Novel Strategy for Alzheimer’s Disease Treatment through Oral Vaccine Therapy with Amyloid Beta
Abstract
1. Introduction
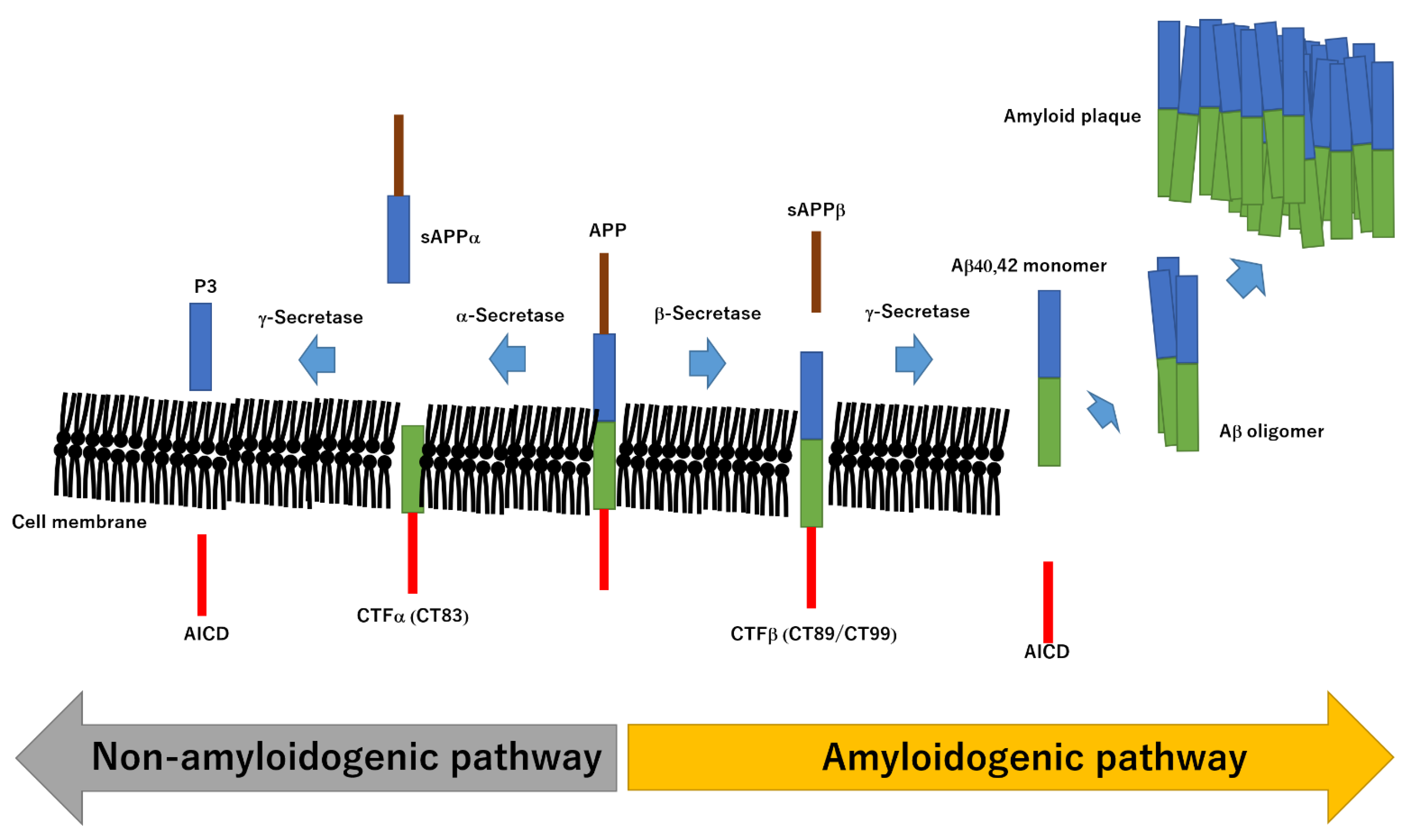
2. Structural Relationship between Aβ and AD
3. Molecular Mechanisms of Pathogenesis in AD
4. Alzheimer’s Vaccine
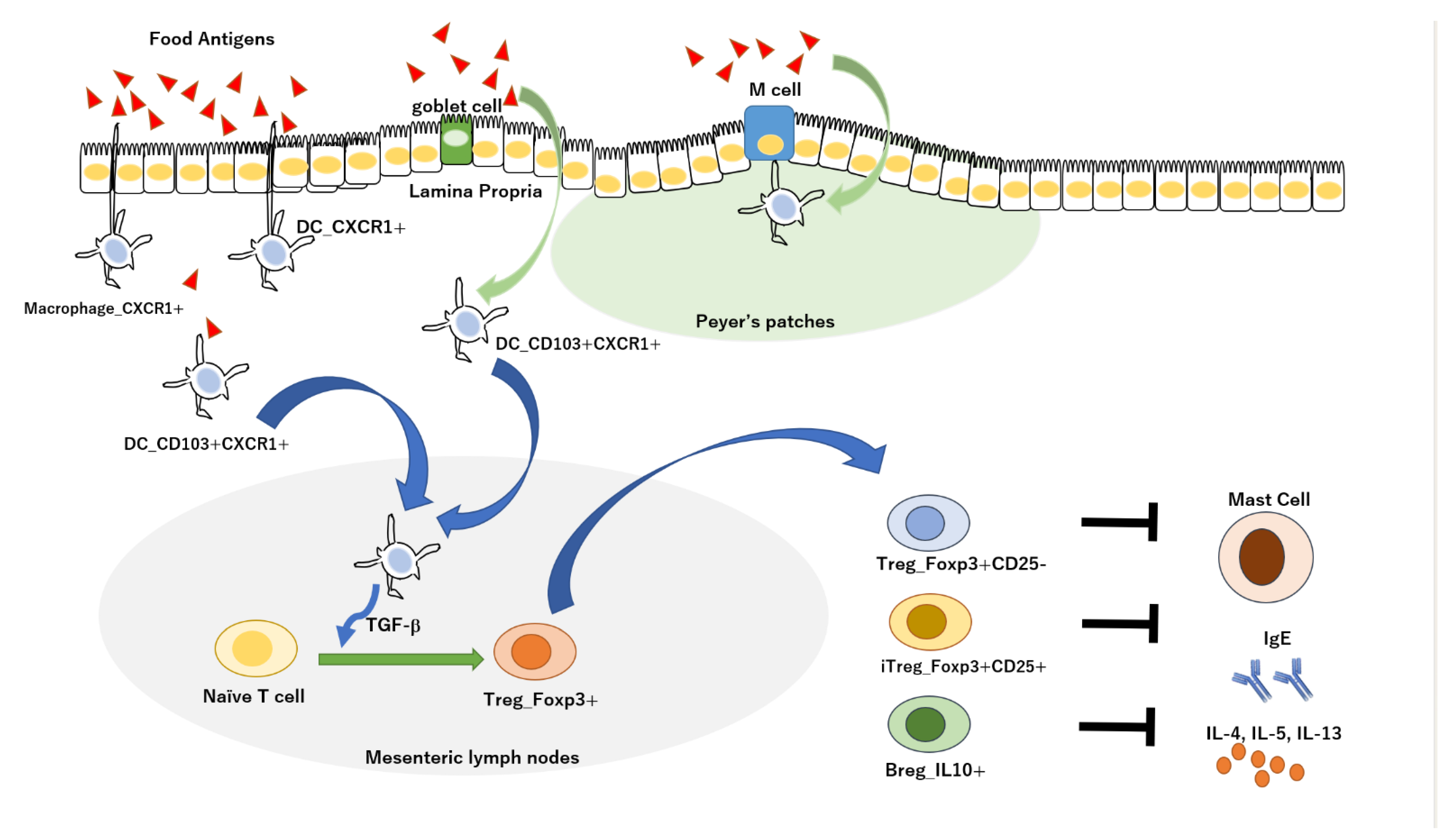
5. Oral Immune Tolerance in Alzheimer’s Disease
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tampi, R.R.; Jeste, D.V. Dementia Is More Than Memory Loss: Neuropsychiatric Symptoms of Dementia and Their Nonpharmacological and Pharmacological Management. Am. J. Psychiatry 2022, 179, 528–543. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.Y.; Cheng, Y.C.; Su, C.H. Exercise Dosage in Reducing the Risk of Dementia Development: Mode, Duration, and Intensity-A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 13331. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafò, A.; Bernardini, R.; Cantarella, G. Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca2+ Homeostasis Dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, S.; Fang, Y.; Qiu, Q.; Zhao, L.; Wei, W.; Tang, Y.; Li, X. Radiomic Features of the Hippocampus for Diagnosing Early-Onset and Late-Onset Alzheimer’s Disease. Front. Aging Neurosci. 2022, 13, 789099. [Google Scholar] [CrossRef]
- Garg, N.; Choudhry, M.S.; Bodade, R.M. A review on Alzheimer’s Disease Classification from Normal Controls and Mild Cognitive Impairment using structural MR Images. J. Neurosci. Methods 2022, 384. in press. [Google Scholar] [CrossRef]
- Folorunso, O.O.; Harvey, T.L.; Brown, S.E.; Chelini, G.; Berretta, S.; Balu, D.T. The D-serine biosynthetic enzyme serine racemase is expressed by reactive astrocytes in the amygdala of human and a mouse model of Alzheimer’s disease. Neurosci. Lett. 2022, 792, 136958. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.F.; Bahia, V.S.; Natividade, J.C.; Bastos, R.V.S.; Shiguti, W.A.; da Silva, K.E.R.; de Souza, W.C. Changes in personality traits in patients with Alzheimer’s Disease. Dement. Neuropsychol. 2022, 16, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.P.; Borghi, I.; Maiella, M.; Pellicciari, M.C.; Bonnì, S.; Mencarelli, L.; Assogna, M.; D’Acunto, A.; Di Lorenzo, F.; Spampinato, D.A.; et al. Regional Precuneus Cortical Hyperexcitability in Alzheimer’s Disease Patients. Ann. Neurol. 2022, 93, 371–383, in press. [Google Scholar] [CrossRef] [PubMed]
- Magham, S.V.; Thaggikuppe Krishnamurthy, P.; Shaji, N.; Mani, L.; Balasubramanian, S. Cannabinoid receptor 2 selective agonists and Alzheimer’s disease: An insight into the therapeutic potentials. J. Neurosci. Res. 2021, 99, 2888–2905. [Google Scholar] [CrossRef]
- Kawakami, I.; Arai, T.; Ikeda, K.; Niizato, K.; Oshima, K.; Akiyama, H. Possible association of limbic tau pathology with psychosis or behavioral disturbances: Studies of two autopsied psychiatric patients. Neuropathology 2022, in press. [Google Scholar] [CrossRef]
- Agüero, P.; Sainz, M.J.; Téllez, R.; Lorda, I.; Ávila, A.; García-Ribas, G.; Rodríguez, P.P.; Gómez-Tortosa, E. De Novo PS1 Mutation (Pro436Gln) in a Very Early-Onset Posterior Variant of Alzheimer’s Disease Associated with Spasticity: A Case Report. J. Alzheimers. Dis. 2021, 83, 1011–1016. [Google Scholar] [CrossRef]
- Yliranta, A.; Nuorva, J.; Karjalainen, V.L.; Ahmasalo, R.; Jehkonen, M. The dementia apraxia test can detect early-onset Alzheimer’s disease. Neuropsychology 2023, 37, 44–51, in press. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Li, Y.; Xu, Z.; Yu, Z.; Peng, B.; Wang, C.; Liu, W.; Li, W.; Ye, Z.; Zhang, G. Applications and research progress of Traditional Chinese medicine delivered via nasal administration. Biomed. Pharmacother. 2022, 157, 113933. [Google Scholar] [CrossRef] [PubMed]
- Coupé, P.; Manjón, J.V.; Mansencal, B.; Tourdias, T.; Catheline, G.; Planche, V. Hippocampal-amygdalo-ventricular atrophy score: Alzheimer disease detection using normative and pathological lifespan models. Hum. Brain Mapp. 2022, 43, 3270–3282. [Google Scholar] [CrossRef]
- Yang, L.; Nao, J. Focus on Alzheimer’s disease: The role of fibroblast growth factor 21 and autophagy. Neuroscience 2022, 511, 13–28, S0306-4522. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Chen, J.; Liu, Y.; Cui, X.; Wang, C.; Zong, S.; Wang, L.; Lu, Z. MicroRNA-22-3p ameliorates Alzheimer’s disease by targeting SOX9 through the NF-κB signaling pathway in the hippocampus. J. Neuroinflamm. 2022, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Uzunhisarcıklı, E.; Yerer, M.B. Neuroprotective Effects of Vapreotide on Tau Transfection-Induced Neurodegeneration. Neurotox. Res. 2022, 40, 1824–1837, in press. [Google Scholar] [CrossRef] [PubMed]
- Basli, A.; Bounaas, J. Pathophysiological mechanism and natural preventive and therapeutic strategies of Alzheimer’s disease. Nutr. Health 2022, in press. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- Nizetic, D.; Chen, C.L.; Hong, W.; Koo, E.H. Inter-Dependent Mechanisms Behind Cognitive Dysfunction, Vascular Biology and Alzheimer’s Dementia in Down Syndrome: Multi-Faceted Roles of APP. Ront. Behav. Neurosci. 2015, 9, 299. [Google Scholar] [CrossRef]
