Polypharmacy and Medication Outcome Reporting Bias in Older Patients with COVID-19
Abstract
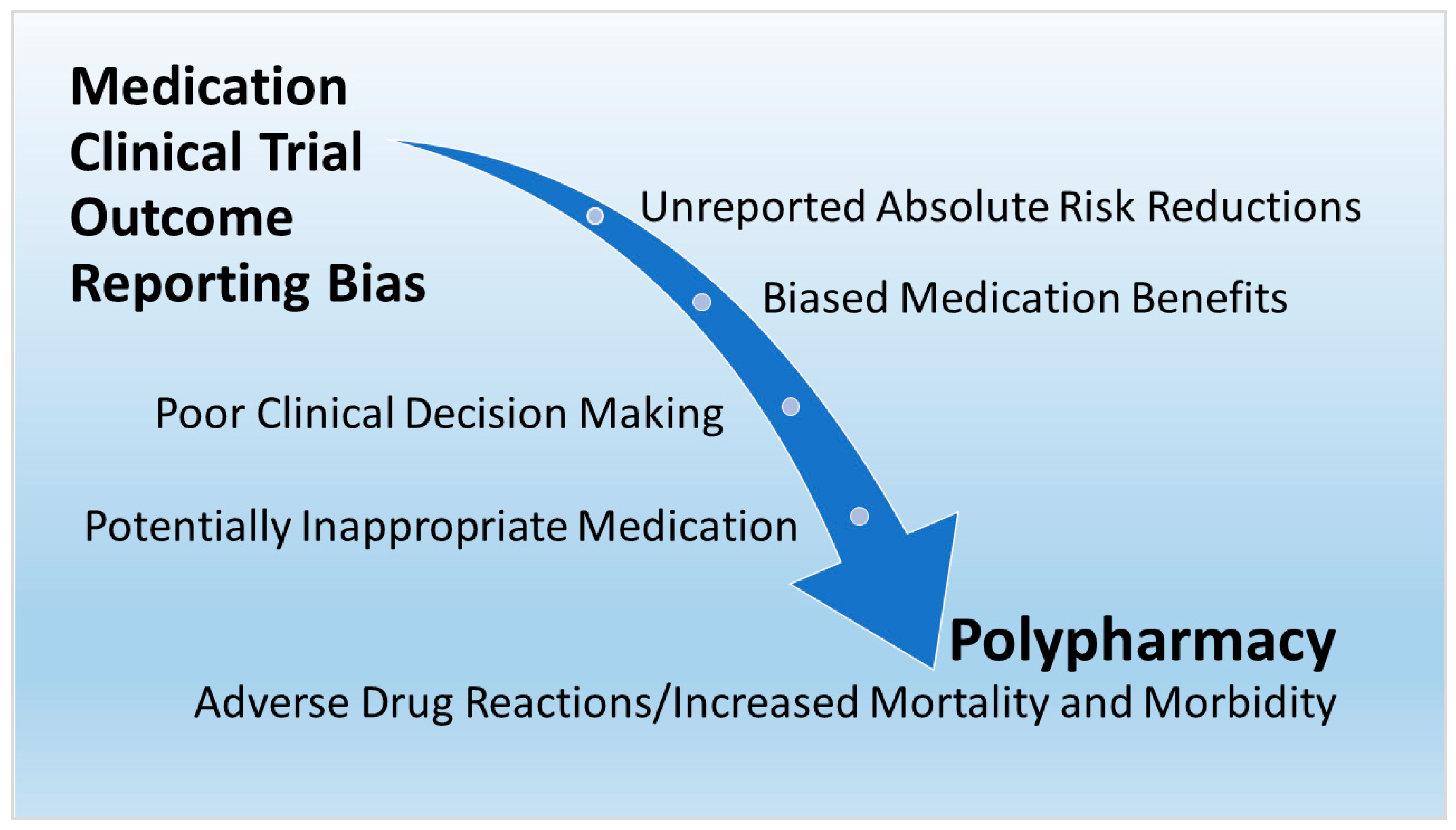
:1. Introduction
2. Polypharmacy in Older Adults with COVID-19
3. RCT Analyses for Medications in Polypharmacy
3.1. Antihypertensive for High Blood Pressure
3.2. Statin for High Cholesterol
3.3. Anticoagulant for Ischemic Heart Disease
3.4. Antihyperglycemic Agent for Diabetes
3.5. Analgesic for Arthritis
4. Determinants and Effects of Polypharmacy
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díez, R.; Cadenas, R.; Susperregui, J.; Sahagún, A.M.; Fernández, N.; García, J.J.; Sierra, M.; López, C. Potentially Inappropriate Medication and Polypharmacy in Nursing Home Residents: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 3808. [Google Scholar] [CrossRef] [PubMed]
- Young, E.H.; Pan, S.; Yap, A.G.; Reveles, K.R.; Bhakta, K. Polypharmacy prevalence in older adults seen in United States physician offices from 2009 to 2016. PLoS ONE 2021, 16, e0255642. [Google Scholar] [CrossRef]
- Kwak, M.J.; Chang, M.; Chiadika, S.; Aguilar, D.; Avritscher, E.; Deshmukh, A.; Goyal, P.; Kim, D.H.; Aparasu, R.; Holmes, H.M. Healthcare Expenditure Associated with Polypharmacy in Older Adults with Cardiovascular Diseases. Am. J. Cardiol. 2022, 169, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.S.; Shati, M.; Malakouti, S.K.; Khankeh, H.R.; Mehravaran, S.; Ahmadi, F. Physicians’ role in the development of inappropriate polypharmacy among older adults in Iran: A qualitative study. BMJ Open 2019, 9, e024128. [Google Scholar] [CrossRef] [Green Version]
- de Godoi Rezende Costa Molino, C.; Chocano-Bedoya, P.O.; Sadlon, A.; Theiler, R.; Orav, J.E.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Kanis, J.A.; Guyonnet, S.; et al. Prevalence of polypharmacy in community-dwelling older adults from seven centres in five European countries: A cross-sectional study of DO-HEALTH. BMJ Open 2022, 12, e051881. [Google Scholar] [CrossRef] [PubMed]
- Lvovschi, V.-E.; Carrouel, F.; du Sartz de Vigneulles, B.; Lamure, M.; Motyka, G.; Fraticelli, L.; Dussart, C. Knowledge, Attitudes and Practices Related to Medication, Antibiotics, and Vaccination among Public Service Population: National Survey Conducted in France. Int. J. Environ. Res. Public Health 2022, 19, 14044. [Google Scholar] [CrossRef]
- Mangin, D.; Bahat, G.; Golomb, B.A.; Mallery, L.H.; Moorhouse, P.; Onder, G.; Petrovic, M.; Garfinkel, D. International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): Position Statement and 10 Recommendations for Action. Drugs Aging 2018, 35, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Mitra-Majumdar, M.; Kesselheim, A.S. Reporting bias in clinical trials: Progress toward transparency and next steps. PLoS Med. 2022, 19, e1003894. [Google Scholar] [CrossRef]
