Abstract
Assessment of rabies virus (RABV) neutralizing antibodies in subjects vaccinated or injected with anti-RABV immunoglobulins is central in determination of rabies protection. The rapid fluorescent focus inhibition test (RFFIT) is used for assessment of anti-RABV activity in serum. The current anti-RABV polyclonal preparations on the market pose difficulties in production and vary in quality. RABV neutralizing monoclonal antibodies (MAbs) are being evaluated as replacements. Different anti-RABV MAbs may neutralize different RABV isolates, thus two or more MAbs directed against different epitopes on the RABV glycoprotein are needed. It is therefore important to ensure neutralizing activity against all RABV isolates in sera of subjects injected with an anti-RABV MAb product consisting of two or more MAbs. The RFFIT, utilizing CVS-11 as challenge virus, cannot discriminate between the activities of different anti-RABV MAbs. We developed and validated two RFFIT methods enabling specific assessment of two different anti-RABV MAbs (CR57 and CR4098) in using two mutant CVS-11 strains resistant to either CR57 or CR4098 neutralization. The validation results demonstrate that both RFFIT assays using MAb resistant RABV are precise, accurate, linear, specific, and stable within the linear range of 0.025 IU/mL to 1.0 IU/mL. This method design can, therefore, be used to determine MAb specific anti-RABV activity in human serum samples.
1. Introduction
The development of human monoclonal antibodies (MAbs) for the protection against infectious diseases is a rapidly growing field [1,2]. Indeed, development of antibodies for the neutralization of pathogens, such as HIV, flu, Marburg and Ebola virus have been reported [3,4,5,6,7,8]. Moreover the neutralizing MAb palivizumab against respiratory syncytial virus (RSV) [9] has already been marketed. Therapeutic use of antibodies to protect from infection is far from new. Since the first use of antisera for protection against diphtheria by Emil von Behring [10], many followed [11]. For example, prophylactic hepatitis A neutralizing polyclonal antibodies can protect after injection [12]. Other polyclonal antibody products, such as Respigam and Cytogam, have been shown effective against RSV and cytomegalovirus infection, respectively [13,14]. A well-known treatment is the post exposure prophylaxis (PEP) upon RABV exposure by multiple vaccinations on subsequent days and injection of anti-RABV immunoglobulins into the wound. The use of pathogen neutralizing polyclonal antibodies has thus been employed for many years.
The use of polyclonal antibodies purified from vaccinated human or equine donors combined with rabies vaccination has been successfully used for the prevention of rabies for many years. Although 100% effective, human or equine rabies immunoglobulins (HRIG or ERIG) have relatively low neutralizing activity, vary in quality, and the availability does not meet the global need for the prevention of rabies, especially in developing countries. New effective products with consistent quality are thus required. The estimated number of deaths annually attributed to canine rabies globally is 59,000 [15]. The use of recombinant RABV neutralizing MAbs provides an opportunity to address the global unmet need for rabies PEP [16].
CL184 is an anti-rabies MAb combination consisting of the two MAbs CR57 and CR4098 capable of neutralizing RABV [17]. Both antibodies are produced using the PER.C6® cell line as a production platform and bind to non-overlapping epitopes present on the RABV glycoprotein [18]. CL184 was shown to be as efficacious in animal models as HRIG and was safe and well tolerated in humans during clinical evaluation [19]. Because of its polyclonal nature, HRIG and ERIG will be effective against a wide variety of RABV isolates. In contrast, CL184 consists of two antibodies each with neutralizing activity against a wide range of RABV isolates, but are complementary to each other. Lacking one of the two antibodies could potentially lead to escape of the infecting RABV once it enters the body. Hence, during clinical evaluation the presence of both CR57 and CR4098 in clinical serum samples of subjects administered with a dose of CL184 needs to be verified, especially during the first 7–10 days when the vaccine-induced immune response has not yet been mounted in full. A reliable assay to detect specific neutralizing activity of either CR57 or CR4098 is therefore necessary to discriminate between both antibodies with respect to RABV neutralizing antibody (RVNA) activity.
