to HS significantly increases affinity for FGFR [34,35]. Cells lacking cell surface HSPGs have reduced proliferation rates that can be restored by exogenous heparin [36]. In addition, interaction with HS protects growth factors from proteolytic degradation, thereby increasing their half-life in the cellular environment. Antithrombin III specifically interacts with heparin, while HGF can bind both HS and dermatan sulfate to exert biological activity and requires a unique pentasaccharide structure containing a rare 3-O-sulfated N-unsubstituted glucosamine (GlcN3S), which induces structural changes in the protein and increases the degree of thrombin inhibition. Glucosamine 3-O-sulfation of the HS chain is a rare modification but critical for several specific HS–protein interactions [37,38].
Because heparin has a high IdoA2S-GlcNS6S content, in the absence of HSPGs, core protein papillomavirus (HPV-16) can infect epidermal cells [39]. Therefore, structural changes in the sulfation pattern of HS can change ligand binding and HS-mediated cellular signaling [23]. The ligand interacts with heparan sulfate on the cell surface in cooperation with high-affinity receptors [36]. The fact that the ligand binding site is the HS sugar side chain means that Syndecan could theoretically serve as a receptor for all heparin-binding molecules [40]. Many extracellular matrix constituent molecules and cell growth factors bind to the HS sugar side chain of Syndecan. Adsorption of Herpes simplex virus type 1 (HSV-1) to the target cell surface via HS occurs when viral glycoproteins gB and gC bind to HS. This binding of HS by gB and gC is involved in the binding of virus particles to the cell surface, but not in the entry of virus particles into cells. For HSV-1 to enter cells, the glycoprotein gD must bind to entry receptors, resulting in membrane fusion between the viral envelope and the host cell membrane [41,42]. In addition to nectin cell adhesion molecule 1 (NECTIN-1), nectin cell adhesion molecule 2 (NECTIN-2), and herpesvirus entry mediator (HVEM), 3-O-sulfated HS has been identified as one of these entry receptors. Interestingly, among the six human 3-O-sulfotransferases (3-OSTs), 3-O-sulfated HS, which is sulfated by six 3-OSTs (3-OST-2, 3-OST-3A, 3-OST-3B, 3-OST-4, 3-OST-5, and 3-OST-6), except 3-OST-1, interacts with gD and functions as an entry receptor [37,43,44]. Multiple sulfotransferases share activity and form sugar chains with more complex sulfation patterns, thereby acting as separate functional domains. In addition to HSV-1, a wide variety of virus particles interact with HS, including human immunodeficiency virus, hepatitis B virus, dengue virus, human papillomavirus, cold coronavirus, vaccinia virus, and Lassa virus [39,45,46]. The electrostatic interaction between the positive charge of the viral protein that makes up the surface of the virus particle and the negative charge of HS is involved in the adsorption of the virus particle to the cell surface. SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), the virus that causes COVID-19 (CoronaVirus Infectious Disease in 2019), also uses HS to bind to target cell surfaces [46,47,48]. SARS-CoV-2 is caused by the Spike protein, an S protein present in the envelope binding to the Angiotensin-converting enzyme 2 (ACE2) receptor present in the cell membrane, entering the cell, when the S protein also binds to HS, increasing its binding to the ACE2 receptor [37,38,39].
3. Structure and Function of Vascular Endothelial Glycocalyx
3.1. Structure and Function of Proteoglycan
Proteoglycan, the main component of the vascular endothelial glycocalyx, which is a structure composed of glycoproteins and polysaccharides that covers the cell surface of the vascular lumen, functions as the most important skeletal molecule of the glycocalyx, which is usually composed of proteoglycan and glycoproteins that are bound to the cell membrane and surface layer, as well as polysaccharides that are not bound to the cell membrane surface in the outer layer [49,50]. The main components of the glycocalyx are glycosaminoglycans such as HS, CS, and hyaluronic acid (HA), and core proteins such as Syndecan that support them, which are also made up of complex bonds of sugar chains. Furthermore, since the presence of glycocalyx in the vascular endothelium has the function of preventing substances from flowing out of the blood vessel and adhering to the vascular endothelium, it is attracting attention in intensive care fields such as infusions and anesthesia [51,52]. Glycocalyx is a jelly-like, band-like structure of glycoprotein that covers the surface of cells such as vascular endothelium and has various physiological functions [53,54]. The glycocalyx has a variety of functions, including maintaining blood flow, protecting the endothelium, antithrombotic effects, binding and signal transmission of hormones and growth factors, and serving as a nutritional source consisting of sugar chains [54,55]. Vascular endothelial glycocalyx is a carbohydrate-rich layer located on the luminal surface of the vascular endothelium [56]. It binds to the endothelium primarily through skeletal molecules such as proteoglycans and glycoproteins and incorporates soluble molecules derived from plasma or endothelium to form a mesh structure [49,54]. The lumen side of the glycocalyx is formed by soluble plasma components bound directly to membrane-bound proteoglycans and glycoproteins or indirectly through sugar chains such as soluble proteoglycans [57]. There is a dynamic equilibrium between the soluble components on the lumen side of the glycocalyx and the flowing blood, and this intermittently influences the composition and thickness of the glycocalyx [57,58].
3.2. Vascular Endothelial Glycocalyx
Glycocalyx is a structure with a thickness of several hundred nm that exists on the vascular endothelium [56,59,60]. It is composed of glycoproteins such as proteoglycans and adhesion molecules that are bound to the cell membrane, and HA that is not directly bound to the cell membrane. Proteoglycan is a core protein with GCG bound to it. Plasma proteins such as albumin and antithrombin bind to the vascular lumen side of glycocalyx, forming an endothelial surface layer (ESL). The physiological functions of glycocalyx and ESL are diverse, including regulation of vascular permeability, mechanoreceptors for shear stress, regulation of leukocyte adhesion and migration, and control of coagulation and fibrinolysis within blood vessels [61]. Glycocalyx is a very fragile structure. Due to various stimuli and pathological conditions, it peels off, releases into the blood, and then regenerates, a process that repeats repeatedly. As one way to recognize glycocalyx damage, blood concentrations of these released glycocalyx components, including Syndecan-1, HS, and HA, etc., are measured for research purposes [62,63]. On the other hand, visualizing glycocalyx is an extremely difficult task, and even with current technology, it is difficult to directly evaluate glycocalyx structure in vivo.
Injury factors for glycocalyx include sepsis, ischemia–reperfusion, hypervolemia due to the use of cardiopulmonary bypass, and hyperglycemia [63]. Among these, hypervolemia damages glycocalyx via atrial natriuretic peptide (ANP) secretion [64]. ANP administration reduces the glycocalyx structure observed by electron microscopy and promotes hydroxyethyl starch (HES) extravasation, even at physiological concentrations. As a result of performing acute normovolemic hemodilution and volume loading (VL) using the same amount of HES (20) mL on surgical patients, only VL increases blood ANP concentration and increases the blood concentration of Syndecan-1 and HA [65]. In addition, in coronary artery bypass surgery, regardless of on-pump or off-pump surgery, blood concentrations of ANP and glycocalyx degradation products increased by several to several dozen times [66]. The increase in blood concentration of decomposition products precedes the increases in inflammatory cytokines and is triggered by the increase in blood ANP concentration [67]. Furthermore, multivariate analysis has shown that there is a relationship between cumulative fluid volume during initial resuscitation and glycocalyx injury in patients with sepsis.
Enzymes such as MMP, heparinase, and hyaluronidase are involved in the decomposition process of glycocalyx [54]. These enzymes are activated in the presence of inflammatory cytokines and reactive oxygen species. Since ANP-induced glycocalyx damage was reduced under the use of MMP inhibitors, activation of MMP by ANP contributes to glycocalyx damage [68].
The vascular endothelial glycocalyx is a complex, self-assembled three-dimensional mesh of various polysaccharide molecules. This is sheared and released by enzyme reactions associated with inflammation and shear stress [69]. In the vascular system, HSPGs, a major component of the glycocalyx, enable the binding of various plasma-derived molecules. Because of their diversity, epimerization of sugar chains, long chains, and sulfation account for approximately 50 to 90% of the total amount of proteoglycans in the glycocalyx [32,70]. The binding of enzymes, ligands, and their receptors to sugar chains locally increases their concentrations, facilitating necessary signaling and enzyme modification reactions, and functions in blood vessel protection [71]. Several important anticoagulant mediators are present in the endothelial glycocalyx, including antithrombin, heparin cofactor II, thrombomodulin, and tissue factor pathway inhibitor (TFPI), which is produced in vascular endothelial cells, is a strong inhibitor of FVIIa and FXa, and exists bound to the glycocalyx via heparan sulfate. Syndecan-4 derived from human vascular endothelial cells binds to heparin-binding proteins FGF-2, midkine, and TFPI via its heparan sulfate sugar chains. Antithrombin, produced and secreted by hepatocytes, is a powerful inhibitor of thrombin and procoagulant proteases such as FXa and FIXa [72]. Their binding to a specific region of heparan sulfate enhances anticoagulant ability hundreds of times [73].
Heparin cofactor II, which is also produced and secreted by hepatocytes, is a specific inhibitor of thrombin and exhibits antithrombin activity by binding to dermatan sulfate in the glycocalyx. Thrombomodulin is a CS-containing glycoprotein expressed on the vascular endothelial cell membrane, and when it binds to thrombin, it abolishes its procoagulant effect [74,75]. On the other hand, it exhibits an anticoagulant effect by activating protein C, a coagulation inhibitor produced by liver cells. However, proteoglycan expression in endothelial cells changes due to various stimuli. For example, Syndecan is changed and regulated by the activation of endothelial cells and stimulated by different chemokines [49,76]. The second most common sugar chain in the vascular endothelial glycocalyx is CS/dermatan sulfate, and the abundance ratio of heparan sulfate and CS in the vascular endothelium is approximately 4:1 [55,60,77]. Another important sugar chain in the vascular endothelial glycocalyx is hyaluronan [60,78,79]. These long polymer molecules with up to 10 kDa differ from other sugar chains in that they are not bound to a core protein, forming surprisingly viscous solutions [80]. Sugar chains have specific binding sites for many plasma proteins, and even slight differences in chain modification can significantly change their function, such as loss of binding ability [81]. Modifications at individual sugar units within sugar chains confer unique functions to proteoglycans, but there are typically 16 to 48 different sulfation patterns per disaccharide chain [82]. The length of the functional domain is typically five to ten glycans. At least 163 different sulfation patterns are theoretically possible, and the various structures correspond to the diverse biological functions of the sugar chains [83]. Indeed, modification patterns change over time and under different pathological and physiological stimuli [81,84,85,86]. The diversity of sugar chain sulfation patterns and their effects on specific protein binding and functional regulation change the thickness of the glycocalyx, sugar chain sulfation patterns, and their charges, regulating vascular permeability, and specific binding proteins and their activities [82,85,87].
In addition, the vascular endothelial glycocalyx sensitively reflects the health status of vascular endothelial cells, and under normal conditions, it remains thick and slippery, but when the vascular endothelium itself is damaged due to diabetes, etc., the glycocalyx becomes thinner and its function decreases [78,88,89]. In particular, the glycocalyx in peripheral capillaries is more important than in large blood vessels because the thickness of the glycocalyx is relatively larger than the vessel diameter [55]. Capillaries, which extend throughout the body’s organs, account for 99% of all blood vessels. This exchanges information substances such as nutrients, oxygen, and hormones with six billion cells, and is not surrounded by smooth muscle, instead consisting of endothelial cells that form the vascular lumen and pericytes that are sparsely present around the periphery [90,91]. In classical theory, the movement of substances from the capillary side to the interstitial side, or from the interstitial side to the capillary side, is caused by the difference in osmotic pressure between the two sides and moves through the small gap between endothelial cells. When many substances, which move due to the difference in osmotic pressure between the upper and lower sides of the glycocalyx covering the capillaries, pass through, the gap in the endothelium opens [54,92,93].