- Chen, X.Q.; Mobley, W.C. Exploring the Pathogenesis of Alzheimer Disease in Basal Forebrain Cholinergic Neurons: Converging Insights From Alternative Hypotheses. Front. Neurosci. 2019, 13, 446. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Melagraki, G.; Zacharia, L.C.; Afantitis, A. Computer-Aided Drug Design of β-Secretase, γ-Secretase and Anti-Tau Inhibitors for the Discovery of Novel Alzheimer’s Therapeutics. Int. J. Mol. Sci. 2020, 21, 703. [Google Scholar] [CrossRef]
- Tang, B.L. Enhancing α-secretase Processing for Alzheimer’s Disease-A View on SFRP1. Brain Sci. 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Sikanyika, N.L.; Parkington, H.C.; Smith, A.I.; Kuruppu, S. Powering Amyloid Beta Degrading Enzymes: A Possible Therapy for Alzheimer’s Disease. Neurochem. Res. 2019, 44, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Mashal, Y.; Abdelhady, H.; Iyer, A.K. Comparison of Tau and Amyloid-β Targeted Immunotherapy Nanoparticles for Alzheimer’s Disease. Biomolecules 2022, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Aillaud, I.; Funke, S.A. Tau Aggregation Inhibiting Peptides as Potential Therapeutics for Alzheimer Disease. Cell Mol. Neurobiol. 2022, in press. [Google Scholar] [CrossRef]
- Pakravan, N.; Abbasi, A.; Hassan, Z.M. New Paradigm in Cell Therapy Using Sperm Head to Restore Brain Function and Structure in Animal Model of Alzheimer’s Disease: Support for Boosting Constructive Inflammation vs. Anti-Inflammatory Approach. J. Immunol. Res. 2022, 2022, 8343763. [Google Scholar] [CrossRef] [PubMed]
- Paramanick, D.; Singh, V.D.; Singh, V.K. Neuroprotective effect of phytoconstituents via nanotechnology for treatment of Alzheimer diseases. J. Control. Release 2022, 351, 638–655. [Google Scholar] [CrossRef]
- Taniguchi, K.; Yamamoto, F.; Arai, T.; Yang, J.; Sakai, Y.; Itoh, M.; Mamada, N.; Sekiguchi, M.; Yamada, D.; Saitoh, A.; et al. Tyrosol Reduces Amyloid-β Oligomer Neurotoxicity and Alleviates Synaptic, Oxidative, and Cognitive Disturbances in Alzheimer’s Disease Model Mice. J. Alzheimers. Dis. 2019, 70, 937–952. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Gene-environment interactions in Alzheimer disease: The emerging role of epigenetics. Nat. Rev. Neurol. 2022, 18, 643–660. [Google Scholar] [CrossRef]
- Ezzati, A.; Pak, V.M. The effects of time-restricted eating on sleep, cognitive decline, and Alzheimer’s disease. Exp. Gerontol. 2022, in press. [Google Scholar] [CrossRef]
- Wright, J.R.; Deen, Q.F.E.; Stevenson, A.; Telford-Cooke, L.L.; Parker, C.; Martin-Ruiz, C.; Steinert, J.R.; Kalaria, R.N.; Mukaetova-Ladinska, E.B. Plasma Myeloperoxidase as a Potential Biomarker of Patient Response to Anti-Dementia Treatment in Alzheimer’s Disease. J. Alzheimers. Dis. 2022, 89, 1483–1492. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Revheim, M.E.; Alavi, A. Alzheimer’s Disease at a Crossroad: Time to Part from Amyloid to More Promising Aspects-Atherosclerosis for a Start. J. Alzheimers. Dis. 2022, 88, 455–458. [Google Scholar] [CrossRef]
- Xu, L.; Li, M.; Wei, A.; Yang, M.; Li, C.; Liu, R.; Zheng, Y.; Chen, Y.; Wang, Z.; Wang, K.; et al. Treadmill exercise promotes E3 ubiquitin ligase to remove amyloid β and P-tau and improve cognitive ability in APP/PS1 transgenic mice. J. Neuroinflamm. 2022, 19, 243. [Google Scholar] [CrossRef]
- Komaki, A.; Shahidi, S.; Hashemi-Firouzi, N.; Rafat, Z.; Keymoradzadeh, A.; Golipoor, Z. Combined Effect of Co-administration of Stromal Cell-Derived Factor-1 and Granulocyte-Colony Stimulating Factor on Rat Model of Alzheimer’s Disease. Front. Behav. Neurosci. 2022, 16, 796230. [Google Scholar] [CrossRef]
- Sanchez-Varo, R.; Mejias-Ortega, M.; Fernandez-Valenzuela, J.J.; Nuñez-Diaz, C.; Caceres-Palomo, L.; Vegas-Gomez, L.; Sanchez-Mejias, E.; Trujillo-Estrada, L.; Garcia-Leon, J.A.; Moreno-Gonzalez, I.; et al. Transgenic Mouse Models of Alzheimer’s Disease: An Integrative Analysis. Int. J. Mol. Sci. 2022, 23, 5404. [Google Scholar] [CrossRef]
- Yasuno, F.; Nakamura, A.; Kato, T.; Iwata, K.; Sakurai, T.; Arahata, Y.; Washimi, Y.; Hattori, H.; Ito, K. An evaluation of the amyloid cascade model using in vivo positron emission tomographic imaging. Psychogeriatrics 2021, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.A.; Koenigsberg, D.G.; McKenzie, A.T.; Kauffman, J.; Hanson, R.W.; Whitney, K.; Signaevsky, M.; Prastawa, M.; Iida, M.A.; White, C.L., 3rd; et al. Artificial intelligence-derived neurofibrillary tangle burden is associated with antemortem cognitive impairment. Acta Neuropathol. Commun. 2022, 10, 157. [Google Scholar] [CrossRef]
- Thal, D.R.; Tomé, S.O. The central role of tau in Alzheimer’s disease: From neurofibrillary tangle maturation to the induction of cell death. Brain Res. Bull. 2022, 90, 204–217. [Google Scholar] [CrossRef]
- Nielsen, R.E.; Grøntved, S.; Lolk, A.; Andersen, K.; Valentin, J.B. Real-world effects of anti-dementia treatment on mortality in patients with Alzheimer’s dementia. Medicine 2022, 101, e31625. [Google Scholar] [CrossRef]
- Pozzi, F.E.; Conti, E.; Appollonio, I.; Ferrarese, C.; Tremolizzo, L. Predictors of response to acetylcholinesterase inhibitors in dementia: A systematic review. Front. Neurosci. 2022, 16, 998224. [Google Scholar] [CrossRef]