The rapid fluorescent focus inhibition test (RFFIT) is routinely used to determine RVNA activity upon vaccination [20]. The test is based on inhibition of infection of baby hamster kidney (BHK) or mouse neuroblastoma (MNA) cells with the challenge virus standard 11 (CVS-11) RABV by serum containing RABV neutralizing antibodies. The RFFIT was validated for use during the CL184 clinical trials [21]. However, because CVS-11 is neutralized by CR57, as well as CR4098, the activities of each individual MAb could not be discriminated. The RFFIT assay was therefore adapted by replacing CVS-11 for two CR57 and CR4098 MAb-resistant CVS-11 isolates. CVS-11-E57 (henceforth called E57) and –E98-4 (henceforth called E98) are escape mutant CVS-11 strains, which can be neutralized by either CR57 or CR4098 [18,22]. CR57 can neutralize E98, but not E57, whereas CR4098 can neutralize E57, but not E98.
Both E57 and E98 RFFIT assays were successfully developed and validated according to International Council for Harmonisation (ICH), United States Food and Drug Administration [23] and European Medicines Agency (EMA) guidelines [23,24,25]. Data show that the assays are specific, with high sensitivity and an acceptable intermediate precision, ranging from 20 to 25% coefficient of variation (CV). The adaptation of the RFFIT assay using two escape mutant CVS-11 strains enables the specific detection of CR57 and CR4098 in human serum samples during clinical studies. The design of these assays could be adapted for specific detection of other RABV neutralizing monoclonal antibodies.
2. Results
2.1. Validation Experiments
Results of experiments performed to assess specificity, repeatability, intermediate precision, lower limit of quantitation (LLOQ), lower limit of detection (LOD), stability, linearity, and accuracy and concordance between the two assays are described in the below. The experiment outline is shown in Table 1. Assays were performed by two operators on different days.

Table 1.
Outline of the validation experiments with experiment subject (see row data collection for variance components (day, operator, cell passage number (CP#)) in the left column; and experiment number across the top. For CP#: early = 10 passages and late = 30 passages. Eight experiments were conducted over eight days for each of the assays.
2.1.1. Specificity
Resistance to neutralization by corresponding escape mutant challenge virus was observed, as expected. No neutralization of E57 by CR57 occurred, while CR4098 was able to neutralize the virus. Similarly, CR57 was able to neutralize E98, whereas CR4098 could not (Figure 1).
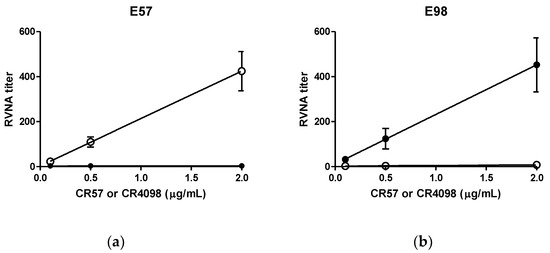
Figure 1.
CVS-11 CR57 and CR4098 escape mutant viruses are MAb specific. Challenge virus was incubated with different concentrations of CR57 and CR4098. Subsequently neutralizing activity was tested. Open circles: incubation with CR4098; closed circles: incubation with CR57. (a) Results of assay performed with E57 escape mutant virus. (b) Results of assay performed with E57 escape mutant virus.
The ratio reduction in RVNA titer signal of the CL184 without pre-incubation and E98 pre-incubated CL184 was 62.4-fold, whereas the ratio reduction calculated for pre-incubation with E57 was 35.3-fold, both above the criterion for specificity (≥4-fold reduction). The negative control result, ratio reduction between CL184 without pre-incubation and VSV pre-incubated CL184, was 1.1-fold. These data show that both assays are specific for the detection of E98 and E57 infection.
2.1.2. Linearity and Accuracy
Linearity was assessed by fitting a regression line through the observed RVNA titer data points covering the range of the RABV neutralizing antibody levels (0.025–2.0 IU/mL). The assumptions of normality were made by default on the log10 values of the observed RVNA titer levels. The area where the assay was assumed linear included data, which are within 30% of the expected result. The regression line through the titers observed in the assay utilizing E57 and E98 virus are shown (Figure 2a,b, respectively). For the E57 virus, the 90% CI of the mean at the 0.05–1.0 IU/mL range was within 30% of the expected result. For the assay utilizing the E98 virus the 90% CI of the mean at the 0.025 to 2.0 IU/mL was within 30% of the expected result (see Table 2). The linear range of the E57 and E98 assays thus were 0.05 IU/mL to 1.0 IU/mL and 0.025 IU/mL to 2.0 IU/mL, respectively.