The microstructure of the lumen of capillaries is classified into three types: the continuous type with almost no gaps in the lumen, the fenestrated type with small holes distributed, and the sinusoidal type, with holes of various sizes. The capillary endothelium in the brain, heart, lungs, etc., is continuous, and its surface is densely covered with a glycocalyx [94,95]. The digestive tract, kidneys, and endocrine organs are fenestrated and have a glycocalyx that covers the holes [55,65,96]. The liver and bone marrow are sinusoidal, with large holes, and are not covered by a glycocalyx [97]. Thus, substances enter and exit the capillaries of the gastrointestinal tract, kidneys, liver, etc., freely, to a certain extent, through holes that are originally opened and are regulated by the function of the glycocalyx [94,98,99]. The permeability of capillaries in each organ can be explained by a combination of the three classifications of their microstructure and the glycocalyx [94,100,101]. Even though the capillary structure is the same, the thickness of the glycocalyx differs depending on the organ [51,59,102]. Comparing their ultrafine three-dimensional structures by organ, the fine structure of the vascular endothelium in the lungs, heart, and brain is classified into the same continuous types, and the same structure was confirmed in electron microscopy images [103].
However, the glycocalyx is thin in the lungs, intermediate in the heart, and very thick in the brain [98,102]. Even if the blood vessels have the same structure, the glycocalyx differs depending on the organ, in which the brain is thick to protect brain cells from cytokines and toxins, and the lungs are thin to carry out the gas exchange of oxygen and carbon dioxide. The vascular endothelial glycocalyx of mouse models of diabetes, aging, and dietary restrictions is thinner than that of normal mice [104]. When inflammation is induced in model mice if the glycocalyx is thick, the endothelium is likely to be protected, but if it is thin, the inflammation will be prolonged [56,105]. Additionally, if infected with COVID-19, people with underlying diseases such as diabetes or cancer are more likely to develop blood clots and become more seriously ill, which may also be due to the thin glycocalyx [106,107]. In addition, when the glycocalyx becomes thinner, damage caused by stimulation and an increase in abnormal signal transmission occurs, leading to the onset or worsening of various diseases such as prolonged inflammation and cancer growth [105]. Elucidation of the mechanisms leading to the onset and deterioration of the disease and the development of treatments targeting the glycocalyx still require further research, but this is an area that is expected to continue to develop [51,55,106]. In addition, blood vessels are essential organs that control circulation throughout the body, as well as inflammatory cytokines such as Tumor Necrosis Factor (TNF) and Interleukin-6 (IL-6) [108,109]. Further, interleukin-1 beta (IL-1beta) and growth factors such as hepatocyte growth factor (HGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) are also transported to organs through blood vessels and are involved in the development of various diseases and tissue repair [110,111].
4. Molecular Mechanisms of the Syndecan Family
4.1. Syndecan-4 Functions
Syndecan-4 plays an important role in biological defense against inflammation and tissue damage [6,76,112]. In addition, the expression of Syndecan-4 is increased in the vascular wall after vascular injury. Syndecan-4 functions in cell proliferation and migration of vascular smooth muscle cells induced by thrombin. For HS–growth factor interactions, it is still unclear whether distinct HS sequences are required. The degree of global sulfation defines HS-FGF interactions in a nonspecific manner. Using FGF-1 and FGF-2, 2-O-sulfation and 6-O-sulfation can be interconverted without loss of physiologically significant interactions. Therefore, for many proteins, what is important is the composition of the HS domain, rather than the precisely determined HS sugar chain sequence. The expression of 3-O-sulfotransferase was regulated through RAS/mitogen-activated protein kinase (MAPK), fibroblast growth factor receptor 2 (FGFR2), and Kit signals [113]. Biotechnically produced 3-O-sulfate-rich HS interacts with FGFR2b, thereby promoting the formation of a ternary complex between fibroblast growth factor receptor 10 (FGF-10) and FGFR2b [114]. Additionally, microRNAs can control HS chain modification, which changes the binding of growth factors to sugar chains [3,22].
Sydecan-4 always exists as a dimer on the cell surface, which becomes multimerized by binding to ligands such as the heparin-binding domain of fibronectin [115]. Furthermore, in focal adhesions, high concentrations of Syndecan-4 increase the probability of oligomer formation that promotes PKC signaling. Membrane-bound HSPGs, including Syndecan, glypican, beta-glycan, neuropilin-1, and CD44v3, an isoform of CD44, are found in the plasma membrane of nearly all eukaryotic tissues and can act as co-receptors that regulate cell signals and behavior, or as receptors for endocytosis and adhesion [3].
4.2. HS Chains and Inflammatory Responses
Syndecan HS chains are also important in inflammatory responses by binding to chemokines [60]. In rheumatoid arthritis (RA), the chemokine CXC chemokine ligand 8 (CXCL8) binds to the HS chain of Syndecan-3, and the excision of the HS chain from Syndecan-3 by bacterial heparanases I and III abolished the binding of CXCL8 to rheumatoid synovial endothelium [116,117]. In addition, the administration of chemokines engineered to have reduced affinity for HS abolishes leukocyte recruitment to RA joints [118]. Chemokine CXC chemokine ligand 1 (CXCL1), a functional rodent homolog of CXCL8, binds to HS on the cell surface of mice [119]. Syndecan-3 knockout mice exhibit reduced neutrophil accumulation in the knee joints due to CXCL1 administration and attenuated inflammatory responses and RA compared to Wt mice [116]. Thus, the role of Syndecan-3 on leukocyte activation and migration via the HS chain is shown [116,120]. Specific deficiency of HS 2-O-sulfotransferase in endothelial and bone marrow cells reveals a specific HS function in inflammatory cell recruitment. Inactivation of this gene in endothelial cells markedly altered the sulfation status of disaccharides, showing a decrease in 2-O-sulfate content as expected, but 6-O-sulfation and N-sulfation increased. This altered sulfation pattern promoted increased binding of cytokines [121,122]. Furthermore, neutrophil recruitment was enhanced in these animals after inflammatory stimuli, leading to intensified neutrophil rolling [123]. The latter is because of increased interaction between neutrophil L-selectin and HS on endothelial cells. In addition to chemokine binding, the pleiotropic roles of HSPGs in immune system signaling are clear. In vitro experiments show that Syndecan-4 HS in CTCL cells acts as a cell surface reservoir for TGFb1 [112]. Additionally, the same HS site was shown to bind dendritic cell HSPG–integrin ligand on activated T cells, in which interactions produce immunosuppressive effects.
4.3. Syndecan and Cell Signals
Syndecan can control cell signals in vitro and in vivo experiments [120]. Several phenotypic changes in FGF-2-mediated signaling induced overexpression of Syndecan-4 in endothelial cells [115,124]. Syndecan-1 null mice were viable and fertile but had reduced mammary branching morphogenesis, in which a reduced number of collaterals characterized the glands of lower morphology [125].
Additionally, by crossing Syndecan-1 null mice with mice expressing constitutively active intracellular beta-catenin, Syndecan-1 was suggested to be required for catenin-induced mammary gland hyperplasia and cancer development in vivo [126]. Furthermore, the expression of Syndecan is enhanced in myocardial infarction models. In patients with myocardial infarction, a marked increase in blood Syndecan-4 was detected, peaking two weeks after onset, and clear Syndecan-4 staining was observed in the tissue repair area in autopsy tissue images, indicating that Syndecan-4 is rapidly expressed during ischemic myocardial injury and function in tissue repair [127,128].
5. Molecular Regulation and Function of Sulfatases In Vivo
5.1. Sulfatases Function and Group
Sulfatases are enzymes that remove sulfate groups from a variety of molecules, including steroids, sulfated lipids, glycoproteins, and proteoglycans. Removal of sulfate groups from HS alters interactions with growth factors and affects downstream signals [10]. Extracellular endosulfatases Sulfatase 1 (Sulf-1) and Sulfatase 2 (sulf-2) selectively remove 6-O-sulfate from glucosamine, thereby changing the binding properties of sugar chains [129]. These sulfatases need to be processed by furin-type proteases to become active enzymes. 6-O-sulfate of the HS chain is important for signals from FGF. Sulf-1 inhibits FGF-2-promoted thymoma viral proto-oncogene 1 (AKT/PKB) and ERK signaling in hepatoma cell lines. Sulf-1 decreases the expression of cyclin D1 and survives, leading to cell cycle arrest and apoptosis. Ultimately, Sulf-1 attenuates hepatoma cell proliferation in vivo. Similarly, Sulf-1 expression is decreased in several types of cancer, including breast cancer. Furthermore, expression of Sulf-1 in MDA-MB-468 breast cancer cells overexpressing epidermal growth factor receptor (EGFR) reduces cell proliferation in vitro and cancer size in vivo. The molecular mechanism underlying these Sulf-1-mediated effects involves the inhibition of autocrine ERK signaling induced by amphiregulin and heparin-binding EGF [130]. Apart from their impact on cancer biology, sulfatases have a role in maintaining tissue homeostasis. Sulf-1 and Sulf-2 knockout mice survived, and no obvious physiological defects were observed [131,132]. On the other hand, double-knockout mice for both sulfatases died around birth. Sulf-1 knockout mice exhibit more severe cartilage degeneration in surgically induced osteoarthritis than Wt mice. In addition, this mouse exhibits high expression levels of matrix metallopeptidase 13 (MMP-13) and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5) and an abnormal extracellular matrix, in which there are low mRNA levels of collagen type 2a1 (col2a1) and aggrecan. This phenotype is associated with increased phosphorylation of Erk1/2, while decreased Smad1 protein expression and Smad1/5 phosphorylation. Sulf-2 knockout mice exhibit a similar phenotype. The degree of sulfation differs between various organs in Sulf-1 and Sulf-2 knockout mice, and each of these sulfatases controls organ-specific sulfation patterns of HS chains [133].
5.2. Sulfatases and Cell Signaling
In addition, alternative splicing of the Sulf-1 gene provides further enzymatic regulation of cellular signals [130]. Short-splicing variant of Sulf-1B suppresses Wnt signaling in osteoblasts, while full-length Sulf-1 induces Wnt signaling [134,135,136]. Furthermore, Sulf-1B induces angiogenesis, whereas Sulf-1 causes the opposite effect. Expression of Sulf-2 is elevated in glioblastoma patients and correlates with cell proliferation and tumorigenesis [137]. The molecular mechanism of Sulf-2-mediated effects involves the regulation of several tyrosine kinase-type receptors, such as Platelet-derived Growth Factor Receptor a (PDGDRa), insulin-like growth factor 1 receptor 1 beta (IGF1Rbeta), and Erythropoietin-producing hepatoma receptor-A2 (EPHA2). Sulf-2 promotes cell proliferation, which was associated with increased expression of glypican-3 [138,139]. This HSPG induces higher binding of FGF-2 and activation of ERK and AKT signaling pathways in hepatoma cell lines [45]. Sulf-2 enhances tumor growth in nude mice and is associated with poor patients’ prognosis [140,141]. Sulf-2 is elevated in breast cancer compared to healthy controls and promotes angiogenesis [142]. Furthermore, the knockdown of Sulf-2 reduces breast cancer proliferation and increases luminal cell apoptosis. Furthermore, tumors lacking Sulf-2 retain lumen formation and basement membrane integrity, which is concomitant with decreased matrix metalloproteinase-9 (MMP-9) expression and activity. On the other hand, when MDA-MB-231 cells, basal-like breast cancer were treated with Sulf-2, phosphorylation of ERK induced by FGF-2 or HB-EGF was reduced, resulting in a decrease in cell invasion and proliferation. Furthermore, heparanase is secreted as an inactive zymogen, taken up through HSPGs such as Syndecan-1, and, finally, transported to lysosomes, where heparanase is converted into an active form by cathepsin-L [143,144]. Heparanase recognizes a specific HS sequence and directly cleaves between GlcA and GlcNS with 3-O- or 6-O-sulfate [145]. On the other hand, polysaccharides containing IdoA2S-GlcNS (2-O-sulfo-α-L-iduronic acid- glucosamine-N-sulfate) inhibit heparanase enzyme activity. The released HS fragments serve as competitors for HSPG–protein interactions but can also bind to Toll-like receptor 4 (TLR-4), eliciting innate immune responses [146,147]. Heparanase induces malignant transformation and is associated with poor prognosis in several cancer types [118]. Therefore, several heparanase inhibitors have been developed, but most of the inhibitors are sulfated sugars or HS analogs and exhibit anti-angiogenic, anti-metastatic, and anticancer activities [148,149].
