Berry Anthocyanins in Rodent and Human Obesity and Diabetes: A Review of the Evidence
Abstract
1. Introduction
2. Epidemiological Studies with Fruits, Vegetables, and Berries
3. Anti-Obesity Effects in Rodent Studies with Berry or Anthocyanin Treatments
3.1. Purple Corn Color: Seminal Work on Anthocyanins and Obesity
3.2. Rodent Studies with Berry Powders or Extracts
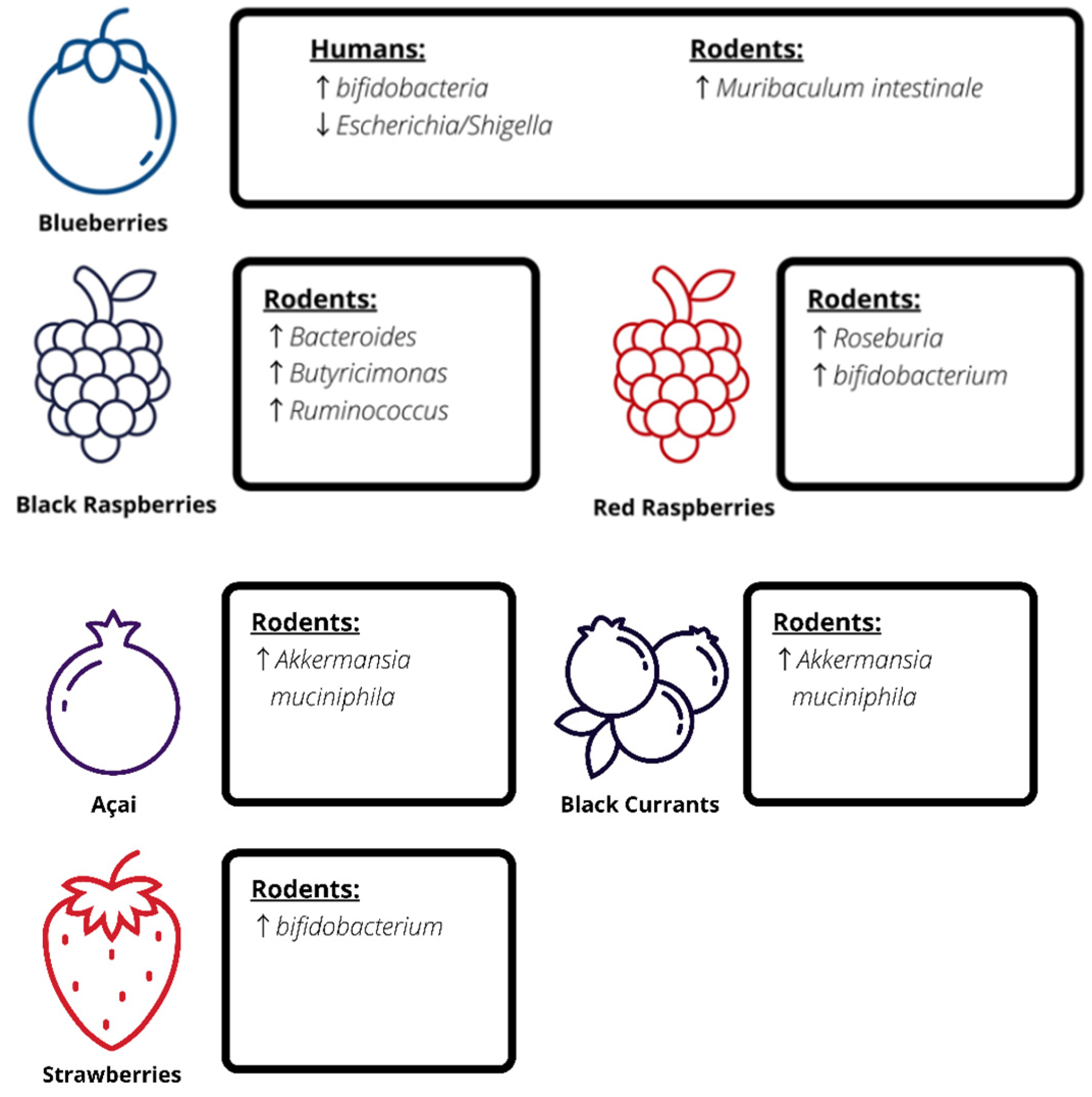
4. Berries and the Gut Microbiota
5. Berries and Anthocyanins vs. Obesity and Metabolic Aberrations: Human Studies
5.1. Meta-Analyses
5.2. Berries and Human Obesity
5.2.1. Human Studies with Anthocyanin Rich Extracts
5.2.2. Human Studies with Freeze-Dried Powders
5.2.3. Human Studies with Whole Berries
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Washington, DC, USA, 2020. [Google Scholar]
- Overweight and Obesity Statistics. 2018. Available online: https://www.niddk.nih.gov/health-information/health-statistics/Pages/overweight-obesity-statistics.aspx (accessed on 1 February 2022).
