Biosurfactants Produced by Yeasts: Environmental Roles and Biotechnological Applications
Abstract
1. Introduction
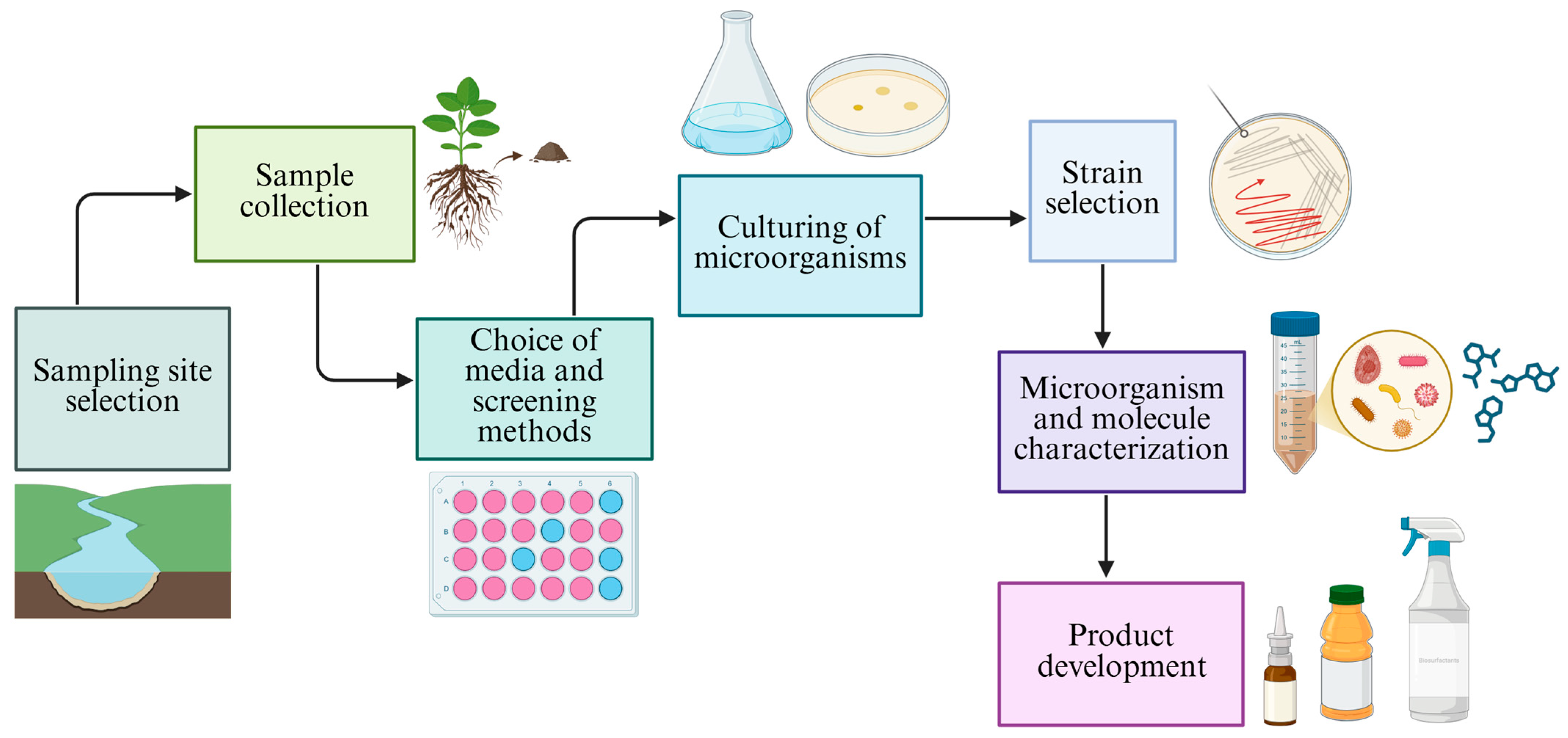
2. Bioprospecting for Biosurfactant-Producing Yeasts
3. Relationship Between Structural Diversity and Biological Role
3.1. Glycolipids and Glycoproteins
3.2. Lipopeptides
4. Biosurfactant Properties and Biotechnological Applications
| Biosurfactant | Yeast | Properties | Applications | References |
|---|---|---|---|---|
| Glycoprotein complex with low lipid content | Yarrowia lipolytica | Emulsification index of 61% or greater, emulsion stability at temperatures of 90–120 °C, stability at pH 2 to 10, and salinity up to 10%. CMC of 15 mg/mL, reduction in surface tension down to 43.8 mN/m. | Enhanced hydrocarbon recovery in contaminated sites. | [26] |
| Sophorolipid | Cutaneotrichosporon mucoides | Emulsification index of up to 70% with an emulsifying activity of 3.0 EU/mL. | NR | [55] |
| Sophorolipid | Metschnikowia dubei Metschnikowia shirgulensis | Surface tension reduction between 34–35.5 mN/m, CMC between 4–5 mg/mL, emulsification index ranging from 82–89%, remaining stable even at 121 °C and 10% NaCl. It possesses antifungal activity against F. solani and F. oxysporum. It induces apoptosis in cancer cells via the generation of reactive oxygen species (ROS). | Antioxidant, anticancer, antifungal (proven by in vitro assays). | [56] |
| Glycolipid | Aureobasidium thailandense | CMC of 550 mg/L, reduction in surface tension down to 31.2 mN/m. | Hydrocarbon recovery in contaminated sites. | [57] |
| Anionic Glycolipid | Geotrichum candidum, Galactomyces pseudocandidum and Candida tropicalis | Reduction in surface tension down to 51.6 mN/m, with emulsification index between 14 and 59.6%. | NR | [6] |