- Hassan, N.A.; Alshamari, A.K.; Hassan, A.A.; Elharrif, M.G.; Alhajri, A.M.; Sattam, M.; Khattab, R.R. Advances on Therapeutic Strategies for Alzheimer’s Disease: From Medicinal Plant to Nanotechnology. Molecules 2022, 27, 4839. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Determination of Anti-Alzheimer’s Disease Activity of Selected Plant Ingredients. Molecules 2022, 27, 3222. [Google Scholar] [CrossRef]
- Wee, A.S.; Nhu, T.D.; Khaw, K.Y.; Tang, K.S.; Yeong, K.Y. Linking Diabetes to Alzheimer’s Disease: Potential Roles of Glucose Metabolism and Alpha-Glucosidase. Curr. Neuropharmacol. 2022, in press. [Google Scholar] [CrossRef]
- Agrawal, M.; Agrawal, A.K. Pathophysiological Association Between Diabetes Mellitus and Alzheimer’s Disease. Cureus 2022, 14, e29120. [Google Scholar] [CrossRef]
- Čater, M.; Hölter, S.M. A Pathophysiological Intersection of Diabetes and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 11562. [Google Scholar] [CrossRef]
- Patel, V.N.; Chorawala, M.R.; Shah, M.B.; Shah, K.C.; Dave, B.P.; Shah, M.P.; Patel, T.M. Emerging Pathophysiological Mechanisms Linking Diabetes Mellitus and Alzheimer’s Disease: An Old Wine in a New Bottle. J. Alzheimers Dis. Rep. 2022, 6, 349–357. [Google Scholar] [CrossRef]
- Das, S.; Ramachandran, A.K.; Halder, D.; Akbar, S.; Ahmed, B.; Joseph, A. Mechanistic and Etiological Similarities in Diabetes Mellitus and Alzheimer’s Disease: Antidiabetic Drugs as Optimistic Therapeutics in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2022, in press. [Google Scholar] [CrossRef]
- Sarkar, P.; Banu, S.; Bhattacharya, S.; Bala, A.; Sur, D. Pathophysiology associated with Diabetes Induced Tauopathy and Development of Alzheimer’s Disease. Curr. Diabetes Rev. 2022, in press. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef]
- Carey, A.; Fossati, S. Hypertension and hyperhomocysteinemia as modifiable risk factors for Alzheimer’s disease and dementia: New evidence, potential therapeutic strategies, and biomarkers. Alzheimers Dement. 2022, in press. [Google Scholar] [CrossRef]
- Valverde, A.; Mitrofanis, J. Photobiomodulation for Hypertension and Alzheimer’s Disease. J. Alzheimers Dis. 2022, in press. [Google Scholar] [CrossRef]
- Malone, J.E.; Elkasaby, M.I.; Lerner, A.J. Effects of Hypertension on Alzheimer’s Disease and Related Disorders. Curr. Hypertens. Rep. 2022, in press. [Google Scholar] [CrossRef]
- Vallée, A.; Vallée, J.N.; Lecarpentier, Y. WNT/β-catenin Pathway: A Possible Link Between Hypertension and Alzheimer’s Disease. Curr. Hypertens. Rep. 2022, 24, 465–475. [Google Scholar] [CrossRef]
- Abdulrahman, H.; van Dalen, J.W.; den Brok, M.; Latimer, C.S.; Larson, E.B.; Richard, E. Hypertension and Alzheimer’s disease pathology at autopsy: A systematic review. Alzheimers Dement. 2022, 18, 2308–2326. [Google Scholar] [CrossRef]
- Bajwa, E.; Klegeris, A. Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease. Neural. Regen. Res. 2022, 17, 2342–2346. [Google Scholar] [CrossRef] [PubMed]
- Schenk, D.; Barbour, R.; Dunn, W.; Gordon, G.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; Khan, K.; et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999, 400, 173–177. [Google Scholar] [CrossRef]
- Meng, Y.; Ding, L.; Zhang, H.; Yin, W.; Yan, Y.; Cao, Y. Immunization of Tg-APPswe/PSEN1dE9 mice with Aβ3-10-KLH vaccine prevents synaptic deficits of Alzheimer’s disease. Behav. Brain Res. 2017, 332, 64–70. [Google Scholar] [CrossRef]
- Ding, L.; Meng, Y.; Zhang, H.Y.; Yin, W.C.; Yan, Y.; Cao, Y.P. Prophylactic active immunization with a novel epitope vaccine improves cognitive ability by decreasing amyloid plaques and neuroinflammation in APP/PS1 transgenic mice. Neurosci. Res. 2017, 119, 7–14. [Google Scholar] [CrossRef]
- Ding, L.; Meng, Y.; Zhang, H.Y.; Yin, W.C.; Yan, Y.; Cao, Y.P. Active immunization with the peptide epitope vaccine Aβ3-10-KLH induces a Th2-polarized anti-Aβ antibody response and decreases amyloid plaques in APP/PS1 transgenic mice. Neurosci. Lett. 2016, 634, 1–6. [Google Scholar] [CrossRef]
- Guan, X.; Yang, J.; Gu, H.; Zou, J.; Yao, Z. Immunotherapeutic efficiency of a tetravalent Aβ1-15 vaccine in APP/PS1 transgenic mice as mouse model for Alzheimer’s disease. Hum. Vaccin. Immunother. 2013, 9, 1643–1653. [Google Scholar] [CrossRef]
- Carrera, I.; Etcheverría, I.; Fernández-Novoa, L.; Lombardi, V.; Cacabelos, R.; Vigo, C. Vaccine Development to Treat Alzheimer’s Disease Neuropathology in APP/PS1 Transgenic Mice. Int. J. Alzheimers Dis. 2012, 2012, 376138. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Gordon, M.N.; Chackerian, B.; Alamed, J.; Ugen, K.E.; Morgan, D. Virus-like peptide vaccines against Abeta N-terminal or C-terminal domains reduce amyloid deposition in APP transgenic mice without addition of adjuvant. J. Neuroimmune Pharmacol. 2010, 5, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.; Tschäpe, J.A.; Kopietz, F.; Braun, G.; Baade, J.K.; Wiederhold, K.H.; Staufenbiel, M.; Prinz, M.; Deller, T.; Kalinke, U.; et al. Vaccination with Abeta-displaying virus-like particles reduces soluble and insoluble cerebral Abeta and lowers plaque burden in APP transgenic mice. J. Immunol. 2009, 182, 7613–7624. [Google Scholar] [CrossRef] [PubMed]