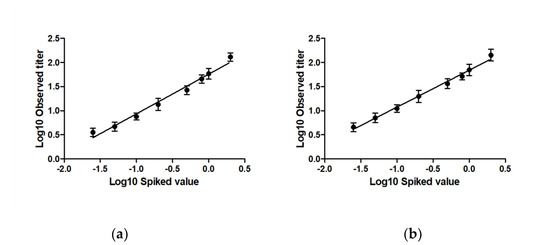
Figure 2.
Linearity between different CL184 RVNA spike levels and observed RVNA titers in the assay utilizing (a) the E57 virus and (b) the E98 virus. Log10 transformed CL184 RVNA levels are plotted as function of the log10 transformed RVNA titers. For E57, a linear range of 0.05 to 1.0 IU/mL was observed, and a linear range of 0.025 to 2.0 IU/mL was observed for the E98 virus.

Table 2.
Linearity range of the RFFIT assays using the E57 and E98 virus. Pred.: predicted rabies virus neutralizing antibody titer; Obs.: observer rabies virus neutralizing antibody titer; L90%CI: lower 90% confidence interval; U90%CI: upper 90% confidence interval.
Accuracy was determined by analysis of the residuals. For both assays, individual log10 observed titers are within total error evaluated as the sum of the precision acceptance limit (30% CV) and the accuracy acceptance limit (30%), i.e., 0.24 in log10 transformed values.
2.1.3. Lower Limit of Quantitation (LLOQ) and Lower Limit of Detection (LOD)
The LLOQ is defined as the lowest RVNA spike level, which is still within 35% CV. At a spiked level of 0.025 IU/mL, the intermediate precision %CV observed for the E57 virus was 19.7% and for the E98 virus 24.4%. Since both values are below 35% CV, the LLOQ of both assays is 0.025 IU/mL.
The LOD was tested using the data of non-spiked sera and is defined as the mean value with three times the standard deviation added to it. A total of 48 data points obtained during the eight experiments were used to set the LOD for both virus strains. For E57, the LOD was at a titer level of 4.5. The minimum RVNA spike level that will give a titer greater than the LOD is 0.049 IU/mL. Thus, although the LLOQ of the assay using this virus strain is 0.025 IU/mL, the sensitivity of this assay is somewhat lower (i.e., 0.049 IU/mL). The assay using E98 as challenge virus, however, showed a LOD at a titer level of 3.2. This corresponded with a RVNA spike level of 0.019 IU/mL, being the first predicted RVNA level above the LOD. The sensitivity of the assay utilizing E98 is therefore set at 0.025 IU/mL (i.e., the LLOQ).
2.1.4. Repeatability (Intra-Assay Variation)
For the E57 virus, an overall repeatability of 13.2% was observed, and the individual CV of the independent RVNA levels ranged from 8.6% to 17.2%. An overall repeatability of 13.5% was observed for the E98 virus. The repeatability CV for this virus at the different RVNA spike levels ranged from 8.1% to 18.6% (Table 3).

Table 3.
Repeatability and intermediate precision observed during assessment of RVNA titers using the E57 and E98 virus. RVNAT: rabies virus neutralizing antibody titer; R: repeatability; IP: intermediate precision. Overall precision of the assay, as well as the precision per spike level, are given.
2.1.5. Intermediate Precision (Inter-Assay Variation)
The overall intermediate precision CV for the E57 virus was 20.5%, and the individual CV observed at the different CL184 RVNA spike levels ranged from 15.6% to 24.1%. In the case of the E98 virus, the overall intermediate precision CV was 24.6% and ranged from 18.1% to 28.0% at the individual CL184 RVNA spike level (Table 3).
2.1.6. Stability
Although antibodies in serum are in general stable at −65 °C [26,27,28], stability of samples may be jeopardized during handling at room temperature (RT), at −20 °C (in case no −65 °C freezer is available), or during freeze/thaw cycles in the case of re-analysis. Therefore, bench top stability, stability at −20 °C, and freeze/thaw stability were tested. No significant difference between the RVNA titer results of samples at RT for 4 h and comparator samples was observed in both assays, with an overall ratio (test/comparator) of 100.3% and 104.6% for E57 and E98 viruses, respectively. (Table 4).

Table 4.