| Cybersan (Glycolipid) | Cyberlindnera saturnus | Antimicrobial activity (200 µg/mL), CMC of 30 mg/mL, reduction in surface tension down to 28 mN/m. Low cytotoxicity with cell viability greater than 70%. | Antimicrobial (proved in vitro assays). | [19] |
| Pullusurfactants A-E (myo-inositol lipids) | Aureobasidium pullulans | Reduction in surface tension from 22.4–32.3 mN/m, low cytotoxicity (>50 ppm). | Pharmaceutical and cosmetic products. | [58] |
| Anionic Lipopeptide | Candida lipolytica | No toxicity in seeds and Artemia salina. CMC of 0.03%, with a surface tension reduction down to 25 mN/m. | Industrial and environmental applications. | [59] |
| Mannosylerythritol lipids-B | NR | At a concentration of 31.6 mg/L, it promotes surface wettability, modifying the contact angle of the water drop. At 158 mg/L, it promotes lettuce seed germination and root growth. | Bioestimulant | [60] |
| Rufisan | Candida lipolytica | CMC of 0.03%, with a surface tension reduction down to 25 mN/m. Antimicrobial activity against Streptococcus agalactiae and Streptococcus mutans strains. Anti-adhesive activity in Lactobacillus, S. epidermidis, E. coli, C. albicans, and P. aeruginosa strains. | Antimicrobial and anti-adhesive (proven by in vitro assays). | [34] |
| Glycolipid | Candida bombicola | Surface tension reduction to 29–30 mN/m, CMC of 0.5%, interfacial tension of 3.5 mN/m, emulsification index between 49–58%. No toxic effects on seeds or significant cytotoxicity. | Thickener for sauces and foods. | [61] |
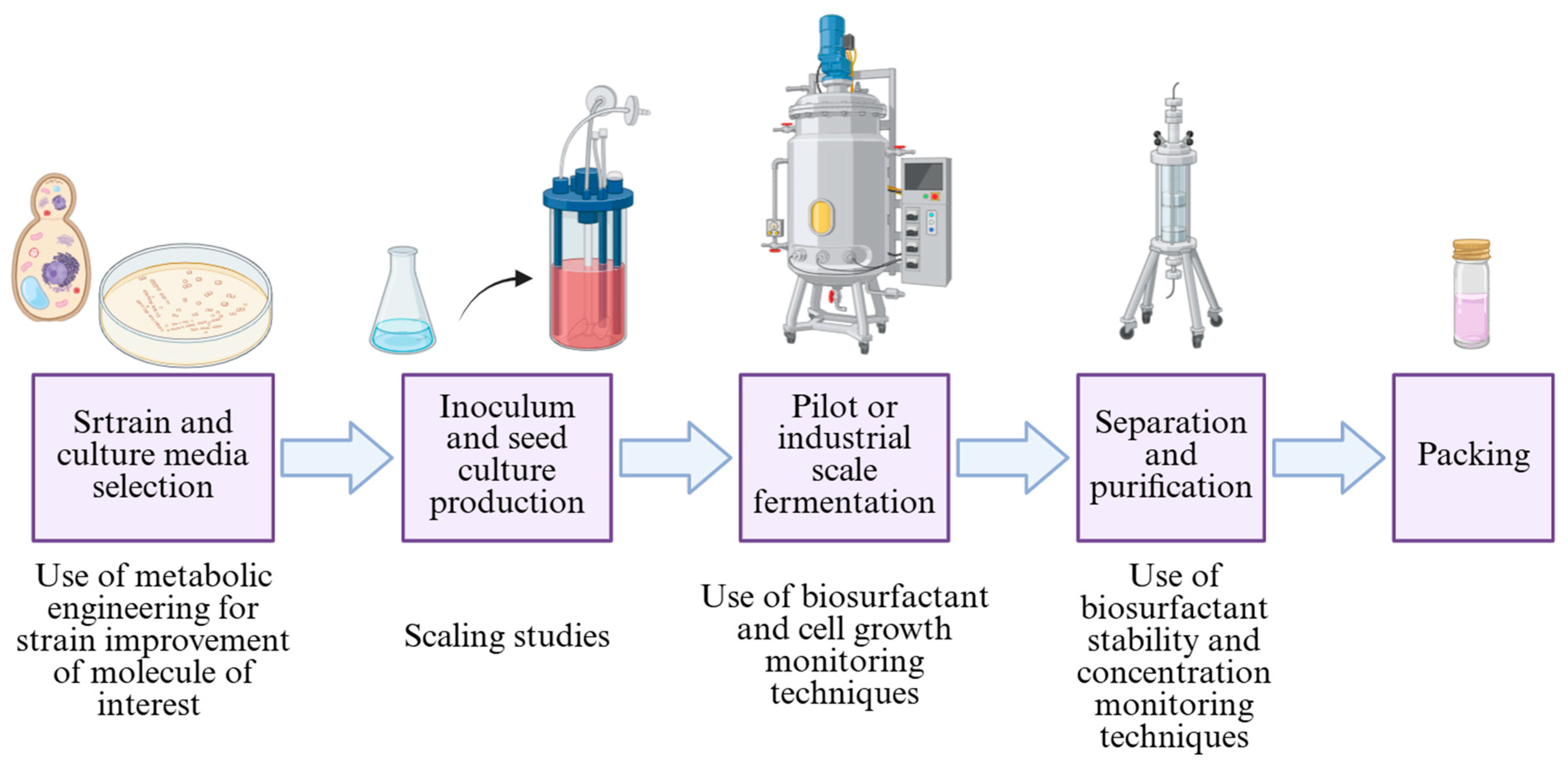
