Augmented Sensitivity of At-Home Rapid SARS-CoV-2 Antigen Test (RAT) Kits with Computer Vision: A Framework and Proof of Concept
Abstract
:1. Introduction
2. Materials and Methods
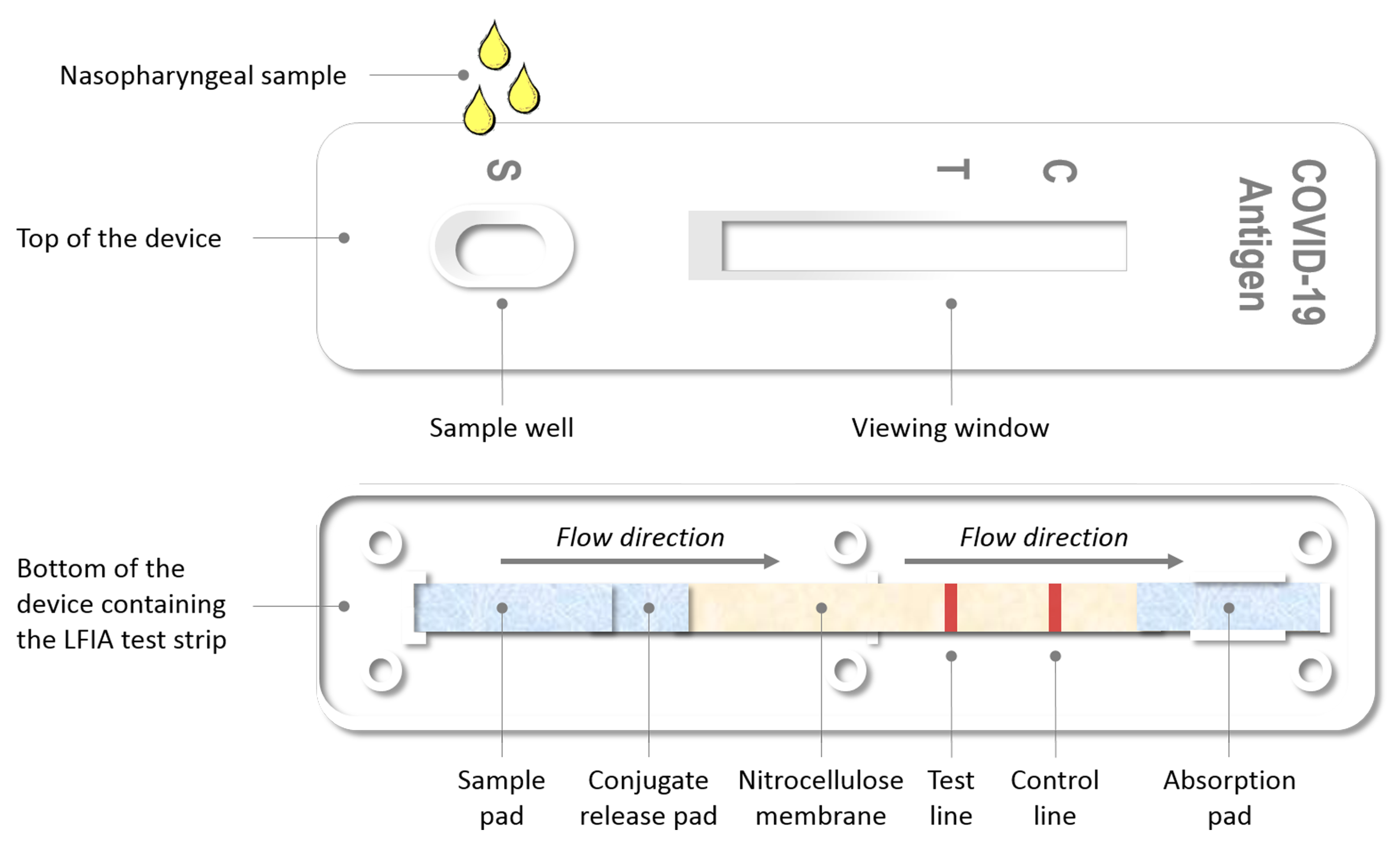
2.1. Foundations of the Evaluated Assays
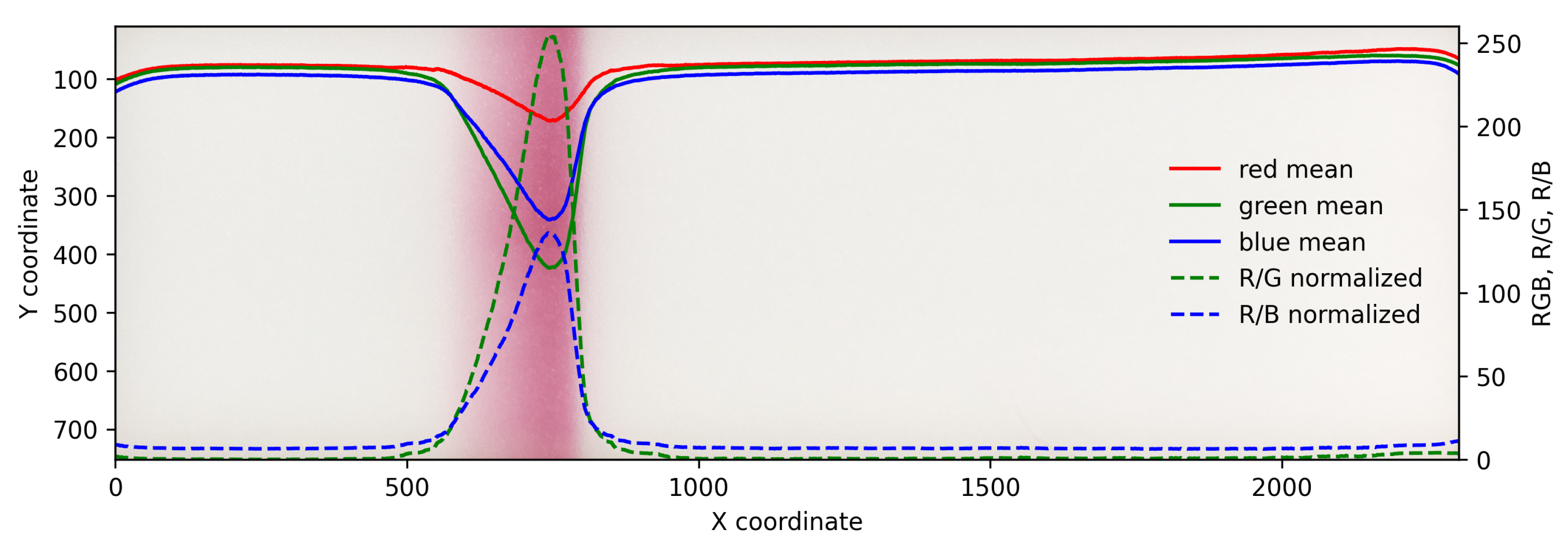
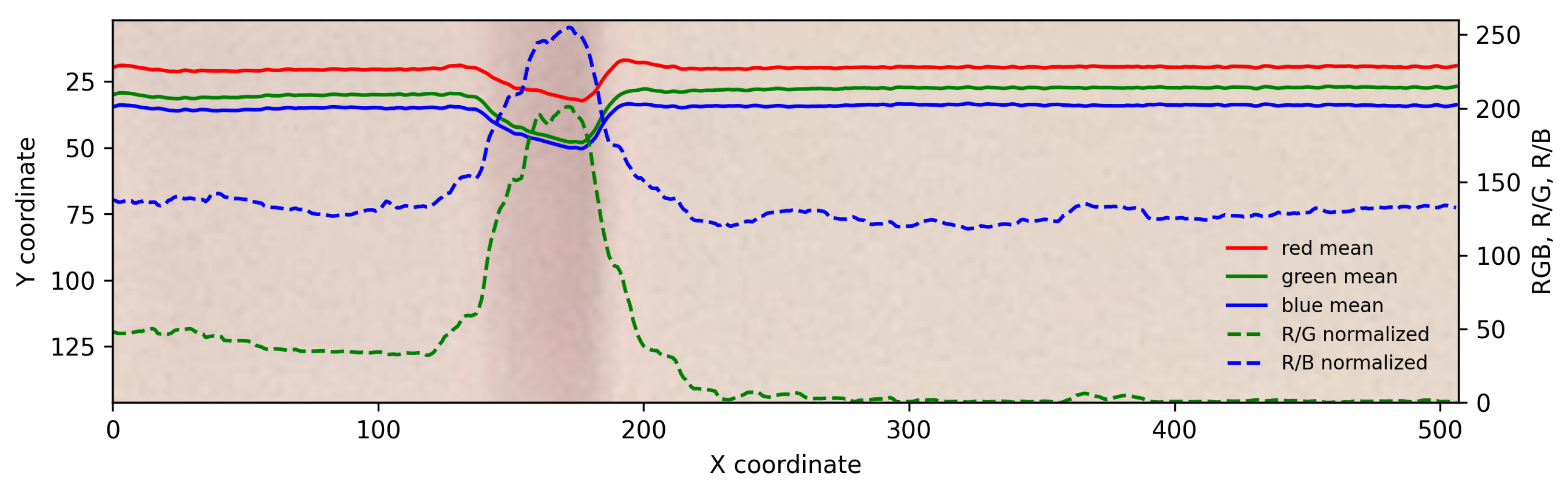
2.2. Sampling and Color Analysis
3. Results
3.1. Detection of Simulated Weak Test Lines
3.2. Detection of C Line
3.3. Case 1 Negative (Reference Case)
3.4. Case 2 False Negative—Very Weak Positive
3.5. Case 3 False Negative—Weak Positive
3.6. Case 4 Positive
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weitzel, T.; Legarraga, P.; Iruretagoyena, M.; Pizarro, G.; Vollrath, V.; Araos, R.; Munita, J.M.; Porte, L. Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples. BioRxiv 2020. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Gianfilippi, G.; Bragantini, D.; Henry, B.M.; Lippi, G. Clinical assessment of the Roche SARS-CoV-2 rapid antigen test. Diagnosis 2021, 8, 322–326. [Google Scholar] [CrossRef]
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Iruretagoyena, M.; Dittrich, S.; et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020, 99, 328–333. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Finco, G.; Demontis, R.; Fossarello, M.; d’Aloja, E. Fear of the COVID-19 and medical liability. Insights from a series of 130 consecutives medico-legal claims evaluated in a single institution during SARS-CoV-2-related pandemic. J. Anesth. Intens. Care Emerg. Pain Med. 2021, 17, 79–85. [Google Scholar]