- Brown, R.B. Absolute Risk Reductions in COVID-19 Antiviral Medication Clinical Trials. Pharmacoepidemiology 2023, 2, 98–105. [Google Scholar] [CrossRef]
- Smyth, R.M.; Kirkham, J.J.; Jacoby, A.; Altman, D.G.; Gamble, C.; Williamson, P.R. Frequency and reasons for outcome reporting bias in clinical trials: Interviews with trialists. BMJ 2011, 342, c7153. [Google Scholar] [CrossRef] [Green Version]
- Butcher, N.J.; Monsour, A.; Mew, E.J.; Chan, A.-W.; Moher, D.; Mayo-Wilson, E.; Terwee, C.B.; Chee-A-Tow, A.; Baba, A.; Gavin, F.; et al. Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension. JAMA 2022, 328, 2252–2264. [Google Scholar] [CrossRef]
- King, N.B.; Harper, S.; Young, M.E. Use of relative and absolute effect measures in reporting health inequalities: Structured review. BMJ Br. Med. J. 2012, 345, e5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfswinkel, J.F.; Furtmueller, E.; Wilderom, C.P.M. Using grounded theory as a method for rigorously reviewing literature. Eur. J. Inf. Syst. 2013, 22, 45–55. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Darvishi, N.; Salari, N.; Hosseinian-Far, A.; Akbari, H.; Mohammadi, M. Global prevalence of polypharmacy among the COVID-19 patients: A comprehensive systematic review and meta-analysis of observational studies. Trop. Med. Health 2022, 50, 60. [Google Scholar] [CrossRef]
- Chang, C.T.; Mohd Shariff, S.M.; Abu Bakar, N.S.; Ramzuzzaman, N.S.; Lim, C.K.; Lim, E.Y.J.; Ong, P.S.; Lee, J.M.; Tan, A.Y.; Kamis, S.F.; et al. Polypharmacy and potentially inappropriate medications among hospitalized older adults with COVID-19 in Malaysian tertiary hospitals. J. Pharm. Policy Pract. 2023, 16, 2. [Google Scholar] [CrossRef]
- Fick, D.; Semla, T.; Steinman, M.; Beizer, J.; Brandt, N.; Dombrowski, R.; DuBeau, C.; Pezzullo, L.; Epplin, J.; Flanagan, N.; et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Gallagher, P.F.; O’Connor, M.N.; O’Mahony, D. Prevention of potentially inappropriate prescribing for elderly patients: A randomized controlled trial using STOPP/START criteria. Clin. Pharm. 2011, 89, 845–854. [Google Scholar] [CrossRef]
- Sirois, C.; Boiteau, V.; Chiu, Y.; Gilca, R.; Simard, M. Exploring the associations between polypharmacy and COVID-19-related hospitalisations and deaths: A population-based cohort study among older adults in Quebec, Canada. BMJ Open 2022, 12, e060295. [Google Scholar] [CrossRef]
- cms.gov. Chronic Conditions among Medicare Beneficiaries, Chartbook, 2012 Edition. Available online: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/chronic-conditions/downloads/2012Chartbook.pdf (accessed on 17 April 2023).