5. Biosurfactant Production and Product Formulations
- Strain selection and bioprospecting: Securing hyper-producing strains, preferably isolated from extreme environments, which impart the capacity to manage them under variable conditions of pH, temperature, salinity, and nutrient scarcity. Although their use is advantageous for industrial-scale conditions, the bioprospecting of these strains poses significant challenges when implementing appropriate isolation and screening methodologies.
- Use of sustainable feedstock: The utilization of media or media components derived from industrial waste streams, while promoting process sustainability, presents substantial challenges in terms of conducting precise media characterization. Furthermore, this complicates the separation of residual matter from the final products, thus increasing the cost of purification procedures.
- Fermentation strategies and bioreactor control: Achieving high biosurfactant concentrations during fermentation is hindered by a scarcity of studies on optimal cultivation strategies (e.g., batch, fed-batch, or continuous cultures) and suitable bioreactor configurations. Most operations involve submerged cultures with mechanical agitation, which introduces a significant technical challenge related to controlling the foaming generated by the biosurfactant itself. This foam phenomenon can significantly impact mass and energy transfer phenomena.
- Process scale-up: The scale-up of bioprocesses constitutes a significant hurdle when aiming to develop a process capable of handling a high volume of culture broth and achieving a high concentration of the final product without the excessive consumption of solvents.