- Ghochikyan, A.; Mkrtichyan, M.; Petrushina, I.; Movsesyan, N.; Karapetyan, A.; Cribbs, D.H.; Agadjanyan, M.G. Prototype Alzheimer’s disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine 2006, 24, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Yokoseki, T.; Shibata, M.; Yamaguchi, H.; Yanagisawa, K. Suppression of Abeta deposition in brain by peripheral administration of Fab fragments of anti-seed antibody. Biochem. Biophys. Res. Commun. 2005, 335, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, J.; Lin, Y.; Su, M.; Lai, L. Administration of anti-ERMAP antibody ameliorates Alzheimer’s disease in mice. J. Neuroinflamm. 2021, 18, 268. [Google Scholar] [CrossRef] [PubMed]
- Takamura, A.; Okamoto, Y.; Kawarabayashi, T.; Yokoseki, T.; Shibata, M.; Mouri, A.; Nabeshima, T.; Sun, H.; Abe, K.; Urisu, T.; et al. Extracellular and intraneuronal HMW-AbetaOs represent a molecular basis of memory loss in Alzheimer’s disease model mouse. Mol. Neurodegener. 2011, 6, 20. [Google Scholar] [CrossRef]
- Urban, A.S.; Pavlov, K.V.; Kamynina, A.V.; Okhrimenko, I.S.; Arseniev, A.S.; Bocharov, E.V. Structural Studies Providing Insights into Production and Conformational Behavior of Amyloid-β Peptide Associated with Alzheimer’s Disease Development. Molecules 2021, 26, 2897. [Google Scholar] [CrossRef]
- Ruiz-Riquelme, A.; Mao, A.; Barghash, M.M.; Lau, H.H.C.; Stuart, E.; Kovacs, G.G.; Nilsson, K.P.R.; Fraser, P.E.; Schmitt-Ulms, G.; Watts, J.C. Aβ43 aggregates exhibit enhanced prion-like seeding activity in mice. Acta Neuropathol. Commun. 2021, 9, 83. [Google Scholar] [CrossRef]
- Jäkel, L.; Biemans, E.A.L.M.; Klijn, C.J.M.; Kuiperij, H.B.; Verbeek, M.M. Reduced Influence of apoE on Aβ43 Aggregation and Reduced Vascular Aβ43 Toxicity as Compared with Aβ40 and Aβ42. Mol. Neurobiol. 2020, 57, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Yi-Bin, W.; Xiang, L.; Bing, Y.; Qi, Z.; Fei-Tong, J.; Minghong, W.; Xiangxiang, Z.; Le, K.; Yan, L.; Ping, S.; et al. Inhibition of the CEBPβ-NFκB interaction by nanocarrier-packaged Carnosic acid ameliorates glia-mediated neuroinflammation and improves cognitive function in an Alzheimer’s disease model. Cell Death Dis. 2022, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Jiang, R.; Zhang, W.; Yang, Z.; Li, L.L.; Shimozawa, M.; Tambaro, S.; Mayer, J.; Zhang, B.; Li, M.; et al. An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res. 2022, 32, 157–175. [Google Scholar] [CrossRef]
- Shi, J.M.; Li, H.Y.; Liu, H.; Zhu, L.; Guo, Y.B.; Pei, J.; An, H.; Li, Y.S.; Li, S.D.; Zhang, Z.Y.; et al. N-terminal Domain of Amyloid-β Impacts Fibrillation and Neurotoxicity. ACS Omega 2022, 7, 38847–38855. [Google Scholar] [CrossRef] [PubMed]
- Aow, J.; Huang, T.R.; Thinakaran, G.; Koo, E.H. Enhanced cleavage of APP by co-expressed Bace1 alters the distribution of APP and its fragments in neuronal and non-neuronal cells. Mol. Neurobiol. 2022, 59, 3073–3090. [Google Scholar] [CrossRef] [PubMed]
- Marttinen, M.; Ferreira, C.B.; Paldanius, K.M.A.; Takalo, M.; Natunen, T.; Mäkinen, P.; Leppänen, L.; Leinonen, V.; Tanigaki, K.; Kang, G.; et al. Presynaptic Vesicle Protein SEPTIN5 Regulates the Degradation of APP C-Terminal Fragments and the Levels of Aβ. Cells 2020, 9, 2482. [Google Scholar] [CrossRef]
- Gehlot, P.; Kumar, S.; Kumar Vyas, V.; Singh Choudhary, B.; Sharma, M.; Malik, R. Guanidine-based β amyloid precursor protein cleavage enzyme 1 (BACE-1) inhibitors for the Alzheimer’s disease (AD): A review. Bioorg. Med. Chem. 2022, 74, 117047. [Google Scholar] [CrossRef] [PubMed]
- Checler, F.; Afram, E.; Pardossi-Piquard, R.; Lauritzen, I. Is γ-secretase a beneficial inactivating enzyme of the toxic APP C-terminal fragment C99? J. Biol. Chem. 2021, 296, 100489. [Google Scholar] [CrossRef]
- Gao, Y.; Pimplikar, S.W. The gamma -secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 14979–14984. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Hata, S.; Omori, C.; Kimura, A.; Saito, H.; Kimura, N.; Gupta, V.; Pedrini, S.; Hone, E.; Chatterjee, P.; Taddei, K.; et al. Decrease in p3-Alcβ37 and p3-Alcβ40, products of Alcadein β generated by γ-secretase cleavages, in aged monkeys and patients with Alzheimer’s disease. Alzheimers Dement. 2019, 5, 740–750. [Google Scholar] [CrossRef]
- Kanagasingam, S.; von Ruhland, C.; Welbury, R.; Chukkapalli, S.S.; Singhrao, S.K. Porphyromonas gingivalis Conditioned Medium Induces Amyloidogenic Processing of the Amyloid-β Protein Precursor upon in vitro Infection of SH-SY5Y Cells. J. Alzheimers. Dis. Rep. 2022, 6, 577–587. [Google Scholar] [CrossRef]
- Khrestchatisky, M.; Baranger, K.; Rivera, S. MT5-MMP controls APP and β-CTF/C99 metabolism through proteolytic-dependent and -independent mechanisms relevant for Alzheimer’s disease. FASEB J. 2021, 35, e21727. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, J.G.; Siddu, A.; Wernig, M.; Südhof, T.C. Synaptogenic effect of APP-Swedish mutation in familial Alzheimer’s disease. Sci. Transl. Med. 2022, 14, eabn9380. [Google Scholar] [CrossRef]
- Kim, M.; Bezprozvanny, I. Conformational Models of APP Processing by Gamma Secretase Based on Analysis of Pathogenic Mutations. Int. J. Mol. Sci. 2021, 22, 13600. [Google Scholar] [CrossRef]