- Gorin, A.A.; Crane, M.M. The obesogenic environment. In Handbook of Childhood and Adolescent Obesity; Springer: Berlin/Heidelberg, Germany, 2008; pp. 145–161. [Google Scholar]
- Ford, E.S.; Cunningham, T.J.; Croft, J.B. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep 2015, 38, 829. [Google Scholar] [CrossRef] [PubMed]
- Mishel, L. Vast Majority of Wage Earners Are Working Harder, and Not for Much More: Trends in US Work Hours and Wages over 1979–2007; Economic Policy Institute Issue Brief: Washington, DC, USA, 2013; p. 348. [Google Scholar]
- Althoff, T.; White, R.W.; Horvitz, E. Influence of Pokemon Go on Physical Activity: Study and Implications. J. Med. Internet Res. 2016, 18, e315. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff. 2009, 28, w822–w831. [Google Scholar] [CrossRef]
- Adult Obesity Causes & Consequences. 2022. Available online: https://www.cdc.gov/obesity/adult/causes.html (accessed on 1 February 2022).
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P. Anthocyanin Bioactivity in Obesity and Diabetes: The Essential Role of Glucose Transporters in the Gut and Periphery. Cells 2020, 9, 2515. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ—Br. Med. J. 2014, 349, g4490. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; López-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef]
- Rissanen, T.H.; Voutilainen, S.; Virtanen, J.K.; Venho, B.; Vanharanta, M.; Mursu, J.; Salonen, J.T. Low intake of fruits, berries and vegetables is associated with excess mortality in men: The Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. J. Nutr. 2003, 133, 199–204. [Google Scholar] [CrossRef]
- Mursu, J.; Virtanen, J.; Tuomainen, T.-P.; Nurmi, T.; Voutilainen, S. Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2014, 99, 328–333. [Google Scholar] [CrossRef]
- Ellingsen, I.; Hjerkinn, E.M.; Seljeflot, I.; Arnesen, H.; Tonstad, S. Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men. Br. J. Nutr. 2008, 99, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Hjerkinn, E.M.; Abdelnoor, M.; Breivik, L.; Bergengen, L.; Ellingsen, I.; Seljeflot, I.; Aase, O.; Klemsdal, T.O.; Hjermann, I.; Arnesen, H. Effect of diet or very long chain omega-3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima-media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 325–333. [Google Scholar] [PubMed]
- Bjelke, E. Epidemiologic studies of cancer of the stomach, colon, and rectum; with special emphasis on the role of diet. Scand. J. Gastroenterol. Suppl. 1974, 31, 1–235. [Google Scholar] [PubMed]
- Magnus, K.; Hougen, A.; Andersen, A.; Pedersen, E. A study of disease in migrants and their siblings: Development of sibling rosters. J. Chronic Dis. 1970, 23, 405–410. [Google Scholar] [CrossRef]
- Hjartåker, A.; Knudsen, M.D.; Tretli, S.; Weiderpass, E. Consumption of berries, fruits and vegetables and mortality among 10,000 Norwegian men followed for four decades. Eur. J. Nutr. 2015, 54, 599–608. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Tsuda, T.; Osawa, T.; Ohshima, K.; Kawakishi, S. Antioxidative Pigments Isolated from the Seeds of Phaseolus-Vulgaris L. J. Agric. Food Chem. 1994, 42, 248–251. [Google Scholar] [CrossRef]
- Tsuda, T.; Watanabe, M.; Ohshima, K.; Norinobu, S.; Choi, S.-W.; Kawakishi, S.; Osawa, T. Antioxidative Activity of the Anthocyanin Pigments Cyanidin 3-O-Beta-D-Glucoside and Cyanidin. J. Agric. Food Chem. 1994, 42, 2407–2410. [Google Scholar] [CrossRef]