- Sen-Crowe, B.; McKenney, M.; Elkbuli, A. Social distancing during the COVID-19 pandemic: Staying home save lives. Am. J. Emerg. Med. 2020, 38, 1519–1520. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M.; Bovo, C.; Sanchis-Gomar, F. Health risks and potential remedies during prolonged lockdowns for coronavirus disease 2019 (COVID-19). Diagnosis 2020, 7, 85–90. [Google Scholar] [CrossRef]
- Bostanci Ceran, B.; Karakoç, A.; Taciroğlu, E. Airborne pathogen projection during ophthalmic examination. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2275–2282. [Google Scholar] [CrossRef]
- Hobbs, J.E. Food supply chains during the COVID-19 pandemic. Can. J. Agric. Econ. Can. D’Agroecon. 2020, 68, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Pujawan, I.N.; Bah, A.U. Supply chains under COVID-19 disruptions: Literature review and research agenda. In Supply Chain Forum: An International Journal; Taylor & Francis: Abingdon, UK, 2021; pp. 1–15. [Google Scholar]
- Crozier, A.; Rajan, S.; Buchan, I.; McKee, M. Put to the test: Use of rapid testing technologies for COVID-19. BMJ 2021, 372, n208. [Google Scholar] [CrossRef]
- Eshghifar, N.; Busheri, A.; Shrestha, R.; Beqaj, S. Evaluation of analytical performance of seven rapid antigen detection kits for detection of SARS-CoV-2 virus. Int. J. Gen. Med. 2021, 14, 435. [Google Scholar] [CrossRef]