- Agüero, P.; Sainz, M.J.; García-Ayllón, M.S.; Sáez-Valero, J.; Téllez, R.; Guerrero-López, R.; Pérez-Pérez, J.; Jiménez-Escrig, A.; Gómez-Tortosa, E. α-Secretase nonsense mutation (ADAM10 Tyr167*) in familial Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 139. [Google Scholar] [CrossRef]
- Tomiyama, T.; Shimada, H. APP Osaka Mutation in Familial Alzheimer’s Disease-Its Discovery, Phenotypes, and Mechanism of Recessive Inheritance. Int. J. Mol. Sci. 2020, 21, 1413. [Google Scholar] [CrossRef] [PubMed]
- Palavicini, J.P.; Wang, C.; Chen, L.; Hosang, K.; Wang, J.; Tomiyama, T.; Mori, H.; Han, X. Oligomeric amyloid-beta induces MAPK-mediated activation of brain cytosolic and calcium-independent phospholipase A2 in a spatial-specific manner. Acta Neuropathol. Commun. 2017, 5, 56. [Google Scholar] [CrossRef]
- Jang, H.; Arce, F.T.; Ramachandran, S.; Kagan, B.L.; Lal, R.; Nussinov, R. Familial Alzheimer’s disease Osaka mutant (ΔE22) β-barrels suggest an explanation for the different Aβ1-40/42 preferred conformational states observed by experiment. J. Phys. Chem. B. 2013, 117, 11518–11529. [Google Scholar] [CrossRef]
- Boopathi, S.; Poma, A.B.; Garduño-Juárez, R. An Overview of Several Inhibitors for Alzheimer’s Disease: Characterization and Failure. Int. J. Mol. Sci. 2021, 22, 10798. [Google Scholar] [CrossRef]
- Wang, Z.X.; Tan, L.; Liu, J.; Yu, J.T. The Essential Role of Soluble Aβ Oligomers in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 1905–1924. [Google Scholar] [CrossRef] [PubMed]
- Barucker, C.; Bittner, H.J.; Chang, P.K.; Cameron, S.; Hancock, M.A.; Liebsch, F.; Hossain, S.; Harmeier, A.; Shaw, H.; Charron, F.M.; et al. Aβ42-oligomer Interacting Peptide (AIP) neutralizes toxic amyloid-β42 species and protects synaptic structure and function. Sci. Rep. 2015, 5, 15410. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Yu, W.C.; Shih, Y.H.; Chen, C.Y.; Guo, Z.H.; Huang, S.J.; Chan, J.C.C.; Chen, Y.R. Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer’s disease. Sci. Rep. 2018, 8, 4772. [Google Scholar] [CrossRef] [PubMed]
- Amar, F.; Sherman, M.A.; Rush, T.; Larson, M.; Boyle, G.; Chang, L.; Götz, J.; Buisson, A.; Lesné, S.E. The amyloid-β oligomer Aβ*56 induces specific alterations in neuronal signaling that lead to tau phosphorylation and aggregation. Sci. Signal 2017, 10, eaal2021. [Google Scholar] [CrossRef]
- Ono, K.; Tsuji, M. Protofibrils of Amyloid-β are Important Targets of a Disease-Modifying Approach for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 952. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lemere, C.A.; Walter, J. Phosphorylated Aβ peptides in human Down syndrome brain and different Alzheimer’s-like mouse models. Acta Neuropathol. Commun. 2020, 8, 118. [Google Scholar] [CrossRef]
- Chikugo, A.; Irie, Y.; Tsukano, C.; Uchino, A.; Maki, T.; Kume, T.; Kawase, T.; Hirose, K.; Kageyama, Y.; Tooyama, I.; et al. Optimization of the Linker Length in the Dimer Model of E22P-Aβ40 Tethered at Position 38. ACS Chem. Neurosci. 2022, 13, 2913–2923. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, S.; Shepardson, N.E.; Walsh, D.M.; Shankar, G.M.; Selkoe, D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 2009, 62, 788–801. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, X.; Fu, Y.; Li, Z.; Gong, R.; Lu, W. NMDAR-dependent somatic potentiation of synaptic inputs is correlated with β amyloid-mediated neuronal hyperactivity. Transl. Neurodegener. 2021, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Goshtasbi, H.; Pakchin, P.S.; Movafeghi, A.; Barar, J.; Castejon, A.M.; Omidian, H.; Omidi, Y. Impacts of oxidants and antioxidants on the emergence and progression of Alzheimer’s disease. Neurochem. Int. 2022, 153, 105268. [Google Scholar] [CrossRef]
- Kelleher, R.J., 3rd; Shen, J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 629–631. [Google Scholar] [CrossRef]
- Walker, E.S.; Martinez, M.; Brunkan, A.L.; Goate, A. Presenilin 2 familial Alzheimer’s disease mutations result in partial loss of function and dramatic changes in Abeta 42/40 ratios. J. Neurochem. 2005, 92, 294–301. [Google Scholar] [CrossRef]
- Bagyinszky, E.; Youn, Y.C.; An, S.S.; Kim, S. Mutations, associated with early-onset Alzheimer’s disease, discovered in Asian countries. Clin. Interv. Aging 2016, 11, 1467–1488. [Google Scholar] [CrossRef] [PubMed]
- Larson, T.; Khandelwal, V.; Weber, M.A.; Leidinger, M.R.; Meyerholz, D.K.; Narayanan, N.S.; Zhang, Q. Mice expressing P301S mutant human tau have deficits in interval timing. Behav. Brain Res. 2022, 432, 113967. [Google Scholar] [CrossRef]
- Polsinelli, A.J.; Logan, P.E.; Lane, K.A.; Manchella, M.K.; Nemes, S.; Sanjay, A.B.; Gao, S.; Apostolova, L.G. APOE ε4 carrier status and sex differentiate rates of cognitive decline in early- and late-onset Alzheimer’s disease. Alzheimers Dement. 2022, in press. [Google Scholar] [CrossRef]
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.L.; Nielsen, H.M.; et al. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 2022, 17, 62. [Google Scholar] [CrossRef]
- Saunders, T.S.; Jenkins, N.; Blennow, K.; Ritchie, C.; Muniz-Terrera, G. Interactions between apolipoprotein E, sex, and amyloid-beta on cerebrospinal fluid p-tau levels in the European prevention of Alzheimer’s dementia longitudinal cohort study (EPAD LCS). EBioMedicine 2022, 83, 104241. [Google Scholar] [CrossRef]