Stability of CL184 in human serum during different stress conditions. RVNA titers upon incubation at different conditions were compared with non-stressed samples. Difference in RVNA titer between stressed and non-stressed condition (ratio TS/CS) should be within 70% and 130%. E57: E57 virus; E98: E98 virus; F/T: freeze/thaw cycle; RVNAT: rabies virus neutralizing antibody titer; CS: comparator (non-stressed sample); TS: test (stressed) sample; CI: confidence interval; P: passed.
Next, stability testing of the samples at −20 °C demonstrated close agreement of results between the two sample sets (stored at −20 °C and comparator) for both assays, with an overall ratio of 97.9% for the E57 virus and 109.8% for the E98 assay (Table 4).
Finally, freeze/thaw stability was assessed. Up to three freeze/thaw cycles were compared with untreated samples in the RFFIT. No significant differences in titers between the test and comparator samples were observed. This was true for the RFFIT utilizing the E57 virus, as well as the assay using the E98 virus (see Table 4). This shows that clinical samples can be held for up to 4 h at RT, stored at −20 °C for up to two weeks and undergo up to three freeze/thaw cycles without loss of activity when anlyzed in both assays.
2.1.7. Concordance between the Two Assays
To ensure CL184 efficacy against different RABV strains, it is of importance that both antibodies are present in serum at similar levels. The concordance in titer level (i.e., whether the titer level of one antibody can be compared with the other) of both assays was assessed.
The results (see Figure 3) indicate that the two measurements are highly correlated (>0.97). The slope is estimated to be 0.93 with the 95% confidence interval (being [0.87, 0.99]) is included within the [0.80, 1.25] indifference zone, suggesting that the ratio between the two measures acceptably close to 1.
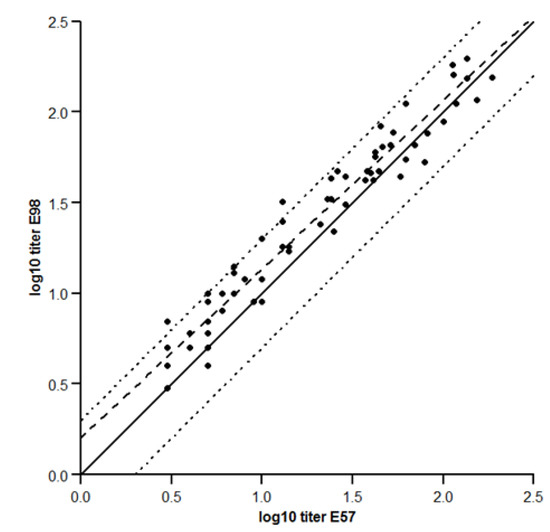
Figure 3.
Concordance between the E57 and –E98 assays. Log10 transformed RVNA titers observed for the E57 virus are plotted against the RVNA titers observed for the E98 virus. Solid line: unit line y = x; dashed line: orthogonal fit with ratio = 1; dotted line: acceptance boundaries.
3. Discussion
CL184 is a combination of two fully human MAbs neutralizing RABV. Since the two antibodies CR57 and CR4098, included in CL184, are complementary in neutralizing RABV isolates, it is of importance that both are actively present in human serum to ensure neutralization of all possible RABV isolates upon exposure. The RFFIT is commonly used to determine anti-RABV activity, but cannot discriminate between activities of different monoclonal anti-RABV antibodies. In order to discriminate between CR57 and CR4098 specific RVNA levels, the RFFIT was customized for the detection of CR57 and CR4098 specific RVNA titers. Two assays utilizing CR57 and CR4098 escape mutant CVS-11 strains were developed and validated enabling the detection of CR57 and CR4098=specific RVNA titers. Specificity experiments confirmed that the two assays can indeed specifically detect CR57 and CR4098 RVNA titer levels in human serum samples. Furthermore, concordance testing indicated that CR57 and CR4098 specific neutralizing activity in serum samples are comparable, enabling the comparison of CR57 and CR4098 activity, which should be present in serum in similar levels.
The standard RFFIT is commonly used to assess RVNA activity upon vaccination or for the determination of rabies vaccine efficacy during clinical studies. The international standard rabies immune globulin (SRIG), with an assigned potency value of 2.0 IU/mL, is used as a reference to calculate the RVNA activity of a test sample. A serum RVNA level ≥0.5 IU/mL is indicative for adequate response to rabies vaccination [29]. During development of the assays, we observed that the two virus strains did not react equally against the polyclonal SRIG. As a consequence, no concordance between the two assays could be obtained upon conversion to IU/mL. Because the two assays are not used to show protective levels against RABV, but rather show that both CR57 and CR4098 are present in serum and available in equal activity, we reported neutralizing titers instead.