- Tsuda, T.; Shiga, K.; Ohshima, K.; Kawakishi, S.; Osawa, T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem. Pharmacol. 1996, 52, 1033–1039. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Osawa, T. Dietary cyanidin 3-O-beta-D-glucoside increases ex vivo oxidation resistance of serum in rats. Lipids 1998, 33, 583–588. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Kitoh, J.; Osawa, T. Protective effects of dietary cyanidin 3-O-beta-D-glucoside on liver ischemia-reperfusion injury in rats. Arch. Biochem. Biophys. 1999, 368, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Kato, Y.; Osawa, T. Cyanidin 3-O-beta-D-glucoside attenuates the hepatic ischemia-reperfusion injury through a decrease in the neutrophil chemoattractant production in rats. J. Nutr. Sci. Vitaminol. 2002, 48, 134–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsuda, T.; Horio, F.; Osawa, T. Cyanidin 3-O-beta-D-glucoside suppresses nitric oxide production during a zymosan treatment in rats. J. Nutr. Sci. Vitaminol. 2002, 48, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Recent Progress in Anti-Obesity and Anti-Diabetes Effect of Berries. Antioxidants 2016, 5, 13. [Google Scholar] [CrossRef]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.; Hager, T.J.; Hager, A.; Howard, L. Dietary Black Raspberry Anthocyanins Do Not Alter Development of Obesity in Mice Fed an Obesogenic High-Fat Diet. J. Agric. Food Chem. 2010, 58, 3977–3983. [Google Scholar] [CrossRef]
- Prior, R.L.; Wilkes, S.E.; Rogers, T.R.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified Blueberry Anthocyanins and Blueberry Juice Alter Development of Obesity in Mice Fed an Obesogenic High-Fat Diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry Intake Alters Skeletal Muscle and Adipose Tissue Peroxisome Proliferator-Activated Receptor Activity and Reduces Insulin Resistance in Obese Rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef]
- Seymour, E.M.; Lewis, S.K.; Urcuyo-Llanes, D.E.; Tanone, I.I.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Regular Tart Cherry Intake Alters Abdominal Adiposity, Adipose Gene Transcription, and Inflammation in Obesity-Prone Rats Fed a High Fat Diet. J. Med. Food 2009, 12, 935–942. [Google Scholar] [CrossRef]
- Seymour, E.M.; Singer, A.A.; Kirakosyan, A.; Urcuyo-Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food 2008, 11, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of Beneficial Metabolic Effects of Berries in High-Fat Fed C57BL/6J Mice. J. Nutr. Metab. 2014, 2014, 403041. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Cragg, M.; deFonseka, J.; Hite, A.; Rosenberg, M.; Zhou, B. Statistical review of US macronutrient consumption data, 1965-2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition 2015, 31, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.; Lila, M.A.; Raskin, I. In Vitro and in Vivo Anti-Diabetic Effects of Anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.F.B.; Dey, M.; Rogers, R.B.; Ribnicky, D.M.; Gipp, D.M.; Cefalu, W.T.; Raskin, I.; Lila, M.A. Phytochemical composition and metabolic performance-enhancing activity of dietary berries traditionally used by native North Americans. J. Agric. Food Chem. 2008, 56, 654–660. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Feo, V.; Battistelli, A.; Da Cruz, A.G.; Coppola, R. Chapter Two—Polyphenols, the new frontiers of prebiotics. In Advances in Food and Nutrition Research; da Cruz, A.G., Prudencio, E.S., Esmerino, E.A., da Silva, M.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 35–89. [Google Scholar]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104 (Suppl. 3), S48–S66. [Google Scholar] [CrossRef]