- Steele, O.G.; Stuart, A.C.; Minkley, L.; Shaw, K.; Bonnar, O.; Anderle, S.; Penn, A.C.; Rusted, J.; Serpell, L.; Hall, C.; et al. A multi-hit hypothesis for an APOE4-dependent pathophysiological state. Eur. J. Neurosci. 2022, 56, 5476–5515. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sajeev, G.; VanderWeele, T.J.; Viswanathan, A.; Sigurdsson, S.; Eiriksdottir, G.; Aspelund, T.; Betensky, R.A.; Grodstein, F.; Hofman, A.; et al. APOE ε4 and late-life cognition: Mediation by structural brain imaging markers. Eur. J. Epidemiol. 2022, 37, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Wang, M.; Yang, Y.; Chen, Y.; Liu, D.; Wang, X.; Song, L.; Wu, J.; Yang, Y. rAAV/ABAD-DP-6His attenuates oxidative stress-induced injury of PC12 cells. Neural. Regen. Res. 2014, 9, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Lim, Y.A.; Mensah-Nyagan, A.G.; Götz, J.; Eckert, A. Alzheimer’s disease, oestrogen and mitochondria: An ambiguous relationship. Mol. Neurobiol. 2012, 46, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ohyagi, Y.; Asahara, H.; Chui, D.H.; Tsuruta, Y.; Sakae, N.; Miyoshi, K.; Yamada, T.; Kikuchi, H.; Taniwaki, T.; Murai, H.; et al. Intracellular Abeta42 activates p53 promoter: A pathway to neurodegeneration in Alzheimer’s disease. FASEB J. 2005, 19, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Izuo, N.; Shimizu, T.; Murakami, K.; Irie, K. Development of a Novel Alzheimer’s Disease Model Based on the Theory of the Toxic-conformer of Amyloid β. Yakugaku Zasshi 2021, 141, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Uda, A.; Mitsubori, M.; Nagashima, S.; Iwasaki, H.; Ito, N.; Shiiba, I.; Ishido, S.; Matsuoka, M.; Inatome, R.; et al. Mitochondrial ubiquitin ligase alleviates Alzheimer’s disease pathology via blocking the toxic amyloid-β oligomer generation. Commun. Biol. 2021, 4, 192. [Google Scholar] [CrossRef]
- Baxter, D. Active and passive immunization for cancer. Hum. Vaccin. Immunother. 2014, 10, 2123–2129. [Google Scholar] [CrossRef]
- Arai, H.; Suzuki, H.; Yoshiyama, T. Vanutide cridificar and the QS-21 adjuvant in Japanese subjects with mild to moderate Alzheimer’s disease: Results from two phase 2 studies. Curr. Alzheimer Res. 2015, 12, 242–254. [Google Scholar] [CrossRef]
- Tizard, I.R. Adjuvants and adjuvanticity. Vacc. Veterin. 2021, 75–86.e1. [Google Scholar] [CrossRef]
- Xing, H.Y.; Li, B.; Peng, D.; Wang, C.Y.; Wang, G.Y.; Li, P.; Le, Y.Y.; Wang, J.M.; Ye, G.; Chen, J.H. A novel monoclonal antibody against the N-terminus of Aβ1-42 reduces plaques and improves cognition in a mouse model of Alzheimer’s disease. PLoS ONE 2017, 12, e0180076. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef]
- Nicoll, J.A.R.; Buckland, G.R.; Harrison, C.H.; Page, A.; Harris, S.; Love, S.; Neal, J.W.; Holmes, C.; Boche, D. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain 2019, 142, 2113–2126. [Google Scholar] [CrossRef]
- Mathis, C.A.; Mason, N.S.; Lopresti, B.J.; Klunk, W.E. Development of positron emission tomography β-amyloid plaque imaging agents. Semin. Nucl. Med. 2012, 42, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Davtyan, H.; Ghochikyan, A.; Cadagan, R.; Zamarin, D.; Petrushina, I.; Movsesyan, N.; Martinez-Sobrido, L.; Albrecht, R.A.; García-Sastre, A.; Agadjanyan, M.G. The immunological potency and therapeutic potential of a prototype dual vaccine against influenza and Alzheimer’s disease. J. Transl. Med. 2011, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Guo, J.; Jia, R. Roles of Regulatory T Cell-Derived Extracellular Vesicles in Human Diseases. Int. J. Mol. Sci. 2022, 23, 11206. [Google Scholar] [CrossRef] [PubMed]
- Montamat, G.; Leonard, C.; Poli, A.; Klimek, L.; Ollert, M. CpG Adjuvant in Allergen-Specific Immunotherapy: Finding the Sweet Spot for the Induction of Immune Tolerance. Front. Immunol. 2021, 12, 590054. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, G.; Chen, M.; Ma, H. Intestinal Uptake and Tolerance to Food Antigens. Front. Immunol. 2022, 13, 906122. [Google Scholar] [CrossRef]
- Takahashi, D.; Kimura, S.; Hase, K. Intestinal immunity: To be, or not to be, induced? That is the question. Int. Immunol. 2021, 33, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tan, J.; Chen, H.; Wu, N.; Su, B. Immune niches orchestrated by intestinal mesenchymal stromal cells lining the crypt-villus. Front. Immunol. 2022, 13, 1057932. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Abdelhafez, E.A. An overview of the structural and functional aspects of immune cells in teleosts. Histol. Histopathol. 2021, 36, 399–414. [Google Scholar] [CrossRef]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal. Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Agulla, B.; García-Sancho, M.; Sainz, Á.; Rodríguez-Franco, F.; Díaz-Regañón, D.; Rodríguez-Bertos, A.; Villaescusa, A. Isolation and immunophenotyping by flow cytometry of canine peripheral blood and intraepithelial and lamina propria duodenal T lymphocytes. Vet. Immunol. Immunopathol. 2021, 239, 110305. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.; Sollid, L.M. The human intestinal B-cell response. Mucosal. Immunol. 2016, 9, 1113–11124. [Google Scholar] [CrossRef] [PubMed]
