Strength in Weakness: The Mutable Collagenous Tissue of Echinoderms
Abstract
1. Introduction
2. History of MCT Research
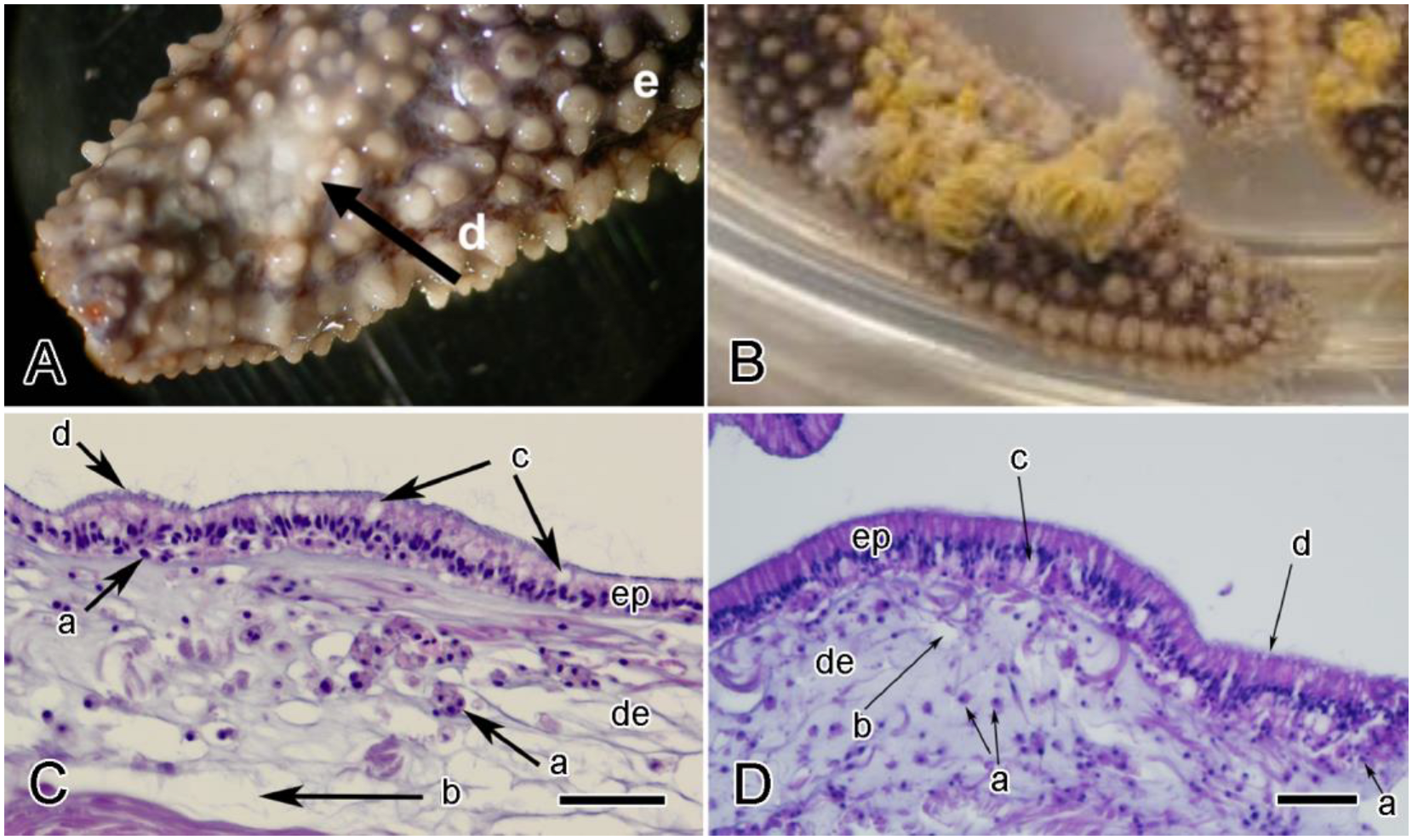
3. Anatomical Distribution of MCT
4. Organization of MCT
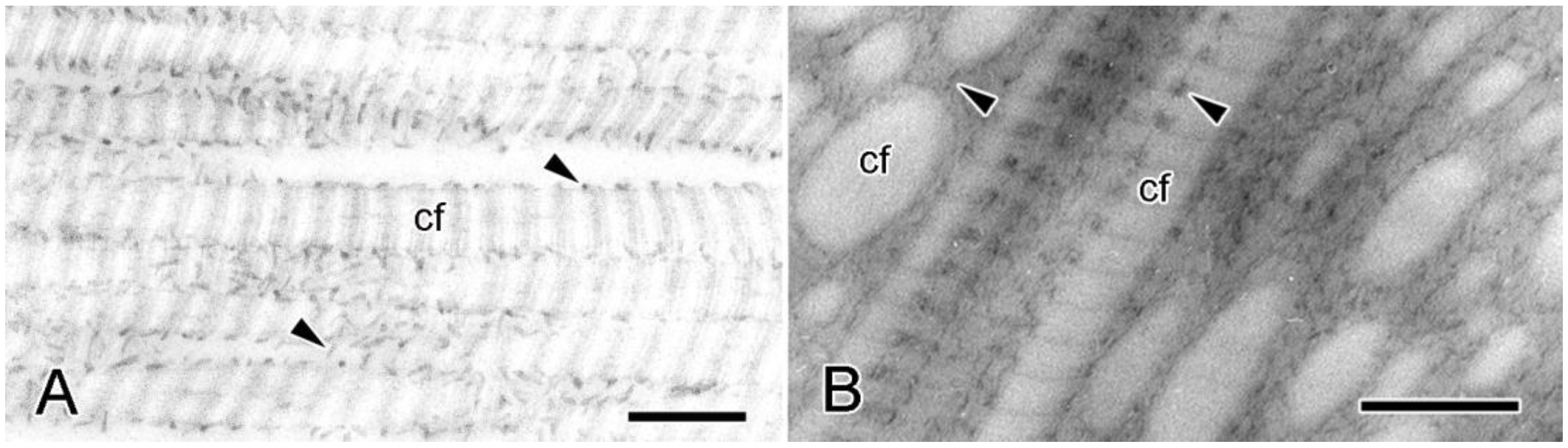
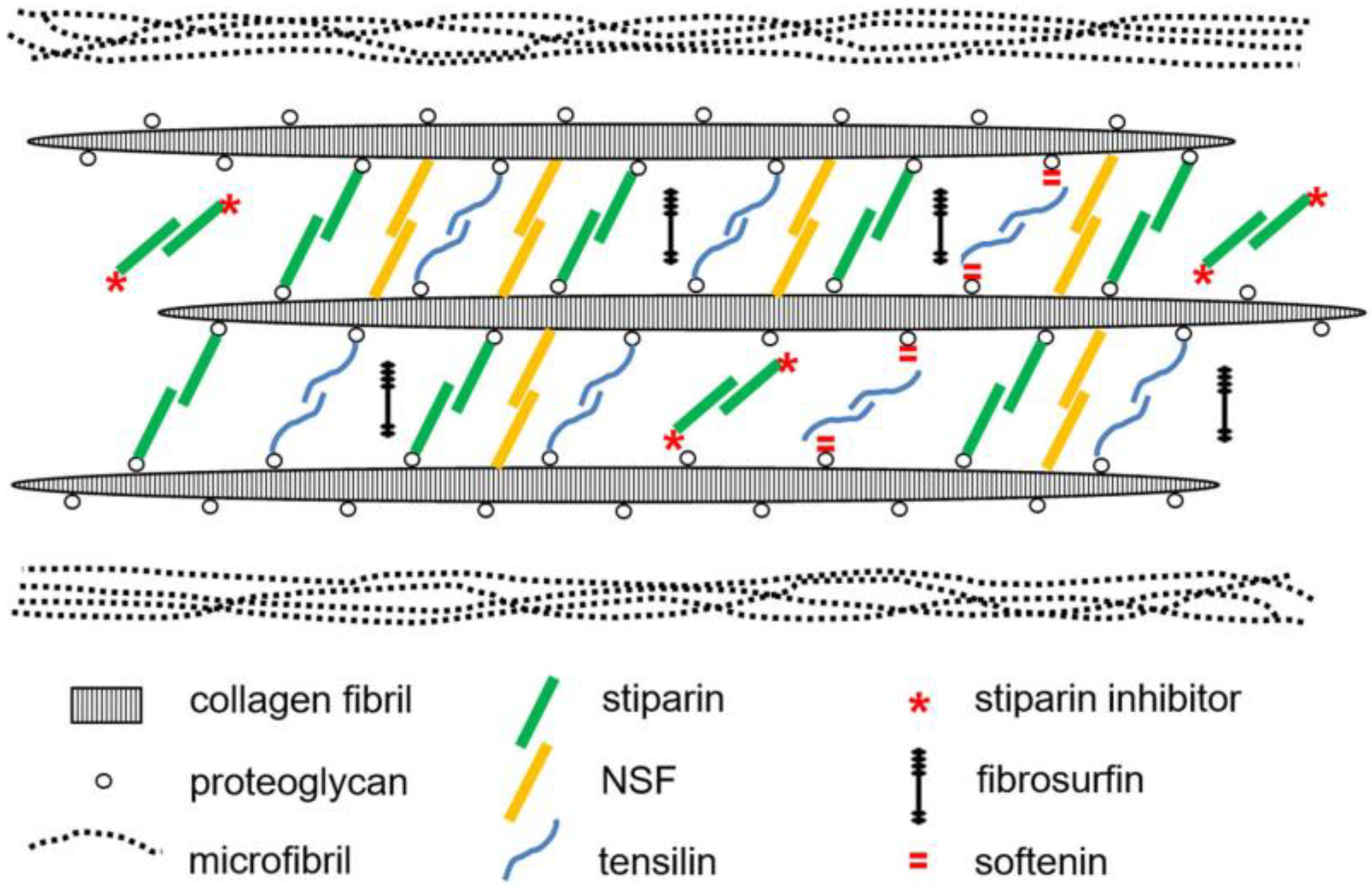
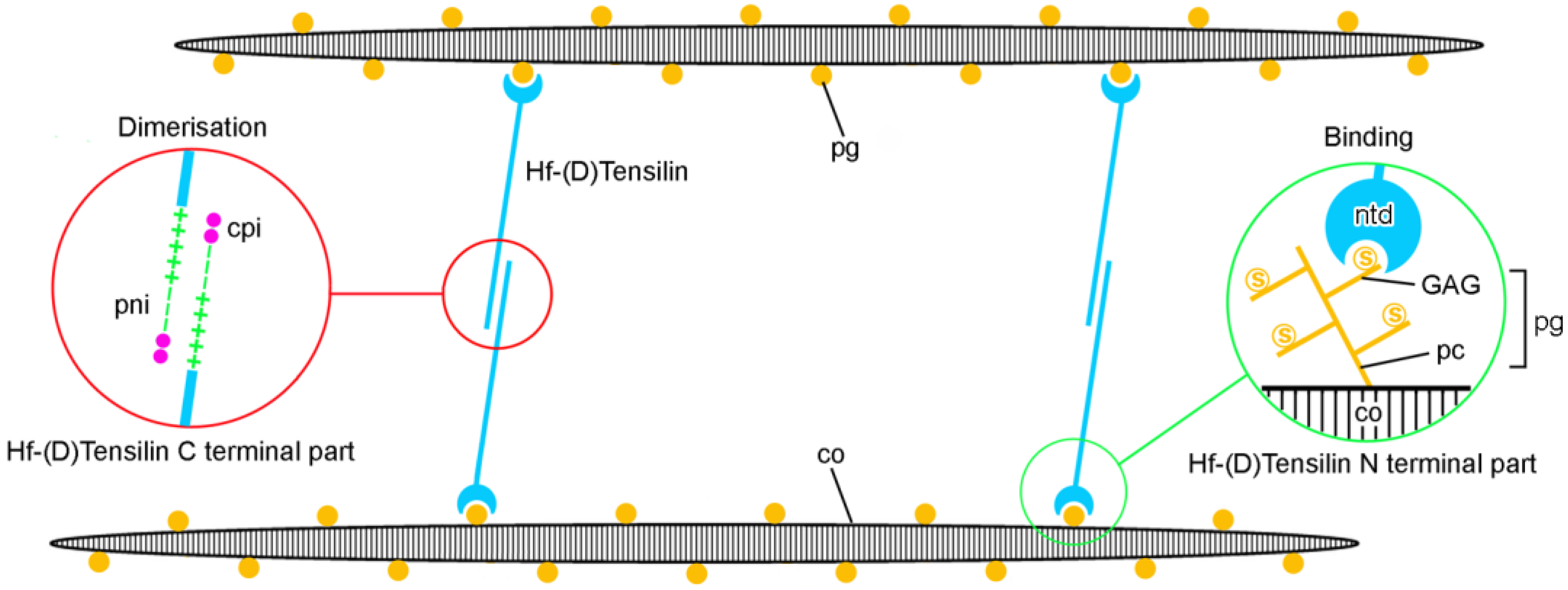
4.1. Extracellular Components
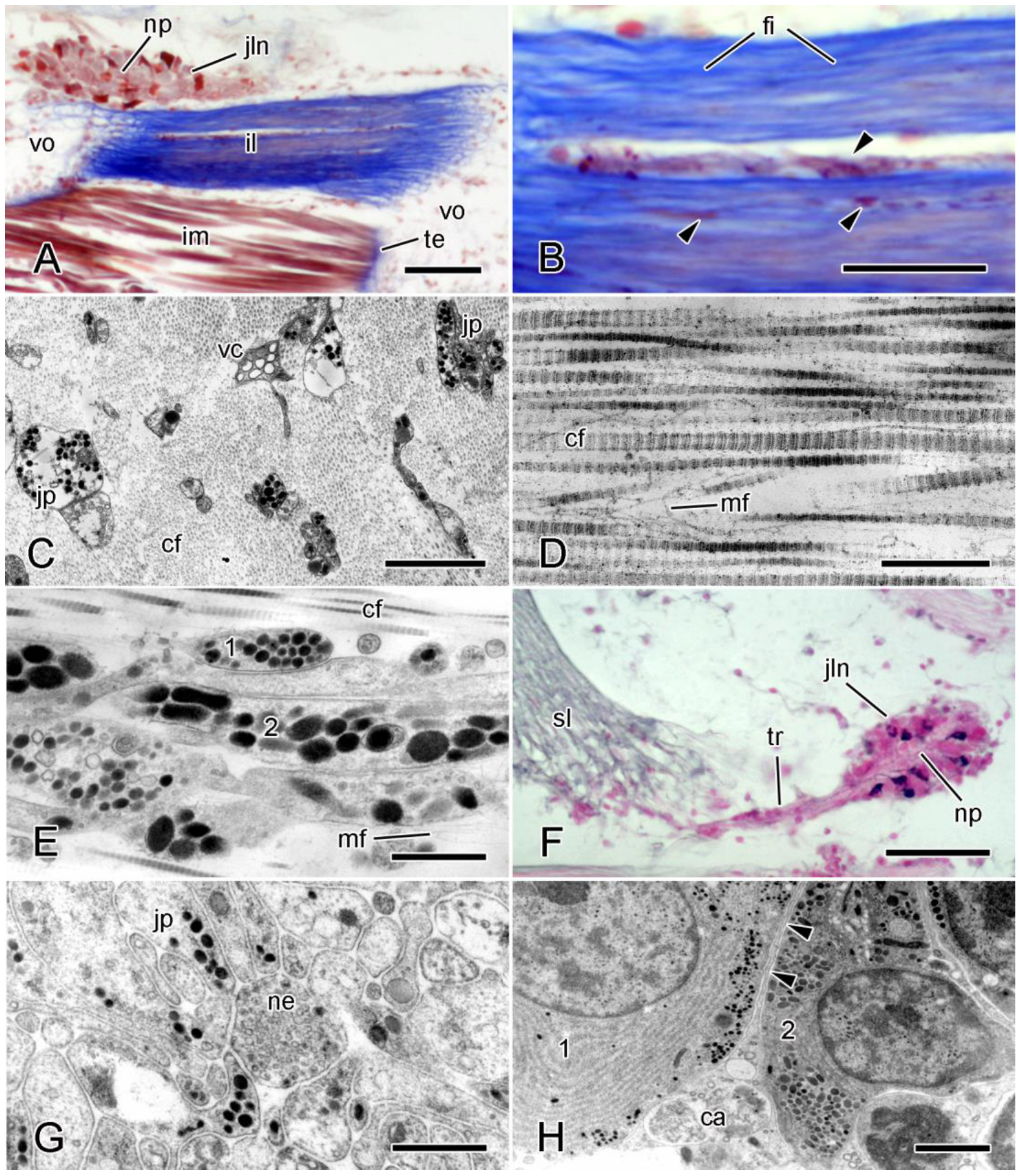
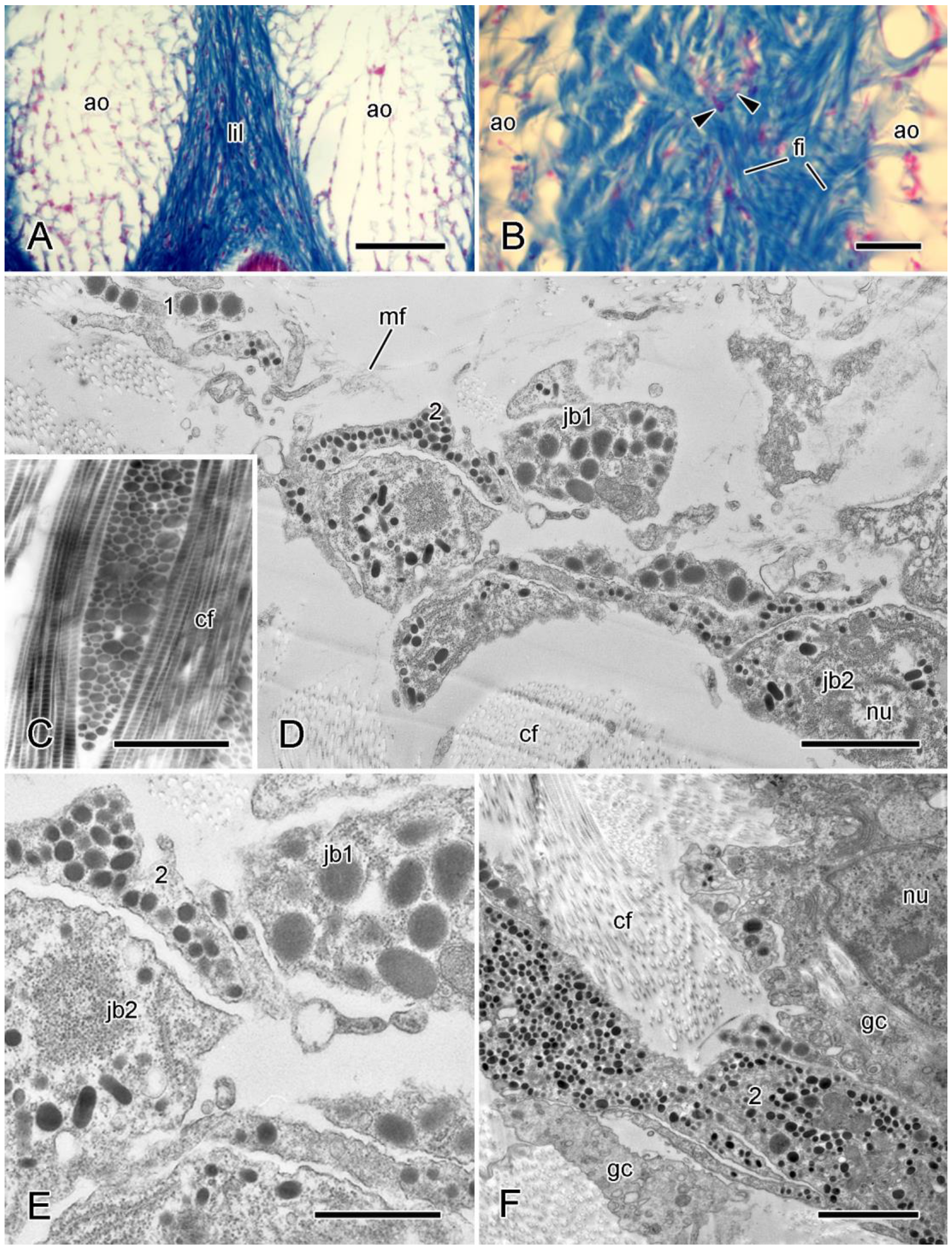
4.2. Cellular Components
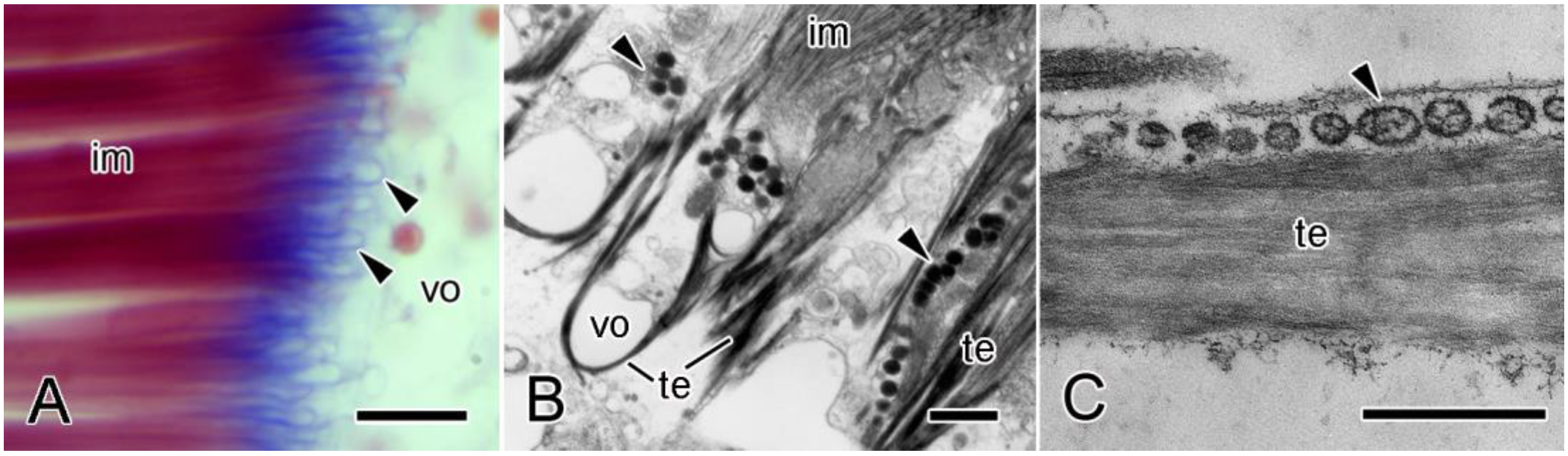
4.2.1. Juxtaligamental Cells
4.2.2. Other Cells
5. Physiology of MCT
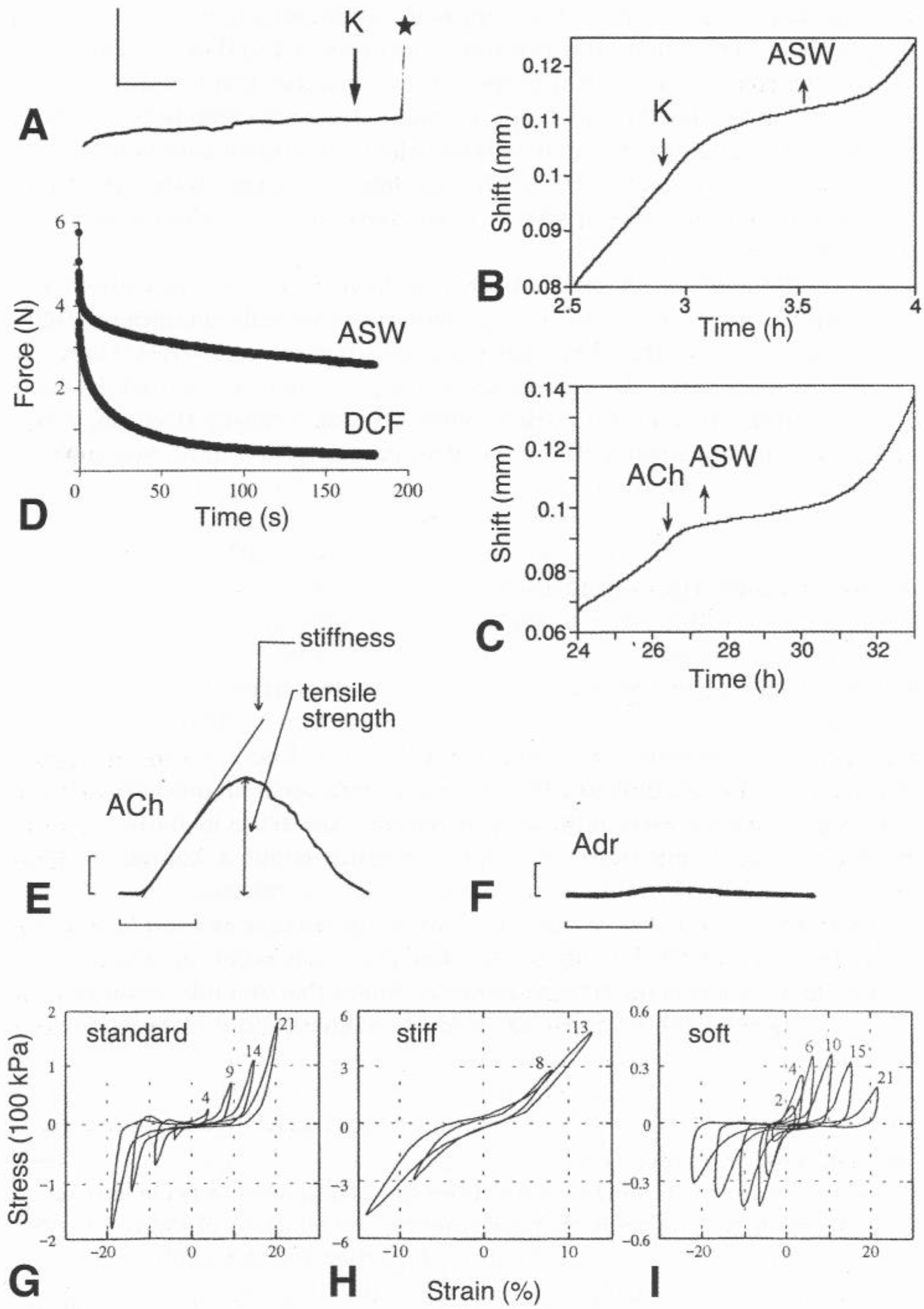
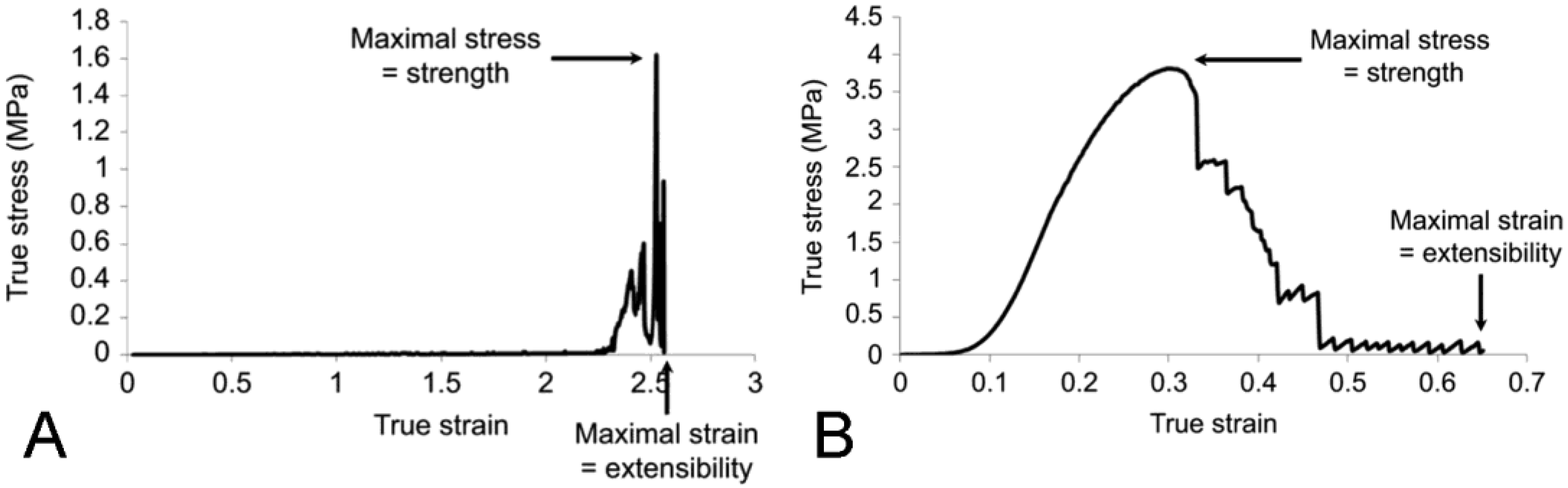
5.1. Passive Mechanical Properties
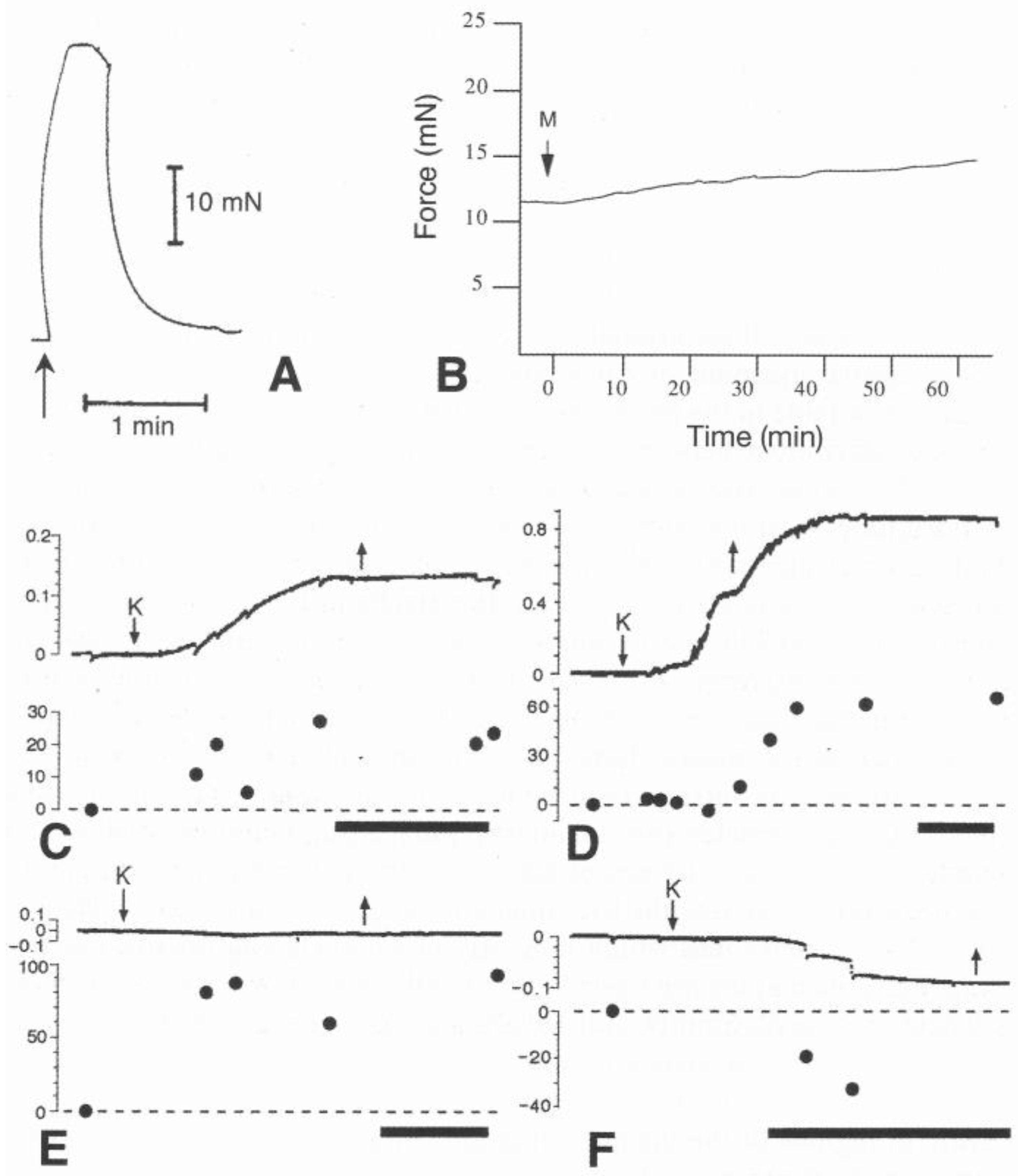
5.1.1. Baseline Mechanical Behavior
5.1.2. Tensile Change
5.2. Molecular Basis of Tensile Change
5.3. Regulation of Mechanical Properties
5.3.1. Juxtaligamental Cells
5.3.2. Nervous System
5.3.3. Neuropeptides and Other Peptides
5.3.4. Celomic Factors
5.4. Force Generation
6. Biological Significance of MCT
6.1. Energy-Sparing Postural Fixation
6.2. Autotomy and Fission
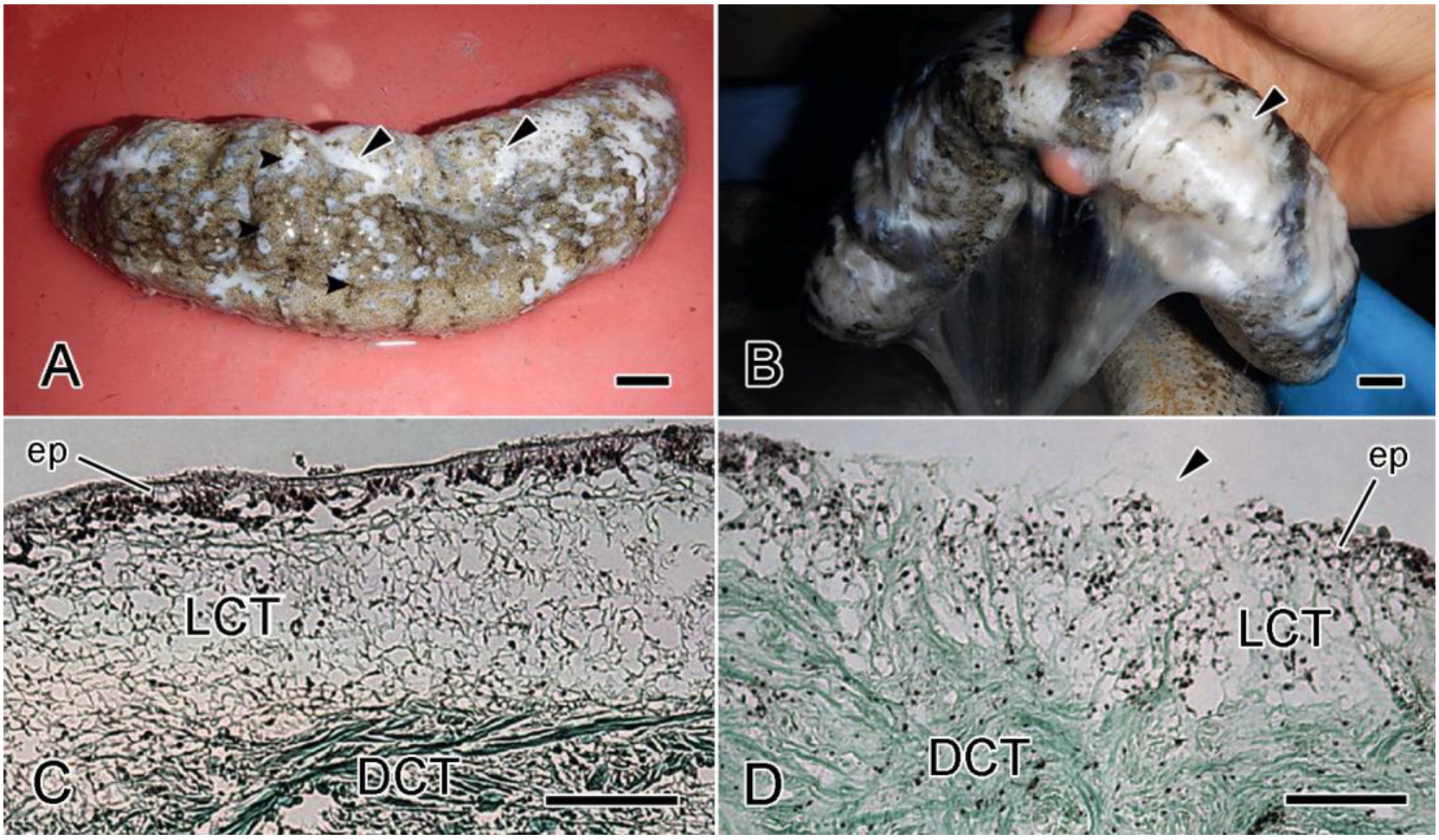
6.3. Sea-Cucumber Dermal Autolysis
6.4. Regeneration
6.5. Pathology
6.5.1. Sea Star Wasting Disease
6.5.2. Sea-Cucumber Skin Ulceration Disease
7. Applications
7.1. Biomimetic Applications Inspired by MCT
7.2. Biotechnological Applications Employing MCT Components
8. Gaps in Knowledge
8.1. Extracellular Components and Mechanisms of Tensile Change
8.2. Juxtaligamental Cells
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| APF | autotomy-promoting factor |
| BM | basement membrane |
| ECM | extracellular matrix |
| GAG | glycosaminoglycan |
| JLC | juxtaligamental cell |
| LDCV | large dense-core vesicle |
| LM | light micrograph |
| MCT | mutable collagenous tissue |
| MMP | matrix metalloproteinase |
| PM | peristomial membrane |
| SCDA | sea-cucumber dermal autolysis |
| SKUD | skin ulceration disease |
| SPM | supramolecular polymeric material |
| SSWD | sea star wasting disease |
| TEM | transmission electron micrograph |
| TIMP | tissue inhibitor of matrix metalloproteinase |
References
- Baccetti, B. Collagen and animal phylogeny. In Biology of Invertebrate and Lower Vertebrate Collagens; Bairati, A., Garrone, R., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 29–47. [Google Scholar]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Gonzales, N.L.; Beall, J.M.; Pechanec, M.Y. Basic structure, physiology, and biochemistry of connective tissues and extracellular matrix collagens. In Progress in Heritable Soft Connective Tissue Diseases, 2nd ed.; Halper, J., Ed.; Springer: Cham, Switzerland, 2021; pp. 5–43. [Google Scholar] [CrossRef]
- Rahman, M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Current Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef]
- Heinoa, J.; Huhtalab, M.; Käpyläa, J.; Johnson, M.S. Evolution of collagen-based adhesion systems. Int. J. Biochem. Cell Biol. 2009, 41, 341–348. [Google Scholar] [CrossRef]
- Rasmussen, C.H.; Petersen, D.R.; Moeller, J.B.; Hansson, M.; Dufva, M. Collagen type I improves the differentiation of human embryonic stem cells towards definitive endoderm. PLoS ONE 2015, 10, e0145389. [Google Scholar] [CrossRef]
- Wareham, L.K.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Collagen in the central nervous system: Contributions to neurodegeneration and promise as a therapeutic target. Mol. Neurodegener. 2024, 19, 11. [Google Scholar] [CrossRef]
- Langevin, H.M. Connective tissue: A body-wide signaling network? Med. Hypotheses 2006, 66, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Landis, W.J. Viscoelasticity, energy storage and transmission and dissipation by extracellular matrices in vertebrates. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer: New York, NY, USA, 2008; pp. 133–154. [Google Scholar]
- Jung, H.J.; Fisher, M.B.; Woo, S.L.Y. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med. Arthrosc. Rehab. Ther. Technol. 2009, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Matson, A.; Konow, N.; Miller, S.; Konow, P.P.; Roberts, T.J. Tendon material properties vary and are interdependent among turkey hindlimb muscles. J. Exp. Biol. 2012, 215, 3552–3558. [Google Scholar] [CrossRef]
- Wilkie, I.C. Basement membranes, brittlestar tendons, and their mechanical adaptability. Biology 2024, 13, 375. [Google Scholar] [CrossRef]
- Svensson, R.B.; Heinemeier, K.M.; Couppé, C.; Kjaer, M.; Magnusson, S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. 2016, 121, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Jayyosi, C.; Lee, N.; Mahendroo, M.; Myers, K.M. Mechanics of cervical remodelling: Insights from rodent models of pregnancy. Interface Focus 2019, 9, 20190026. [Google Scholar] [CrossRef] [PubMed]
- Khalilgharibi, N.; Mao, Y. To form and function: On the role of basement membrane mechanics in tissue development, homeostasis and disease. Open Biol. 2021, 11, 200360. [Google Scholar] [CrossRef]
- Candia Carnevali, M.D.; Sugni, M.; Bonasoro, F.; Wilkie, I.C. Mutable collagenous tissue: A concept generator for biomimetic materials and devices. Mar. Drugs 2024, 22, 37. [Google Scholar] [CrossRef]
- Motokawa, T. Skin of sea cucumbers: The smart connective tissue that alters mechanical properties in response to external stimuli. J. Aero Aqua Bio-Mech. 2019, 8, 2–5. [Google Scholar] [CrossRef]
- Tamori, M.; Yamada, A. Possible mechanisms of stiffness changes induced by stiffeners and softeners in catch connective tissue of echinoderms. Mar. Drugs 2023, 21, 140. [Google Scholar] [CrossRef]
- Sugi, H.; Ohno, T.; Moriya, M. Mechanism and function of the catch state in molluscan smooth muscle: A historical perspective. Int. J. Mol. Sci. 2020, 21, 7576. [Google Scholar] [CrossRef]
- Saleh, F.; Lefebvre, B.; Dupichaud, C.; Martin, E.L.O.; Nohejlová, M.; Spaccesi, L. Skeletal elements controlled soft-tissue preservation in echinoderms from the Early Ordovician Fezouata Biota. Geobios 2023, 81, 51–66. [Google Scholar] [CrossRef]
- Waters, J.A.; Bohatý, J.; Macurda, D.B. Feeding postures as indicators of mutable collagenous tissue in extinct echinoderms. Comm. Biol. 2024, 7, 1516. [Google Scholar] [CrossRef]
- Rahman, I.A.; Zamora, S. Origin and early evolution of echinoderms. Ann. Rev. Earth Planet. Sci. 2024, 52, 295–320. [Google Scholar] [CrossRef]
- Emson, R.H. Bone idle—A recipe for success? In Echinodermata; Keegan, B.F., O’Connor, B.D.S., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1985; pp. 25–30. [Google Scholar]
- Motokawa, T. Catch connective tissue: A key character for echinoderms’ success. In Echinoderm Biology; Burke, R.D., Mladenov, P.V., Lambert, P., Parsley, R.L., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1988; pp. 39–54. [Google Scholar]
- Wilkie, I.C.; Emson, R.H. Mutable collagenous tissues and their significance for echinoderm palaeontology and phylogeny. In Echinoderm Phylogeny and Evolutionary Biology; Paul, C.R.C., Smith, A.B., Eds.; Clarendon Press: Oxford, UK, 1988; pp. 311–330. [Google Scholar]
- Wollman, A.J.M.; Nudd, R.; Hedlund, E.G.; Leake, M.C. From Animaculum to single molecules: 300 years of the light microscope. Open Biol. 2015, 5, 150019. [Google Scholar] [CrossRef] [PubMed]
- Jickeli, C.F. Vorläufige Mittheilungen über den Bau der Echinodermen. Zool. Anz. 1884, 7, 346–349, 366–370. [Google Scholar]
- Perrier, E. Mémoire sur l’organisation et le développement de la comatule de la Méditerranée (Antedon rosacea Linck). Part 3. Organisation de l’Antedon adulte. I–IX. Nouv. Arch. Mus. D’Hist. Nat. Paris (3) 1889, 1, 169–286. [Google Scholar]
- Bosshard, H. Zur Kenntnis der Verbindungsweise der Skelettstücke der Arme und Ranken von Antedon rosacea Linck (Comatula mediterranea Lam.). Jena. Z. Naturw. 1900, 34, 65–112. [Google Scholar]
- Reichensperger, A. Beiträge zur Histologie und zum Verlauf der Regeneration bei Crinoiden. Z. Wiss. Zool. 1912, 101, 1–69. [Google Scholar]
- Lindemann, W. Über einige Eigenschaften der Holothurienhaut. Z. Biol. 1900, 39, 18–36. [Google Scholar]
- Jordan, H. Über ‘reflexarme’ Tiere. IV. Die Holothurien. 1 Mitteil. Die Reizbarkeit und der Einfluss des zentralen Nervensystems auf die Muskulatur und die muskulähnlichen Fasern der Haut (auf Erregbarkeit und Tonusfunktion). Zool. Jarhrb. Jena. Allg. Zool. Physiol. 1914, 34, 365–436. [Google Scholar]
- Jordan, H. Über ‘reflexarme’ Tiere. IV. Die Holothurien. 2 Mitteil. Die Holothurien als hohlorganartige Tiere und die Tonusfunktion ihrer Muskulatur. Zool. Jarhrb. Jena. Allg. Zool. Physiol. 1919, 36, 109–156. [Google Scholar]
- von Uexküll, J. Die Sperrmuskulatur der Holothurien. Pflüg. Arch. Ges. Physiol. 1926, 212, 1–14. [Google Scholar] [CrossRef]
- von Uexküll, J. Die Physiologie des Seeigelstachels. Z. Biol. 1900, 39, 73–112. [Google Scholar]
- Hamann, O. Beiträge zur Histologie der Echinodermen. Jena. Z. Med. Naturw. 1887, 21, 87–266. [Google Scholar]
- Prouho, H. Recherches sur le Dorocidaris papillata et quelques autres échinides de la Méditerranée. Arch. Zool. Exp. Gén. 1887, 5, 213–380. [Google Scholar]
- Serra-von Buddenbrock, E. Etudes physiologiques et histologiques sur le tégument des holothuries (Holothuria tubulosa). Vie Milieu 1963, 14, 55–70. [Google Scholar]
- Takahashi, K. The catch apparatus of the sea-urchin spine I. Gross histology. J. Fac. Sci. Univ. Tokyo Sec. IV 1967, 11, 110–120. [Google Scholar]
- Takahashi, K. The catch apparatus of the sea-urchin spine II. Responses to stimuli. J. Fac. Sci. Univ. Tokyo Sec. IV 1967, 11, 122–130. [Google Scholar]
- Meyer, D.L. The collagenous nature of problematical ligaments in crinoids (Echinodermata). Mar. Biol. 1971, 9, 235–241. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Sugni, M.; Gupta, H.S.; Candia Carnevali, M.D.; Elphick, M.R. The mutable collagenous tissue of echinoderms: From biology to biomedical applications. In Soft Matter for Biomedical Applications; Azevedo, H.S., Mano, J.F., Borges, J., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 3–33. [Google Scholar]