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBLs | Cellobiose lipids |
| CMC | Critical Micelle Concentration |
| C/N | Carbon/nitrogen |
| GRAS | Generally Recognized As Safe |
| IC50 | Half Maximal Inhibitory Concentration |
| MELs | Mannosylerythritol lipids |
References
- Konishi, M.; Maruoka, N.; Furuta, Y.; Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Biosurfactant-producing yeasts widely inhabit various vegetables and fruits. Biosci. Biotechnol. Biochem. 2014, 78, 516–523. [Google Scholar] [CrossRef]
- Deak, T. Environmental factors influencing yeasts. In Biodiversity and Ecophysiology of Yeasts; Springer: Berlin/Heidelberg, Germany, 2006; pp. 155–174. [Google Scholar]
- Rawat, G.; Dhasmana, A.; Kumar, V. Biosurfactants: The next generation biomolecules for diverse applications. Environ. Sustain. 2020, 3, 353–369. [Google Scholar] [CrossRef]
- Amaral, P.F.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A. Biosurfactants from yeasts: Characteristics, production and application. In Biosurfactants; Springer: New York, NY, USA, 2010; pp. 236–249. [Google Scholar]
- Souza, K.S.T.; Gudiña, E.J.; Azevedo, Z.; de Freitas, V.; Schwan, R.F.; Rodrigues, L.R.; Teixeira, J.A. New glycolipid biosurfactants produced by the yeast strain Wickerhamomyces anomalus CCMA 0358. Colloids Surf. B Biointerfaces 2017, 154, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Eldin, A.M.; Kamel, Z.; Hossam, N. Isolation and genetic identification of yeast producing biosurfactants, evaluated by different screening methods. Microchem. J. 2019, 146, 309–314. [Google Scholar] [CrossRef]
- Sakaki, T.; Zähringer, U.; Warnecke, D.C.; Fahl, A.; Knogge, W.; Heinz, E. Sterol glycosides and cerebrosides accumulate in Pichia pastoris, Rhynchosporium secalis and other fungi under normal conditions or under heat shock and ethanol stress. Yeast 2001, 18, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Campos-Takaki, G.M.; Sarubbo, L.A.; Albuquerque, C.D.C. Environmentally friendly biosurfactants produced by yeasts. In Biosurfactants; Springer: New York, NY, USA, 2010; pp. 250–260. [Google Scholar]
- Ramdass, A.C.; Rampersad, S.N. Detection and diversity of the mannosylerythritol lipid (MEL) gene cluster and lipase A and B genes of Moesziomyces antarcticus isolated from terrestrial sites chronically contaminated with crude oil in Trinidad. BMC Microbiol. 2022, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef]
- Indira, M.; Venkateswarulu, T.C.; Krupanidhi, S.; Peele, K.A. Microbial Production of Antimicrobial and Anticancerous Biomolecules. In Bioprospecting of Microorganism-Based Industrial Molecules; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 147–169. [Google Scholar]
- Kaur, K.; Sangwan, S.; Kaur, H. Biosurfactant production by yeasts isolated from hydrocarbon polluted environments. Environ. Monit. Assess. 2017, 189, 603. [Google Scholar] [CrossRef]
- Songdech, P.; Jayasekara, L.C.B.; Watchaputi, K.; Butkinaree, C.; Yingchutrakul, Y.; Soontorngun, N. Elucidating a novel metabolic pathway for enhanced antimicrobial glycolipid biosurfactant production in the yeast Meyerozyma guilliermondii. Sci. Rep. 2025, 15, 18233. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Waghmode, S.R.; Hulkane, S.; Bagayatkar, M.; Chakraborty, R. Screening strategies and production of biosurfactants (BSs)/bioemulsifiers (BEs) from marine yeasts and fungi. AIMS Microbiol. 2025, 11, 542–573. [Google Scholar] [CrossRef]
- Georgescu, A.M.; Corbu, V.M.; Csutak, O. Molecular basis of yeasts antimicrobial activity—Developing innovative strategies for biomedicine and biocontrol. Curr. Issues Mol. Biol. 2024, 46, 4721–4750. [Google Scholar] [CrossRef]
- Mamaev, D.; Zvyagilskaya, R. Yarrowia lipolytica: A multitalented yeast species of ecological significance. FEMS Yeast Res. 2021, 21, foab008. [Google Scholar] [CrossRef]
- Segal-Kischinevzky, C.; Romero-Aguilar, L.; Alcaraz, L.D.; López-Ortiz, G.; Martínez-Castillo, B.; Torres-Ramírez, N.; González, J. Yeasts inhabiting extreme environments and their biotechnological applications. Microorganisms 2022, 10, 794. [Google Scholar] [CrossRef]