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BioFactors 2017, 43, 507–516. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Fracassetti, D.; Taverniti, V.; Del Bo’, C.; Vendrame, S.; Klimis-Zacas, D.; Arioli, S.; Riso, P.; Porrini, M. Differential modulation of human intestinal bifidobacterium populations after consumption of a wild blueberry (Vaccinium angustifolium) drink. J. Agric. Food Chem. 2013, 61, 8134–8140. [Google Scholar] [CrossRef]
- Ntemiri, A.; Ghosh, T.; Gheller, M.; Tran, T.; Blum, J.; Pellanda, P.; Vlckova, K.; Neto, M.; Howell, A.; Thalacker-Mercer, A.; et al. Whole Blueberry and Isolated Polyphenol-Rich Fractions Modulate Specific Gut Microbes in an In Vitro Colon Model and in a Pilot Study in Human Consumers. Nutrients 2020, 12, 2800. [Google Scholar] [CrossRef]
- Rock, C.; Donnenberg, M.S. Human Pathogenic Enterobacteriaceae. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Volk, J.K.; Nyström, E.E.; van der Post, S.; Abad, B.M.; Schroeder, B.O.; Johansson, Å.; Svensson, F.; Jäverfelt, S.; Johansson, M.E.; Hansson, G.C.; et al. The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function. J. Exp. Med. 2019, 216, 2602–2618. [Google Scholar] [CrossRef]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef]
- Xian, Y. Polyphenolic fractions isolated from red raspberry whole fruit, pulp, and seed differentially alter the gut microbiota of mice with diet-induced obesity. J. Funct. Foods 2021, 76, 104288. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. eBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Lee, H. Amelioration of hyperglycemia by Rubus occidentalis (black raspberry) and increase in short-chain fatty acids producing bacteria. J. Funct. Foods 2019, 54, 433–439. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Shaping the Metabolism of Intestinal Bacteroides Population through Diet to Improve Human Health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of Obesity and Glucose Tolerance by Acetate in Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef]
- Petersen, C.; Wankhade, U.D.; Bharat, D.; Wong, K.; Mueller, J.E.; Chintapalli, S.V.; Piccolo, B.D.; Jalili, T.; Jia, Z.; Symons, J.D.; et al. Dietary supplementation with strawberry induces marked changes in the composition and functional potential of the gut microbiome in diabetic mice. J. Nutr. Biochem. 2019, 66, 63–69. [Google Scholar] [CrossRef]
- Upadhyaya, S.; Banerjee, G. Type 2 diabetes and gut microbiome: At the intersection of known and unknown. Gut Microbes 2015, 6, 85–92. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Deng, R.; Zhang, Y.; Zheng, X. Dietary anthocyanin-rich extract of açai protects from diet-induced obesity, liver steatosis, and insulin resistance with modulation of gut microbiota in mice. Nutrition 2021, 86, 111176. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Wang, F.; Li, Y.; Zheng, X. Black Current Anthocyanins Improve Lipid Metabolism and Modulate Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2021, 65, e2001090. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of Berries Consumption on Cardiovascular Risk Factors: A Meta-analysis with Trial Sequential Analysis of Randomized Controlled Trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef]
- Montonen, J.; Järvinen, R.; Heliövaara, M.; Reunanen, A.; Aromaa, A.; Knekt, P. Food consumption and the incidence of type II diabetes mellitus. Eur. J. Clin. Nutr. 2005, 59, 441–448. [Google Scholar] [CrossRef]
- Muraki, I.; Imamura, F.; Manson, J.E.; Hu, F.B.; Willett, W.C.; van Dam, R.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M.; et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef]
- USDA. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-database-for-the-flavonoid-content-of-selected-foods-release-31-december-2013/ (accessed on 1 February 2022).