5.3. HSPGs and Reservoir for Bioactive Molecules
HSPGs present in the extracellular matrix serve as a reservoir for bioactive molecules (Figure 2) [150]. In a physiological situation using a model of mammary duct branching morphogenesis, heparanase activity promotes the release of fibroblast growth factor-10 (FGF-10) bound to the HS of perlecan. This allows growth factors to bind to fibroblast growth factor receptors (FGFRs), increasing mitogen-activated protein kinase (MAPK) activation, epithelial invasion, and lateral branch formation [151]. Similarly, fibroblast growth factor-2 (FGF-2) and VEGF are released from the extracellular matrix by the action of heparanase and control the angiogenic response [152], while heparanase can regulate cellular signals independently of its enzymatic activity. For example, mutated and inactive heparanase promotes VEGF activation mediated by proto-oncogene tyrosine-protein kinase Src and p38 mitogen-activated protein kinase activation. Besides hydrolyzing glycosidic bounds, heparanase promotes changes in the sulfation pattern of HS, which, in turn, modulates cellular signals [23,153]. Heparanase overexpression increases HS turnover as well as N- and O-sulfate content in the liver [154]. Additionally, HS6-O-sulfation is higher in other organs of mice overexpressing heparanase. Similarly, cancers that highly express heparanase correlate with increased HS 6-O-sulfation [22]. This change in sulfation promotes the formation of a tripartite complex between fibroblast growth factor (FGF) and FGFR1 and modulates signal transduction.
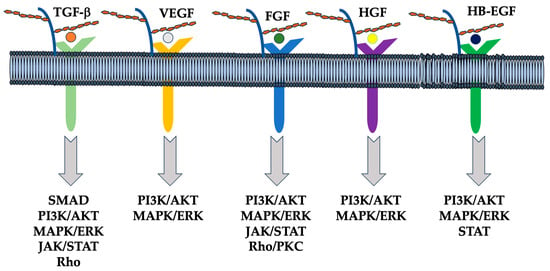
Figure 2.
Schematic representation of the interaction between HSPGs, growth factors, receptors, and downstream signaling pathways. HSPGs bind several other growth factors such as hepatocyte growth factor (HGF), fibroblast growth factor (FGF), heparin-binding epidermal growth factor-like growth factor (HB-EGF), transforming growth factor (TGF) beta, and vascular endothelial growth factor (VEGF), and modulate their signaling, including SMAD, PI3K/AKT, MAPK/ERK, JAK/STAT, and Rho/PKC [155].
6. Function of Syndecan via Extracellular Vesicle (EV) and miRNAs
6.1. EVs and Heparanase
EVs are extracellular granules that represent important communication pathways between cells, transporting molecules that affect cellular signals, such as proteins, lipids, microRNAs, and mRNAs [156,157]. Most cells produce EVs, but under pathological conditions, EV secretion is increased and correlated with cancer progression [158,159,160]. Myeloma cells that highly express heparanase secrete significantly more EVs than control cells [161]. In addition, exogenously applied heparanase enhances EV secretion in other types of cancer cells [162,163]. These effects depend on the enzymatic activity of catalytically inactivated heparanase, as it did not induce EV release [164]. EVs derived from cells with high heparanase expression contain increased amounts of Syndecan-1, VEGF, and HGF, which regulate cancer and host cell behavior [128,165,166]. Syndecan core protein is key to EV formation through interaction with the syntenin/ALIX (ALG-2-interacting protein X) complex [167,168]. Besides controlling EV biogenesis, HSPGs are important for EV uptake and biological activities, e.g., EV-induced ERK1/2 signal activation [169,170]. Poor prognosis in myeloma patients correlates with high HGF levels [171,172]. In this context, heparanase influences cellular signals by regulating growth factor expression and activity [147,173]. Analysis of bone marrow biopsies from myeloma patients shows a positive correlation between heparanase and HGF levels [161]. In vitro experiments showed that heparanase induces the expression and secretion of active HGF in myeloma cells, and that enzyme activity is not required for this effect. Furthermore, heparanase increases the formation of active complexes between HGF and Syndecan-1 released from the membrane [165].
6.2. Sydecans Control Cellular Functions
Transmembrane Sydecans control cell adhesion and migration through interactions with signal molecules and the cytoskeleton. On the other hand, it interacts with extracellular matrix molecules, integrins, growth factors, and other ligands [66]. For example, Syndecan-2, as a receptor for the cell adhesion substrate fibronectin, controls the fibronectin-binding information of integrin a5b1 and induces different cytoskeleton formation. Thus, Syndecan mediates cell adhesion and migration via cytoskeleton architecture as an extracellular matrix receptor and cell proliferation as a growth factor low-affinity receptor. Syndecan shedding is an important mechanism that controls the amount of Syndecan on the cell surface.
Furthermore, the shedding of Syndecan is an important phenomenon because shed Syndecan can serve as a soluble ligand [174]. Although the Syndecan extracellular domain may be constitutively shed into the extracellular space, the rate at which this occurs in vivo is poorly understood. However, pathological conditions and some stimuli such as growth factors, chemokines, microbial toxins, insulin, heparanase, and cellular stress, increase the rate of shedding. Matrix metallopeptidase (MMPs) primarily catalyze Syndecan shedding, usually cleaving the extracellular domain of Syndecan close to the transmembrane domain. Shredded Syndecan-1 acts as a paracrine factor during cancer progression [127,165]. Syndecan-1 shed from fibroblasts induces cell division response in human breast cancer cells using a three-dimensional co-culture model. HS chains, FGF-1, and stromal-derived factor 1 are key factors in this paracrine phenomenon [1,114].
Furthermore, in MCF-7 breast cancer cells, overexpression of Syndecan-1 ectodomain rapidly promotes the acquisition of invasiveness in a normally poorly invasive cell line [175]. Apart from metalloproteases, heparanase promotes the shedding of Syndecan-1 from myeloma cells by several mechanisms [166]. First, heparanase cleaves HS chains and enhances the sensitivity of core proteins to matrix metalloproteinases (MMPs). Increased heparanase maintains ERK signaling [176,177]. This increases the expression level of MMP-9, potentially increasing the shedding rate of Syndecan-1, which is shed from myeloma cells and acts in a paracrine manner [166]. For example, culture supernatants from myeloma cells that highly express heparanase contain shed Syndecan-1 and VEGF.
Chemotherapy and radiotherapy, the most common treatments for cancer patients, induce Syndecan shedding [127,165]. However, this can potentially be considered a negative side effect as it creates a favorable microenvironment for cancer progression [178,179]. In addition, treatment of myeloma mice with chemotherapeutic drugs increases the amount of shed Syndecan-1 compared to control animals. The molecular mechanism involved in chemotherapy-induced shedding of Syndecan-1 involves the activation of caspases and ADAM metallopeptidase domain (ADAMs) consisting of disintegrin and metal proteinase domains [127,166]. MMP was the metalloprotease that mediated Syndecan-1 shedding in this case [22]. Furthermore, miR-494 was identified as a microRNA involved in Syndecan-1 shedding [127].
6.3. Diversity in HS Chain Length and Modification
The great diversity in HS chain length and modifications contributes to a high degree of complexity, but important aspects remain unclear, especially in vivo [180,181]. This suggests a mechanism by which heparanase expression is linked to subsequent HS sulfation and ultrastructural changes [1]. It involves sensitivity to changes in the pericellular environment, such as from interactions between growth factors and HS [182]. In cancer tissues, aberrant expression of HSPGs, heparanase, and sulfatase is observed and influences disease progression and outcome [10,131,183]. There is increasing interest in developing HS and HS-modifying enzymes as useful targets for novel cancer therapeutics, such as inhibitors of heparanase [10,184,185]. HSPGs are expressed on accessible cell surfaces, but it remains unclear how abnormalities in HSPG expression affect changes in cell physiology and how different cell types in the cancer microenvironment are affected [10,55,186]. To achieve an understanding of the function of HSPGs in cancer progression, it is necessary to clarify how the expression of HSPG is regulated and maintained during normal development and how these molecules are deregulated during disease [9,10,55,187]. In addition, since HS is abundant on the cell surface, viruses use it as a scaffold for adsorption to target cells [29,38,188]. On the other hand, the structure of HS changes depending on the cell type, individual, age, etc., and there is various structural diversity of HS, which determines the infection tropism of viruses and the infection susceptibility of cells [4,29,39,189]. More detailed analysis of the interaction between virus particles and HS may lead to a better understanding of the various HS [37,38].
7. Extracellular Matrix (ECM) HSPGs
7.1. Cell Surface Type and Extracellular Matrix Type of HSPGs
With very few exceptions, HS is covalently bound to core proteins, and together they form HSPG, which are classified into two types: cell surface type and extracellular matrix type. The two main cell surface HSPGs are glypicans and Syndecans. As mentioned above, Syndecan has an extracellular region where HS chains and chondroitin sulfate chains bind, as well as a transmembrane region and an intracellular region. Glypicans are bound to membranes via glycosylphosphatidylinositol. Other cell surface HSPGs include CD44 and beta glycan [190]. On the other hand, the main HSPG in the extracellular matrix is perlecan. Cell surface HSPGs may also be secreted into the matrix because of enzymatic cleavage of core proteins.
7.2. Structure, Expression, and Functions of CD44
CD44 on the surface of lymphocytes and fibroblasts binds to HA, transmits signals by phosphorylating a kinase-like protein kinase C and phosphorylating serine/threonine residues within the CD44 molecules, and controls cell adhesion, proliferation, connective tissue component synthesis, etc. [191,192]. CD44 is a family with multiple isotopes and is a type 1 transmembrane glycoprotein that is expressed in a wide range of tissues and cells in normal living organisms [193,194]. These molecules bind to the extracellular matrix and are involved in cell movement and aggregation. Abnormal expression of these molecules has been reported to play an important role in tumor invasion and metastasis.
The human CD44 gene locus is in the p13 region of chromosome 11, and its genetic structure consists of at least 20 exons. Some exons undergo alternative splicing, resulting in multiple isotopes and isoforms, and, to date, more than 20 combinations of isotopes have been reported. In the upstream region of the CD44 gene, a typical TATA box sequence is not observed, but an AP-1 binding site, TPA (12-O-tetradecanoylphorbol 13-acetate) response element (TRE), and epidermal growth factor (EGF) response element (ERE) is present, leading to activation by intracellular signals. In addition to sugar chains, CS and HS are added to this gene product, and it is finally localized in the cell membrane. These molecules are classified into two major forms and variants. One of the major forms has a molecular weight of 80 to 90 kDa and is also called the standard form (CD44s) or the hematopoietic form (CD44H) since it was first discovered in blood cells. In particular, the role of binding to HA and its association with ankyrin in the intracellular domain in cell movement has been pointed out. The second major form has a molecular weight of 110 to 160 kDa and is called the epithelial form (CD44E) because it was first discovered in epithelial cells. Isotopes other than these two are collectively called variant forms (CD44v). Five well-conserved N-glycosylation sites exist in the N-terminal 120 amino acid region. The intracellular region of the CD44 molecules is usually selected from exon 20 and is called the long form (CD44-L) [195]. Exon 19 is rarely selected, and when it is selected, the molecule is called short form CD44 (CD44s), and this isotope exists at a ratio of about 1/100 to 1/200 times less. The intracytoplasmic region contains ezrin, radixin, moesin (ERM family) binding sites, ankyrin binding sites, and two phosphorylation sites (Ser325 and Ser327).
CD44 is expressed in many cell types, including blood cells, fibroblasts, epithelial cells, vascular endothelial cells, muscle cells, and neuroglial cells. It also appears and disappears during the differentiation and proliferation processes of each cell lineage, and changes dynamically [191,195]. In intestinal mucosa, gastric mucosa, and squamous epithelial mucosa, CD44 expression is enhanced in areas with strong basal proliferation, but the expression is weak or absent in superficial areas. When T cells are activated by antigen stimulation, etc., they begin to express isotopes containing v6, which are not observed in the resting phase and are involved in T cell migration to tissues.