Because RABV neutralizing immunoglobulins are usually injected along with rabies vaccine, it must be noted that the CR57 and CR4098 specific RFFIT can only be used for sera of subjects injected with CL184 only, or serum isolated no later than three days upon vaccine administration. The antibody response against the vaccine will obscure the CR57 and CR4098 specific RVNA levels. The RVNA levels typically observed one to three days after injection and determined using the standard RFFIT in serum of subjects participating in CL184 clinical trials ranged from 0.2 (LLOQ) to 3 IU/mL (Bakker et al. 2008, and unpublished data). Since the standard RFFIT cannot discriminate between the two antibodies present in CL184, these levels reflect both CR57 and CR4098 neutralizing activity. CL184 consists of equal amounts of CR57 and CR4098. The expected RVNA levels in these samples are therefore 0.1 to 1.5 IU/mL. Given the observed sensitivity and stable linear range of the assays of 0.049 IU/mL to 1.0 IU/mL (CR4098 specific assay) and 0.025 IU/mL to 2.0 IU/mL (CR57 specific assay), both assays are capable to measure in the desired range.
In conclusion, the results of this validation demonstrate that these RFFITs are precise, accurate, linear, specific, and stable within the linear range of 0.025 IU/mL to 2.0 IU/mL. The assays are suitable for their intended use to detect CR57 and CR4098 RVNA titers in sera of subjects injected with CL184 during clinical trials.
4. Materials and Methods
- RFFIT protocol
The RFFIT procedure [30] was modified to measure the level of RVNA titers in human serum against the rabies CVS-11 CR57 escape mutant E57 or the rabies CVS-11 CR4098 escape mutant E98. Two-fold serial dilutions of heat inactivated serum spiked with different levels CL184 were incubated with the E57 or E98 strain in 8-well tissue chamber slides for 90 min at 37 °C. Subsequently, BHK-21 cells were added to the serum–virus mixture and incubated for an additional 20 to 24 h at 37 °C with 2 to 5% CO2. Next slides were acetone fixed and stained with an anti-rabies N-FITC conjugate (Chemicon).
Twenty distinct microscopic fields per well were examined using a fluorescence microscope at ×100 magnification to score the virus infected cells (foci) by one independent reader, and one of every 8 slides was read by another independent reader for slide reading quality control. The number of positive fields with RABV infected cells per well were recorded. The neutralization endpoint titer was defined as the highest sample dilution at which 50% of the observed microscopic fields contain one or more infected cells. The RVNA titers are mathematically interpolated using the Reed and Muench method for calculating a RVNA titer [31].
- Validation samples
Experiments were performed using normal human serum spiked with CL184. Pooled normal human serum (Fisher Scientific, Fair Lawn, NJ) was spiked with different concentrations of CL184 to obtain a range of validation samples. Serial dilutions of CL184 in serum were calculated based on the clinical dosages as specified on the label (i.e., 500 IU/mL). Spike levels were 0.025, 0.05, 0.1, 0.2, 0.5, 0.8, 1.0 and 2.0 IU/mL. At the start of the study, a pool of each validation sample was prepared, aliquoted, and frozen at −80 °C until use. To assess the serum background and LOD, a non-spiked serum sample was included. For specificity testing, the single MAbs CR57 and CR4098 were diluted in normal human serum on basis of their protein concentration. Hereto CR57 and CR4098 concentrations of 0.1, 0.5, and 2.0 μg/mL were prepared. All tested sera were stored at −80 °C (nominal), similar to the clinical sample storage temperature, unless stated otherwise.
- Challenge viruses
Preparation of the escape mutant viruses have been previously described [16,20]. Stocks of E57 and E98 prepared for the validation experiment were grown over two days, resulting in similar endpoint titers that met acceptance criteria for use as challenge virus in the RFFIT. Both stock virus preparations were used in working dilutions to produce a dose of 30-100 TCID50 in the RFFIT, as required.