- Esterházy, D.; Canesso, M.C.C.; Mesin, L.; Muller, P.A.; de Castro, T.B.R.; Lockhart, A.; ElJalby, M.; Faria, A.M.C.; Mucida, D. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 2019, 569, 126–130. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J. Impact of food-derived bioactive peptides on gut function and health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. Resilience of the oral microbiome. Periodontology 2021, 86, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Selmi, C.; Meyers, F.J.; Keen, C.L.; Gershwin, M.E. Probiotics and immunity. J. Gastroenterol. 2009, 44, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Orsini Delgado, M.L.; Rizzo, G.P.; Fossati, C.A.; Pasquevich, K.A.; Cassataro, J.; Smaldini, P.L.; Docena, G.H. Sublingual Omp16-driven redirection of the allergic intestinal response in a pre-clinical model of food allergy. Clin. Exp. Allergy 2020, 50, 954–963. [Google Scholar] [CrossRef]
- Jia, Z.; Wignall, A.; Prestidge, C.; Thierry, B. An ex vivo investigation of the intestinal uptake and translocation of nanoparticles targeted to Peyer’s patches microfold cells. Int. J. Pharm. 2021, 594, 120167. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Fan, R.; He, H.; Cui, Q.; Liang, X.; Liu, Q.; Liu, T.; Lin, K.; Zhang, Z.; Yi, H.; et al. Bifidobacterium animalis KV9 and Lactobacillus vaginalis FN3 alleviated β-lactoglobulin-induced allergy by modulating dendritic cells in mice. Front. Immunol. 2022, 13, 992605. [Google Scholar] [CrossRef]
- Mason, K.L.; Huffnagle, G.B.; Noverr, M.C.; Kao, J.Y. Overview of gut immunology. Adv. Exp. Med. Biol. 2008, 635, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wosen, J.E.; Mukhopadhyay, D.; Macaubas, C.; Mellins, E.D. Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front. Immunol. 2018, 9, 2144. [Google Scholar] [CrossRef]
- Yu, L.C. Intestinal epithelial barrier dysfunction in food hypersensitivity. J. Allergy 2012, 2012, 596081. [Google Scholar] [CrossRef]
- Knoop, K.A.; Kulkarni, D.H.; McDonald, K.G.; Gustafsson, J.K.; Davis, J.E.; Floyd, A.N.; Newberry, R.D. In vivo labeling of epithelial cell-associated antigen passages in the murine intestine. Lab. Anim. 2020, 49, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; Masli, S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS ONE 2015, 10, e0120284. [Google Scholar] [CrossRef]
- Tang, M.; Mei, J.; Sun, M.; Ma, K.; Zhao, A.; Fu, X. An optimized method to visualize the goblet cell-associated antigen passages and identify goblet cells in the intestine, conjunctiva, and airway. Immunobiology 2022, 227, 152260. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Davis, J.E.; Rappai, T.; McDonald, K.G.; Kulkarni, D.H.; Knoop, K.A.; Hogan, S.P.; Fitzpatrick, J.A.; Lencer, W.I.; Newberry, R.D. Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis. eLife 2021, 10, e67292. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.M.; Onufer, E.J.; McDonald, K.G.; Steinberger, A.E.; Sescleifer, A.M.; Seiler, K.M.; Tecos, M.E.; Newberry, R.D.; Warner, B.W. Small Bowel Resection Increases Paracellular Gut Barrier Permeability via Alterations of Tight Junction Complexes Mediated by Intestinal TLR4. J. Surg. Res. 2021, 258, 73–81. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; McDonald, K.G.; Knoop, K.A.; Gustafsson, J.K.; Kozlowski, K.M.; Hunstad, D.A.; Miller, M.J.; Newberry, R.D. Goblet cell associated antigen passages are inhibited during Salmonella typhimurium infection to prevent pathogen dissemination and limit responses to dietary antigens. Mucosal. Immunol. 2018, 11, 1103–1113. [Google Scholar] [CrossRef]
- Knoop, K.A.; Gustafsson, J.K.; McDonald, K.G.; Kulkarni, D.H.; Kassel, R.; Newberry, R.D. Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes 2017, 8, 400–411. [Google Scholar] [CrossRef] [PubMed]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.H.; Gustafsson, J.K.; Knoop, K.A.; McDonald, K.G.; Bidani, S.S.; Davis, J.E.; Floyd, A.N.; Hogan, S.P.; Hsieh, C.S.; Newberry, R.D. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal. Immunol. 2020, 13, 271–282. [Google Scholar] [CrossRef]
- Weiner, H.L. Oral tolerance: Immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001, 3, 947–954. [Google Scholar] [CrossRef]
- Bilsborough, J.; Viney, J.L. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology 2004, 127, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdi, Z.; Fitzpatrick, D.; Boisvert, A.; Baba, N.; Bouguermouh, S.; Sarfati, M.; Delespesse, G. Expression of CD103 identifies human regulatory T-cell subsets. J. Allergy Clin. Immunol. 2006, 118, 1342–1349. [Google Scholar] [CrossRef]
- Matteoli, G.; Mazzini, E.; Iliev, I.D.; Mileti, E.; Fallarino, F.; Puccetti, P.; Chieppa, M.; Rescigno, M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 2010, 59, 595–604. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Chen, W.; Konkel, J.E. Development of thymic Foxp3+ regulatory T cells: TGF-β matters. Eur. J. Immunol. 2015, 45, 958–965. [Google Scholar] [CrossRef]
- Husain, I.; Luo, X. Apoptotic Donor Cells in Transplantation. Front. Immunol. 2021, 12, 626840. [Google Scholar] [CrossRef] [PubMed]