HA is a long-chain glycosaminoglycan consisting of repeating disaccharides and is involved as an extracellular matrix in cell migration during morphogenesis, wound healing, and tumor progression. Cell surface molecules such as CD44 and RHAMM mediate cell movement by binding to HA as a ligand [196]. The binding of CD44 to HA is influenced not only by the amount of CD44 expressed but also by its distribution density and activation state [197]. Covalent homodimerization of CD44 via cysteine 286 in the transmembrane region is important for high-level binding of HA [198,199]. Furthermore, since it has been suggested that five well-conserved N-glycosylation sites present in the N-terminal 120 amino acid region play an important role in this region, the HA-binding ability is attenuated by these N-glycosylation and sialic acid additions. This also provides the basis for explaining the diversity of CD44 function across different tissues and cell types. Furthermore, the presence of a motif (B(7x)B) consisting of two basic amino acids into which seven non-acidic amino acids are inserted is important for binding to HA. In wildtype CD44, this motif exists repeatedly, once in the cartilage attachment region and twice in the center of the extracellular domain. Serglycin, a new ligand for CD44s, is a proteoglycan secreted by immune hematopoietic cells and is involved in the activation of immune cells. Osteopontin is an extracellular phosphorylated protein that is secreted by activated T cells, osteoblasts, histiocytes, etc. In addition to interacting with integrins, it also binds to CD44, an isotope containing v7 to v10, and the pathological conditions caused by this interaction are chemotaxis rather than motility.
7.3. Therapeutic Application of HSPGs
HA, whose functions are being further elucidated, is expected to be applied to a wide range of fields beyond the areas in which it has been primarily used, such as musculoskeletal diseases and ophthalmology. In addition, sugar chains such as heparin and CS, which belong to the same proteoglycan region as HA, have also been widely used as medicines. Heparin has a unique function of expressing anticoagulant activity through antithrombin III in the blood and has long held an unwavering position as a drug that maintains blood circulation. Low-molecular-weight heparin, an improved version of heparin with reduced bleeding effects, is an antithrombotic drug. Furthermore, as the oligosaccharide structure of the active moiety is elucidated, progress is being made in the development of antithrombotic agents using this active oligosaccharide chain. CS has been used as a medical drug for more than half a century. Its efficacy has been recognized for joint pain, neuralgia, frozen shoulders, etc. It is also used to prevent corneal dryness and is included in medical and non-prescription eye drops. In addition, polysaccharides such as chitin and chitosan, which are proteoglycan analogs, are being developed for use in medical devices [200,201]. In addition, polysaccharides such as dextran produced by microorganisms have long been used in infusions as plasma expanders, and polysaccharides derived from plants are used in anticancer drugs and healthy foods [202,203,204].
There are still not many examples of applying knowledge from glycoscience to pharmaceuticals. However, carbohydrates play unique roles in developmental and aging processes and diseases such as infection, immunity, inflammation, and cancer, and, as the elucidation of their mechanisms is rapidly progressing, there is no doubt that new opportunities for clinical application will open [205]. For example, influenza treatments, neuraminidase inhibitors, and other drugs are developed based on the knowledge and technology of glycoscience. The various cell surface sugar chain molecules involved in viral and microbial infections and the mechanisms involved in these infections have been elucidated, which is expected to further accelerate the development of infection prevention and treatment agents. Elucidation of the changes in sugar chains on the cell surface associated with cancerization and their mechanisms have become increasingly useful as an indicator for cancer diagnosis and treatment [206,207]. Furthermore, progress has been made in elucidating the involvement of HS and HA in cancer metastasis, implantation, and proliferation.
7.4. Molecular Mechanism of CD44 and Tumor Invasion and Metastasis
In metastatic breast cancer cell lines, CD44v3m v8 to V10 and MMP-9 combine and localize on the cell surface, contributing to invadopodia formation and cell migration [208]. In mouse breast cancer cell lines and human melanoma cell lines, CD44 binds to active MMP-9, localizes on the cell membrane, and contributes to tumor invasion by promoting the degradation of ECM collagen IV. This binding between CD44 and MMP-9 is due to the ability of CD44 to bind to HA. The migration ability of cancer cells via HA, a ligand for CD44, is related to the cleavage of CD44 by membrane-type metalloproteases [209]. In lung cancer cell lines, CD44 expression induces and enhances the expression of membrane type 2-matrix metalloproteinase (MT2-MMP) is induced and enhanced, promoting the activation of proMMP-2, contributing to the decomposition of the surrounding ECM, and being involved in tumor invasion [210,211]. Thus, in elucidating the molecular mechanism of cancer invasion and metastasis involving CD44, an adhesion molecule, not only its ligand binding ability but also MMPs, which are ECM degrading enzymes, are involved [191].
7.5. CD44 and Angiogenesis
The growth of tumors larger than one to two mm requires blood vessel induction and maintenance, and tumor metastasis is dependent on angiogenesis. Angiogenesis is controlled by the balance between promoting and suppressing factors secreted by tumors. Inside a tumor, microvessels supply the oxygen and nutrients necessary for tumor cell growth and maintenance and serve as passageways for tumor cells to migrate away from their primary tumor [212]. Factors that promote this include bFGF, VEGF, and IL-8, and some of these growth factors can bind to the HS chain of CD44, and cytokines have an affinity for heparin and can bind to HS. Vascular endothelial cells displaying growth factors on the CD44 molecule promote the survival, proliferation, and migration of tumor cells out of the blood vessels. The expression of CD44 is enhanced in vascular endothelial cells in tumors compared to endothelial cells in normal tissues, in which enhanced expression of CD44 in endothelial cells is induced by stimulation with bFGF or VEGF [213]. In addition, by blocking CD44 expressed on endothelial cells with an anti-CD44 monoclonal antibody, proliferation, migration, and lumen formation of endothelial cells are inhibited. In fibroblasts and alveolar macrophages, CD44 binds to HA, takes it into the cells, and is degraded by hyaluronidase. These degraded products of HA act on tyrosine phosphorylation in endothelial cells, promoting endothelial cell proliferation. On the other hand, when CD44 isotopes containing v10 were introduced into human mammary gland epithelial cells expressing CD44s, the expression of bFGF and IL-8 was enhanced. In addition, introducing CD44E into CD44-negative human lung cancer cells enhances VEGF production and induces angiogenesis in vitro and in vivo.
7.6. CD44 and Cell Motility via Cell Skeletal Protein
Ezrin/radixin/moesin (ERM) protein family are localized directly beneath protoplasmic membranes such as microvilli, ruffling membranes, cleavage furrows, and adherens junctions, and are involved in their formation and function. These regions are where the actin filament is tightly bound to the membrane, and the ERM family is directly involved in the molecular mechanisms that control the interaction of the actin filament with the plasma membrane. One of the major membrane proteins that this ERM protein binds to is CD44. In addition, CD44 and ERM proteins show strong affinity and form a complex in the presence of phosphoinositides. When the CD44/ERM complex was immunoprecipitated, this complex contained a Rho-GDP dissociation inhibitor (GDI). Furthermore, C3 toxin, a specific inhibitor of Rho, inhibits the binding of recombinant ERM proteins to CD44, reducing motor performance. Therefore, Rho and phosphorylation play a critical role in the formation of the CD44/ERM complex and are also involved in the regulation of locomotor performance.
7.7. Soluble CD44
CD44 can be rapidly downregulated in response to signals inside and outside the cell, but this is mainly due to the dissociation of soluble CD44 from the extracellular region. The CD44 released in this way can be found in the serum, and soluble CD44 is elevated in the serum of patients with malignant tumors. Serum CD44 and CD44v6 levels in patients with malignant lymphoma are both increased compared to healthy individuals and, as these values decreased in response to treatment, they can be used to monitor the effectiveness of treatment. Furthermore, soluble CD44s-Ig fusion protein suppresses the metastasis of lymphoid malignant tumors that highly express CD44s and can be applied to therapy.
8. Conclusions
HSPGs have been involved in developmental and homeostatic processes, as well as in many pathological conditions. Therefore, it can be applied to a wide range of fields for therapeutic strategies, including Alzheimer’s disease, heart failure, cancer, organ transplants, diabetes, chronic inflammation, aging, and autoimmune diseases. HSPGs, a major component of the glycocalyx, enable the binding of various plasma-derived molecules due to their diversity, epimerization of sugar chains, long chains, and sulfation. The glycocalyx has a variety of functions, including maintaining blood flow, protecting the endothelium, antithrombotic effects, binding and signal transmission of hormones and growth factors, and serving as a nutritional source consisting of sugar chains. The various structural diversity of HS determines the infection tropism of viruses and the infection susceptibility of cells.
Author Contributions
Writing—review and editing, Y.M.; supervision, R.Y.; funding acquisition, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not appliable.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Ravikumar, M.; Smith, R.A.A.; Nurcombe, V.; Cool, S.M. Heparan Sulfate Proteoglycans: Key Mediators of Stem Cell Function. Front. Cell Dev. Biol. 2020, 8, 581213. [Google Scholar] [CrossRef]
- Moon, S.; Zhao, Y.T. Spatial, temporal and cell-type-specific expression profiles of genes encoding heparan sulfate biosynthesis enzymes and proteoglycan core proteins. Glycobiology 2021, 31, 1308–1318. [Google Scholar] [CrossRef]