- Bonneel, M.; Hennebert, E.; Aranko, A.S.; Hwang, D.S.; Lefevre, M.; Pommier, V.; Wattiez, R.; Delroisse, J.; Flammang, P. Molecular mechanisms mediating stiffening in the mechanically adaptable connective tissues of sea cucumbers. Matrix Biol. 2022, 108, 39–54. [Google Scholar] [CrossRef]
- Barbieri, E.; Gupta, H.S. Is stress relaxation in sea cucumber dermis chemoelastic? Mar. Drugs 2023, 21, 610. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D. The juxtaligamental cells of echinoderms and their role in the mechano-effector function of connective tissue. In Frontiers in Invertebrate Physiology: A Collection of Reviews, Volume 3: Annelida and Echinodermata; Saleuddin, A.S., Leys, S., Roer, R., Wilkie, I.C., Eds.; Apple Academic Press: Point Pleasant, NJ, USA, 2024; pp. 345–430. [Google Scholar]
- Nouri, R.; Mashanov, V.; Harris, A.; New, G.; Taylor, W.; Janies, D.; Reid, R.W.; Machado, D.J. Unveiling putative modulators of mutable collagenous tissue in the brittle star Ophiomastix wendtii: An RNA-Seq analysis. BMC Genom. 2024, 25, 1013. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and bioactive natural products from marine invertebrates: From basic research to innovative applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef]
- Atanassova, M.R.; Kolden Midtbø, L.; Mildenberger, J.; Friðjónsson, Ó.H. Novel biomaterials and biotechnological applications derived from North Atlantic sea cucumbers: A systematic review. In The World of Sea Cucumbers; Mercier, A., Hamel, J.F., Suhrbier, A., Pearce, C., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 585–609. [Google Scholar] [CrossRef]
- Raman; Labisch, S.; Dirks, J.H. A starfish-inspired 4D self-healing morphing structure. Sci. Rep. 2024, 14, 22024. [Google Scholar] [CrossRef]
- Wilkie, I.C. Arm autotomy in brittlestars. J. Zool. Lond. 1978, 186, 311–330. [Google Scholar] [CrossRef]
- Motokawa, T. Rapid change in mechanical properties of echinoderm connective tissues caused by coelomic fluid. Comp. Biochem. Physiol. C 1982, 73, 223–229. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Griffiths, G.V.R.; Glennie, S.F. Morphological and physiological aspects of the autotomy plane in the aboral integument of Asterias rubens L. (Echinodermata). In Echinoderm Research; De Ridder, C., Dubois, P., Lahaye, M., Jangoux, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1990; pp. 301–313. [Google Scholar]
- Wilkie, I.C.; Emson, R.H.; Mladenov, P.V. Autotomy mechanism and its control in the starfish Pycnopodia helianthoides (Brandt). In Echinoderm Research 1995; Emson, R.H., Smith, A.B., Campbell, A.C., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1995; pp. 137–146. [Google Scholar]
- Santos, R.; Haesaerts, D.; Jangoux, M.; Flammang, P. The tube feet of sea urchins and sea stars contain functionally different mutable collagenous tissues. J. Exp. Biol. 2005, 208, 2277–2288. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, I.C. Nervously mediated change in the mechanical properties of the cirral ligaments of a crinoid. Mar. Behav. Physiol. 1983, 9, 229–248. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D.; Bonasoro, F. Evidence for the ‘cellular regulation hypothesis’ from ‘simple’ mutable collagenous structures: The brachial and cirral syzygial ligaments of Antedon mediterranea (Lam.). In Echinoderm Research 1998; Candia Carnevali, M.D., Bonasoro, F., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1999; pp. 119–125. [Google Scholar]
- Wilkie, I.C.; Emson, R.H.; Young, C.M. Variable tensility of the ligaments in the stalk of a sea-lily. Comp. Biochem. Physiol. A 1994, 109, 633–641. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Barbaglio, A.; Maclaren, W.M.; Candia Carnevali, M.D. Physiological and immunocytochemical evidence that glutamatergic neurotransmission is involved in the activation of arm autotomy in the featherstar Antedon mediterranea (Echinodermata: Crinoidea). J. Exp. Biol. 2010, 213, 2104–2115. [Google Scholar] [CrossRef]
- Bobrovskaya, N.V.; Dolmatov, I.Y. Autotomy of the visceral mass in the feather star Himerometra robustipinna (Crinoidea, Comatulida). Biol. Bull. 2014, 226, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, M.; Takahashi, K. Fine structure and mechanical properties of the catch apparatus of the sea-urchin spine, a collagenous connective tissue with muscle-like holding capacity. J. Exp. Biol. 1983, 103, 1–14. [Google Scholar] [CrossRef]
- Rodríguez-Barreras, R.; Sabat, A.M. Evaluation of three tagging methods in the sea urchin Diadema antillarum. J. Mar. Biol. Ass. UK 2015, 95, 1255–1260. [Google Scholar] [CrossRef]
- Motokawa, T. Mechanical properties and structure of the spine-joint central ligament of the sea urchin, Diadema setosum (Echinodermata, Echinoidea). J. Zool. Lond. 1983, 201, 223–235. [Google Scholar] [CrossRef]
- Birenheide, R.; Tsuchi, A.; Motokawa, T. To be stiff or to be soft—The dilemma of the echinoid tooth ligament. II. Mechanical properties. Biol. Bull. 1996, 190, 231–236. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D.; Bonasoro, F. The compass depressors of Paracentrotus lividus (Echinodermata: Echinoida): Ultrastructural and mechanical aspects of their variable tensility and contractility. Zoomorphology 1992, 112, 142–153. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D.; Andrietti, F. Variable tensility of the peristomial membrane of the sea-urchin Paracentrotus lividus (Lamarck). Comp. Biochem. Physiol. A 1993, 105, 493–501. [Google Scholar] [CrossRef]
- Seagar, L. The Mechanical Properties of the Sea-Urchin Periproct. Bachelor’s Thesis, Glasgow Caledonian University, Glasgow, Scotland, 1994. [Google Scholar]
- Bretschneider, A.; Pomory, C.M. Autotomy of globiferous pedicellariae in the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Gulf. Carib. Res. 2024, 35, 16–22. [Google Scholar] [CrossRef]
- Motokawa, T. The stiffness change of the holothurian dermis caused by chemical and electrical stimulation. Comp. Biochem. Physiol. C 1981, 73, 41–48. [Google Scholar] [CrossRef]
- Byrne, M. The mechanical properties of the autotomy tissues of the holothurian Eupentacta quinquesemita and the effects of certain physico-chemical agents. J. Exp. Biol. 1985, 117, 69–86. [Google Scholar] [CrossRef]
- Demeuldre, M.; Hennebert, E.; Bonneel, M.; Lengerer, B.; Van Dyck, S.; Wattiez, R.; Ladurner, P.; Flammang, P. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J. Exp. Biol. 2017, 220, 2108–2119. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Emson, R.H.; Mladenov, P.V. Morphological and mechanical aspects of fission in Ophiocomella ophiactoides. Zoomorphology 1984, 104, 310–322. [Google Scholar] [CrossRef]
- Motokawa, T. Connective tissue catch in echinoderms. Biol. Rev. 1984, 59, 255–270. [Google Scholar] [CrossRef]
- Wilkie, I.C. Design for disaster: The ophiuroid intervertebral ligament as a typical mutable collagenous structure. In Echinoderm Biology; Burke, R.D., Mladenov, P.V., Lambert, P., Parsley, R.L., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1988; pp. 25–38. [Google Scholar]
- Wilkie, I.C.; Emson, R.H. The tendons of Ophiocomina nigra and their role in autotomy (Echinodermata, Ophiuroida). Zoomorphology 1987, 107, 33–44. [Google Scholar] [CrossRef]
- Wilkie, I.C. Variable tensility of the oral arm plate ligaments of the brittlestar Ophiura ophiura (Echinodermata: Ophiuroidea). J. Zool. Lond. 1992, 228, 5–26. [Google Scholar] [CrossRef]
- Dobson, W.E.; Turner, R.L. Morphology and histology of the disc autotomy plane in Ophiophragmus filograneus (Echinodermata, Ophiurida). Zoomorphology 1989, 108, 323–332. [Google Scholar] [CrossRef]
- Wilkie, I.C. Functional morphology of the arm spine joint and adjacent structures of the brittlestar Ophiocomina nigra (Echinodermata: Ophiuroidea). PLoS ONE 2016, 11, e0167533. [Google Scholar] [CrossRef]
- Candia Carnevali, M.D.; Bonasoro, F.; Wilkie, I.C. Structural and mechanical aspects of the mouth-frame of the brittlestar Ophioderma longicaudum (Retz.). In Echinoderms through Time; David, B., Guille, A., Féral, J.P., Roux, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1988; pp. 387–392. [Google Scholar]
- Wilkie, I.C.; McKew, M.; Candia Carnevali, M.D. Functional morphology of the compass-rotular ligament of Echinus esculentus (Echinodermata: Echinoida): A non-mutable collagenous component of Aristotle’s lantern. Zoomorphology 2005, 124, 9–26. [Google Scholar] [CrossRef]
- Del Castillo, J.; Smith, D.S.; Vidal, A.M.; Sierra, C. Catch in the primary spines of the sea urchin Eucidaris tribuloides: A brief review and a new interpretation. Biol. Bull. 1995, 188, 120–127. [Google Scholar] [CrossRef]
- Smith, D.S.; del Castillo, J.; Morales, M.; Luke, B. The attachment of collagenous ligament to stereom in primary spine of the sea-urchin Eucidaris tribuloides. Tissue Cell 1990, 22, 157–176. [Google Scholar] [CrossRef]
- O’Neill, P.L. Structure and mechanics of starfish body wall. J. Exp. Biol. 1989, 147, 53–89. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D.; Andrietti, F. Microarchitecture and mechanics of the sea-urchin peristomial membrane. Boll. Zool. 1994, 61, 39–51. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: Structure and Biomechanics; Springer: Berlin, Germany, 2008. [Google Scholar]
- Wilkie, I.C.; Candia Carnevali, M.D. Morphological and physiological aspects of mutable collagenous tissue at the autotomy plane of the starfish Asterias rubens L. (Echinodermata, Asteroidea): An echinoderm paradigm. Mar. Drugs 2023, 21, 138. [Google Scholar] [CrossRef]