- Yang, R.; Gui, X.; Xiong, Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw. Open 2020, 3, e2010182. [Google Scholar] [CrossRef]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; Navarin, N.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020, 584, 425–429. [Google Scholar] [CrossRef]
- Khan, S.; Ali, A.; Shi, H.; Siddique, R.; Nabi, G.; Hu, J.; Wang, T.; Dong, M.; Zaman, W.; Han, G.; et al. COVID-19: Clinical aspects and therapeutics responses. Saudi Pharm. J. 2020, 28, 1004–1008. [Google Scholar] [CrossRef]
- Burki, T.K. Testing for COVID-19. Lancet Respir. Med. 2020, 8, e63–e64. [Google Scholar] [CrossRef]
- Nalumansi, A.; Lutalo, T.; Kayiwa, J.; Watera, C.; Balinandi, S.; Kiconco, J.; Nakaseegu, J.; Olara, D.; Odwilo, E.; Serwanga, J.; et al. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. Int. J. Infect. Dis. 2021, 104, 282–286. [Google Scholar] [CrossRef]
- Vashist, S.K. In vitro diagnostic assays for COVID-19: Recent advances and emerging trends. Diagnostics 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.F.; Selvam, K.; Jeffry, A.J.N.; Salmi, M.F.; Najib, M.A.; Norhayati, M.N.; Aziah, I. Performance of Rapid Antigen Tests for COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 110. [Google Scholar] [CrossRef]