- Condomitti, G.; de Wit, J. Heparan Sulfate Proteoglycans as Emerging Players in Synaptic Specificity. Front. Mol. Neurosci. 2018, 11, 14. [Google Scholar] [CrossRef]
- De Pasquale, V.; Quiccione, M.S.; Tafuri, S.; Avallone, L.; Pavone, L.M. Heparan Sulfate Proteoglycans in Viral Infection and Treatment: A Special Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 6574. [Google Scholar] [CrossRef]
- Sparn, C.; Dimou, E.; Meyer, A.; Saleppico, R.; Wegehingel, S.; Gerstner, M.; Klaus, S.; Ewers, H.; Nickel, W. Glypican-1 drives unconventional secretion of fibroblast growth factor 2. eLife 2022, 11, e75545. [Google Scholar] [CrossRef]
- Gopal, S.; Arokiasamy, S.; Pataki, C.; Whiteford, J.R.; Couchman, J.R. Syndecan receptors: Pericellular regulators in development and inflammatory disease. Open Biol. 2021, 11, 200377. [Google Scholar] [CrossRef]
- Gondelaud, F.; Ricard-Blum, S. Structures and interactions of syndecans. FEBS J. 2019, 286, 2994–3007. [Google Scholar] [CrossRef]
- Jang, B.; Kim, A.; Hwang, J.; Song, H.K.; Kim, Y.; Oh, E.S. Emerging Role of Syndecans in Extracellular Matrix Remodeling in Cancer. J. Histochem. Cytochem. 2020, 68, 863–870. [Google Scholar] [CrossRef]
- Onyeisi, J.O.S.; Ferreira, B.Z.F.; Nader, H.B.; Lopes, C.C. Heparan sulfate proteoglycans as targets for cancer therapy: A review. Cancer Biol. Ther. 2020, 21, 1087–1094. [Google Scholar] [CrossRef]
- Nagarajan, A.; Malvi, P.; Wajapeyee, N. Heparan Sulfate and Heparan Sulfate Proteoglycans in Cancer Initiation and Progression. Front. Endocrinol. 2018, 9, 483. [Google Scholar] [CrossRef]
- Crespo, A.; García-Suárez, O.; Fernández-Vega, I.; Solis-Hernandez, M.P.; García, B.; Castañón, S.; Quirós, L.M. Heparan sulfate proteoglycans undergo differential expression alterations in left sided colorectal cancer, depending on their metastatic character. BMC Cancer 2018, 18, 687. [Google Scholar] [CrossRef]
- Thota, L.N.R.; Chignalia, A.Z. The role of the glypican and syndecan families of heparan sulfate proteoglycans in cardiovascular function and disease. Am. J. Physiol. Cell Physiol. 2022, 323, C1052–C1060. [Google Scholar] [CrossRef]
- Guss, E.J.; Akbergenova, Y.; Cunningham, K.L.; Littleton, J.T. Loss of the extracellular matrix protein Perlecan disrupts axonal and synaptic stability during Drosophila development. eLife 2023, 12, RP88273. [Google Scholar] [CrossRef]
- Hayes, A.J.; Farrugia, B.L.; Biose, I.J.; Bix, G.J.; Melrose, J. Perlecan, A Multi-Functional, Cell-Instructive, Matrix-Stabilizing Proteoglycan with Roles in Tissue Development Has Relevance to Connective Tissue Repair and Regeneration. Front. Cell Dev. Biol. 2022, 10, 856261. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nakada, S.; Yoshihara, T.; Nara, T.; Furuya, N.; Miida, T.; Hattori, N.; Arikawa-Hirasawa, E. Perlecan, a heparan sulfate proteoglycan, regulates systemic metabolism with dynamic changes in adipose tissue and skeletal muscle. Sci. Rep. 2018, 8, 7766. [Google Scholar] [CrossRef]
- Bai, G.; Zhang, M. Clustering acetylcholine receptors in neuromuscular junction by phase-separated Rapsn condensates. Neuron 2021, 109, 1907–1909. [Google Scholar] [CrossRef]
- Guarino, S.R.; Canciani, A.; Forneris, F. Dissecting the Extracellular Complexity of Neuromuscular Junction Organizers. Front. Mol. Biosci. 2020, 6, 156. [Google Scholar] [CrossRef]
- Dong, C.; Choi, Y.K.; Lee, J.; Zhang, X.F.; Honerkamp-Smith, A.; Widmalm, G.; Lowe-Krentz, L.J.; Im, W. Structure, Dynamics, and Interactions of GPI-Anchored Human Glypican-1 with Heparan Sulfates in a Membrane. Glycobiology 2021, 31, 593–602. [Google Scholar] [CrossRef]
- Yu, P.; Pearson, C.S.; Geller, H.M. Flexible Roles for Proteoglycan Sulfation and Receptor Signaling. Trends Neurosci. 2018, 41, 47–61. [Google Scholar] [CrossRef]
- Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004, 131, 6009–6021. [Google Scholar] [CrossRef]
- Park, P.W. Isolation and functional analysis of syndecans. Methods Cell Biol. 2018, 143, 317–333. [Google Scholar] [CrossRef]
- Annaval, T.; Wild, R.; Crétinon, Y.; Sadir, R.; Vivès, R.R.; Lortat-Jacob, H. Heparan Sulfate Proteoglycans Biosynthesis and Post Synthesis Mechanisms Combine Few Enzymes and Few Core Proteins to Generate Extensive Structural and Functional Diversity. Molecules 2020, 25, 4215. [Google Scholar] [CrossRef]
- Marques, C.; Reis, C.A.; Vivès, R.R.; Magalhães, A. Heparan Sulfate Biosynthesis and Sulfation Profiles as Modulators of Cancer Signalling and Progression. Front. Oncol. 2021, 11, 778752. [Google Scholar] [CrossRef]
- Leisico, F.; Omeiri, J.; Le Narvor, C.; Beaudouin, J.; Hons, M.; Fenel, D.; Schoehn, G.; Couté, Y.; Bonnaffé, D.; Sadir, R.; et al. Structure of the human heparan sulfate polymerase complex EXT1-EXT2. Nat. Commun. 2022, 13, 7110. [Google Scholar] [CrossRef]
- Niwa, A.; Taniguchi, T.; Tomita, H.; Okada, H.; Kinoshita, T.; Mizutani, C.; Matsuo, M.; Imaizumi, Y.; Kuroda, T.; Ichihashi, K.; et al. Conditional ablation of heparan sulfate expression in stromal fibroblasts promotes tumor growth in vivo. PLoS ONE 2023, 18, e0281820. [Google Scholar] [CrossRef]
- Hwang, J.; Jang, B.; Kim, A.; Lee, Y.; Lee, J.; Kim, C.; Kim, J.; Moon, K.M.; Kim, K.; Wagle, R.; et al. Syndecan Transmembrane Domain Specifically Regulates Downstream Signaling Events of the Transmembrane Receptor Cytoplasmic Domain. Int. J. Mol. Sci. 2021, 22, 7918. [Google Scholar] [CrossRef]
- Hara, T.; Sato, A.; Yamamoto, C.; Kaji, T. Syndecan-1 downregulates syndecan-4 expression by suppressing the ERK1/2 and p38 MAPK signaling pathways in cultured vascular endothelial cells. Biochem. Biophys. Rep. 2021, 26, 101001. [Google Scholar] [CrossRef]
- Ren, Z.; Spaargaren, M.; Pals, S.T. Syndecan-1 and stromal heparan sulfate proteoglycans: Key moderators of plasma cell biology and myeloma pathogenesis. Blood 2021, 137, 1713–1718. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.T.; Pan, Y.; Liu, X.F.; Xu, J.W.; Cui, W.J.; Qiao, X.R.; Dong, L. Syndecan-1 Shedding by Matrix Metalloproteinase-9 Signaling Regulates Alveolar Epithelial Tight Junction in Lipopolysaccharide-Induced Early Acute Lung Injury. J. Inflamm. Res. 2021, 14, 5801–5816. [Google Scholar] [CrossRef]
- Jang, B.; Yun, J.H.; Choi, S.; Park, J.; Shin, D.H.; Lee, S.T.; Lee, W.; Oh, E.S. Tyrosine 51 residue of the syndecan-2 extracellular domain is involved in the interaction with and activation of pro-matrix metalloproteinase-7. Sci. Rep. 2019, 9, 10625. [Google Scholar] [CrossRef]
- De Pasquale, V.; Sarogni, P.; Pistorio, V.; Cerulo, G.; Paladino, S.; Pavone, L.M. Targeting Heparan Sulfate Proteoglycans as a Novel Therapeutic Strategy for Mucopolysaccharidoses. Mol. Ther. Methods Clin. Dev. 2018, 10, 8–16. [Google Scholar] [CrossRef]
- Pretorius, D.; Richter, R.P.; Anand, T.; Cardenas, J.C.; Richter, J.R. Alterations in heparan sulfate proteoglycan synthesis and sulfation and the impact on vascular endothelial function. Matrix Biol. Plus 2022, 16, 100121. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.; Ying, H.; Yao, W. Targeting syndecan-1: New opportunities in cancer therapy. Am. J. Physiol. Cell Physiol. 2022, 323, C29–C45. [Google Scholar] [CrossRef]
- Garcia, J.; Patel, N.; Basehore, S.; Clyne, A.M. Fibroblast Growth Factor-2 Binding to Heparan Sulfate Proteoglycans Varies with Shear Stress in Flow-Adapted Cells. Ann. Biomed. Eng. 2019, 47, 1078–1093. [Google Scholar] [CrossRef]
- Koledova, Z.; Sumbal, J.; Rabata, A.; de La Bourdonnaye, G.; Chaloupkova, R.; Hrdlickova, B.; Damborsky, J.; Stepankova, V. Fibroblast Growth Factor 2 Protein Stability Provides Decreased Dependence on Heparin for Induction of FGFR Signaling and Alters ERK Signaling Dynamics. Front. Cell Dev. Biol. 2019, 7, 331. [Google Scholar] [CrossRef]
- Hayashida, K.; Aquino, R.S.; Park, P.W. Coreceptor functions of cell surface heparan sulfate proteoglycans. Am. J. Physiol. Cell Physiol. 2022, 322, C896–C912. [Google Scholar] [CrossRef]
- Chopra, P.; Joshi, A.; Wu, J.; Lu, W.; Yadavalli, T.; Wolfert, M.A.; Shukla, D.; Zaia, J.; Boons, G.J. The 3-O-sulfation of heparan sulfate modulates protein binding and lyase degradation. Proc. Natl. Acad. Sci. USA 2021, 118, e2012935118. [Google Scholar] [CrossRef]
- Ferreira, A.; Timmerman, E.; Staes, A.; Vuylsteke, M.; De Muynck, L.; Gevaert, K. Protein interactors of 3-O sulfated heparan sulfates in human MCI and age-matched control cerebrospinal fluid. Sci. Data 2023, 10, 121. [Google Scholar] [CrossRef]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef]
- Gómez Toledo, A.; Sorrentino, J.T.; Sandoval, D.R.; Malmström, J.; Lewis, N.E.; Esko, J.D. A Systems View of the Heparan Sulfate Interactome. J. Histochem. Cytochem. 2021, 69, 105–119. [Google Scholar] [CrossRef]
- Pataki, Z.; Rebolledo Viveros, A.; Heldwein, E.E. Herpes Simplex Virus 1 Entry Glycoproteins Form Complexes before and during Membrane Fusion. mBio 2022, 13, e0203922. [Google Scholar] [CrossRef]
- Weed, D.J.; Nicola, A.V. Herpes simplex virus Membrane Fusion. Adv. Anat. Embryol. Cell Biol. 2017, 223, 29–47. [Google Scholar] [CrossRef]
- Elste, J.; Chan, A.; Patil, C.; Tripathi, V.; Shadrack, D.M.; Jaishankar, D.; Hawkey, A.; Mungerson, M.S.; Shukla, D.; Tiwari, V. Archaic connectivity between the sulfated heparan sulfate and the herpesviruses—An evolutionary potential for cross-species interactions. Comput. Struct. Biotechnol. J. 2023, 21, 1030–1040. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Song, X.; Xiao, Y.; Su, G.; Liu, X.; Wang, Z.; Xu, Y.; Liu, J.; Eliezer, D.; et al. 3-O-Sulfation of Heparan Sulfate Enhances Tau Interaction and Cellular Uptake. Angew. Chem. Int. Ed. Engl. 2020, 59, 1818–1827. [Google Scholar] [CrossRef]
- Kearns, F.L.; Sandoval, D.R.; Casalino, L.; Clausen, T.M.; Rosenfeld, M.A.; Spliid, C.B.; Amaro, R.E.; Esko, J.D. Spike-heparan sulfate interactions in SARS-CoV-2 infection. Curr. Opin. Struct. Biol. 2022, 76, 102439. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.Z.; Swaroop, M.; Xu, M.; Wang, L.; Lee, J.; Wang, A.Q.; Pradhan, M.; Hagen, N.; Chen, L.; et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020, 6, 80. [Google Scholar] [CrossRef]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, W.; Stancanelli, E.; Jung, E.; Syed, Z.; Pagadala, V.; Saidi, L.; Chen, C.Z.; Gao, P.; Xu, M.; et al. Host heparan sulfate promotes ACE2 super-cluster assembly and enhances SARS-CoV-2-associated syncytium formation. Nat. Commun. 2023, 14, 5777. [Google Scholar] [CrossRef]
- Villalba, N.; Baby, S.; Yuan, S.Y. The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front. Cell Dev. Biol. 2021, 9, 711003. [Google Scholar] [CrossRef]