- Cluzel, C.; Lethias, C.; Garrone, R.; Exposito, J.-Y. Distinct maturations of N-propeptide domains in fibrillar procollagen molecules involved in the formation of heterotypic fibrils in adult sea urchin collagenous tissues. J. Biol. Chem. 2004, 279, 9811–9817. [Google Scholar] [CrossRef]
- Cui, F.; Xue, C.; Li, Z.; Zhang, Y.; Dong, P.; Fu, X.; Gao, X. Characterization and subunit composition of collagen from the body wall of sea cucumber Stichopus japonicus. Food Chem. 2007, 100, 1120–1125. [Google Scholar] [CrossRef]
- Tian, M.; Xue, C.; Chang, Y.; Shen, J.; Zhang, Y.; Li, Z.; Wang, Y. Collagen fibrils of sea cucumber (Apostichopus japonicus) are heterotypic. Food Chem. 2020, 316, 126272. [Google Scholar] [CrossRef]
- Hulmes, D.J.S. Collagen diversity, synthesis and assembly. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 15–47. ISBN 978-0-387-73906-9. [Google Scholar]
- Bisgrove, B.W.; Andrews, M.E.; Raff, R.A. Fibropellins, products of an EGF repeat-containing gene, form a unique extracellular matrix structure that surrounds the sea-urchin embryo. Dev. Biol. 1991, 146, 89–99. [Google Scholar] [CrossRef]
- Cluzel, C.; Lethias, C.; Humbert, F.; Garronne, R.; Exposito, J.-Y. Characterization of fibrosurfin, an interfibrillar component of sea urchin catch connective tissues. J. Biol. Chem. 2001, 276, 18108–18114. [Google Scholar] [CrossRef]
- Dolmatov, I.Y.; Afanasyev, S.V.; Boyko, A.V. Molecular mechanisms of fission in echinoderms: Transcriptome analysis. PLoS ONE 2018, 13, e0195836. [Google Scholar] [CrossRef]
- Sea Urchin Genome Sequencing Consortium; Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef]
- Whittaker, C.A.; Bergeron, K.-F.; Whittle, J.; Brandhorst, B.P.; Burke, R.D.; Hynes, R.O. The echinoderm adhesome. Dev. Biol. 2006, 300, 252–266. [Google Scholar] [CrossRef]
- Dolmatov, I.Y.; Nizhnichenko, V.A. Extracellular matrix of echinoderms. Mar. Drugs 2023, 21, 417. [Google Scholar] [CrossRef]
- Shi, F.; Liu, K.; Chen, G.; Chang, Y.; Xue, C. Investigation of the presence of fibrillin in sea cucumber (Apostichopus japonicus) body wall by utilizing targeted proteomics and visualization strategies. J. Agr. Food Chem. 2024, 72, 8798–8804. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Barbaglio, A.; Benedetto, C.D.; Ribeiro, C.C.; Wilkie, I.C.; Candia Carnevali, M.D.; Barbosa, M.A. New insights into mutable collagenous tissue: Correlations between the microstructure and mechanical state of a sea-urchin ligament. PLoS ONE 2011, 6, e24822. [Google Scholar] [CrossRef]
- Wilkie, I.C. Mutable collagenous tissue: Overview and biotechnological perspective. In Echinodermata (Progress in Molecular and Subcellular Biology 39); Matranga, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 221–250. ISBN 978-3-540-24402-8. [Google Scholar] [CrossRef]
- Erlinger, R.; Welsch, U.; Scott, J.E. Ultrastructural and biochemical observations on proteoglycans and collagen in the mutable connective tissue of the feather star Antedon bifida (Echinodermata, Crinoidea). J. Anat. 1993, 183, 1–11. [Google Scholar]
- Wang, J.; Chang, Y.; Wu, F.; Xu, X.; Xue, C. Fucosylated chondroitin sulfate is covalently associated with collagen fibrils in sea cucumber Apostichopus japonicus body wall. Carbohyd. Polym. 2018, 186, 439–444. [Google Scholar] [CrossRef]
- Felix, A.L.; Penno, S.M.; Bezerra, F.F.; Mourão, P.A.S. Fucosylated chondroitin sulfate, an intriguing polysaccharide from sea cucumber: Past, present, and future. Glycobiology 2025, 35, cwae098. [Google Scholar] [CrossRef]
- Exposito, J.Y.; D’Alessio, M.; Di Liberto, M.; Ramirez, F. Complete primary structure of a sea urchin type IV collagen α chain and analysis of the 5’ end of its gene. J. Biol. Chem. 1993, 268, 5249–5254. [Google Scholar] [CrossRef]
- Bechtel, M.; Keller, M.V.; Bloch, W.; Sasaki, T.; Boukamp, P.; Zaucke, F.; Paulsson, M.; Nischt, R. Different domains in nidogen-1 and nidogen-2 drive basement membrane formation in skin organotypic cocultures. FASEB J. 2012, 26, 3637–3648. [Google Scholar] [CrossRef]
- Hohenester, E.; Yurchenco, P.D. Laminins in basement membrane assembly. Cell Adhes. Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef]
- Wilkie, I.C. The juxtaligamental cells of Ophiocomina nigra and their possible role in mechano-effector function of collagenous tissue. Cell Tissue Res. 1979, 197, 515–530. [Google Scholar] [CrossRef]
- Motokawa, T. Fine structure of the dermis of the body wall of the sea cucumber, Stichopus chloronotus, a connective tissue which changes its mechanical properties. Galaxea 1982, 1, 55–64. [Google Scholar]
- Koob, T.J.; Koob-Emunds, M.M.; Trotter, J.A. Cell-derived stiffening and plasticizing factors in sea cucumber (Cucumaria frondosa) dermis. J. Exp. Biol. 1999, 202, 2291–2301. [Google Scholar] [CrossRef]
- Byrne, M. The morphology of autotomy structures in the sea cucumber Eupentacta quinquesemita before and during evisceration. J. Exp. Biol. 2001, 204, 848–863. [Google Scholar] [CrossRef]
- Byrne, M. Morphological, physiological and mechanical features of the mutable collagenous tissues associated with autotomy and evisceration in dendrochirotid holothuroids. Mar. Drugs 2023, 21, 134. [Google Scholar] [CrossRef]
- Chia, F.S.; Koss, R. Asteroidea. In Microscopic Anatomy of Invertebrates, Volume 14. Echinodermata; Harrison, F.W., Chia, F.S., Eds.; Wiley-Liss: New York, NY, USA, 1994; pp. 169–245. [Google Scholar]
- Byrne, M. Ophiuroidea. In Microscopic Anatomy of Invertebrates, Volume 14. Echinodermata; Harrison, F.W., Chia, F.S., Eds.; Wiley-Liss: New York, NY, USA, 1994; pp. 247–343. [Google Scholar]
- Hennebert, E.; Haesaerts, D.; Dubois, P.; Flammang, P. Evaluation of the different forces brought into play during tube foot activities in sea stars. J. Exp. Biol. 2010, 213, 1162–1174. [Google Scholar] [CrossRef]
- Welsch, U.; Heinzeller, T.; Cobb, J.L.S. Ultrastructure and innervation of ligament connective tissue in Antedon bifida and Decametra sp. In Echinoderm Research; De Ridder, C., Dubois, P., LaHaye, M.C., Jangoux, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1990; pp. 295–300. [Google Scholar]
- Grimmer, J.C.; Holland, N.D.; Hayami, I. Fine structure of the stalk of an isocrinid sea lily (Metacrinus rotundus) (Echinodermata, Crinoidea). Zoomorphologie 1985, 105, 39–50. [Google Scholar] [CrossRef]
- Smith, D.S.; Wainwright, S.A.; Baker, J.; Cayer, M.L. Structural features associated with movement and ‘catch’ of sea-urchin spines. Tissue Cell 1981, 13, 299–320. [Google Scholar] [CrossRef]
- Welsch, U.; Heinzeller, T.; Cobb, J.L.S. Histochemical and finestructural observations on the nervous tissue of Antedon bifida and Decametra spec. (Echinodermata: Crinoidea). Biomed. Res. 1989, 10, 145–154. [Google Scholar]
- Zueva, O.; Khoury, M.; Heinzeller, T.; Mashanova, D.; Mashanov, V. The complex simplicity of the brittle star nervous system. Front. Zool. 2018, 15, 1. [Google Scholar] [CrossRef]
- Kreiner, T.; Sossin, W.; Scheller, R.H. Localization of Aplysia neurosecretory peptides to multiple populations of dense core vesicles. J. Cell Biol. 1986, 102, 769–782. [Google Scholar] [CrossRef]
- Plattner, H.; Artalejo, A.R.; Neher, E. Ultrastructural organization of bovine chromaffin cell cortex—Analysis by cryofixation and morphometry of aspects pertinent to exocytosis. J. Cell Biol. 1997, 139, 1709–1717. [Google Scholar] [CrossRef]
- Smith, C.L.; Varoqueaux, F.; Kittelmann, M.; Azzam, R.N.; Cooper, B.; Winters, C.A.; Eitel, M.; Fasshauer, D.; Reese, T.S. Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Current Biol. 2014, 24, 1565–1572. [Google Scholar] [CrossRef]
- Welsch, U.; Lange, A.; Bals, R.; Heinzeller, T. Juxtaligamental cells in feather stars and isocrinids. In Echinoderm Research 1995; Emson, R.H., Smith, A.B., Campbell, A.C., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1995; pp. 129–135. [Google Scholar]
- Peters, B.H. The innervation of spines in the sea-urchin Echinus esculentus L. An electron-microscopic study. Cell Tissue Res. 1985, 239, 219–228. [Google Scholar] [CrossRef]
- Birenheide, R.; Motokawa, T. To be stiff or to be soft—The dilemma of the echinoid tooth ligament. I. Morphology. Biol. Bull. 1996, 190, 218–230. [Google Scholar] [CrossRef]
- Mashanov, V.; Zueva, O.; Rubilar, T.; Epherra, L.; García-Arrarás, J.E. Echinodermata. In Structure and Evolution of Invertebrate Nervous Systems; Schmidt-Rhaesa, A., Harzsch, S., Purschke, G., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 665–688. [Google Scholar]
- Grimmer, J.C.; Holland, N.D.; Kubota, H. The fine structure of the stalk of the pentacrinoid larva of a feather star, Comanthus japonica. Acta Zool. Stockh. 1984, 65, 41–58. [Google Scholar] [CrossRef]
- Wilkie, I.C.; McKew, M.; Candia Carnevali, M.D. Unusual morphological features of the compass-rotular ligament of Echinus esculentus L. In Echinoderms: München; Heinzeller, T., Nebelsick, J.H., Eds.; Taylor & Francis Group: London, UK, 2004; pp. 379–385. [Google Scholar]
- Byrne, M. The ultrastructure of the morula cells of Eupentacta quinquesemita (Echinodermata: Holothuroidea) and their role in the maintenance of the extracellular matrix. J. Exp. Biol. 1986, 188, 179–189. [Google Scholar] [CrossRef]
- Motokawa, T. Morphology of spines and spine joint in the crown-of-thorns starfish Acanthaster planci (Echinodermata, Asteroida). Zoomorphology 1986, 106, 247–253. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D.; Bonasoro, F. Organization and mechanical behaviour of myocyte-ligament composites in a sea-urchin lantern: The compass depressors of Stylocidaris affinis. Zoomorphology 1998, 118, 87–101. [Google Scholar] [CrossRef]
- Frank, C.B.; Shrive, N.G. Ligament. In Biomechanics of the Musculo-Skeletal System; Nigg, B.M., Herzog, W., Eds.; Wiley: Chichester, NH, USA, 1994; pp. 106–131. [Google Scholar]
- Wang, J.H.C. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef]