- Validation experiments
The validation plan was based on the guidelines on bioanalytical method validation available and ICH Q2(R1) [21,22,23]. Experiments were performed to assess specificity, repeatability, intermediate precision, lower limit of quantitation (LLOQ), lower limit of detection (LOD), stability, linearity and accuracy and concordance between the two assays. Validation samples were spiked with different RVNA levels of CL184 containing equal amounts of CR57 and CR4098. The samples were tested three times in eight independent experiments over eight days by two operators using early (10) and late (30) BHK cell passages for each of the virus strains. During these experiments, a total of eight RVNA levels were observed. For specificity, regarding experiments involving the MAbs separately, normal human serum was spiked with 0.1, 0.5, and 2.0 μg/mL CR57 or CR4098. The experiment outline is shown in Table 1. The following validation parameters were considered:
- (1)
- Specificity. Specificity was tested two ways: First, the two MAbs, CR57 and CR4098, were incubated separately with the challenge virus E57 or E98. Criteria were that E57 should be neutralized by CR4098, but not by CR57, whereas E98 should be neutralized by CR57, but not by CR4098. Second, the specificity of the virus in context of CL184 was tested. Hereto inactivated virus was incubated with CL184 to adsorb either CR57 or CR4098. Subsequently the mixture was incubated with live challenge virus and compared with CL184 not adsorbed to the inactivated virus. As a negative control inactivated irrelevant rhabdovirus (Vesicular Stomatitis Virus-Indiana, (VSV-IN)) was used. The ratio of the RVNA titer signal observed using the unabsorbed CL184 and the adsorbed CL184 was calculated. A reduction of ≥4 fold in signal indicates specificity of the assay.
- (2)
- Linearity and Accuracy. To assess linearity, the ICH guidelines on validation of analytical procedures (Q2(R1)) were followed. A regression line was fitted through the observed RVNA titer level data points (i.e., the log10 transformed antibody measured titers as a function of the log10 transformed potency level of CL184) using maximum likelihood method. Linearity was analyzed using a linear regression model and accuracy assessed via analysis of the residuals. The 90% confidence interval at each level should be included within ±0.114 log-units (corresponding with 30% CV on the original scale) from the expected result, (i.e., the regression line). The range where the data follows a linear regression model constitutes the linear range of the assay.
- (3)
- Precision. Repeatability (intra-assay variation) and intermediate precision (the sum of the inter-assay and intra-assay variation) of the assays were determined by assessment of eight CL184 validation samples covering the RVNA range of 0.025 to 2 IU/mL. Guidelines recommend a CV of 20% to 25% for ligand binding assays (LBA), such as enzyme-linked immunosorbent assays (ELISA). However, since bioassays, such as the RFFIT, make use of biological agents such as cells and virus, which are prone to a higher variation, a higher variability is expected, as acknowledged by the World Health Organization [32,33]. A larger CV limit criterion of <30% is therefore accepted. More variation is expected for the LLOQ and ULOQ [21], thus acceptance criteria for the LLOQ is 35% instead of 30%. We accept this higher variability because data in this range are considered relevant in the clinical trials in which, at early time points, relative low levels of passively administered RIG are expected. RVNA titer data were first log10 transformed to achieve a normal distribution. The repeatability and intermediate precision of the RVNA titer values was estimated in a nested error variance components model by analysis of variance (ANOVA) with experiment as random term. To express precision in percent CV, the formulawas used to translate the standard deviation (σ) on a log10 scale to a percent CV, which can be compared with the acceptance criteria.
- (4)
- Stability. Stability was assessed as described in the EMA and FDA guidelines on bioanalytical method validation. The stability items tested reflect the routine handling of clinical samples. During all stability experiments, 3 RVNA spike levels (0.1, 0.5 and 2.0 IU/mL) were tested. During each stability test, a total of 7 aliquots per spike level were stressed and compared to the same number of non-stressed comparator samples. Freeze thaw testing was performed as follows: 3 sets of 7 aliquots were taken from the −80 °C freezer and allowed to thaw at room temperature (RT) for 4 h. Next, the samples were placed back and allowed to freeze for at least 24 h. Subsequently, all samples were taken from the freezer and allowed to thaw at RT for 4 h (freeze/thaw cycle 1). One set was tested in the RFFIT assays, and the remaining 2 sets were placed back in the −80 °C freezer for at least 24 h. This procedure was repeated for a total of 3 cycles. The RVNA titer levels for each stability sample were log10 transformed and compared with the log10 transformed comparator samples by ANOVA, with spiked value as covariate to correct for differences in concentration. Appropriate estimate statements in the ANOVA were used to estimate the mean differences and the corresponding 90% confidence intervals of various stability conditions to the comparator. As a criterion, no significant differences of more 30% on original scale between stability samples and comparator samples as determined by ANOVA were allowed.