- Jääskeläinen, A.; Ahava, M.J.; Jokela, P.; Szirovicza, L.; Pohjala, S.; Vapalahti, O.; Lappalainen, M.; Hepojoki, J.; Kurkela, S. Evaluation of three rapid lateral flow antigen detection tests for the diagnosis of SARS-CoV-2 infection. J. Clin. Virol. 2021, 137, 104785. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [Green Version]
- ECDC. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK; Technical Report; ECDC: Solna, Sweden, 2020. [Google Scholar]
- Kretzschmar, M.E.; Rozhnova, G.; Bootsma, M.C.; van Boven, M.; van de Wijgert, J.H.; Bonten, M.J. Impact of delays on effectiveness of contact tracing strategies for COVID-19: A modelling study. Lancet Public Health 2020, 5, e452–e459. [Google Scholar] [CrossRef]
- Thornton, J. Covid-19: Delays in getting tests are keeping doctors from work, health leaders warn. BMJ 2020, 370, m3755. [Google Scholar] [CrossRef]
- Merckx, J.; Wali, R.; Schiller, I.; Caya, C.; Gore, G.C.; Chartrand, C.; Dendukuri, N.; Papenburg, J. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: A systematic review and meta-analysis. Ann. Intern. Med. 2017, 167, 394–409. [Google Scholar] [CrossRef]
- Pray, I.W. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses—Wisconsin, September–October 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 69, 1642–1647. [Google Scholar] [CrossRef]
- Embrett, M.; Sim, M. At-Home COVID-19 Testing: A Rapid Scoping Review. 2021. Available online: https://sporevidencealliance.ca/wp-content/uploads/2021/05/At-home-COVID-19-testing_Full-Report_2021.02.12.pdf (accessed on 8 May 2021).