- Birenheide, R.; Yokoyama, K.; Motokawa, T. Cirri of the stalked crinoid Metacrinus rotundus: Neural elements and the effect of cholinergic agonists on mechanical properties. Proc. R. Soc. Lond. B 2000, 267, 7–16. [Google Scholar] [CrossRef]
- Ellers, O.; Telford, M. Advancement mechanics of growing teeth in sand dollars (Echinodermata, Echinoidea): A role for mutable collagenous tissue. Proc. R. Soc. Lond. B 1996, 263, 39–44. [Google Scholar]
- Motokawa, T.; Tsuchi, A. Dynamic mechanical properties of the body-wall dermis in various mechanical states and their implications for the behavior of sea cucumbers. Biol. Bull. 2003, 205, 261–275. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Fassini, D.; Cullorà, E.; Barbaglio, A.; Tricarico, S.; Sugni, M.; Del Giacco, L.; Candia Carnevali, M.D. Mechanical properties of the compass depressors of the sea-urchin Paracentrotus lividus (Echinodermata, Echinoidea) and the effects of enzymes, neurotransmitters and synthetic tensilin-like protein. PLoS ONE 2015, 10, e0120339. [Google Scholar] [CrossRef]
- Motokawa, T. Viscoelasticity of holothurian body wall. J. Exp. Biol. 1984, 109, 63–75. [Google Scholar] [CrossRef]
- Motokawa, T. The viscosity change of the body-wall dermis of the sea cucumber Stichopus japonicus caused by mechanical and chemical stimulation. Comp. Biochem. Physiol. A 1984, 77, 419–423. [Google Scholar]
- Motokawa, T. Factors regulating the mechanical properties of holothurian dermis. J. Exp. Biol. 1982, 99, 29–41. [Google Scholar] [CrossRef]
- Eylers, J.P. Ion-dependent viscosity of holothurian body wall and its implications for the functional morphology of echinoderms. J. Exp. Biol. 1982, 99, 1–8. [Google Scholar] [CrossRef]
- Pioletti, D.P.; Rakotomanana, L.R.; Benvenuti, J.F.; Leyvraz, P.F. Viscoelastic constitutive law in large deformations: Application to human knee ligaments and tendons. J. Biomech. 1998, 31, 753–757. [Google Scholar] [CrossRef]
- Del Prete, Z.; Antoniucci, S.; Hoffman, A.H.; Grigg, P. Viscoelastic properties of skin in Mov-13 and Tsk mice. J. Biomech. 2004, 37, 1491–1497. [Google Scholar] [CrossRef]
- Harkness, M.L.R.; Harkness, R.D. Changes in the physical properties of the uterine cervix of the rat during pregnancy. J. Physiol. 1959, 148, 524–547. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic properties of human skin and processed dermis. Skin Res. Technol. 2001, 7, 18–23. [Google Scholar] [CrossRef]
- Marrs, J.; Wilkie, I.C.; Sköld, M.; Maclaren, W.M.; McKenzie, J.D. Size-related aspects of arm damage, tissue mechanics, and autotomy in the starfish Asterias rubens. Mar. Biol. 2000, 137, 59–70. [Google Scholar] [CrossRef]
- O’Neill, P.L.; Withers, P.C. An analysis of the load curve of the body wall of Coscinasterias calamaria (Echinodermata: Asteroidea). Mar. Fresh. Behav. Physiol. 1995, 25, 245–260. [Google Scholar] [CrossRef]
- Motokawa, T. Mechanical mutability in connective tissue of starfish body wall. Biol. Bull. 2011, 221, 280–291. [Google Scholar] [CrossRef]
- Birenheide, R.; Motokawa, T. Morphological basis and mechanics of arm movement in the stalked crinoid Metacrinus rotundus (Echinodermata, Crinoida). Mar. Biol. 1994, 121, 273–283. [Google Scholar] [CrossRef]
- Szulgit, G.K.; Shadwick, R.E. The effects of calcium chelation and cell perforation on the mechanical properties of sea urchin ligaments. In Echinoderms Through Time; David, B., Guille, A., Féral, J.P., Roux, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1988; pp. 887–892. [Google Scholar]
- Trotter, J.A.; Koob, T.J. Collagen and proteoglycan in a sea urchin ligament with mutable mechanical properties. Cell Tissue Res. 1989, 258, 527–539. [Google Scholar] [CrossRef]
- Legerlotz, K.; Riley, G.P.; Screen, H.R.C. GAG depletion increases the stress-relaxation response of tendon fascicles, but does not influence recovery. Acta Biomater. 2013, 9, 6860–6866. [Google Scholar] [CrossRef]
- Silver, F.H.; Seehra, G.P.; Freeman, J.W.; DeVore, D.P. Viscoelastic properties of young and old human dermis: A proposed molecular mechanism for elastic energy storage in collagen and elastin. J. App. Polym. Sci. 2002, 86, 1978–1985. [Google Scholar] [CrossRef]
- Mattucci, S.F.E.; Moulton, J.A.; Chandrashekar, N.; Cronin, D.S. Strain rate dependent properties of younger human cervical spine ligaments. J. Mech. Behav. Biomed. Mater. 2012, 10, 216–226. [Google Scholar] [CrossRef]
- Kastelic, J.; Baer, E. Deformation in tendon collagen. Symp. Soc. Exp. Biol. 1980, 34, 397–435. [Google Scholar]
- Wilkie, I.C. Autotomy as a prelude to autotomy in echinoderms. Microscop. Res. Technol. 2001, 55, 369–396. [Google Scholar] [CrossRef]
- Ciarletta, P.; Ben Amar, M. A finite dissipative theory of temporary interfibrillar bridges in the extracellular matrix of ligaments and tendons. J. R. Soc. Interface 2009, 6, 909–924. [Google Scholar] [CrossRef][Green Version]
- Szczesny, S.E.; Elliott, D.M. Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomater. 2014, 10, 2582–2590. [Google Scholar] [CrossRef]
- Tricarico, S.; Barbaglio, A.; Burlini, N.; Del Giacco, L.; Ghilardi, A.; Sugni, M.; Di Benedetto, C.; Bonasoro, F.; Wilkie, I.C.; Candia Carnevali, M.D. New insights into the mutable collagenous tissue of Paracentrotus lividus: Preliminary results. Zoosymposia 2012, 7, 279–285. [Google Scholar] [CrossRef]
- Barbaglio, A.; Tricarico, S.; Ribeiro, A.R.; Di Benedetto, C.; Barbato, M.; Dessì, D.; Fugnanesi, V.; Magni, S.; Mosca, F.; Sugni, M.; et al. Ultrastructural and biochemical characterization of mechanically adaptable collagenous structures in the edible sea urchin Paracentrotus lividus. Zoology 2015, 118, 147–160. [Google Scholar] [CrossRef]
- Birk, D.E.; Nurminskaya, M.V.; Zycband, E.I. Collagen fibrillogenesis in situ: Fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev. Dyn. 1995, 202, 229–243. [Google Scholar] [CrossRef] [PubMed]
- deVente, J.E.; Lester, G.E.; Trotter, J.A.; Dahners, L.E. Isolation of intact collagen fibrils from healing ligament. J. Electron Microsc. 1997, 46, 353–356. [Google Scholar] [CrossRef]
- Liu, Y.; Andarawis-Puri, N.; Eppell, S.J. Method to extract minimally damaged collagen fibrils from tendon. J. Biol. Methods 2016, 3, e54. [Google Scholar] [CrossRef]
- Wilkie, I.C. Variable tensility in echinoderm collagenous tissues: A review. Mar. Behav. Physiol. 1984, 11, 1–34. [Google Scholar] [CrossRef]
- Wilkie, I.C. Mutable collagenous tissues: Extracellular matrix as mechano-effector. In Echinoderm Studies 5; Jangoux, M., Lawrence, J.M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1996; pp. 61–102. [Google Scholar]
- Wilkie, I.C. Is muscle involved in the mechanical adaptability of echinoderm mutable collagenous tissue? J. Exp. Biol. 2002, 205, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Bonneel, M.; Hennebert, E.; Byrne, M.; Flammang, P. Mutable collagenous tissues in sea cucumbers. In The World of Sea Cucumbers; Mercier, A., Hamel, J.-F., Suhrbier, A., Pearce, C., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 573–584. [Google Scholar] [CrossRef]
- Tamori, M.; Yamada, A.; Nishida, N.; Motobayashi, Y.; Oiwa, K.; Motokawa, T. Tensilin-like stiffening protein from Holothuria leucospilota does not induce the stiffest state of catch connective tissue. J. Exp. Biol. 2006, 209, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Tamori, M.; Iketani, T.; Oiwa, K.; Motokawa, T. A novel stiffening factor inducing the stiffest state of holothurian catch connective tissue. J. Exp. Biol. 2010, 213, 3416–3422. [Google Scholar] [CrossRef]
- Trotter, J.A.; Tipper, J.; Lyons-Levy, G.; Chino, K.; Heuer, A.H.; Liu, Z.; Mrksich, M.; Hodneland, C.; Dillmore, W.S.; Koob, T.J.; et al. Towards a fibrous composite with dynamically controlled stiffness: Lessons from echinoderms. Biochem. Soc. Trans. 2000, 28, 357–362. [Google Scholar] [CrossRef]
- Takehana, Y.; Yamada, A.; Tamori, M.; Motokawa, T. Softenin, a novel protein that softens the connective tissue of sea cucumbers through inhibiting interaction between collagen fibrils. PLoS ONE 2014, 9, e85644. [Google Scholar] [CrossRef]
- Ingersoll, E.P.; Wilt, F.H. Matrix metalloproteinase inhibitors disrupt spicule formation by primary mesenchyme cells in the sea urchin embryo. Dev. Biol. 1998, 196, 95–106. [Google Scholar] [CrossRef]
- Sharpe, C.; Robinson, J.J. Characterization of matrix metalloprotease activities induced in the sea urchin extraembryonic matrix, the hyaline layer. Biochem. Cell Biol. 2001, 79, 461–468. [Google Scholar] [CrossRef]
- Quiñones, J.L.; Rosa, R.; Ruiz, D.L.; García-Arrarás, J.E. Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev. Biol. 2002, 250, 181–197. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Barbaglio, A.; Oliveira, M.J.; Ribeiro, C.C.; Wilkie, I.C.; Candia Carnevali, M.D.; Barbosa, M.A. Matrix metalloproteinases in a sea urchin ligament with adaptable mechanical properties. PLoS ONE 2012, 7, e49016. [Google Scholar] [CrossRef]
- Elphick, M.R. The protein precursors of peptides that affect the mechanics of connective tissue and/or muscle in the echinoderm Apostichopus japonicus. PLoS ONE 2012, 7, e44492. [Google Scholar] [CrossRef]
- Tamori, M.; Takemae, C.; Motokawa, T. Evidence that water exudes when holothurian connective tissue stiffens. J. Exp. Biol. 2010, 213, 1960–1966. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Barbaglio, A.; Oliveira, M.J.; Santos, R.; Coelho, A.V.; Ribeiro, C.C.; Wilkie, I.C.; Candia Carnevali, M.D.; Barbosa, M.A. Correlations between the biochemistry and mechanical states of a sea-urchin ligament: A mutable collagenous structure. Biointerphases 2012, 7, 38. [Google Scholar] [CrossRef]
- Tamori, M.; Ishida, K.; Matsuura, E.; Ogasawara, K.; Hanasaka, T.; Takehana, Y.; Motokawa, T.; Osawa, T. Ultrastructural changes associated with reversible stiffening in catch connective tissue of sea cucumbers. PLoS ONE 2016, 11, e0155673. [Google Scholar] [CrossRef]