- Claus, S.; Jezierska, S.; Elbourne, L.D.; Van Bogaert, I. Exploring the transportome of the biosurfactant producing yeast Starmerella bombicola. BMC Genom. 2022, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Physicochemical, structural and biological evaluation of Cybersan (trigalactomargarate), a new glycolipid biosurfactant produced by a marine yeast, Cyberlindnera saturnus strain SBPN-27. Process. Biochem. 2019, 80, 171–180. [Google Scholar] [CrossRef]
- Perfumo, A.; Banat, I.M.; Marchant, R. Going green and cold: Biosurfactants from low-temperature environments to biotechnology applications. Trends Biotechnol. 2018, 36, 277–289. [Google Scholar] [CrossRef]
- Martorell, M.M.; Ruberto, L.A.M.; Fernández, P.M.; Castellanos de Figueroa, L.I.; Mac Cormack, W.P. Bioprospection of cold-adapted yeasts with biotechnological potential from Antarctica. J. Basic Microbiol. 2017, 57, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Chaves, F.D.S.; Brumano, L.P.; Franco Marcelino, P.R.; da Silva, S.S.; Sette, L.D.; Felipe, M.D.G.D.A. Biosurfactant production by Antarctic-derived yeasts in sugarcane straw hemicellulosic hydrolysate. Biomass Convers. Biorefinery 2023, 13, 5295–5305. [Google Scholar] [CrossRef]
- Bueno, J.D.L.; Santos, P.A.D.; da Silva, R.R.; Moguel, I.S.; Pessoa, A., Jr.; Vianna, M.V.; Gurpilhares, D.D.B. Biosurfactant production by yeasts from different types of soil of the South Shetland Islands (Maritime Antarctica). J. Appl. Microbiol. 2019, 126, 1402–1413. [Google Scholar] [CrossRef]
- Handa, S.; Aggarwal, Y.; Puri, S.; Chatterjee, M. Pharmaceutical prospects of biosurfactants produced from fungal species. J. Basic Microbiol. 2022, 62, 1307–1318. [Google Scholar] [CrossRef]
- Yalçın, H.T.; Ergin-Tepebaşı, G.; Uyar, E. Isolation and molecular characterization of biosurfactant producing yeasts from the soil samples contaminated with petroleum derivatives. J. Basic Microbiol. 2018, 58, 782–792. [Google Scholar] [CrossRef]
- de Oliveira Barros, V.P.; Silva, J.R.M.; Melo, V.M.M.; Terceiro, P.S.; de Oliveira, I.N.; de Freitas, J.D.; Landell, M.F. Biosurfactants production by marine yeasts isolated from zoanthids and characterization of an emulsifier produced by Yarrowia lipolytica LMS 24B. Chemosphere 2024, 355, 141807. [Google Scholar] [CrossRef] [PubMed]
- Loeto, D.; Jongman, M.; Lekote, L.; Muzila, M.; Mokomane, M.; Motlhanka, K.; Ndlovu, T.; Zhou, N. Biosurfactant production by halophilic yeasts isolated from extreme environments in Botswana. FEMS Microbiol. Lett. 2021, 368, fnab146. [Google Scholar] [CrossRef]
- Sharma, J.; Sundar, D.; Srivastava, P. Biosurfactants: Potential agents for controlling cellular communication, motility, and antagonism. Front. Mol. Biosci. 2021, 8, 727070. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants: Minireview. Environ. Microbiol. 2001, 3, 229–236. [Google Scholar] [CrossRef]
- Coelho, A.L.S.; Feuser, P.E.; Carciofi, B.A.M.; de Andrade, C.J.; de Oliveira, D. Mannosylerythritol lipids: Antimicrobial and biomedical properties. Appl. Microbiol. Biotechnol. 2020, 104, 2297–2318. [Google Scholar] [CrossRef]
- Olasanmi, I.O.; Thring, R.W. The role of biosurfactants in the continued drive for environmental sustainability. Sustainability 2018, 10, 4817. [Google Scholar] [CrossRef]
- Matvyeyeva, O.L.; Vasylchenko, O.A.; Aliieva, O.R. Microbial biosurfactants role in oil products biodegradation. Int. J. Environ. Bioremediation Biodegrad. 2014, 2, 69–74. [Google Scholar]
- Kubicki, S.; Bollinger, A.; Katzke, N.; Jaeger, K.E.; Loeschcke, A.; Thies, S. Marine biosurfactants: Biosynthesis, structural diversity and biotechnological applications. Mar. Drugs 2019, 17, 408. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; Campos-Takaki, D.G. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Colloids Surf. B Biointerfaces 2011, 84, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.; Jenkins Sánchez, L.; Van Bogaert, I.N.A. The role of transport proteins in the production of microbial glycolipid biosurfactants. Appl. Microbiol. Biotechnol. 2021, 105, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Stephanopoulos, G. Simple glycolipids of microbes: Chemistry, biological activity and metabolic engineering. Synth. Syst. Biotechnol. 2018, 3, 3–19. [Google Scholar]