- CDC. Interim Guidance for Antigen Testing for SARS-CoV-2; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- Weitzel, T.; Legarraga, P.; Iruretagoyena, M.; Pizarro, G.; Vollrath, V.; Araos, R.; Munita, J.M.; Porte, L. Comparative evaluation of four rapid SARS-CoV-2 antigen detection tests using universal transport medium. Travel Med. Infect. Dis. 2021, 39, 101942. [Google Scholar] [CrossRef]
- European Commission. COVID-19 In Vitro Diagnostic Devices and Test Methods Database; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Ciotti, M.; Maurici, M.; Pieri, M.; Andreoni, M.; Bernardini, S. Performance of a rapid antigen test in the diagnosis of SARS-CoV-2 infection. J. Med. Virol. 2021, 93, 2988–2991. [Google Scholar] [CrossRef]
- Mendels, D.A.; Dortet, L.; Emeraud, C.; Oueslati, S.; Girlich, D.; Ronat, J.B.; Bernabeu, S.; Bahi, S.; Atkinson, G.J.; Naas, T. Using artificial intelligence to improve COVID-19 rapid diagnostic test result interpretation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019893118. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; International Version; W. H. Freeman: New York, NY, USA, 2002; pp. 921–950. [Google Scholar]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Hansson, J.; Yasuga, H.; Haraldsson, T.; Van der Wijngaart, W. Synthetic microfluidic paper: High surface area and high porosity polymer micropillar arrays. Lab Chip 2016, 16, 298–304. [Google Scholar] [CrossRef] [Green Version]
- ECDC. Options for the Use of Rapid Antigen Detection Tests for COVID-19 in the EU/EEA—First Update; Technical Report; ECDC: Solna, Sweden, 2021. [Google Scholar]
- Miikki, K.; Karakoç, A.; Rafiee, M.; Lee, D.W.; Vapaavuori, J.; Tersteegen, J.; Lemetti, L.; Paltakari, J. An open-source camera system for experimental measurements. SoftwareX 2021, 14, 100688. [Google Scholar] [CrossRef]
- Miikki, K. Raspberry Pi High Speed Camera. Available online: https://github.com/kmiikki/rpi-camera (accessed on 26 February 2022).
- Iancu, R.I.; Zară, A.D.; Mireștean, C.C.; Iancu, D.P.T. Radiomics in COVID-19: The Time for (R) evolution Has Came. BioMed 2022, 2, 60–68. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Henry, B.M.; Lippi, G. Making sense of rapid antigen testing in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnostics. Diagnosis 2021, 8, 27–31. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miikki, K.; Miikki, L.; Wiklund, J.; Karakoç, A. Augmented Sensitivity of At-Home Rapid SARS-CoV-2 Antigen Test (RAT) Kits with Computer Vision: A Framework and Proof of Concept. BioMed 2022, 2, 199-209. https://doi.org/10.3390/biomed2020018
Miikki K, Miikki L, Wiklund J, Karakoç A. Augmented Sensitivity of At-Home Rapid SARS-CoV-2 Antigen Test (RAT) Kits with Computer Vision: A Framework and Proof of Concept. BioMed. 2022; 2(2):199-209. https://doi.org/10.3390/biomed2020018
Chicago/Turabian StyleMiikki, Kim, Lars Miikki, Jenny Wiklund, and Alp Karakoç. 2022. "Augmented Sensitivity of At-Home Rapid SARS-CoV-2 Antigen Test (RAT) Kits with Computer Vision: A Framework and Proof of Concept" BioMed 2, no. 2: 199-209. https://doi.org/10.3390/biomed2020018
APA StyleMiikki, K., Miikki, L., Wiklund, J., & Karakoç, A. (2022). Augmented Sensitivity of At-Home Rapid SARS-CoV-2 Antigen Test (RAT) Kits with Computer Vision: A Framework and Proof of Concept. BioMed, 2(2), 199-209. https://doi.org/10.3390/biomed2020018