- Mo, J.; Prévost, S.F.; Blowes, L.M.; Egertová, M.; Terrill, N.J.; Wang, W.; Elphick, M.R.; Gupta, H.S. Interfibrillar stiffening of echinoderm mutable collagenous tissue demonstrated at the nanoscale. Proc. Natl. Acad. Sci. USA 2016, 113, E6362–E6371. [Google Scholar] [CrossRef]
- Gupta, H.S.; Barbieri, E.; Inamdar, S.R.; Mo, J. Synchrotron X-ray imaging combined with multiscale modelling applied to biological soft tissues. In Soft Matter for Biomedical Applications; Azevedo, H.S., Mano, J.F., Borges, J., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 34–60. [Google Scholar]
- Barbieri, E.; Mo, J.; Gupta, H.S. Chemoviscoelasticity of the interfibrillar matrix of the dermis of the black sea cucumber Holothuria atra. Mech. Mater. 2022, 168, 104252. [Google Scholar] [CrossRef]
- Charlina, N.A.; Dolmatov, I.Y.; Wilkie, I.C. Juxtaligamental system of the disc and oral frame of the ophiuroid Amphipholis kochii (Echinodermata: Ophiuroidea) and its role in autotomy. Invert. Biol. 2009, 128, 145–156. [Google Scholar] [CrossRef]
- Scalettar, B.A. How neurosecretory vesicles release their cargo. Neuroscientist 2006, 12, 164–176. [Google Scholar] [CrossRef]
- Imamura, Y.; Morita, S.; Nakatani, Y.; Okada, K.; Ueshima, S.; Matsuo, O.; Miyata, S. Tissue plasminogen activator and plasminogen are critical for osmotic homeostasis by regulating vasopressin secretion. J. Neurosci. Res. 2010, 88, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Nangia, S.; Parmer, R.J. The plasminogen activation system and the regulation of catecholaminergic function. J. Biomed. Biotechnol. 2012, 2012, 721657. [Google Scholar] [CrossRef]
- Díaz-Balzac, C.A.; Santacana-Laffitte, G.; San Miguel-Ruíz, J.E.; Tossas, K.; Valentín-Tirado, G.; Rives-Sánchez, M.; Mesleh, A.; Torres, I.I.; García-Arrarás, J.E. Identification of nerve plexi in connective tissues of the sea cucumber Holothuria glaberrima by using a novel nerve-specific antibody. Biol. Bull. 2007, 213, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, A.B.; Barreiro-Iglesias, A.; Yañez-Guerra, L.A.; Delroisse, J.; Zhang, Y.; Gunner, E.F.; Zampronio, C.G.; Jones, A.M.; Egertová, M.; Elphick, M.R. Ancient role of sulfakinin/cholecystokinin-type signalling in inhibitory regulation of feeding processes revealed in an echinoderm. eLife 2021, 10, e65667. [Google Scholar] [CrossRef]
- Cobb, J.L.S. The motor innervation of the oral plate ligament in the brittlestar Ophiura ophiura. Cell Tissue Res. 1985, 242, 685–688. [Google Scholar] [CrossRef]
- Cobb, J.L.S. Neurobiology of the Echinodermata. In Nervous Systems in Invertebrates; Ali, M.A., Ed.; Plenum Press: New York, NY, USA, 1987; pp. 483–525. [Google Scholar]
- Inoue, M.; Birenheide, R.; Koizumi, O.; Kobayakawa, Y.; Muneoka, Y.; Motokawa, T. Localization of the neuropeptide NGIWYamide in the holothurian nervous system and its effects on muscular contraction. Proc. R. Soc. Lond. B 1999, 266, 993–1000. [Google Scholar] [CrossRef]
- Cobb, J.L.S.; Begbie, K.M. Aspects of the hyponeural nervous system. In Echinoderms Through Time; David, B., Guille, A., Féral, J.P., Roux, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1988; pp. 25–29. [Google Scholar]
- Díaz-Balzac, C.A.; García-Arrarás, J.E. Echinoderm nervous system. In Oxford Research Encyclopedia, Neuroscience; Sherman, S.M., Ed.; Oxford University Press: New York, NY, USA, 2019; pp. 1–31. [Google Scholar]
- Motokawa, T. Cholinergic control of the mechanical properties of the catch connective tissue in the holothurian body wall. Comp. Biochem. Physiol. C 1987, 86, 333–337. [Google Scholar] [CrossRef]
- Morales, M.; Sierra, C.; Vidal, A.; Del Castillo, J.; Smith, D.S. Pharmacological sensitivity of the articular capsule of the primary spines of Eucidaris tribuloides. Comp. Biochem. Physiol. C 1993, 105, 25–30. [Google Scholar] [CrossRef]
- Motokawa, T.; Fuchigama, Y. Coordination between catch connective tissue and muscles through nerves in the spine joint of the sea urchin Diadema setosum. J. Exp. Biol. 2015, 218, 703–710. [Google Scholar] [CrossRef]
- Birenheide, R.; Tamori, T.; Motokawa, T.; Ohtani, M.; Iwakoshi, E.; Muneoka, Y.; Fujita, T.; Minakata, H.; Nomoto, K. Peptides controlling stiffness of connective tissue in sea cucumbers. Biol. Bull. 1998, 194, 253–259. [Google Scholar] [CrossRef]
- Tamori, M.; Saha, A.K.; Matsuno, A.; Noskor, S.C.; Koizumi, O.; Kobayakawa, Y.; Nakajima, Y.; Motokawa, T. Stichopin-containing nerves and secretory cells specific to connective tissues of the sea cucumber. Proc. R. Soc. B 2007, 274, 2279–2285. [Google Scholar] [CrossRef]
- Aleotti, A.; Wilkie, I.C.; Yañez-Guerra, L.A.; Gattoni, G.; Rahman, T.A.; Wademan, R.; Ahmad, Z.; Ivanova, D.A.; Semmens, D.C.; Delroisse, J.; et al. Discovery and functional characterization of neuropeptides in crinoid echinoderms. Front. Neurosci. 2022, 16, 1006594. [Google Scholar] [CrossRef]
- Tinoco, A.B.; Kirupakaran, V.; Capatina, D.; Egertová, M.; Elphick, M.R. Discovery of a neuropeptide that acts as an autotomy-promoting factor. Curr. Biol. 2024, 34, 4325–4331. [Google Scholar] [CrossRef]
- Smith, G.N.; Greenberg, M.J. Chemical control of the evisceration process in Thyone briareus. Biol. Bull. 1973, 144, 421–436. [Google Scholar] [CrossRef]
- Byrne, M. Induction of evisceration in the holothurian Eupentacta quinquesemita and evidence for the existence of an endogenous evisceration factor. J. Exp. Biol. 1986, 120, 25–40. [Google Scholar] [CrossRef]
- Chaet, A.B. A toxin in the coelomic fluid of scalded starfish (Asterias forbesi). Proc. Soc. Exp. Biol. Med. 1962, 109, 791–794. [Google Scholar] [CrossRef]
- Mladenov, P.V.; Igdoura, S.; Asotra, S.; Burke, R.D. Purification and partial characterization of an autotomy-promoting factor from the sea star Pycnopodia helianthoides. Biol. Bull. 1989, 176, 169–175. [Google Scholar] [CrossRef]
- Murphy, C.J.; Mladenov, P.V. Autotomy promoting factor (APF) in south west Pacific sea stars. In Troisième Symposium Sur Substances Naturelles D’Intérét Biologique de la Région Pacifique-Asie; Debitus, C., Amade, P., Laurent, D., Cosson, J.P., Eds.; ORSTOM: Paris, France, 1992; pp. 411–420. [Google Scholar]
- Morales, M.; del Castillo, J.; Smith, D.S. Acetylcholine sensitivity of the spine-test articular capsule of the sea urchin Eucidaris tribuloides. Comp. Biochem. Physiol. C 1989, 94, 547–554. [Google Scholar] [CrossRef]
- Vidal, A.M.; del Castillo, J.; Smith, D.S. Contractile properties of the articular capsule or ligament, in the primary spines of the sea urchin Eucidaris tribuloides. Comp. Biochem. Physiol. C 1993, 106, 643–647. [Google Scholar] [CrossRef]
- Takemae, N.; Motokawa, T. Mechanical properties of the isolated catch apparatus of the sea urchin spine joint: Muscle fibers do not contribute to passive stiffness changes. Biol. Bull. 2005, 208, 29–35. [Google Scholar] [CrossRef]
- Motokawa, T.; Shintani, O.; Birenheide, R. Contraction and stiffness changes in collagenous arm ligaments of the stalked crinoid Metacrinus rotundus (Echinodermata). Biol. Bull. 2004, 206, 4–12. [Google Scholar] [CrossRef]
- Birenheide, R.; Motokawa, T. Motility and stiffness of cirri of the stalked crinoid Metacrinus rotundus. In Echinoderm Research 1995; Emson, R.H., Smith, A.B., Campbell, A.C., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1995; pp. 91–94. [Google Scholar]
- Birenheide, R.; Motokawa, T. Crinoid ligaments: Catch and contractility. In Echinoderms: San Francisco; Mooi, R., Telford, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1998; pp. 139–144. [Google Scholar]
- Birenheide, R.; Motokawa, T. Contractile connective tissue in crinoids. Biol. Bull. 1996, 191, 1–4. [Google Scholar] [CrossRef]
- Li, W.; Guan, Q.; Li, M.; Saiz, E.; Hou, X. Nature-inspired strategies for the synthesis of hydrogel actuators and their applications. Prog. Polym. Sci. 2023, 140, 101665. [Google Scholar] [CrossRef]
- Meyer, D.L. Feeding behavior and ecology of shallow-water unstalked crinoids (Echinodermata) in the Caribbean Sea. Mar. Biol. 1973, 22, 105–129. [Google Scholar] [CrossRef]
- Macurda, D.B.; Meyer, D.L. Feeding posture of modern stalked crinoids. Nature 1974, 247, 394–396. [Google Scholar] [CrossRef]
- Meyer, D.L.; Veitch, M.; Messing, C.G.; Stevenson, A. Crinoid Feeding Strategies: New Insights from Subsea Video and Time-Lapse; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Warner, G.F. Food and feeding mechanisms: Ophiuroidea. In Echinoderm Nutrition; Jangoux, M., Lawrence, J.M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1982; pp. 161–181. [Google Scholar]
- Emson, R.H.; Young, C.M. Feeding mechanism of the brisingid starfish Novodinia antillensis. Mar. Biol. 1994, 118, 433–442. [Google Scholar] [CrossRef]
- Mitsukuri, K. Notes on the habits and life-history of Stichopus japonicus Selenka. Annot. Zool. Japon. 1903, 5, 1–21. [Google Scholar]
- Chen, M.; Li, X.; Zhu, A.; Storey, K.B.; Sun, L.; Gao, T.; Wang, T. Understanding mechanism of sea cucumber Apostichopus japonicus aestivation: Insights from TMT-based proteomic study. Comp. Biochem. Physiol. D 2016, 19, 78–89. [Google Scholar] [CrossRef]
- Otter, G.W. Rock-burrowing echinoids. Biol. Bull. 1932, 7, 89–107. [Google Scholar] [CrossRef]
- Cobb, J.L.S. A physiological study of the correlation between motor output in hyponeural nerves and arm movements in the brittlestar Ophiura ophiura. Mar. Behav. Physiol. 1990, 17, 147–157. [Google Scholar] [CrossRef]
- Grimmer, J.C.; Holland, N.D.; Messing, C.G. Fine structure of the stalk of the bourgueticrinid sea lily Democrinus conifer (Echinodermata: Crinoidea). Mar. Biol. 1984, 81, 163–176. [Google Scholar] [CrossRef]
- Holland, N.D.; Grimmer, J.C. Fine structure of the cirri and a possible mechanism for their motility in stalkless crinoids (Echinodermata). Cell Tissue Res. 1981, 214, 207–217. [Google Scholar] [CrossRef]