- Kulakovskaya, E.; Kulakovskaya, T. Chapter 5—Metabolism of Yeast Extracellular Glycolipids. In Extracellular Glycolipids of Yeasts; Kulakovskaya, E., Kulakovskaya, T., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 65–74. ISBN 9780124200692. [Google Scholar]
- Joshi-Navare, K.; Singh, P.K.; Prabhune, A.A. New yeast isolate Pichia caribbica synthesizes xylolipid biosurfactant with enhanced functionality. Eur. J. Lipid Sci. Technol. 2014, 116, 1070–1079. [Google Scholar] [CrossRef]
- de Almeida, J.D.; Nascimento, M.F.; Keković, P.; Ferreira, F.C.; Faria, N.T. Unlocking the potential of mannosylerythritol lipids: Properties and industrial applications. Fermentation 2024, 10, 246. [Google Scholar] [CrossRef]
- Ceresa, C.; Hutton, S.; Lajarin-Cuesta, M.; Heaton, R.; Hargreaves, I.; Fracchia, L.; De Rienzo, M.A.D. Production of mannosylerythritol lipids (MELs) to be used as antimicrobial agents against S. aureus ATCC 6538. Curr. Microbiol. 2020, 77, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.H.; Lee, E.S.; Yoo, J.W.; Lee, S.H.; Ko, J.Y.; Kim, Y.J.; Lee, T.R.; Kim, D.Y.; Lee, C.S. Mannosylerythritol lipids inhibit melanogenesis via suppressing ERK-CREB-MiTF-tyrosinase signalling in normal human melanocytes and a three-dimensional human skin equivalent. Exp. Dermatol. 2019, 28, 738–741. [Google Scholar]
- Beck, A.; Haitz, F.; Thier, I.; Siems, K.; Jakupovic, S.; Rupp, S.; Zibek, S. Novel mannosylerythritol lipid biosurfactant structures from castor oil revealed by advanced structure analysis. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab042. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.; Liu, S. Promising application, efficient production, and genetic basis of mannosylerythritol lipids. Biomolecules 2024, 14, 557. [Google Scholar] [CrossRef]
- KaKachrimanidou, V.; Alexandri, M.; Nascimento, M.F.; Alimpoumpa, D.; Faria, N.T.; Papadaki, A.; Ferreira, F.C.; Kopsahelis, N. Lactobacilli and moesziomyces biosurfactants: Toward a closed-loop approach for the dairy industry. Fermentation 2022, 8, 517. [Google Scholar] [CrossRef]
- Sunde, M.; Pham, C.L.; Kwan, A.H. Molecular characteristics and biological functions of surface-active and surfactant proteins. Annu. Rev. Biochem. 2017, 86, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Kulakovskaya, T.V.; Shashkov, A.S.; Kulakovskaya, E.V.; Golubev, W.I. Characterization of an antifungal glycolipid secreted by the yeast Sympodiomycopsis paphiopedili. FEMS Yeast Res. 2004, 5, 247–252. [Google Scholar] [CrossRef]
- Sen, S.; Borah, S.N.; Bora, A.; Deka, S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb. Cell Factories 2017, 16, 95. [Google Scholar] [CrossRef]
- Jamal, A.; Ali, M.I.; Badshah, M.; Masood, A.B. Role of biosurfactants in agriculture management. In Advancements in Biosurfactants Research; Springer International Publishing: Cham, Switzerland, 2023; pp. 277–308. [Google Scholar]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Rufino, R.D.; Rodrigues, G.I.B.; Campos-Takaki, G.M.; Sarubbo, L.A.; Ferreira, S.R.M. Application of a yeast biosurfactant in the removal of heavy metals and hydrophobic contaminant in a soil used as slurry barrier. Appl. Environ. Soil Sci. 2011, 2011, 939648. [Google Scholar] [CrossRef]
- Keković, P.; Borges, M.; Faria, N.T.; Ferreira, F.C. Towards Mannosylerythritol Lipids (MELs) for Bioremediation: Effects of NaCl on M. antarcticus Physiology and Biosurfactant and Lipid Production; Ecotoxicity of MELs. J. Mar. Sci. Eng. 2022, 10, 1773. [Google Scholar] [CrossRef]