- Hu, Z.; Cano, I.; D’Amore, P.A. Update on the Role of the Endothelial Glycocalyx in Angiogenesis and Vascular Inflammation. Front. Cell Dev. Biol. 2021, 9, 734276. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: A review. Ann. Intensive Care 2020, 10, 85. [Google Scholar] [CrossRef]
- Möckl, L. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front. Cell Dev. Biol. 2020, 8, 253. [Google Scholar] [CrossRef]
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier. Front. Cell. Neurosci. 2021, 15, 739699. [Google Scholar] [CrossRef]
- Moore, K.H.; Murphy, H.A.; George, E.M. The glycocalyx: A central regulator of vascular function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R508–R518. [Google Scholar] [CrossRef]
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087. [Google Scholar] [CrossRef]
- Schött, U.; Solomon, C.; Fries, D.; Bentzer, P. The endothelial glycocalyx and its disruption, protection and regeneration: A narrative review. Scand. J. Trauma. Resusc. Emerg. Med. 2016, 24, 48. [Google Scholar] [CrossRef]
- Kundra, P.; Goswami, S. Endothelial glycocalyx: Role in body fluid homeostasis and fluid management. Indian J. Anaesth. 2019, 63, 6–14. [Google Scholar] [CrossRef]
- Haymet, A.B.; Bartnikowski, N.; Wood, E.S.; Vallely, M.P.; McBride, A.; Yacoub, S.; Biering, S.B.; Harris, E.; Suen, J.Y.; Fraser, J.F. Studying the Endothelial Glycocalyx in vitro: What Is Missing? Front. Cardiovasc. Med. 2021, 8, 647086. [Google Scholar] [CrossRef]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781–3811. [Google Scholar] [CrossRef]
- Jiang, X.Z.; Goligorsky, M.S. Biomechanical properties of endothelial glycocalyx: An imperfect pendulum. Matrix Biol. Plus 2021, 12, 100087. [Google Scholar] [CrossRef]
- Bol, M.E.; Huckriede, J.B.; van de Pas, K.G.H.; Delhaas, T.; Lorusso, R.; Nicolaes, G.A.F.; Sels, J.E.M.; van de Poll, M.C.G. Multimodal measurement of glycocalyx degradation during coronary artery bypass grafting. Front. Med. 2022, 9, 1045728. [Google Scholar] [CrossRef]
- Patterson, E.K.; Cepinskas, G.; Fraser, D.D. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front. Med. 2022, 9, 898592. [Google Scholar] [CrossRef]
- Damén, T.; Kolsrud, O.; Dellgren, G.; Hesse, C.; Ricksten, S.E.; Nygren, A. Atrial natriuretic peptide does not degrade the endothelial glycocalyx: A secondary analysis of a randomized porcine model. Acta. Anaesthesiol. Scand. 2021, 65, 1305–1312. [Google Scholar] [CrossRef]
- Hahn, R.G.; Patel, V.; Dull, R.O. Human glycocalyx shedding: Systematic review and critical appraisal. Acta. Anaesthesiol. Scand. 2021, 65, 590–606. [Google Scholar] [CrossRef]
- Passov, A.; Schramko, A.; Salminen, U.S.; Aittomäki, J.; Andersson, S.; Pesonen, E. Endothelial glycocalyx during early reperfusion in patients undergoing cardiac surgery. PLoS ONE 2021, 16, e0251747. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, W.; Miao, C.; Zhong, J. Hypercatabolism and Anti-catabolic Therapies in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Nutr. 2022, 9, 941097. [Google Scholar] [CrossRef]
- Zha, D.; Fu, M.; Qian, Y. Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19. Cells 2022, 11, 1972. [Google Scholar] [CrossRef]
- Wang, G.; Kostidis, S.; Tiemeier, G.L.; Sol, W.M.P.J.; de Vries, M.R.; Giera, M.; Carmeliet, P.; van den Berg, B.M.; Ton, J.; Rabelink, T.J. Shear Stress Regulation of Endothelial Glycocalyx Structure Is Determined by Glucobiosynthesis. Arterioscler. Thromb. Vasc Biol. 2020, 40, 350–364. [Google Scholar] [CrossRef]
- Oshima, K.; King, S.I.; McMurtry, S.A.; Schmidt, E.P. Endothelial Heparan Sulfate Proteoglycans in Sepsis: The Role of the Glycocalyx. Semin. Thromb. Hemost. 2021, 47, 274–282. [Google Scholar] [CrossRef]
- Wight, T.N. A role for proteoglycans in vascular disease. Matrix Biol. 2018, 71–72, 396–420. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Du, H.; Ye, Y.; Miu, Y.; Su, T.; Guo, X.; Wang, S.; Qiu, Y.; Wang, J.; et al. Antithrombin III activity is associated with prognosis, infection, and inflammation in patients with hepatitis B virus-related acute-on-chronic liver failure. Eur. J. Gastroenterol. Hepatol. 2023, 35, 914–920. [Google Scholar] [CrossRef]
- Arnold, K.; Xu, Y.; Liao, Y.E.; Cooley, B.C.; Pawlinski, R.; Liu, J. Synthetic anticoagulant heparan sulfate attenuates liver ischemia reperfusion injury. Sci. Rep. 2020, 10, 17187. [Google Scholar] [CrossRef]
- Ito, T.; Thachil, J.; Asakura, H.; Levy, J.H.; Iba, T. Thrombomodulin in disseminated intravascular coagulation and other critical conditions-a multi-faceted anticoagulant protein with therapeutic potential. Crit. Care 2019, 23, 280. [Google Scholar] [CrossRef]
- Watanabe-Kusunoki, K.; Nakazawa, D.; Ishizu, A.; Atsumi, T. Thrombomodulin as a Physiological Modulator of Intravascular Injury. Front. Immunol. 2020, 11, 575890. [Google Scholar] [CrossRef]
- Gopal, S. Syndecans in Inflammation at a Glance. Front. Immunol. 2020, 11, 227. [Google Scholar] [CrossRef]
- Ahn, S.J.; Le Master, E.; Granados, S.T.; Levitan, I. Impairment of endothelial glycocalyx in atherosclerosis and obesity. Curr. Top. Membr. 2023, 91, 1–19. [Google Scholar] [CrossRef]
- Wang, G.; Tiemeier, G.L.; van den Berg, B.M.; Rabelink, T.J. Endothelial Glycocalyx Hyaluronan: Regulation and Role in Prevention of Diabetic Complications. Am. J. Pathol. 2020, 190, 781–790. [Google Scholar] [CrossRef]
- Dogné, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Shi, D.; Sheng, A.; Chi, L. Glycosaminoglycan-Protein Interactions and Their Roles in Human Disease. Front. Mol. Biosci. 2021, 8, 639666. [Google Scholar] [CrossRef]
- Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Lepedda, A.J.; Nieddu, G.; Formato, M.; Baker, M.B.; Fernández-Pérez, J.; Moroni, L. Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 2021, 9, 680836. [Google Scholar] [CrossRef]
- Zapp, C.; Mundinger, P.; Boehm, H. Natural Presentation of Glycosaminoglycans in Synthetic Matrices for 3D Angiogenesis Models. Front. Cell Dev. Biol. 2021, 9, 729670. [Google Scholar] [CrossRef]
- Wang, Q.; Chi, L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers 2022, 14, 5014. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Wang, J.; Zhang, Q. Roles of Endovascular Calyx Related Enzymes in Endothelial Dysfunction and Diabetic Vascular Complications. Front. Pharmacol. 2020, 1, 590614. [Google Scholar] [CrossRef]
- Dogné, S.; Flamion, B.; Caron, N. Endothelial Glycocalyx as a Shield Against Diabetic Vascular Complications: Involvement of Hyaluronan and Hyaluronidases. Arterioscler. Thromb. Vasc Biol. 2018, 38, 1427–1439. [Google Scholar] [CrossRef]
- van Splunder, H.; Villacampa, P.; Martínez-Romero, A.; Graupera, M. Pericytes in the disease spotlight. Trends Cell Biol. 2023, 34, 58–71. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Escribano, J.; Chen, M.B.; Moeendarbary, E.; Cao, X.; Shenoy, V.; Garcia-Aznar, J.M.; Kamm, R.D.; Spill, F. Balance of mechanical forces drives endothelial gap formation and may facilitate cancer and immune-cell extravasation. PLoS Comput. Biol. 2019, 15, e1006395. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Okamoto, T.; Kawamoto, E.; Takagi, Y.; Akita, N.; Hayashi, T.; Park, E.J.; Suzuki, K.; Shimaoka, M. Gap junction-mediated regulation of endothelial cellular stiffness. Sci. Rep. 2017, 7, 6134. [Google Scholar] [CrossRef]
- Zhao, F.; Zhong, L.; Luo, Y. Endothelial glycocalyx as an important factor in composition of blood-brain barrier. CNS Neurosci. Ther. 2021, 27, 26–35. [Google Scholar] [CrossRef]
- Yu, H.; Song, Y.Y.; Li, X.H. Early diabetic kidney disease: Focus on the glycocalyx. World J. Diabetes. 2023, 14, 460–480. [Google Scholar] [CrossRef]
- Suzuki, K.; Miura, T.; Okada, H. The endothelial glycocalyx-All the same? No, it is not. Acute Med. Surg. 2023, 10, e896. [Google Scholar] [CrossRef]
- Kong, C.; Elderman, M.; Cheng, L.; de Haan, B.J.; Nauta, A.; de Vos, P. Modulation of Intestinal Epithelial Glycocalyx Development by Human Milk Oligosaccharides and Non-Digestible Carbohydrates. Mol. Nutr. Food Res. 2019, 63, e1900303. [Google Scholar] [CrossRef]
- Sun, W.W.; Krystofiak, E.S.; Leo-Macias, A.; Cui, R.; Sesso, A.; Weigert, R.; Ebrahim, S.; Kachar, B. Nanoarchitecture and dynamics of the mouse enteric glycocalyx examined by freeze-etching electron tomography and intravital microscopy. Commun. Biol. 2020, 3, 5. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Salazar-Peláez, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300. [Google Scholar] [CrossRef]
- Dull, R.O.; Hahn, R.G. The glycocalyx as a permeability barrier: Basic science and clinical evidence. Crit. Care 2022, 26, 273. [Google Scholar] [CrossRef]
- Suzuki, A.; Tomita, H.; Okada, H. Form follows function: The endothelial glycocalyx. Transl. Res. 2022, 247, 158–167. [Google Scholar] [CrossRef]
- Trimm, E.; Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. 2023, 20, 197–210. [Google Scholar] [CrossRef]
- Lim, J.; Machin, D.R.; Donato, A.J. The role of hyaluronan in endothelial glycocalyx and potential preventative lifestyle strategy with advancing age. Curr. Top. Membr. 2023, 91, 139–156. [Google Scholar] [CrossRef]
- Qu, J.; Cheng, Y.; Wu, W.; Yuan, L.; Liu, X. Glycocalyx Impairment in Vascular Disease: Focus on Inflammation. Front. Cell Dev. Biol. 2021, 9, 730621. [Google Scholar] [CrossRef]
- Zavras, P.D.; Mehta, V.; Goel, S.; Billett, H.H. Increased incidence of thrombosis in a cohort of cancer patients with COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Levi, M.; van Es, N. COVID-19 associated coagulopathy and thrombosis in cancer. Thromb. Res. 2022, 213 (Suppl. S1), S72–S76. [Google Scholar] [CrossRef]
- Villar-Fincheira, P.; Sanhueza-Olivares, F.; Norambuena-Soto, I.; Cancino-Arenas, N.; Hernandez-Vargas, F.; Troncoso, R.; Gabrielli, L.; Chiong, M. Role of Interleukin-6 in Vascular Health and Disease. Front. Mol. Biosci. 2021, 8, 641734. [Google Scholar] [CrossRef]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- Wilson, S.E. Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response to Injury. Investig. Ophthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar] [CrossRef]
- Tanino, Y.; Wang, X.; Nikaido, T.; Misa, K.; Sato, Y.; Togawa, R.; Kawamata, T.; Kikuchi, M.; Frevert, C.W.; Tanino, M.; et al. Syndecan-4 Inhibits the Development of Pulmonary Fibrosis by Attenuating TGF-β Signaling. Int. J. Mol. Sci. 2019, 20, 4989. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Chen, L.; Fu, L.; Sun, J.; Huang, Z.; Fang, M.; Zinkle, A.; Liu, X.; Lu, J.; Pan, Z.; Wang, Y.; et al. Structural basis for FGF hormone signalling. Nature 2023, 618, 862–870. [Google Scholar] [CrossRef]