- Takemae, N.; Nakaya, F.; Motokawa, T. Low oxygen consumption and high body content of catch connective tissue contribute to low metabolic rate of sea cucumbers. Biol. Bull. 2009, 216, 45–54. [Google Scholar] [CrossRef]
- Motokawa, T.; Sato, E.; Umeyama, K. Energy expenditure associated with softening and stiffening of echinoderm connective tissue. Biol. Bull. 2012, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Fredericq, L. Sur l’autotomie ou mutilation par voie refléxe comme moyen de défense chez les animaux. Arch. Zool. Exp. Gén. 1883, 1, 413–426. [Google Scholar]
- Kubota, T.; Iwamuro, H.; Miyahara, R. Autotomy of the apodid sea cucumber Polycheira fusca (rufescens): Observations and surgical experiments. Rep. Fac. Sci. Kagoshima Univ. 2001, 34, 25–33. [Google Scholar]
- Anderson, J.M. Observations on autotomy in the starfish, Asterias forbesi. Biol. Bull. 1956, 111, 297. [Google Scholar]
- Jobson, S.; Penney, H.D.; Hamel, J.F.; Mercier, A. Split personality. Front. Ecol. Environ. 2020, 18, 557. [Google Scholar] [CrossRef]
- Emson, R.H.; Wilkie, I.C. Fission and autotomy in echinoderms. Oceanogr. Mar. Biol. Ann. Rev. 1980, 18, 155–250. [Google Scholar]
- Jaeckle, W.B. Multiple modes of asexual reproduction by tropical and subtropical sea star larvae: An unusual adaptation for genet dispersal and survival. Biol. Bull. 1994, 186, 62–71. [Google Scholar] [CrossRef]
- Uthicke, S. The process of asexual reproduction by transverse fission in Stichopus chloronotus (greenfish). SPC Bech-De-Mer Inform. Bull. 2001, 14, 23–25. [Google Scholar]
- Rubilar, T.; Pastor, C.; Díaz de Vivar, E. Timing of fission in the starfish Allostichaster capensis (Echinodermata: Asteroidea) in the laboratory. Rev. Biol. Trop. 2005, 53 (Suppl. S3), 299–303. [Google Scholar]
- Crawford, B.J. Changes in the arrangement of the extracellular matrix, larval shape, and mesenchyme cell migration during asteroid larval development. J. Morphol. 1990, 206, 147–161. [Google Scholar] [CrossRef]
- Hill, R.B. Role of Ca2+ in excitation-contraction coupling in echinoderm muscle: Comparison with role in other tissues. J. Exp. Biol. 2001, 204, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Montes, G.S.; Mourão, P.A.S.; Carneiro, J.; Salles, L.M.M.; Bonetti, S.S. Collagen-proteoglycans interaction during autotomy in the sea cucumber, Stichopus badionotus. Rev. Can. Biol. 1980, 39, 157–164. [Google Scholar]
- Hill, R.B. Propylene phenoxetol as a “preservative” for living holothurians. Nature 1966, 211, 204–205. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhou, D.Y.; Liu, Y.X.; Yu, M.M.; Liu, B.; Song, L.; Dong, X.P.; Qi, H.; Shahidi, F. Inhibitory effect of natural metal ion chelators on the autolysis of sea cucumber (Stichopus japonicus) and its mechanism. Food Res. Int. 2020, 133, 109205. [Google Scholar] [CrossRef]
- Fan, X.; Wu, K.; Tian, X.; Benjakul, S.; Li, Y.; Sang, X.; Zhao, Q.; Zhang, J. Endogenous proteases in sea cucumber (Apostichopus japonicus): Deterioration and prevention during handling, processing, and preservation. Foods 2024, 13, 2153. [Google Scholar] [CrossRef]
- Kropp, R.K. Responses of five holothurian species to attacks by a predatory gastropod, Tonna perdix. Pacific Sci. 1982, 36, 445–452. [Google Scholar]
- Muzahar, M.; Wulandari, R.; Putri, D.S.; Yulianto, T.; Irawan, H.; Bakkara, O.R. Fission technique on sea cucumbers (Holothuroidea): An alternative method for seed production. BIO Web Conf. 2024, 134, 06005. [Google Scholar] [CrossRef]
- Zhu, B.; Zheng, J.; Zhang, Z.; Dong, X.; Zhao, L.; Tada, M. Autophagy plays a potential role in the process of sea cucumber body wall “melting” induced by UV irradiation. Wuhan Univ. J. Nat. Sci. 2008, 13, 232–238. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhou, D.Y.; Liu, Y.X.; Liu, X.Y.; Liu, Y.; Liu, B.; Song, L.; Shahidi, F. In vivo mechanism of action of matrix metalloprotease (MMP) in the autolysis of sea cucumber (Stichopus japonicus). J. Food Process. Preserv. 2020, 44, e14383. [Google Scholar] [CrossRef]
- Sun, L.M.; Wang, T.T.; Zhu, B.W.; Niu, H.L.; Zhang, R.; Hou, H.M.; Zhang, G.L.; Murata, Y. Effect of matrix metalloproteinase on autolysis of sea cucumber Stichopus japonicus. Food Sci. Biotechnol. 2013, 22, 1–3. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhou, D.Y.; Liu, Z.Q.; Lu, T.; Song, L.; Li, D.M.; Dong, X.P.; Qi, H.; Zhu, B.W.; Shahidi, F. Structural and biochemical changes in dermis of sea cucumber (Stichopus japonicus) during autolysis in response to cutting the body wall. Food Chem. 2018, 240, 1254–1261. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zhou, D.; Liu, X.; Dong, X.; Li, D.; Shahidi, F. The role of matrix metalloprotease (MMP) to the autolysis of sea cucumber (Stichopus japonicus). J. Sci. Food Agric. 2019, 99, 5752–5759. [Google Scholar] [CrossRef]
- Yan, L.J.; Sun, L.; Cao, K.Y.; Chen, Y.L.; Zhang, L.J.; Liu, G.M.; Jin, T.; Cao, M.J. Type I collagen from sea cucumber (Stichopus japonicus) and the role of matrix metalloproteinase-2 in autolysis. Food Biosci. 2021, 41, 100959. [Google Scholar] [CrossRef]
- Yan, T.; Sun, J.; Zheng, J.; Yang, J. An analysis combining proteomics and transcriptomics revealed a regulation target of sea cucumber autolysis. Comp. Biochem. Physiol. D 2024, 52, 101274. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhou, D.Y.; Ma, D.D.; Liu, Y.F.; Li, D.M.; Dong, X.P.; Tan, M.Q.; Du, M.; Zhu, B.W. Changes in collagenous tissue microstructures and distributions of cathepsin L in body wall of autolytic sea cucumber (Stichopus japonicus). Food Chem. 2016, 212, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, A.; Motokawa, T. Evidence for calcium translocation in catch connective tissue of the sea cucumber Stichopus chloronotus. Cell Tissue Res. 1992, 267, 307–312. [Google Scholar] [CrossRef]
- Hill, R.B.; Reinschmidt, D. Relative importance of the antioxidant and anesthetic properties of propylene phenoxetol in its action as a “preservative” for living holothurians. J. Invert. Pathol. 1976, 28, 131–135. [Google Scholar] [CrossRef]
- Candia Carnevali, M.D.; Bonasoro, F. Microscopic overview of crinoid regeneration. Microscop. Res. Technol. 2001, 55, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Biressi, A.C.M.; Zou, T.; Dupont, S.; Dahlberg, C.; Di Benedetto, C.; Bonasoro, F.; Thorndyke, M.C.; Candia Carnevali, M.D. Wound healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): Comparative morphogenesis and histogenesis. Zoomorphology 2010, 129, 1–19. [Google Scholar] [CrossRef]
- Ben Khadra, Y.; Sugni, M.; Ferrario, C.; Bonasoro, F.; Coelho, A.V.; Martinez, P.; Candia Carnevali, M.D. An integrated view of asteroid regeneration: Tissues, cells and molecules. Cell Tissue Res. 2017, 370, 13–28. [Google Scholar] [CrossRef]
- Ferrario, C.; Sugni, M.; Somorjai, I.M.L.; Ballarin, L. Beyond adult stem cells: Dedifferentiation as a unifying mechanism underlying regeneration in invertebrate deuterostomes. Front. Cell Dev. Biol. 2020, 8, 587320. [Google Scholar] [CrossRef]
- Kalacheva, N.V.; Eliseikina, M.G.; Frolova, I.Y.; Dolmatov, I.Y. Regeneration of the digestive system in the crinoid Himerometra robustipinna occurs by transdifferentiation of neurosecretory-like cells. PLoS ONE 2017, 12, e0182001. [Google Scholar] [CrossRef]
- Kalacheva, N.V.; Kamenev, Y.O.; Dolmatov, I.Y. Regeneration of the digestive system in the crinoid Lamprometra palmata (Mariametridae, Comatulida). Cell Tissue Res. 2021, 391, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Dolmatov, I.Y.; Kalacheva, N.V.; Mekhova, E.S.; Frolova, L.T. Autotomy and regeneration of the visceral mass in feather stars. Zoomorphology 2020, 139, 171–187. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Yu, M.K.; Dong, J.; Zhang, X.; Tsang, L.L.; Chung, Y.W.; Li, T.; Chan, H.C. Switching from bone marrow-derived neurons to epithelial cells through dedifferentiation and translineage redifferentiation. Cell Biol. Int. 2010, 34, 1075–1083. [Google Scholar] [CrossRef]
- Mozzi, D.; Dolmatov, I.Y.; Bonasoro, F.; Candia Carnevali, M.D. Visceral regeneration in the crinoid Antedon mediterranea: Basic mechanisms, tissues and cells involved in gut regrowth. Central Eur. J. Biol. 2006, 1, 609–635. [Google Scholar] [CrossRef]
- Candia Carnevali, M.D.; Bonasoro, F. Arm regeneration and pattern formation in crinoids. In Echinoderm Research 1995; Emson, R.H., Smith, A.B., Campbell, A.C., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1995; pp. 245–253. [Google Scholar]
- Byrne, M. The link between autotomy and CNS regeneration: Echinoderms as non-model species for regenerative biology. BioEssays 2020, 42, 1900219. [Google Scholar] [CrossRef]
- Oulhen, N.; Byrne, M.; Duffin, P.; Gomez-Chiarri, M.; Hewson, I.; Hodin, J.; Konar, B.; Lipp, E.K.; Miner, B.G.; Newton, A.L.; et al. A review of asteroid biology in the context of sea star wasting: Possible causes and consequences. Biol. Bull. 2022, 243, 50–75. [Google Scholar] [CrossRef]
- Hewson, I.; Johnson, M.R.; Reyes-Chavez, B. Lessons learned from the sea star wasting disease investigation. Annu. Rev. Mar. Sci. 2025, 17, 257–279. [Google Scholar] [CrossRef]
- Bucci, C.; Francoeur, M.; McGreal, J.; Smolowitz, R.; Zazueta-Novoa, V.; Wessel, G.M.; Gomez-Chiarri, M. Sea star wasting disease in Asterias forbesi along the Atlantic Coast of North America. PLoS ONE 2017, 12, e0188523. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Work, T.M.; Angulo-Preckler, C.; Moles, J.; Avila, C. Exploring the pathology of an epidermal disease affecting a circum-Antarctic sea star. Sci. Rep. 2018, 8, 11353. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M.; Weatherby, T.M.; DeRito, C.M.; Besemer, R.M.; Hewson, I. Sea star wasting disease pathology in Pisaster ochraceus shows a basal-to-surface process affecting color phenotypes differently. Dis. Aquat. Org. 2021, 145, 21–33. [Google Scholar] [CrossRef]