- Kuang, R.; Perruche, S.; Chen, W. Apoptotic cell-linked immunoregulation: Implications for promoting immune tolerance in transplantation. Cell Biosci. 2015, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A. Mechanisms of tolerance. Immunol. Rev. 2011, 241, 5–19. [Google Scholar] [CrossRef]
- Dubois, B.; Chapat, L.; Goubier, A.; Papiernik, M.; Nicolas, J.F.; Kaiserlian, D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood 2003, 102, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.S.; Rosenberg, A.S.; Miah, S.M.S.; Skowron, G.; Roberts, B.J.; Lélias, S.; Terry, F.E.; Martin, W.D. Identification of a potent regulatory T cell epitope in factor V that modulates CD4+ and CD8+ memory T cell responses. Clin. Immunol. 2021, 224, 108661. [Google Scholar] [CrossRef] [PubMed]
- Eggenhuizen, P.J.; Ng, B.H.; Ooi, J.D. Treg Enhancing Therapies to Treat Autoimmune Diseases. Int. J. Mol. Sci. 2020, 21, 7015. [Google Scholar] [CrossRef]
- Moorman, C.D.; Bastian, A.G.; DeOca, K.B.; Mannie, M.D. A GM-CSF-neuroantigen tolerogenic vaccine elicits inefficient antigen recognition events below the CD40L triggering threshold to expand CD4+ CD25+ FOXP3+ Tregs that inhibit experimental autoimmune encephalomyelitis (EAE). J. Neuroinflamm. 2020, 17, 180. [Google Scholar] [CrossRef]
- Singh, R.; Alape, D.; de Lima, A.; Ascanio, J.; Majid, A.; Gangadharan, S.P. Regulatory T Cells in Respiratory Health and Diseases. Pulm. Med. 2019, 2019, 1907807. [Google Scholar] [CrossRef]
- Okamura, T.; Yamamoto, K.; Fujio, K. Early Growth Response Gene 2-Expressing CD4+LAG3+ Regulatory T Cells: The Therapeutic Potential for Treating Autoimmune Diseases. Front. Immunol. 2018, 9, 340. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhu, Y.; Liu, X.; Gu, Y.; Dai, X.; Li, B. Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. Eur. J. Immunol. 2021, 51, 2137–2150. [Google Scholar] [CrossRef]
- Irla, M. Instructive Cues of Thymic T Cell Selection. Annu. Rev. Immunol. 2022, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Guerder, S.; Hassel, C.; Carrier, A. Thymus-specific serine protease, a protease that shapes the CD4 T cell repertoire. Immunogenetics 2019, 71, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Dhamne, C.; Chung, Y.; Alousi, A.M.; Cooper, L.J.; Tran, D.Q. Peripheral and thymic foxp3+ regulatory T cells in search of origin, distinction, and function. Front. Immunol. 2013, 4, 253. [Google Scholar] [CrossRef] [PubMed]
- Povoleri, G.A.; Scottà, C.; Nova-Lamperti, E.A.; John, S.; Lombardi, G.; Afzali, B. Thymic versus induced regulatory T cells—Who regulates the regulators? Front. Immunol. 2013, 4, 169. [Google Scholar] [CrossRef] [PubMed]
- Siller, M.; Zeng, Y.; Hinterleitner, R. Can Microbes Boost Tregs to Suppress Food Sensitivities? Trends Immunol. 2020, 41, 967–971. [Google Scholar] [CrossRef]
- Kim, K.S.; Hong, S.W.; Han, D.; Yi, J.; Jung, J.; Yang, B.G.; Lee, J.Y.; Lee, M.; Surh, C.D. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016, 351, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Taitz, J.; Sun, S.M.; Langford, L.; Ni, D.; Macia, L. Your Regulatory T Cells Are What You Eat: How Diet and Gut Microbiota Affect Regulatory T Cell Development. Front. Nutr. 2022, 9, 878382. [Google Scholar] [CrossRef] [PubMed]
- van Sadelhoff, J.H.J.; Wiertsema, S.P.; Garssen, J.; Hogenkamp, A. Free Amino Acids in Human Milk: A Potential Role for Glutamine and Glutamate in the Protection against Neonatal Allergies and Infections. Front. Immunol. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Wu, R.; Yuan, X.; Li, X.; Ma, N.; Jiang, H.; Tang, H.; Xu, G.; Liu, Z.; Zhang, Z. The bile acid-activated retinoic acid response in dendritic cells is involved in food allergen sensitization. Allergy 2022, 77, 483–498. [Google Scholar] [CrossRef]
- Tulyeu, J.; Kumagai, H.; Jimbo, E.; Watanabe, S.; Yokoyama, K.; Cui, L.; Osaka, H.; Mieno, M.; Yamagata, T. Probiotics Prevents Sensitization to Oral Antigen and Subsequent Increases in Intestinal Tight Junction Permeability in Juvenile-Young Adult Rats. Microorganisms 2019, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, S.; Kaneko, K. Gut microbiota and allergic diseases in children. Allergol. Int. 2022, 71, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, C.; Zou, M.; Galvez, E.; Beckstette, M.; Ebel, M.; Strowig, T.; Huehn, J.; Pezoldt, J. The microbiota is dispensable for the early stages of peripheral regulatory T cell induction within mesenteric lymph nodes. Cell Mol. Immunol. 2021, 18, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaka, Y.; Yashiro, R. Novel Strategy for Alzheimer’s Disease Treatment through Oral Vaccine Therapy with Amyloid Beta. Biologics 2023, 3, 23-39. https://doi.org/10.3390/biologics3010003
Matsuzaka Y, Yashiro R. Novel Strategy for Alzheimer’s Disease Treatment through Oral Vaccine Therapy with Amyloid Beta. Biologics. 2023; 3(1):23-39. https://doi.org/10.3390/biologics3010003
Chicago/Turabian StyleMatsuzaka, Yasunari, and Ryu Yashiro. 2023. "Novel Strategy for Alzheimer’s Disease Treatment through Oral Vaccine Therapy with Amyloid Beta" Biologics 3, no. 1: 23-39. https://doi.org/10.3390/biologics3010003
APA StyleMatsuzaka, Y., & Yashiro, R. (2023). Novel Strategy for Alzheimer’s Disease Treatment through Oral Vaccine Therapy with Amyloid Beta. Biologics, 3(1), 23-39. https://doi.org/10.3390/biologics3010003