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Vitale, M.; Vaccaro, O.; Masulli, M.; Bonora, E.; Del Prato, S.; Giorda, C.B.; Nicolucci, A.; Squatrito, S.; Auciello, S.; Babini, A.C.; et al. Polyphenol intake and cardiovascular risk factors in a population with type 2 diabetes: The TOSCA.IT study. Clin. Nutr. 2017, 36, 1686–1692. [Google Scholar] [CrossRef]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef]
- Brown, B.G.; Stukovsky, K.H.; Zhao, X.Q. Simultaneous low-density lipoprotein-C lowering and high-density lipoprotein-C elevation for optimum cardiovascular disease prevention with various drug classes, and their combinations: A meta-analysis of 23 randomized lipid trials. Curr. Opin. Lipidol. 2006, 17, 631–636. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Paraoxonase, a cardioprotective enzyme: Continuing issues. Curr. Opin. Lipidol. 2004, 15, 261–267. [Google Scholar] [CrossRef]
- Hoggard, N.; Cruickshank, M.; Moar, K.-M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J. Nutr. Sci. 2013, 2, e22. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Zhang, Y.; Sun, R.; Xia, M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E975–E988. [Google Scholar] [CrossRef]
- Kianbakht, S.; Abasi, B.; Hashem Dabaghian, F. Improved lipid profile in hyperlipidemic patients taking Vaccinium arctostaphylos fruit hydroalcoholic extract: A randomized double-blind placebo-controlled clinical trial. Phytother. Res. 2014, 28, 432–436. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.; Champagne, C.M.; Cefalu, W.T. Bioactives in Blueberries Improve Insulin Sensitivity in Obese, Insulin-Resistant Men and Women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Basu, A.; Wilkinson, M.; Penugonda, K.; Simmons, B.; Betts, N.M.; Lyons, T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr. J. 2009, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef]
- Moazen, S.; Amani, R.; Rad, A.H.; Shahbazian, H.; Ahmadi, K.; Jalali, M.-T. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: A randomized double-blind controlled trial. Ann. Nutr. Metab. 2013, 63, 256–264. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef]
- Lehtonen, H.-M.; Suomela, J.-P.; Tahvonen, R.; Yang, B.; Venojärvi, M.; Viikari, J.; Kallio, H. Different berries and berry fractions have various but slightly positive effects on the associated variables of metabolic diseases on overweight and obese women. Eur. J. Clin. Nutr. 2011, 65, 394–401. [Google Scholar] [CrossRef]
- Solverson, P.M.; Henderson, T.R.; Debelo, H.; Ferruzzi, M.G.; Baer, D.J.; Novotny, J.A. An Anthocyanin-Rich Mixed-Berry Intervention May Improve Insulin Sensitivity in a Randomized Trial of Overweight and Obese Adults. Nutrients 2019, 11, 2876. [Google Scholar] [CrossRef]
- Solverson, P.M.; Rumpler, W.V.; Leger, J.L.; Redan, B.W.; Ferruzzi, M.G.; Baer, D.J.; Castonguay, T.W.; Novotny, J.A. Blackberry Feeding Increases Fat Oxidation and Improves Insulin Sensitivity in Overweight and Obese Males. Nutrients 2018, 10, 1048. [Google Scholar] [CrossRef]

| Intervention | Duration | Method | Species | Variables | Results | References |
|---|---|---|---|---|---|---|
| Purple corn color (PCC) | 12 weeks |
| 4-wk old diet-induced obese C57BL/6J mice |
| In HFD + PCC:
| [21] |
| Blueberry juice or purified blueberry anthocyanins in drinking water | 72 days |
| 25-d old C57BL/6J mice |
|
| [32] |
| Freeze-dried blueberry powder (BBP) | 90 days |
| 7-w old diabetic Zucker fatty rat |
|
| [34] |
Freeze-dried berry powders:
| 13 weeks |
| 6-wk old diet-induced obese C57BL/6J mice |
| For lingonberry, blackcurrant, and bilberry:
| [37] |