- Smith, S.; Kunc, H.P.; Hewson, I.; Collins, P.C. Elevated temperature linked to signs associated with sea star wasting disease in a keystone European, Asterias rubens. Mar. Ecol. Prog. Ser. 2023, 724, 97–109. [Google Scholar] [CrossRef]
- Gudenkauf, B.M.; Hewson, I. Metatranscriptomic analysis of Pycnopodia helianthoides (Asteroidea) affected by sea star wasting disease. PLoS ONE 2015, 10, e0128150. [Google Scholar] [CrossRef]
- Pespeni, M.H.; Lloyd, M.M. Sea stars resist wasting through active immune and collagen systems. Proc. R. Soc. B 2023, 290, 20230347. [Google Scholar] [CrossRef]
- Fuess, L.E.; Eisenlord, M.E.; Closek, C.J.; Tracy, A.M.; Mauntz, R.; Gignoux-Wolfsohn, S.; Moritsch, M.M.; Yoshioka, R.; Burge, C.A.; Harvell, C.D.; et al. Up in arms: Immune and nervous system response to sea star wasting disease. PLoS ONE 2015, 10, e0133053. [Google Scholar] [CrossRef]
- Deng, H.; He, C.; Zhou, Z.; Liu, C.; Tan, K.; Wang, N.; Jiang, B.; Gao, X.; Liu, W. Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumber Apostichopus japonicus. Aquaculture 2009, 287, 18–27. [Google Scholar] [CrossRef]
- Delroisse, J.; Wayneberghe, K.V.; Flammang, P.; Gillan, D.; Gerbaux, P.; Opina, N.; Todinanahary, G.G.B.; Eeckhaut, I. Epidemiology of Skin Ulceration Disease in the sea cucumber Holothuria scabra with a review on the SKUDs in Holothuroidea (Echinodermata). Sci. Rep. 2020, 10, 22150. [Google Scholar] [CrossRef]
- Becker, P.; Gillan, D.; Lanterbecq, D.; Jangoux, M.; Rasolofonirina, R.; Rakotovao, J.; Eeckhaut, I. The skin ulceration disease in cultivated juveniles of Holothuria scabra (Holothuroidea, Echinodermata). Aquaculture 2004, 242, 13–30. [Google Scholar] [CrossRef]
- Scott, M.M.F.; Ma, K.C.K.; Wolvin, S.; Hamel, J.F.; Mercier, A. First report of skin ulceration disease from temperate waters of the Northwest Atlantic: The case of the sea cucumber Cucumaria frondosa. SPC Beche-de-mer Inform. Bull. 2024, 44, 22–26. [Google Scholar]
- Arjona-Cambranes, K.A.; Olvera-Novoa, M.A.; Cerqueda-García, D.; Arjona-Torres, M.G.; Aguirre-Macedo, M.L.; Vidal-Martínez, V.M.; García-Maldonado, J.Q. Characterization of microbiota and histology of cultured sea cucumber Isostichopus badionotus juveniles during an outbreak of skin ulceration syndrome. PLoS ONE 2024, 19, e0303480. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, J.; Pan, Y.; Sun, H.; Guan, X.; Gao, S.; Chen, Z.; Dong, Y.; Zhou, Z. Proteomic analysis reveals the important roles of alpha-5-collagen and ATP5β during skin ulceration syndrome progression of sea cucumber Apostichopus japonicus. J. Proteomics 2018, 175, 136–143. [Google Scholar] [CrossRef]
- Westerlund, B.; Korhonen, T.K. Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 1993, 9, 687–694. [Google Scholar] [CrossRef]
- Arora, S.; Gordon, J.; Hook, M. Collagen binding proteins of gram-positive pathogens. Front. Microbiol. 2021, 12, 628798. [Google Scholar] [CrossRef]
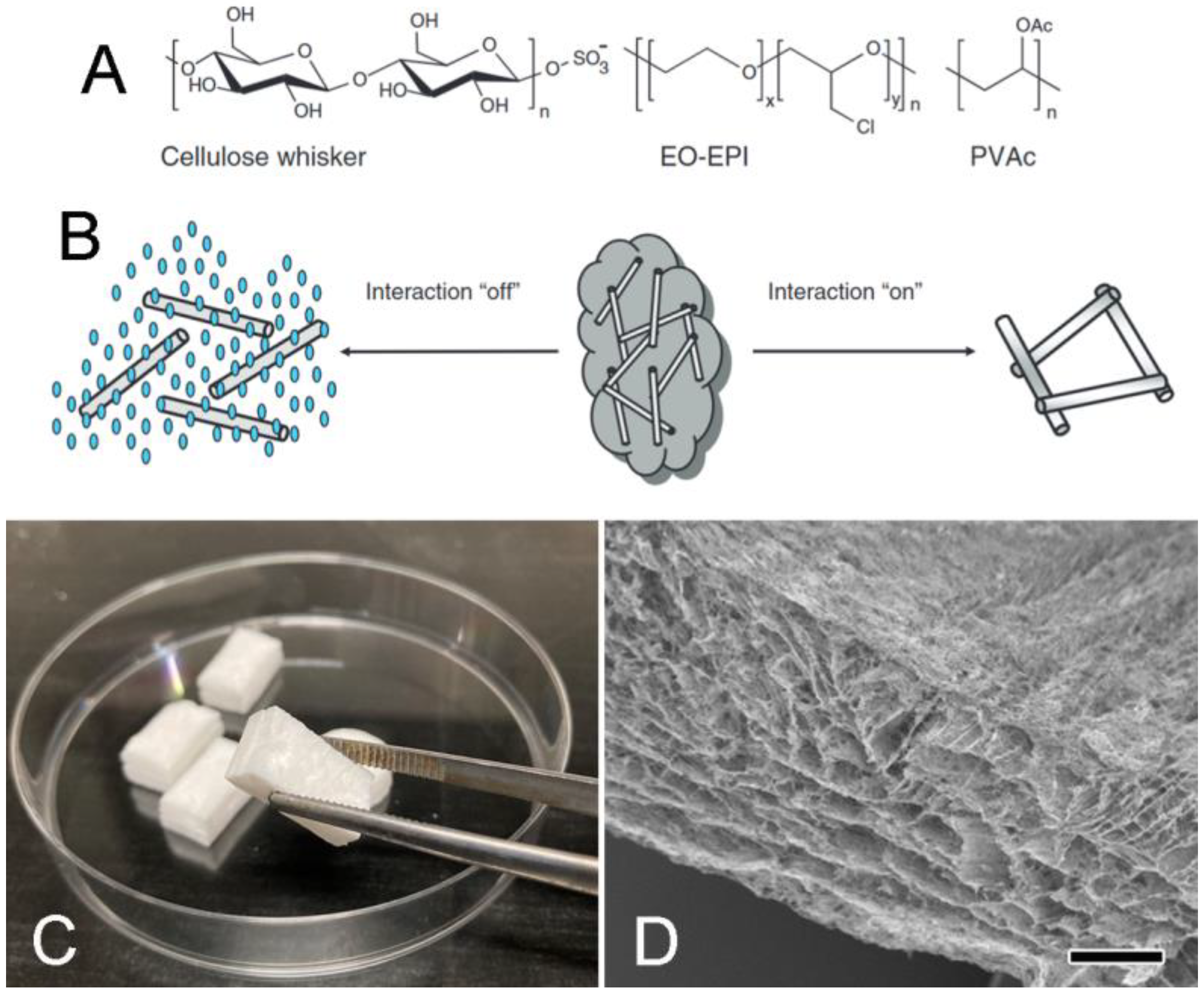
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; István, F.; Berglund, L.A.; Wohlert, J. Cellulose and the role of hydrogen bonds: Not in charge of everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Mendez, J.; Annamalai, P.K.; Eichhorn, S.J.; Rusli, R.; Rowan, S.J.; Foster, E.J.; Weder, C. Bioinspired mechanically adaptive polymer nanocomposites with water-activated shape-memory effect. Macromolecules 2011, 44, 6827–6835. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Cai, Z.; Qu, L.; Yu, T. Self-reinforced calcium phosphate cement inspired by sea cucumber dermis. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2024, 39, 682–688. [Google Scholar] [CrossRef]
- Ciulla, M.G.; Massironi, A.; Sugni, M.; Ensign, M.A.; Marzorati, S.; Forouharshad, M. Recent advances in the development of biomimetic materials. Gels 2023, 9, 833. [Google Scholar] [CrossRef]
- Lim, H.L.; Hwang, Y.; Kar, M.; Varghese, S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014, 2, 603–618. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Li, Y.; Xu, B.; Cao, Z.; Liu, W. Sea cucumber-inspired autolytic hydrogels exhibiting tunable high mechanical performances, repairability, and reusability. ACS Appl. Mater. Interfaces 2016, 8, 8956–8966. [Google Scholar] [CrossRef]
- Studart, A.R.; Erb, R.M. Bioinspired materials that self-shape through programmed microstructures. Soft Matter 2014, 10, 1284–1294. [Google Scholar] [CrossRef]
- Honda, S.; Toyota, T. Photo-triggered solvent-free metamorphosis of polymeric materials. Nat. Commun. 2017, 8, 502. [Google Scholar] [CrossRef]
- Montero De Espinosa, L.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired polymer systems with stimuli-responsive mechanical properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef]
- Moatsou, D.; Weder, C. Mechanically adaptive nanocomposites inspired by sea cucumbers. In Bio-Inspired Polymers; Bruns, N., Kilbinger, A.F.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2017; pp. 402–428. [Google Scholar]
- Charaklias, D.; Qiang, D.; Dorey, R.; Mohagheghian, I. Rapid de-stiffening of multilayer transparent structures using controlled thermoplastic softening. Smart Mater. Struct. 2023, 32, 115020. [Google Scholar] [CrossRef]
- Choi, A.; Han, H.; Kim, D.S. A programmable powerful and ultra-fast water-driven soft actuator inspired by the mutable collagenous tissue of the sea cucumber. J. Mater. Chem. A 2021, 9, 15937–15947. [Google Scholar] [CrossRef]
- Liu, K.; Cheng, L.; Zhang, N.; Pan, H.; Fan, X.; Li, G.; Zhang, Z.; Zhao, D.; Zhao, J.; Yang, X.; et al. Biomimetic impact protective supramolecular polymeric materials enabled by quadruple H-bonding. J. Am. Chem. Soc. 2021, 143, 1162–1170. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Coccè, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Candia Carnevali, M.D.; et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef]
- Di Benedetto, C.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Candia Carnevali, M.D.; et al. Production, characterization and biocompatibility of marine collagen matrices from an alternative and sustainable source: The sea urchin Paracentrotus lividus. Mar. Drugs 2014, 12, 4912–4933. [Google Scholar] [CrossRef]
- Sisican, K.M.D.; Torreno, V.P.M.; Yu, E.T.; Conato, M.T. Physicochemical and biochemical characterization of collagen from Stichopus cf. horrens tissues for use as stimuli-responsive thin films. ACS Omega 2023, 8, 35791–35799. [Google Scholar] [CrossRef]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From food waste to innovative biomaterial: Sea urchin-derived collagen for applications in skin regenerative medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef]
- Melotti, L.; Martinello, T.; Perazzi, A.; Iacopetti, I.; Ferrario, C.; Sugni, M.; Sacchetto, R.; Patruno, M. A prototype skin substitute, made of recycled marine collagen, improves the skin regeneration of sheep. Animals 2021, 11, 1219. [Google Scholar] [CrossRef]
- Carolo, A.; Melotti, L.; Zivelonghi, G.; Sacchetto, R.; Akyürek, E.E.; Martinello, T.; Venerando, A.; Iacopetti, I.; Sugni, M.; Martinelli, G.; et al. Mutable collagenous tissue isolated from echinoderms leads to the production of a dermal template that is biocompatible and effective for wound healing in rats. Mar. Drugs 2023, 21, 506. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioactive Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Goh, K.L.; Holmes, D.F. Collagenous extracellular matrix biomaterials for tissue engineering: Lessons from the common sea urchin tissue. Int. J. Mol. Sci. 2017, 18, 901. [Google Scholar] [CrossRef]
- Di Benedetto, C. Progenitor Cells and Regenerative Potential in Echinoderms: An In Vivo and In Vitro Approach; Lambert Academic Publishing: Saarbrücken, Germany, 2011. [Google Scholar]
- Clouse, R.M.; Linchangco, G.V.; Kerr, A.M.; Reid, R.W.; Janies, D.A. Phylotranscriptomic analysis uncovers a wealth of tissue inhibitor of metalloproteinases variants in echinoderms. R. Soc. Open Sci. 2015, 2, 150377. [Google Scholar] [CrossRef]
- Emlet, R.B. Morphological evolution of newly metamorphosed sea urchins—A phylogenetic and functional analysis. Integr. Comp. Biol. 2010, 50, 571–588. [Google Scholar] [CrossRef]
- Hamel, J.F.; Mercier, A. Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea). Can. J. Fish. Aquat. Sci. 1996, 53, 253–271. [Google Scholar] [CrossRef]
- Formery, L.; Peluso, P.; Rank, D.R.; Rokhsar, D.S.; Lowe, C.J. Anterio-posterior patterning in the brittle star Amphipholis squamata and the evolution of echinoderm body plans. EvoDevo 2025, 16, 7. [Google Scholar] [CrossRef]
- Brown, N.H. Extracellular matrix in development: Insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb. Perspect. Biol. 2011, 3, a005082. [Google Scholar] [CrossRef]
- Long, K.R.; Huttner, W.B. How the extracellular matrix shapes neural development. Open Biol. 2019, 9, 180216. [Google Scholar] [CrossRef]
- Nemes, P.; Knolhoff, A.M.; Rubakhin, S.S.; Sweedler, J.V. Single-cell metabolomics: Changes in the metabolome of freshly isolated and cultured neurons. ACS Chem. Neurosci. 2012, 3, 782–792. [Google Scholar] [CrossRef]
- McGrath, L.L.; Vollmer, S.V.; Kaluziak, S.T.; Ayers, J. De novo transcriptome assembly for the lobster Homarus americanus and characterization of differential gene expression across nervous system tissues. BMC Genom. 2016, 17, 63. [Google Scholar] [CrossRef] [PubMed]

| Class and Species | Anatomical Component | Mutable Collagenous Structure | Tensile Change | Reference |
|---|---|---|---|---|
| Asteroidea | ||||
| Acanthaster planci | Spine joint | Dermis and SP ligament | R | [52] |
| Asterias rubens | Body wall | Dorsolateral dermis | R,Id | [53] |
| Pycnopodia helianthoides | Body wall | LI ligament | R,Id | [54] |
| Marthasterias glacialis | Tube foot | Dermis | R | [55] |