- (5)
- Concordance between the two assays was performed using an orthogonal regression model [34], assuming the error on both measurements was the same, as suggested in the precision section.
- Calculations
All computations have been performed using SAS 9.4 and JMP 10.0.
Author Contributions
Conceptualization, W.E.M., A.C., S.M.M. and S.K.; methodology, W.E.M., A.C., S.M.M. and S.K.; software, B.B.; validation, A.C., S.M.M., B.B. and S.K.; formal analysis, B.B.; investigation, S.M.M.; resources, S.M.M.; data curation, S.M.M. and B.B.; writing—original draft preparation, W.E.M., A.C, S.M.M. and S.K.; writing—review and editing, W.E.M., A.C., S.M.M., B.B. and S.K.; visualization, A.C. and B.B.; supervision, W.E.M.; project administration, S.M.M.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Perceval Sondag for preparing the figures and Samantha Pralle for excellent technical assistance with the analysis of the assays.
Conflicts of Interest
W.E. Marissen, S. Kostense, and A. Companjen were employees of Crucell Holland BV at the time of the study. B. Boulanger and S.M. Moore declare no conflict of interest.
References
- Both, L.; Banyard, A.C.; van Dolleweerd, C.; Wright, E.; Ma, J.K.-C.; Fooks, A.R. Monoclonal antibodies for prophylactic and therapeutic use against viral infections. Vaccine 2013, 31, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Marasco, W.A.; Sui, J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007, 25, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Bhabha, G.; Elsliger, M.-A.; Friesen, R.H.E.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Friesen, R.H.; Bhabha, G.; Kwaks, T.; Jongeneelen, M.; Yu, W.; Ophorst, C.; Cox, F.; Korse, H.J.; Brandenburg, B.; et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 2011, 333, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Friesen, R.H.; Lee, P.S.; Stoop, E.J.; Hoffman, R.M.; Ekiert, D.C.; Bhabha, G.; Yu, W.; Juraszek, J.; Koudstaal, W.; Jongeneelen, M.; et al. A common solution to group 2 influenza virus neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, M.; Marzi, A.; Nakayama, E.; Noda, T.; Kuroda, M.; Manzoor, R.; Matsuno, K.; Feldmann, H.; Yoshida, R.; Kawaoka, Y.; et al. Inhibition of Marburg virus budding by nonneutralizing antibodies to the envelope glycoprotein. J. Virol. 2012, 86, 13467–13474. [Google Scholar] [CrossRef]
- Qiu, X.; Audet, J.; Wong, G.; Fernando, L.; Bello, A.; Pillet, S.; Alimonti, J.B.; Kobinger, G.P. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci. Rep. 2013, 3, 3365. [Google Scholar] [CrossRef]
- Ruprecht, R.M. Passive immunization with human neutralizing monoclonal antibodies against HIV-1 in macaque models: Experimental approaches. Methods Mol. Biol. 2009, 525, 559–566. [Google Scholar]
- Llorens, X.S.; Castaño, E.; Null, D.; Steichen, J.; Sánchez, P.J.; Ramilo, O.; Top, F.H.; Connor, E. Safety and pharmacokinetics of an intramuscular humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. The MEDI-493 Study Group. Pediatr. Infect. Dis. J. 1998, 17, 787–791. [Google Scholar] [CrossRef]
- Behring, E.A.; Kitasato, S. Ueber das zustandekommen der diptherie-immunität und der tetanus-immunität bei thieren. Deutch. Med. Woch. 1890, 49, 1113–1114. [Google Scholar]
- Casadevall, A.; Dadachova, E.; Pirofski, L.-A. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004, 2, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Kluge, T. Gamma-Globulin in the Prevention on Viral Hepatitis: A Study of the Effect of Medium-Size Doses. Acta Med. Scand. 1963, 174, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D. CytoGam infusions at home. J. Infus. Nurs. 1999, 22, 331–335. [Google Scholar]
- Oertel, M.D. RespiGam: An RSV immune globulin. Pediatr. Nurs. 1996, 22, 525–528. [Google Scholar] [PubMed]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- WHO. Rabies Vaccines: WHO Position Paper—April 2018; Abela Ridder, B., Ed.; World Health Organization: Geneva, Switzerland, 2018; pp. 201–220. [Google Scholar]