- Chaprão, M.J.; Ferreira, I.N.; Correa, P.F.; Rufino, R.D.; Luna, J.M.; Silva, E.J.; Sarubbo, L.A. Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand. Electron. J. Biotechnol. 2015, 18, 471–479. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, H.; Zeng, F.; Jiang, L.; Atakpa, E.O.; Chen, G.; Xie, Q. A biosurfactant-producing yeast Rhodotorula sp. CC01 utilizing landfill leachate as nitrogen source and its broad degradation spectra of petroleum hydrocarbons. World J. Microbiol. Biotechnol. 2022, 38, 68. [Google Scholar] [CrossRef]
- Derguine-Mecheri, L.; Kebbouche-Gana, S.; Djenane, D. Biosurfactant production from newly isolated Rhodotorula sp. YBR and its great potential in enhanced removal of hydrocarbons from contaminated soils. World J. Microbiol. Biotechnol. 2021, 37, 18. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, P.R.F.; Peres, G.F.D.; Terán-Hilares, R.; Pagnocca, F.C.; Rosa, C.A.; Lacerda, T.M.; Da Silva, S.S. Biosurfactants production by yeasts using sugarcane bagasse hemicellulosic hydrolysate as new sustainable alternative for lignocellulosic biorefineries. Ind. Crops Prod. 2019, 129, 212–223. [Google Scholar] [CrossRef]
- Kumari, S.; Kumari, A.; Dhiman, A.; Mihooliya, K.N.; Raje, M.; Prasad, G.S.; Pinnaka, A.K. Unveiling the potential of novel Metschnikowia yeast biosurfactants: Triggering oxidative stress for promising antifungal and anticancer activity. Microb. Cell Factories 2024, 23, 245. [Google Scholar] [CrossRef]
- Meneses, D.P.; Gudiña, E.J.; Fernandes, F.; Gonçalves, L.R.; Rodrigues, L.R.; Rodrigues, S. The yeast-like fungus Aureobasidium thailandense LB01 produces a new biosurfactant using olive oil mill wastewater as an inducer. Microbiol. Res. 2017, 204, 40–47. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, I.K.; Yun, B.S. Pullusurfactans A–E, new biosurfactants produced by Aureobasidium pullulans A11211-4-57 from a fleabane, Erigeron annus (L.) pers. J. Antibiot. 2018, 71, 920–926. [Google Scholar] [CrossRef]
- Rufino, R.D.; de Luna, J.M.; de Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. [Google Scholar] [CrossRef]
- Matosinhos, R.D.; Cesca, K.; Carciofi, B.A.M.; de Oliveira, D.; de Andrade, C.J. The biosurfactants mannosylerythritol lipids (mels) as stimulant on the germination of Lactuca sativa L. Agriculture 2023, 13, 1646. [Google Scholar]
- Pinto, M.I.S.; Guerra, J.M.C.; Meira, H.M.; Sarubbo, L.A.; de Luna, J.M. A biosurfactant from Candida bombicola: Its synthesis, characterization and its application as a food emulsions. Foods 2022, 11, 561. [Google Scholar] [CrossRef]
- Kumari, A.; Kumari, S.; Prasad, G.S.; Pinnaka, A.K. Production of sophorolipid biosurfactant by insect derived novel yeast Metschnikowia churdharensis fa, sp. nov., and its antifungal activity against plant and human pathogens. Front. Microbiol. 2021, 12, 678668. [Google Scholar] [CrossRef]
- Manjusha, P.G.S.; Anannya, A.P.; Hameeda, B. Isolation and Characterization of Pichia Occidentalis MHY1: Biosurfactant-Producing Yeast with Plant Growth-Promoting Traits. Asian J. Microbiol. Biotechnol. Environ. Sci. 2025, 27, 359–367. [Google Scholar]
- Sharma, P.; Sangwan, S.; Singh, S.; Kaur, H. Microbial biosurfactants: An eco-friendly perspective for soil health management and environmental remediation. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 277–298. [Google Scholar]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure. Pharmaceutics 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska, E.; Adamczak, M. Evaluation of waste products in the synthesis of surfactants by yeasts. Chem. Pap. 2013, 67, 1113–1122. [Google Scholar] [CrossRef]
- Kitamoto, D.; Fukuoka, T.; Saika, A.; Morita, T. Glycolipid biosurfactants, mannosylerythritol lipids: Distinctive interfacial properties and applications in cosmetic and personal care products. J. Oleo Sci. 2022, 71, 1–13. [Google Scholar]
- Tancredi, M.; Carandente Coscia, C.; Russo Krauss, I.; D’Errico, G. Antioxidant Properties of Biosurfactants: Multifunctional Biomolecules with Added Value in Formulation Chemistry. Biomolecules 2025, 15, 308. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Potential food application of a biosurfactant produced by Saccharomyces cerevisiae URM 6670. Front. Bioeng. Biotechnol. 2020, 8, 434. [Google Scholar] [CrossRef]