- Onyeisi, J.O.S.; Lopes, C.C.; Götte, M. Syndecan-4 as a Pathogenesis Factor and Therapeutic Target in Cancer. Biomolecules 2021, 11, 503. [Google Scholar] [CrossRef]
- Eustace, A.D.; McNaughton, E.F.; King, S.; Kehoe, O.; Kungl, A.; Mattey, D.; Nobbs, A.H.; Williams, N.; Middleton, J. Soluble syndecan-3 binds chemokines, reduces leukocyte migration in vitro and ameliorates disease severity in models of rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 172. [Google Scholar] [CrossRef]
- Gerlza, T.; Nagele, M.; Mihalic, Z.; Trojacher, C.; Kungl, A. Glycosaminoglycans located on neutrophils and monocytes impact on CXCL8- and CCL2-induced cell migration. Cytokine 2021, 142, 155503. [Google Scholar] [CrossRef]
- Crijns, H.; Vanheule, V.; Proost, P. Targeting Chemokine-Glycosaminoglycan Interactions to Inhibit Inflammation. Front. Immunol. 2020, 11, 483. [Google Scholar] [CrossRef]
- Sepuru, K.M.; Rajarathnam, K. Structural basis of chemokine interactions with heparan sulfate, chondroitin sulfate, and dermatan sulfate. J. Biol. Chem. 2019, 294, 15650–15661. [Google Scholar] [CrossRef]
- Jones, F.K.; Stefan, A.; Kay, A.G.; Hyland, M.; Morgan, R.; Forsyth, N.R.; Pisconti, A.; Kehoe, O. Syndecan-3 regulates MSC adhesion, ERK and AKT signalling in vitro and its deletion enhances MSC efficacy in a model of inflammatory arthritis in vivo. Sci. Rep. 2020, 10, 20487. [Google Scholar] [CrossRef]
- Hachim, D.; Whittaker, T.E.; Kim, H.; Stevens, M.M. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices. J. Control. Release 2019, 313, 131–147. [Google Scholar] [CrossRef]
- Shamdani, S.; Chantepie, S.; Flageollet, C.; Henni-Chebra, N.; Jouan, Y.; Eymard, F.; Hay, E.; Cohen-Solal, M.; Papy-Garcia, D.; Chevalier, X.; et al. Heparan sulfate functions are altered in the osteoarthritic cartilage. Arthritis Res. Ther. 2020, 22, 283. [Google Scholar] [CrossRef]
- Margraf, A.; Lowell, C.A.; Zarbock, A. Neutrophils in acute inflammation: Current concepts and translational implications. Blood 2022, 139, 2130–2144. [Google Scholar] [CrossRef]
- Hara, T.; Yabushita, S.; Yamamoto, C.; Kaji, T. Cell Density-Dependent Fibroblast Growth Factor-2 Signaling Regulates Syndecan-4 Expression in Cultured Vascular Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 3698. [Google Scholar] [CrossRef]
- Huang, X.; Reye, G.; Momot, K.I.; Blick, T.; Lloyd, T.; Tilley, W.D.; Hickey, T.E.; Snell, C.E.; Okolicsanyi, R.K.; Haupt, L.M.; et al. Heparanase Promotes Syndecan-1 Expression to Mediate Fibrillar Collagen and Mammographic Density in Human Breast Tissue Cultured ex vivo. Front. Cell Dev. Biol. 2020, 8, 599. [Google Scholar] [CrossRef]
- Guo, S.; Wu, X.; Lei, T.; Zhong, R.; Wang, Y.; Zhang, L.; Zhao, Q.; Huang, Y.; Shi, Y.; Wu, L. The Role and Therapeutic Value of Syndecan-1 in Cancer Metastasis and Drug Resistance. Front. Cell Dev. Biol. 2022, 9, 784983. [Google Scholar] [CrossRef]
- Strand, M.E.; Vanhaverbeke, M.; Henkens, M.T.H.M.; Sikking, M.A.; Rypdal, K.B.; Braathen, B.; Almaas, V.M.; Tønnessen, T.; Christensen, G.; Heymans, S.; et al. Inflammation and Syndecan-4 Shedding from Cardiac Cells in Ischemic and Non-Ischemic Heart Disease. Biomedicines 2023, 11, 1066. [Google Scholar] [CrossRef]
- Shaik, F.; Balderstone, M.J.M.; Arokiasamy, S.; Whiteford, J.R. Roles of Syndecan-4 in cardiac injury and repair. Int. J. Biochem. Cell Biol. 2022, 146, 106196. [Google Scholar] [CrossRef]
- El Masri, R.; Seffouh, A.; Roelants, C.; Seffouh, I.; Gout, E.; Pérard, J.; Dalonneau, F.; Nishitsuji, K.; Noborn, F.; Nikpour, M.; et al. Extracellular endosulfatase Sulf-2 harbors a chondroitin/dermatan sulfate chain that modulates its enzyme activity. Cell Rep. 2022, 38, 110516. [Google Scholar] [CrossRef]
- Dao, D.T.; Anez-Bustillos, L.; Adam, R.M.; Puder, M.; Bielenberg, D.R. Heparin-Binding Epidermal Growth Factor-Like Growth Factor as a Critical Mediator of Tissue Repair and Regeneration. Am. J. Pathol. 2018, 188, 2446–2456. [Google Scholar] [CrossRef]
- Jayatilleke, K.M.; Hulett, M.D. Heparanase and the hallmarks of cancer. J. Transl. Med. 2020, 18, 453. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Liu, B.; Xie, W. Estrogen sulfotransferase and sulfatase in steroid homeostasis, metabolic disease, and cancer. Steroids 2023, 201, 109335. [Google Scholar] [CrossRef]
- Okada, T.; Keino-Masu, K.; Nagamine, S.; Kametani, F.; Ohto, T.; Hasegawa, M.; van Kuppevelt, T.H.; Kunita, S.; Takahashi, S.; Masu, M. Desulfation of Heparan Sulfate by Sulf1 and Sulf2 Is Required for Corticospinal Tract Formation. Sci. Rep. 2017, 7, 13847. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, N.; Fu, Z.; Zhang, Q. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules 2023, 13, 483. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Zhou, J.; Liang, Q.; He, F.; Li, F.; Li, Y.; Chen, J.; Zhang, F.; Han, C.; et al. Fibronectin 1 activates WNT/β-catenin signaling to induce osteogenic differentiation via integrin β1 interaction. Lab. Investig. 2020, 100, 1494–1502. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Kinoshita, T.; Tomita, H.; Okada, H.; Niwa, A.; Hyodo, F.; Kanayama, T.; Matsuo, M.; Imaizumi, Y.; Kuroda, T.; Hatano, Y.; et al. Endothelial cell-specific reduction of heparan sulfate suppresses glioma growth in mice. Discov. Oncol. 2021, 12, 50. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer. 2018, 17, 58. [Google Scholar] [CrossRef]
- Lanzi, C.; Cassinelli, G. Receptor tyrosine kinases and heparan sulfate proteoglycans: Interplay providing anticancer targeting strategies and new therapeutic opportunities. Biochem. Pharmacol. 2020, 178, 114084. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, Z.H.; Chen, Z.; Tan, D. SULF2 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells through the ERK/AKT signaling pathway. Braz. J. Med. Biol. Res. 2020, 53, e8901. [Google Scholar] [CrossRef]
- Wang, C.; Shang, C.; Gai, X.; Song, T.; Han, S.; Liu, Q.; Zheng, X. Sulfatase 2-Induced Cancer-Associated Fibroblasts Promote Hepatocellular Carcinoma Progression via Inhibition of Apoptosis and Induction of Epithelial-to-Mesenchymal Transition. Front. Cell Dev. Biol. 2021, 9, 631931. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Jaradat, S.K.; Al-Shami, K.M.; Alkhalifa, A.E. Targeting Angiogenesis in Breast Cancer: Current Evidence and Future Perspectives of Novel Anti-Angiogenic Approaches. Front. Pharmacol. 2022, 13, 838133. [Google Scholar] [CrossRef]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The Key Role of Lysosomal Protease Cathepsins in Viral Infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef]
- Gomes, C.P.; Fernandes, D.E.; Casimiro, F.; da Mata, G.F.; Passos, M.T.; Varela, P.; Mastroianni-Kirsztajn, G.; Pesquero, J.B. Cathepsin L in COVID-19: From Pharmacological Evidences to Genetics. Front. Cell. Infect. Microbiol. 2020, 10, 589505. [Google Scholar] [CrossRef]
- Yu, Y.; Williams, A.; Zhang, X.; Fu, L.; Xia, K.; Xu, Y.; Zhang, F.; Liu, J.; Koffas, M.; Linhardt, R.J. Specificity and action pattern of heparanase Bp, a β-glucuronidase from Burkholderia pseudomallei. Glycobiology 2019, 29, 572–581. [Google Scholar] [CrossRef]
- Collins, L.E.; Troeberg, L. Heparan sulfate as a regulator of inflammation and immunity. J. Leukoc. Biol. 2019, 105, 81–92. [Google Scholar] [CrossRef]
- Farrugia, B.L.; Lord, M.S.; Melrose, J.; Whitelock, J.M. The Role of Heparan Sulfate in Inflammation, and the Development of Biomimetics as Anti-Inflammatory Strategies. J. Histochem. Cytochem. 2018, 66, 321–336. [Google Scholar] [CrossRef]
- de Boer, C.; Armstrong, Z.; Lit, V.A.J.; Barash, U.; Ruijgrok, G.; Boyango, I.; Weitzenberg, M.M.; Schröder, S.P.; Sarris, A.J.C.; Meeuwenoord, N.J.; et al. Mechanism-based heparanase inhibitors reduce cancer metastasis in vivo. Proc. Natl. Acad. Sci. USA 2022, 119, e2203167119. [Google Scholar] [CrossRef]
- He, P.; Zhang, X.; Xia, K.; Green, D.E.; Baytas, S.; Xu, Y.; Pham, T.; Liu, J.; Zhang, F.; Almond, A.; et al. Chemoenzymatic synthesis of sulfur-linked sugar polymers as heparanase inhibitors. Nat. Commun. 2022, 13, 7438. [Google Scholar] [CrossRef]
- Hassan, N.; Greve, B.; Espinoza-Sánchez, N.A.; Götte, M. Cell-surface heparan sulfate proteoglycans as multifunctional integrators of signaling in cancer. Cell Signal. 2021, 77, 109822. [Google Scholar] [CrossRef]
- Latko, M.; Czyrek, A.; Porębska, N.; Kucińska, M.; Otlewski, J.; Zakrzewska, M.; Opaliński, Ł. Cross-Talk between Fibroblast Growth Factor Receptors and Other Cell Surface Proteins. Cells 2019, 8, 455. [Google Scholar] [CrossRef]
- Jia, T.; Jacquet, T.; Dalonneau, F.; Coudert, P.; Vaganay, E.; Exbrayat-Héritier, C.; Vollaire, J.; Josserand, V.; Ruggiero, F.; Coll, J.L.; et al. FGF-2 promotes angiogenesis through a SRSF1/SRSF3/SRPK1-dependent axis that controls VEGFR1 splicing in endothelial cells. BMC Biol. 2021, 19, 173. [Google Scholar] [CrossRef]
- Ferreira, A.; Royaux, I.; Liu, J.; Wang, Z.; Su, G.; Moechars, D.; Callewaert, N.; De Muynck, L. The 3-O sulfation of heparan sulfate proteoglycans contributes to the cellular internalization of tau aggregates. BMC Mol. Cell Biol. 2022, 23, 61. [Google Scholar] [CrossRef]
- Poli, M.; Anower-E-Khuda, F.; Asperti, M.; Ruzzenenti, P.; Gryzik, M.; Denardo, A.; Gordts, P.L.S.M.; Arosio, P.; Esko, J.D. Hepatic heparan sulfate is a master regulator of hepcidin expression and iron homeostasis in human hepatocytes and mice. J. Biol. Chem. 2019, 294, 13292–13303. [Google Scholar] [CrossRef]
- De Pasquale, V.; Pavone, L.M. Heparan Sulfate Proteoglycan Signaling in Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 6588. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal. 2023, 21, 77. [Google Scholar] [CrossRef]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef]
- Chang, W.H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar] [CrossRef]
- Hu, W.; Liu, C.; Bi, Z.Y.; Zhou, Q.; Zhang, H.; Li, L.L.; Zhang, J.; Zhu, W.; Song, Y.Y.; Zhang, F.; et al. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol. Cancer 2020, 19, 102. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, H.; Sun, S.; Wang, L.; Sun, S. Extracellular vesicles and immunogenic stress in cancer. Cell Death Dis. 2021, 12, 894. [Google Scholar] [CrossRef]
- Purushothaman, A.; Sanderson, R.D. Heparanase: A Dynamic Promoter of Myeloma Progression. Adv. Exp. Med. Biol. 2020, 1221, 331–349. [Google Scholar] [CrossRef]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; de Jong, O.G.; Schiffelers, R.M. Exploring interactions between extracellular vesicles and cells for innovative drug delivery system design. Adv. Drug Deliv. Rev. 2021, 173, 252–278. [Google Scholar] [CrossRef]