- De Kruif, J.; Bakker, A.B.; Marissen, W.E.; Kramer, R.A.; Throsby, M.; Rupprecht, C.E.; Goudsmit, J. A human monoclonal antibody cocktail as a novel component of rabies postexposure prophylaxis. Annu. Rev. Med. 2007, 58, 359–368. [Google Scholar] [CrossRef]
- Bakker, A.B.H.; Marissen, W.E.; Kramer, R.A.; Rice, A.B.; Weldon, W.C.; Niezgoda, M.; Hanlon, C.A.; Thijsse, S.; Backus, H.H.J.; de Kruif, J.; et al. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J. Virol. 2005, 79, 9062–9068. [Google Scholar] [CrossRef]
- Bakker, A.; Python, C.; Kissling, C.; Pandya, P.; Marissen, W.; Brink, M.; Lagerwerf, F.; Worst, S.; van Corven, E.; Kostense, S.; et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: Safety, tolerability, and neutralizing activity. Vaccine 2008, 26, 5922–5927. [Google Scholar] [CrossRef]
- Smith, J.S.; Yager, P.A.; Baer, G.M. A rapid reproducible test for determining rabies neutralizing antibody. Bull. World Health Organ. 1973, 48, 535–541. [Google Scholar]
- Kostense, S.; Moore, S.; Companjen, A.; Bakker, A.B.H.; Marissen, W.E.; von Eyben, R.; Weverling, G.J.; Hanlon, C.; Goudsmit, J. Validation of the rapid fluorescent focus inhibition test for rabies virus-neutralizing antibodies in clinical samples. Antimicrob. Agents Chemother. 2012, 56, 3524–3530. [Google Scholar] [CrossRef]
- Marissen, W.E.; Kramer, R.A.; Rice, A.; Weldon, W.C.; Niezgoda, M.; Faber, M.; Slootstra, J.W.; Meloen, R.H.; der Horst, M.C.-V.; Visser, T.J.; et al. Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: Fine mapping and escape mutant analysis. J. Virol. 2005, 79, 4672–4678. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry, Bioanalytical Method Validation; FDA: Silver Spring, MD, USA, 2001.
- EMA. Guideline on Bioanalytical Method Validation, in EMEA/CHMP/EWP/192217/2009; EMA: Amsterdam, The Netherlands, 2012. [Google Scholar]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2(R1); ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Hendriks, J.; Stals, C.; Versteilen, A.; Mommaas, B.; Verhoeven, M.; Tirion, F.; ter Haak, M.; Ribbens, W.; Bosch, M.; Trommel, M.; et al. Stability studies of binding and functional anti-vaccine antibodies. Bioanalysis 2014, 6, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Michaut, L.; Laurent, N.; Kentsch, K.; Spindeldreher, S.; Deckert-Salva, F. Stability of anti-immunotherapeutic antibodies in frozen human serum samples. Bioanalysis 2014, 6, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Pihl, S.; Michaut, L.; Hendriks, J.; Loebbert, R.; Ryding, J.; Nemansky, M.; Vermet, L.; Companjen, A. EBF recommendation for stability testing of anti-drug antibodies; lessons learned from anti-vaccine antibody stability studies. Bioanalysis 2014, 6, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Expert Consultation on Rabies. In WHO Technical Report Series; No. 982; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Smith, J.S.; Yager, P.A.; Baer, G.M. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibody. In Laboratory Techniques in Rabies; Meslin, F.X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organization: Geneva, Switzerland, 1996; pp. 181–192. [Google Scholar]
- Habel, K. Habel test for potency. In Laboratory Techniques in Rabies; Meslin, F.X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organization: Geneva, Switzerland, 1996; pp. 369–373. [Google Scholar]
- WHO. WHO Expert Committee on Biological Standardization, Sixty-First Report; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Chaloner-Larsson, G.; Anderson, R.; Egan, A. A WHO Guide to Goo Manufacturing Practice (GMP) Requirements; Part: Validation; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Tan, C.Y.; Iglewicz, B. Measurement-methods comparisions and linear statistical relationship. Technometrics 1999, 41, 192–201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).