| Crinoidea | ||||
| Antedon bifida | Cirrus | Synathrial ligament | R | [56] |
| Antedon mediterranea | Cirrus | Synostosal ligament | Id | [57] |
| Cenocrinus asterius | Stalk | Symplexal/PTG ligaments | R | [58] |
| Cenocrinus asterius | Stalk | Synostosal ligament | Id | [58] |
| Antedon mediterranea | Arm | Diarthrial ligament | R | [59] |
| Antedon mediterranea | Arm | Syzygial ligament | Id | [59] |
| Himerometra robustipinna | Visceral mass | Tegmen and septa | Id | [60] |
| Echinoidea | ||||
| Anthocidaris crassispina | Spine joint | Capsular ligament | R | [61] |
| Diadema antillarum | Spine joint | Capsular ligament | Id* | [62] |
| Diadema setosum | Spine joint | Central ligament | R | [63] |
| Diadema setosum | Aristotle’s lantern | Tooth ligament | R | [64] |
| Paracentrotus lividus | Aristotle’s lantern | Compass depressor ligament | R | [65] |
| Paracentrotus lividus | Tube foot | Dermis | R | [55] |
| Paracentrotus lividus | Body wall | Peristomial membrane | R | [66] |
| Echinus esculentus | Body wall | Periproctal dermis | R | [67] |
| Lytechinus variegatus | Globiferous pedicellaria | Head-stalk ligament | Id | [68] |
| Holothuroidea | ||||
| Stichopus chloronotus | Main body wall | Dermis | R | [69] |
| Stichopus chloronotus | Main body wall | Dermis | Id* | [25] |
| Eupentacta quinquesemita | Introvert body wall | Dermis | Id | [70] |
| Eupentacta quinquesemita | LBWM | PRM-LBWM tendon | Id | [70] |
| Holothuria forskali | Cuvierian tubule | Inner connective tissue layer | Is | [71] |
| Ophiuroidea | ||||
| Ophiocomella ophiactoides | Dorsal disk body wall | Dermis | Id | [72] |
| Ophiomastix lütkeni | Arm | Intervertebral ligament | R | [73] |
| Ophiocomina nigra | Arm | Intervertebral ligament | R,Id | [74] |
| Ophiocomina nigra | Arm | IM autotomy tendon | Id | [75] |
| Ophiura ophiura | Arm | Distal ventral APL | R,Id | [76] |
| Ophiura ophiura | Arm | Proximal ventral APL | R | [76] |
| Ophiophragmus filograneus | Arm | Genital plate-lateral APL | Id | [77] |
| Ophiocomina nigra | Arm spine joint | Spine ligament | R,Id* | [78] |
| Ophioderma longicaudum | Ventral disk body wall | Oral shield-oral plate ligament | R | [79] |
| Class and Species | Anatomical Component | Collagenous Structure | Reference |
|---|---|---|---|
| Echinoidea | |||
| Echinus esculentus | Aristotle’s lantern | Compass-rotular ligament | [80] |
| Eucidaris tribuloides | Spine joint | Central ligament | [81] |
| Ophiuroidea | |||
| Ophiocomina nigra | Arm | Intervertebral muscle non-autotomy tendon | [75] |
| Ophioderma longicaudum | Interbrachial frame | Radial and interradial oral plate ligaments | [79] |
| Class and Species | JLC Component | Location | LDCV Type (a) | LDCV Type (b) | Reference | ||
|---|---|---|---|---|---|---|---|
| Shape | Size (nm) | Shape | Size (nm) | ||||
| Asteroidea | |||||||
| Asterias rubens | Somata | LIL | c-o | ≤250 × 380 | c | ≤540 | [86] |
| Processes | LIL | c-o | ≤230 × 360 | c | ≤540 | ||
| Marthasterias glacialis | Somata | TF outer CT sheath | ca. 150 | ca. 250 | [55] [113] | ||
| Processes | TF outer CT sheath | c-o | ca. 150 | ca. 250 | |||
| Crinoidea | |||||||
| Decametra sp. | Somata | Stereom spaces | c | ca. 100 | o | ca. 180 | [114] |
| Processes | Diarthrial arm ligaments | c | ca. 100 | o | ca. 180 | ||
| Metacrinus rotundus | Somata | Stereom spaces | c | 150–200 | c | 600 | [115] |
| Processes | Stalk ligaments | c | 150–200 | c | 600 | ||
| Echinoidea | |||||||
| Arbacia punctulata | Somata | Inside + outside CL | c-o | ≤320 | [116] | ||
| Processes | Inside + outside CL | c-o | ≤220/330 | ||||
| Diadema setosum | Somata | Inside CSL | c-o | 100–500 | [63] | ||
| Processes | Inside CSL | c-o | 100–500 | ||||
| Holothuroidea | |||||||
| Holothuria forskali | Somata | Cuvierian tubule CT | c | 200–400 | c-o | 300–600 | [71] |
| Processes | Cuvierian tubule CT | c | 200–400 | c-o | 300–600 | ||
| Stichopus chloronotus | Somata | Body wall dermis | o-cr | ≤1400 | [107] | ||
| Processes | Body wall dermis | c-o | ≤300 × 700 | ||||
| Ophiuroidea | |||||||
| Ophiocomina nigra | Somata | IL JLNs | c | ≤160 | c-o | ≤210 × 460 | [74,106] |
| Processes | Intervertebral ligament | c | ≤300–400 | c-o | ≤250 × 750 | ||
| Ophiocomina nigra | Somata | Spine ligament JLN | c | ≤150 | c-o | ≤220 × 310 | [78] |
| Processes | Spine ligament | c | ≤200 | c-o | ≤270 × 520 | ||
| Class and Species | Anatomical Component | Collagenous Structure | CSA (%) | Reference |
|---|---|---|---|---|
| Asteroidea | ||||
| Acanthaster planci | Spine joint | Dermis and SP ligament | <2–ca. 30 1 | [129] |
| Echinoidea | ||||
| Anthocidaris crassispina | Spine joint | Capsular ligament | 3 | [61] |
| Eucidaris tribuloides | Spine joint | Capsular ligament | 1.5 | [81] |
| Paracentrotus lividus | Aristotle’s lantern | Compass depressor ligament | 8 | [65] |
| Stylocidaris affinis | Aristotle’s lantern | Compass depressor ligament | 13 | [130] |
| Holothuroidea | ||||
| Eupentacta quinquesemita | Introvert body wall | Dermis | 1–4 | [110] |
| Class and Species | Mutable Collagenous Structure | Stress 1 MPa | Coefficient of Viscosity MPa·s | Strain at Breakage | Reference |
|---|---|---|---|---|---|
| A. Echinodermata | |||||
| Asteroidea | |||||
| Asterias rubens | Basal aboral body wall 2 | --- | 0.5 | --- | [53] |
| Asterias rubens | Distal aboral body wall 2 | --- | 1.4 | --- | [53] |
| Echinoidea | |||||
| Diadema setosum | Central spine ligament | ca. 0.1 | 20–6000 | 0.8–3.7 | [63] |
| Paracentrotus lividus | Compass depressor ligament | 0.17–1.16 | 104–1477 | 1.41–4.54 | [136] |
| Holothuroidea | |||||
| Actinopyga echinites | Dermis | 0.056 | 100 | --- | [137] |
| Apostichopus japonicus | Dermis | 0.3–3.0 | 0.076–35 | --- | [138] |
| Eupentacta quinquesemita | Introvert dermis | 0.03 | ca. 100 | ≤9 | [70] |
| Holothuria leucospilota | Dermis | 0.056 | 11 | --- | [137] |
| Stichopus chloronotus | Dermis (untreated, K-stiffened) | 6 × 10−5 | 0.06, 1.25 | --- | [139] |
| Thyone inermis | Dermis 3 (untreated, DW-stiffened) | --- | 5100, 15,800 | --- | [140] |
| Ophiuroidea | |||||
| Ophiocomella ophiactoides | Aboral disk body wall 2 | --- | ca. 1000 | --- | [72] |
| Ophiocomina nigra | Intervertebral ligament | 0.2–1.1 | 500–7100 | 0.6–3.0 | [74] |
| B. Chordata | |||||
| Mammalia | |||||
| Homo sapiens | Anterior CL, posterior CL | --- | 39.29, 48.67 | --- | [141] |
| Homo sapiens | Patellar tendon | --- | 438.1 | --- | [141] |
| Mus musculus | Skin 4 | --- | 1375 | --- | [142] |
| Rattus norvegicus | Uterine cervix (pregnant) | 0.029 | 128.9 | --- | [143] |
| Class and Species | Mutable Collagenous Structure | Strain Rate s−1 | Elastic Stiffness MPa | Ultimate Stress MPa | Ultimate Strain | Reference |
|---|---|---|---|---|---|---|
| A. Echinodermata | ||||||
| Asteroidea | ||||||
| Asterias rubens | Aboral body wall 1 (anesthetized) | 0.03 | 2.48 | 0.68 | 0.49 | [145] |
| Echinaster spinulosus | Aboral body wall 1 (stiffened by handling) | 0.01–0.02 | 249–353 | 37–45 | 12–15 | [83] |
| Coscinasterias calamaria | Aboral body wall 1 (anesthetized) | 0.09–0.24 | 8.6–10.6 | 3.27 | 0.43 | [146] |
| Linckia laevigata | Dermis 1 | 0.0004, 0.004 | 20.9, 36.0 | 3.65, 6.27 | 30.5, 23.0 | [147] |
| Crinoidea | ||||||
| Metacrinus rotundus | Aboral brachial ligament | --- | 0.4–15.9 | --- | --- | [148] |
| Echinoidea | ||||||
| Anthocidaris crassispina | Capsular spine ligament (ACh-stiffened) | ca. 0.003–0.29 | 230–420 | 18–38 | ca. 0.3 | [61] |
| Eucidaris tribuloides | Capsular spine ligament | 0.006 | 25 | 8 | --- | [149] |
| Eucidaris tribuloides | Capsular spine ligament (stiff state) | --- | 200 | --- | --- | [150] |
| Paracentrotus lividus | Compass depressor ligament | 0.003–0.250 | 3.3–44.2 | 1.5–23.2 | 0.56–6.50 | [136] |
| Holothuroidea | ||||||
| Actinopyga echinites | Dermis (untreated, K-stiffened) | 1.5 | 1.67, 2.08 | --- | --- | [138] |
| Holothuria forskali | Cuvierian tubule (quiescent, elongated) | --- | 0.044, 19.7 | 0.88, 3.2 | 2.0, 0.27 | [71] |
| Holothuria leucospilota | Dermis (untreated, K-stiffened) | 1.5 | 0.42, 0.59 | --- | --- | [138] |
| Stichopus chloronotus | Dermis (untreated, K-stiffened) | 1.5 | 0.024, 0.128 | 0.03, 0.115 | >12–13, --- | [139] |
| Ophiuroidea | ||||||
| Ophiocomina nigra | Intervertebral ligament (anesthetized) | --- | --- | 6.17 | --- | [74] |
| B. Chordata | ||||||
| Mammalia | ||||||
| Bos taurus | Digital extensor tendon | 0.1 | 639 | 95.7 | 0.231 | [151] |
| Homo sapiens | Dermis (cryopreserved) | 0.1, 10 | 15–41, 7–45 | --- | --- | [152] |
| Homo sapiens | Spine anterior longitudinal ligament | 0.5 | 50 | 32 | 1.15 | [153] |
| Rattus norvegicus | Tail tendon | 0.0013 | 1304 | 40–80 | 0.05–0.17 | [154] |
| Species | Average VO2 (µL·g−1·h−1) ± SD | |||
|---|---|---|---|---|
| Dermis | Muscle | |||
| Standard | Stiff | Relaxed | Contracted | |
| Actinopyga mauritiana | 1.61 ± 0.51 | 2.45 ± 1.34 | 9.21 ± 3.28 | 23.5 ± 5.8; 18.4 ± 3.6 * |
| Linckia laevigata | 0.56 ± 0.12 | 0.78 ± 0.19 | 12.44 ± 5.54 | 29.30 ± 17.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkie, I.C.; Carnevali, M.D.C. Strength in Weakness: The Mutable Collagenous Tissue of Echinoderms. Encyclopedia 2025, 5, 185. https://doi.org/10.3390/encyclopedia5040185
Wilkie IC, Carnevali MDC. Strength in Weakness: The Mutable Collagenous Tissue of Echinoderms. Encyclopedia. 2025; 5(4):185. https://doi.org/10.3390/encyclopedia5040185
Chicago/Turabian StyleWilkie, Iain C., and M. Daniela Candia Carnevali. 2025. "Strength in Weakness: The Mutable Collagenous Tissue of Echinoderms" Encyclopedia 5, no. 4: 185. https://doi.org/10.3390/encyclopedia5040185
APA StyleWilkie, I. C., & Carnevali, M. D. C. (2025). Strength in Weakness: The Mutable Collagenous Tissue of Echinoderms. Encyclopedia, 5(4), 185. https://doi.org/10.3390/encyclopedia5040185