- Mahmoud, M.; Carmisciano, L.; Tagliafico, L.; Muzyka, M.; Rosa, G.; Signori, A.; Bassetti, M.; Nencioni, A.; Monacelli, F. Patterns of Comorbidity and In-Hospital Mortality in Older Patients with COVID-19 Infection. Front. Med. 2021, 8, 726837. [Google Scholar] [CrossRef]
- Brown, R.B. Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials. Medicina 2021, 57, 199. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B. Relative risk reduction: Misinformative measure in clinical trials and COVID-19 vaccine efficacy. Dialogues Health 2022, 1, 100074. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.M., Jr.; Davis, B.R.; Price, T.R.; Applegate, W.B.; Fields, W.S.; Guralnik, J.M.; Kuller, L.; Pressel, S.; Stamler, J.; Probstfield, J.L.; et al. Effect of Treating Isolated Systolic Hypertension on the Risk of Developing Various Types and Subtypes of StrokeThe Systolic Hypertension in the Elderly Program (SHEP). JAMA 2000, 284, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanic, C.M.; Barbour, K.E.; Liu, Y.; Fang, J.; Lu, H.; Schieb, L.; Greenlund, K.J. Prevalence of Self-Reported Hypertension and Antihypertensive Medication Use Among Adults—United States, 2017. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klungel, O.H.; Heckbert, S.R.; Longstreth, W.T., Jr.; Furberg, C.D.; Kaplan, R.C.; Smith, N.L.; Lemaitre, R.N.; Leufkens, H.G.M.; de Boer, A.; Psaty, B.M. Antihypertensive Drug Therapies and the Risk of Ischemic Stroke. Arch. Intern. Med. 2001, 161, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravenni, R.; Jabre, J.F.; Casiglia, E.; Mazza, A. Primary stroke prevention and hypertension treatment: Which is the first-line strategy? Neurol. Int. 2011, 3, e12. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Lin, S.-y.; Baumann, K.; Zhou, C.; Zhou, W.; Cuellar, A.E.; Xue, H. Trends in Use and Expenditures for Brand-name Statins After Introduction of Generic Statins in the US, 2002–2018. JAMA Netw. Open 2021, 4, e2135371. [Google Scholar] [CrossRef]
- Byrne, P.; Demasi, M.; Jones, M.; Smith, S.M.; O’Brien, K.K.; DuBroff, R. Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2022, 182, 474–481. [Google Scholar] [CrossRef]
- Zipkin, D.A.; Umscheid, C.A.; Keating, N.L.; Allen, E.; Aung, K.; Beyth, R.; Kaatz, S.; Mann, D.M.; Sussman, J.B.; Korenstein, D.; et al. Evidence-based risk communication: A systematic review. Ann. Intern. Med. 2014, 161, 270–280. [Google Scholar] [CrossRef]
- Diamond, D.M.; Ravnskov, U. How statistical deception created the appearance that statins are safe and effective in primary and secondary prevention of cardiovascular disease. Expert Rev. Clin. Pharm. 2015, 8, 201–210. [Google Scholar] [CrossRef]
- Singer, D.E.; Hughes, R.A.; Gress, D.R.; Sheehan, M.A.; Oertel, L.B.; Maraventano, S.W.; Blewett, D.R.; Rosner, B.; Kistler, J.P. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N. Engl. J. Med. 1990, 323, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Colacci, M.; Tseng, E.K.; Sacks, C.A.; Fralick, M. Oral Anticoagulant Utilization in the United States and United Kingdom. J. Gen. Intern. Med. 2020, 35, 2505–2507. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.; Holman, R.; Stratton, I.; Cull, C.; Matthews, D.; Manley, S.; Frighi, V.; Wright, D.; Neil, A.; Kohner, E.; et al. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 854–865. [Google Scholar]