| Black elderberry extract (BEE) | 16 weeks |
| 6-wk old C57BL/6J mice |
|
| [38] |
| Berry | Study Design | Methods | Variables | Results | References |
|---|---|---|---|---|---|
| Freeze-dried wild blueberry powder |
|
|
|
| [48] |
| Isolated blueberry polyphenol fractions |
|
|
|
| [49] |
| Intervention | Duration | Method | Species | Variables | Results | References |
|---|---|---|---|---|---|---|
| 12-week diet intervention + 8-week FMT follow up |
| 8-wk old C57BL/6J male mice |
|
| [51] |
Red raspberry (RR) polyphenolic extract from:
| 16 weeks |
| C57BL/6 male mice |
|
| [53] |
| Black raspberry extract (RO) | 16 weeks |
| C57BL/6N male diet-induced obese and hyperglycemic mice |
|
| [55] |
| Freeze-dried strawberry | 10 weeks |
| 6-week-old diabetic and non-diabetic C57BL/6J mice |
|
| [61] |
| Acai anthocyanin extract (AEA) | 14 weeks |
| 4-week-old Male C57BL/6J mice |
|
| [63] |
| Black currant (BC) | 12 weeks |
| 4-week-old Male SPF C57BL/6J |
|
| [64] |
| Study Type | Berry | Methods | Duration | Variables | Results | References |
|---|---|---|---|---|---|---|
| Randomized, double-blind placebo-control | Bilberry/black currant extract | Groups:
| 12 weeks |
|
| [76] |
| Randomized, double-blind placebo-control | Bilberry/black currant extract |
| 12 weeks |
|
| [78] |
| Randomized, double-blind, placebo-control, crossover | Bilberry extract (36% anthocyanins by weight) |
| <1 day |
|
| [82] |
| Randomized, placebo-controlled | Bilberry/black currant extract |
| 12 weeks |
|
| [83] |
| Randomized, parallel-arm, double-blind, placebo-control | Whortleberry extract |
| 2 months |
| In treatment group:
| [84] |
| Randomized, parallel-arm, double-blind, placebo-control | Freeze-dried blueberry powder |
| 6 weeks |
|
| [85] |
| Randomized, parallel-arm, double-blind, placebo-control | Freeze-dried blueberry powder |
| 6 weeks |
|
| [86] |
| Randomized, placebo-controlled | Blueberry powder |
| 8 weeks |
|
| [87] |
| Double-blind, placebo-controlled, parallel arm | Blueberry powder |
| 8 weeks |
| In treatment group:
| [88] |
| Intervention study | Freeze-dried strawberry powder |
| 4 weeks |
|
| [89] |
| Randomized, parallel arm | Freeze-dried strawberry powder | Obese participants with metabolic syndrome + 50 g/day of powder | 8 weeks | Blood lipids |
| [90] |
| Randomized, single-blind, crossover | Strawberry powder | Insulin resistant subjects with central obesity + HF-HC breakfast + 1 of 4 doses of strawberry powder | 6-hrs | Postprandial changes on:
|
| [91] |
| Randomized, double-blind, parallel arm | Strawberry powder |
| 6 weeks |
|
| [92] |
| Intervention study | Whole strawberries | Healthy participants consumed 500 g/day of strawberries | 30 days |
|
| [93] |
| Randomized, crossover trial |
| Obese women consumed one of the following:
| 30 days/study + washout period |
|
| [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riordan, J.; Solverson, P. Berry Anthocyanins in Rodent and Human Obesity and Diabetes: A Review of the Evidence. BioMed 2022, 2, 210-237. https://doi.org/10.3390/biomed2020019
Riordan J, Solverson P. Berry Anthocyanins in Rodent and Human Obesity and Diabetes: A Review of the Evidence. BioMed. 2022; 2(2):210-237. https://doi.org/10.3390/biomed2020019
Chicago/Turabian StyleRiordan, Joseph, and Patrick Solverson. 2022. "Berry Anthocyanins in Rodent and Human Obesity and Diabetes: A Review of the Evidence" BioMed 2, no. 2: 210-237. https://doi.org/10.3390/biomed2020019
APA StyleRiordan, J., & Solverson, P. (2022). Berry Anthocyanins in Rodent and Human Obesity and Diabetes: A Review of the Evidence. BioMed, 2(2), 210-237. https://doi.org/10.3390/biomed2020019