- Coombe, D.R.; Gandhi, N.S. Heparanase: A Challenging Cancer Drug Target. Front. Oncol. 2019, 9, 1316. [Google Scholar] [CrossRef]
- Rangarajan, S.; Richter, J.R.; Richter, R.P.; Bandari, S.K.; Tripathi, K.; Vlodavsky, I.; Sanderson, R.D. Heparanase-enhanced Shedding of Syndecan-1 and Its Role in Driving Disease Pathogenesis and Progression. J. Histochem. Cytochem. 2020, 68, 823–840. [Google Scholar] [CrossRef]
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.C. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer. 2019, 18, 55. [Google Scholar] [CrossRef]
- Lee, K.M.; Seo, E.C.; Lee, J.H.; Kim, H.J.; Hwangbo, C. The Multifunctional Protein Syntenin-1: Regulator of Exosome Biogenesis, Cellular Function, and Tumor Progression. Int. J. Mol. Sci. 2023, 24, 9418. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Cerezo-Magaña, M.; Bång-Rudenstam, A.; Belting, M. The pleiotropic role of proteoglycans in extracellular vesicle mediated communication in the tumor microenvironment. Semin. Cancer Biol. 2020, 62, 99–107. [Google Scholar] [CrossRef]
- Castro-Cruz, M.; Hyka, L.; Daaboul, G.; Leblanc, R.; Meeussen, S.; Lembo, F.; Oris, A.; Van Herck, L.; Granjeaud, S.; David, G.; et al. PDZ scaffolds regulate extracellular vesicle production, composition, and uptake. Proc. Natl. Acad. Sci. USA 2023, 120, e2310914120. [Google Scholar] [CrossRef]
- Klotz, D.M.; Link, T.; Wimberger, P.; Kuhlmann, J.D. Prognostic relevance of longitudinal HGF levels in serum of patients with ovarian cancer. Mol. Oncol. 2021, 15, 3626–3638. [Google Scholar] [CrossRef]
- Li, F.; Liu, P.; Huang, Y.; Li, L.; Zhang, S.; Yang, Z.; Wang, R.; Tao, Z.; Han, Z.; Fan, J.; et al. The Incremental Prognostic Value of Hepatocyte Growth Factor in First-Ever Acute Ischemic Stroke: An Early Link Between Growth Factor and Interleukins. Front. Neurol. 2021, 12, 691886. [Google Scholar] [CrossRef]
- Belvedere, R.; Novizio, N.; Palazzo, M.; Pessolano, E.; Petrella, A. The pro-healing effects of heparan sulfate and growth factors are enhanced by the heparinase enzyme: New association for skin wound healing treatment. Eur. J. Pharmacol. 2023, 960, 176138. [Google Scholar] [CrossRef]
- Bertrand, J.; Bollmann, M. Soluble syndecans: Biomarkers for diseases and therapeutic options. Br. J. Pharmacol. 2019, 176, 67–81. [Google Scholar] [CrossRef]
- Hassan, N.; Bückreiß, N.; Efing, J.; Schulz-Fincke, M.; König, P.; Greve, B.; Bendas, G.; Götte, M. The Heparan Sulfate Proteoglycan Syndecan-1 Triggers Breast Cancer Cell-Induced Coagulability by Induced Expression of Tissue Factor. Cells 2023, 12, 910. [Google Scholar] [CrossRef]
- Nadanaka, S.; Bai, Y.; Kitagawa, H. Cleavage of Syndecan-1 Promotes the Proliferation of the Basal-Like Breast Cancer Cell Line BT-549 Via Akt SUMOylation. Front. Cell Dev. Biol. 2021, 9, 659428. [Google Scholar] [CrossRef]
- Koganti, R.; Suryawanshi, R.; Shukla, D. Heparanase, cell signaling, and viral infections. Cell. Mol. Life Sci. 2020, 77, 5059–5077. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, F.; Zhao, T.; Zhao, H.; Wang, X.; Chen, L.; Li, J.P.; Luo, S.Z. Phase separation on cell surface facilitates bFGF signal transduction with heparan sulphate. Nat. Commun. 2022, 13, 1112. [Google Scholar] [CrossRef]
- Jang, B.; Song, H.K.; Hwang, J.; Lee, S.; Park, E.; Oh, A.; Hwang, E.S.; Sung, J.Y.; Kim, Y.N.; Park, K.; et al. Shed syndecan-2 enhances colon cancer progression by increasing cooperative angiogenesis in the tumor microenvironment. Matrix Biol. 2022, 107, 40–58. [Google Scholar] [CrossRef]
- Czarnowski, D. Syndecans in cancer: A review of function, expression, prognostic value, and therapeutic significance. Cancer Treat. Res. Commun. 2021, 27, 100312. [Google Scholar] [CrossRef]
- Hadigal, S.; Koganti, R.; Yadavalli, T.; Agelidis, A.; Suryawanshi, R.; Shukla, D. Heparanase-Regulated Syndecan-1 Shedding Facilitates Herpes Simplex Virus 1 Egress. J. Virol. 2020, 94, e01672-19. [Google Scholar] [CrossRef]
- Schultheis, N.; Jiang, M.; Selleck, S.B. Putting the brakes on autophagy: The role of heparan sulfate modified proteins in the balance of anabolic and catabolic pathways and intracellular quality control. Matrix Biol. 2021, 100–101, 173–181. [Google Scholar] [CrossRef]
- Makarova, N.; Lekka, M.; Gnanachandran, K.; Sokolov, I. Mechanical Way to Study Molecular Structure of Pericellular Layer. ACS Appl. Mater. Interfaces 2023, 15, 35962–35972. [Google Scholar] [CrossRef]
- Alshehri, M.A.; Alshehri, M.M.; Albalawi, N.N.; Al-Ghamdi, M.A.; Al-Gayyar, M.M.H. Heparan sulfate proteoglycans and their modification as promising anticancer targets in hepatocellular carcinoma. Oncol. Lett. 2021, 21, 173. [Google Scholar] [CrossRef]
- Lanzi, C.; Yates, E.A.; Cassinelli, G. Editorial: Heparan Sulfate Proteoglycans and Their Endogenous Modifying Enzymes: Cancer Players, Biomarkers and Therapeutic Targets. Front. Oncol. 2020, 10, 195. [Google Scholar] [CrossRef]
- Furini, S.; Falciani, C. Expression and Role of Heparan Sulfated Proteoglycans in Pancreatic Cancer. Front. Oncol. 2021, 11, 695858. [Google Scholar] [CrossRef]
- Hoffmann, M.; Snyder, N.L.; Hartmann, L. Polymers Inspired by Heparin and Heparan Sulfate for Viral Targeting. Macromolecules 2022, 55, 7957–7973. [Google Scholar] [CrossRef]
- Volland, A.; Lohmüller, M.; Heilmann, E.; Kimpel, J.; Herzog, S.; von Laer, D. Heparan sulfate proteoglycans serve as alternative receptors for low affinity LCMV variants. PLoS Pathog. 2021, 17, e1009996. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, T.; Zhang, W.; Sun, Q.; Li, H.; Li, J.P. Elucidating the Interactions Between Heparin/Heparan Sulfate and SARS-CoV-2-Related Proteins-An Important Strategy for Developing Novel Therapeutics for the COVID-19 Pandemic. Front. Mol. Biosci. 2021, 7, 628551. [Google Scholar] [CrossRef]
- Kamimura, K.; Maeda, N. Glypicans and Heparan Sulfate in Synaptic Development, Neural Plasticity, and Neurological Disorders. Front. Neural Circuits 2021, 15, 595596. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Weng, X.; Maxwell-Warburton, S.; Hasib, A.; Ma, L.; Kang, L. The membrane receptor CD44: Novel insights into metabolism. Trends Endocrinol. Metab. 2022, 33, 318–332. [Google Scholar] [CrossRef]
- Gaiteiro, C.; Soares, J.; Relvas-Santos, M.; Peixoto, A.; Ferreira, D.; Paulo, P.; Brandão, A.; Fernandes, E.; Azevedo, R.; Palmeira, C.; et al. Glycoproteogenomics characterizes the CD44 splicing code associated with bladder cancer invasion. Theranostics 2022, 12, 3150–3177. [Google Scholar] [CrossRef]
- Liao, C.; Wang, Q.; An, J.; Chen, J.; Li, X.; Long, Q.; Xiao, L.; Guan, X.; Liu, J. CD44 Glycosylation as a Therapeutic Target in Oncology. Front. Oncol. 2022, 12, 883831. [Google Scholar] [CrossRef]
- Patil, S. CD44 Sorted Cells Have an Augmented Potential for Proliferation, Epithelial-Mesenchymal Transition, Stemness, and a Predominantly Inflammatory Cytokine and Angiogenic Secretome. Curr. Issues Mol. Biol. 2021, 43, 423–433. [Google Scholar] [CrossRef]
- Hinneh, J.A.; Gillis, J.L.; Moore, N.L.; Butler, L.M.; Centenera, M.M. The role of RHAMM in cancer: Exposing novel therapeutic vulnerabilities. Front. Oncol. 2022, 12, 982231. [Google Scholar] [CrossRef]
- Chaudhry, G.E.; Akim, A.; Naveed Zafar, M.; Safdar, N.; Sung, Y.Y.; Muhammad, T.S.T. Understanding Hyaluronan Receptor (CD44) Interaction, HA-CD44 Activated Potential Targets in Cancer Therapeutics. Adv. Pharm. Bull. 2021, 11, 426–438. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, S.; Ren, M.; Pang, C.; Zhan, Y.; An, H.; Sun, F. Molecular mechanism of CD44 homodimerization modulated by palmitoylation and membrane environments. Biophys. J. 2022, 121, 2671–2683. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, C.; Gao, F. The state of CD44 activation in cancer progression and therapeutic targeting. FEBS J. 2022, 89, 7970–7986. [Google Scholar] [CrossRef]
- Sanjanwala, D.; Londhe, V.; Trivedi, R.; Bonde, S.; Sawarkar, S.; Kale, V.; Patravale, V. Polysaccharide-based hydrogels for medical devices, implants and tissue engineering: A review. Int. J. Biol. Macromol. 2024, 256, 128488. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A Review on Biomedical Application of Polysaccharide-Based Hydrogels with a Focus on Drug Delivery Systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Wang, A.; Liu, Y.; Zeng, S.; Liu, Y.; Li, W.; Wu, D.; Wu, X.; Zou, L.; Chen, H. Dietary Plant Polysaccharides for Cancer Prevention: Role of Immune Cells and Gut Microbiota, Challenges and Perspectives. Nutrients 2023, 15, 3019. [Google Scholar] [CrossRef]
- Khan, R.; Shah, M.D.; Shah, L.; Lee, P.C.; Khan, I. Bacterial polysaccharides-A big source for prebiotics and therapeutics. Front. Nutr. 2022, 9, 1031935. [Google Scholar] [CrossRef]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as Potential Anti-tumor Biomacromolecules—A Review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef]
- Zhao, S.; Han, T.; Pei, X.; Song, Y.; Zhang, Y.; Liu, L.; Wang, X.; Hou, W.; Sun, C. The association of diet carbohydrates consumption with cognitive function among US older adults modification by daily fasting duration. Front. Aging Neurosci. 2022, 4, 991007. [Google Scholar] [CrossRef]
- Čaval, T.; Alisson-Silva, F.; Schwarz, F. Roles of glycosylation at the cancer cell surface: Opportunities for large scale glycoproteomics. Theranostics 2023, 13, 2605–2615. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, J.; Chae, J.; Hong, J.; Park, J.; Jeong, E.; Kim, H.; Tanaka, M.; Okochi, M.; Choi, J. Surface glycan targeting for cancer nano-immunotherapy. Control. Release 2022, 342, 321–336. [Google Scholar] [CrossRef]
- Perrin, L.; Belova, E.; Bayarmagnai, B.; Tüzel, E.; Gligorijevic, B. Invadopodia enable cooperative invasion and metastasis of breast cancer cells. Commun. Biol. 2022, 5, 758. [Google Scholar] [CrossRef]
- Medrano-González, P.A.; Rivera-Ramírez, O.; Montaño, L.F.; Rendón-Huerta, E.P. Proteolytic Processing of CD44 and Its Implications in Cancer. Stem Cells Int. 2021, 2021, 6667735. [Google Scholar] [CrossRef]
- Wei, C. The multifaceted roles of matrix metalloproteinases in lung cancer. Front. Oncol. 2023, 13, 1195426. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Vadhan, A.; Chen, P.H.; Lee, Y.L.; Chao, C.Y.; Cheng, K.H.; Chang, Y.C.; Hu, S.C.; Yuan, S.F. CD44 Promotes Lung Cancer Cell Metastasis through ERK-ZEB1 Signaling. Cancers 2021, 13, 4057. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef]
- Essa, A.A.M.; Deraz, E.M. Expression of CD44 (NKI-P1) in oral squamous cell carcinoma associated vascular endothelial cells: A relationship to tumor angiogenesis. Saudi Dent. J. 2022, 34, 21–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).