- Le, S.; Lee, G.C. Emerging Trends in Metformin Prescribing in the United States from 2000 to 2015. Clin. Drug Investig. 2019, 39, 757–763. [Google Scholar] [CrossRef]
- Megale, R.Z.; Deveza, L.A.; Blyth, F.M.; Naganathan, V.; Ferreira, P.H.; McLachlan, A.J.; Ferreira, M.L. Efficacy and Safety of Oral and Transdermal Opioid Analgesics for Musculoskeletal Pain in Older Adults: A Systematic Review of Randomized, Placebo-Controlled Trials. J. Pain 2018, 19, e471–e475. [Google Scholar] [CrossRef]
- Taghy, N.; Ramel, V.; Rivadeneyra, A.; Carrouel, F.; Cambon, L.; Dussart, C. Exploring the Determinants of Polypharmacy Prescribing and Dispensing Behaviors in Primary Care for the Elderly—Qualitative Study. Int. J. Environ. Res. Public Health 2023, 20, 1389. [Google Scholar]
- lowninstitute.org. Eliminating Medication Overload: A National Action Plan. Working Group on Medication Overload. The Lown Institute. Available online: https://lowninstitute.org/reports/eliminating-medication-overload-a-national-action-plan/ (accessed on 19 April 2023).
- Ioannidis, J.P.; Caplan, A.L.; Dal-Ré, R. Outcome reporting bias in clinical trials: Why monitoring matters. BMJ 2017, 356, j408. [Google Scholar] [CrossRef]
- deprescribingresearch.org. U.S. Deprescribing Research Network—National Institue of Aging. Available online: https://deprescribingresearch.org/ (accessed on 19 April 2023).
- Pahus, L.; Suehs, C.M.; Halimi, L.; Bourdin, A.; Chanez, P.; Jaffuel, D.; Marciano, J.; Gamez, A.S.; Vachier, I.; Molinari, N. Patient distrust in pharmaceutical companies: An explanation for women under-representation in respiratory clinical trials? BMC Med. Ethics 2020, 21, 72. [Google Scholar] [CrossRef]
- Baker, D.W. Trust in Health Care in the Time of COVID-19. JAMA 2020, 324, 2373–2375. [Google Scholar] [CrossRef]
| Antihypertensive | Stroke | RRR | 95% CI | ARR | 95% CI | NNT |
|---|---|---|---|---|---|---|
| Chlorthalidone/atenolol/reserpine (2365) | 103 | 35.06% | 17.33–48.98% | 2.35% | 1.05–3.65% | 43 |
| Placebo (2371) | 159 |
| Statin | CHD Death/Non-Fatal MI | RRR | 95% CI | ARR | 95% CI | NNT |
|---|---|---|---|---|---|---|
| Pravastatin (2891) | 292 | 17.35% | 4.37–28.58% | 2.12% | 0.50–3.74% | 48 |
| Placebo (2913) | 356 |
| Anticoagulant | Ischemic Stroke | RRR | 95% CI | ARR | 95% CI | NNT |
|---|---|---|---|---|---|---|
| Warfarin (212) | 2 | 84.91% | 33.93–96.55% | 5.31% | 1.77–8.84% | 19 |
| Placebo (208) | 13 |
| Antihyperglycemic Agent | Diabetes Death | RRR | 95% CI | ARR | 95% CI | NNT |
|---|---|---|---|---|---|---|
| Metformin (342) | 28 | 38.82% | 5.78–60.27% | 5.19% | 0.80–9.59% | 20 |
| Conventional Dietary Treatment (411) | 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.B. Polypharmacy and Medication Outcome Reporting Bias in Older Patients with COVID-19. BioMed 2023, 3, 320-328. https://doi.org/10.3390/biomed3030027
Brown RB. Polypharmacy and Medication Outcome Reporting Bias in Older Patients with COVID-19. BioMed. 2023; 3(3):320-328. https://doi.org/10.3390/biomed3030027
Chicago/Turabian StyleBrown, Ronald B. 2023. "Polypharmacy and Medication Outcome Reporting Bias in Older Patients with COVID-19" BioMed 3, no. 3: 320-328. https://doi.org/10.3390/biomed3030027
APA StyleBrown, R. B. (2023). Polypharmacy and Medication Outcome Reporting Bias in Older Patients with COVID-19. BioMed, 3(3), 320-328. https://doi.org/10.3390/biomed3030027