- Campos, J.M.; Montenegro Stamford, T.L.; Sarubbo, L.A.; de Luna, J.M.; Rufino, R.D.; Banat, I.M. Microbial biosurfactants as additives for food industries. Biotechnol. Prog. 2013, 29, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Bippus, L.; Briem, A.K.; Beck, A.; Zibek, S.; Albrecht, S. Life cycle assessment for early-stage process optimization of microbial biosurfactant production using kinetic models—A case study on mannosylerythritol lipids (MEL). Front. Bioeng. Biotechnol. 2024, 12, 1347452. [Google Scholar] [CrossRef]
- Brumano, L.P.; Antunes, F.A.F.; Souto, S.G.; Dos Santos, J.C.; Venus, J.; Schneider, R.; da Silva, S.S. Biosurfactant production by Aureobasidium pullulans in stirred tank bioreactor: New approach to understand the influence of important variables in the process. Bioresour. Technol. 2017, 243, 264–272. [Google Scholar] [CrossRef]
- Sharma, P.; Sangwan, S.; Kaur, H. Process parameters for biosurfactant production using yeast Meyerozyma guilliermondii YK32. Environ. Monit. Assess. 2019, 191, 531. [Google Scholar] [CrossRef] [PubMed]
- Alfian, A.R.; Watchaputi, K.; Sooklim, C.; Soontorngun, N. Production of new antimicrobial palm oil-derived sophorolipids by the yeast Starmerella riodocensis sp. nov. against Candida albicans hyphal and biofilm formation. Microb. Cell Factories 2022, 21, 163. [Google Scholar] [CrossRef]
- Jezierska, S.; Claus, S.; Van Bogaert, I. Yeast glycolipid biosurfactants. FEBS Lett. 2018, 592, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, A.D.; Teke, G.M.; van Rensburg, E.; Pott, R.W. Bioprocess development for microbial production and purification of cellobiose lipids by the smut fungus Ustilago maydis DSM 4500. Bioprocess Biosyst. Eng. 2025, 48, 509–520. [Google Scholar] [CrossRef]
- Faria, N.T.; Nascimento, M.F.; Ferreira, F.A.; Esteves, T.; Santos, M.V.; Ferreira, F.C. Substrates of Opposite Polarities and Downstream Processing for Efficient Production of the Biosurfactant Mannosylerythritol Lipids from Moesziomyces spp. Appl. Biochem. Biotechnol. 2023, 195, 6132–6149. [Google Scholar]
- Valkenburg, A.D.; Ncube, M.Z.; Teke, G.M.; van Rensburg, E.; Pott, R.W. A review on the upstream production and downstream purification of mannosylerythritol lipids. Biotechnol. Bioeng. 2024, 121, 853–876. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, A.D.; Ncube, M.Z.; Teke, G.M.; van Rensburg, E.; Pott, R.W. Cellobiose lipids: Applications, production, and downstream processing. Trends Biotechnol. 2025, 43, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Luft, L.; Confortin, T.C.; Todero, I.; Zabot, G.L.; Mazutti, M.A. An overview of fungal biopolymers: Bioemulsifiers and biosurfactants compounds production. Crit. Rev. Biotechnol. 2020, 40, 1059–1080. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.G.; Soares da Silva, R.D.C.F.; Meira, H.M.; Brasileiro, P.P.F.; Silva, E.J.; Luna, J.M.; Sarubbo, L.A. Production, characterization and commercial formulation of a biosurfactant from Candida tropicalis UCP0996 and its application in decontamination of petroleum pollutants. Processes 2021, 9, 885. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Błońska-Sikora, E.M.; Kulik-Siarek, K.; Zhussupova, A.; Wrzosek, M. Bioferments and Biosurfactants as New Products with Potential Use in the Cosmetic Industry. Appl. Sci. 2024, 14, 3902. [Google Scholar] [CrossRef]
- Global Market Insights, Inc. 2025. Available online: https://www.gminsights.com/industry-analysis/biosurfactants-market-report (accessed on 30 September 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holguín-Salas, A.; Enríquez-Núñez, C.A.; Sáenz-Marta, C.I.; Nevárez-Moorillón, G.V. Biosurfactants Produced by Yeasts: Environmental Roles and Biotechnological Applications. Encyclopedia 2025, 5, 172. https://doi.org/10.3390/encyclopedia5040172
Holguín-Salas A, Enríquez-Núñez CA, Sáenz-Marta CI, Nevárez-Moorillón GV. Biosurfactants Produced by Yeasts: Environmental Roles and Biotechnological Applications. Encyclopedia. 2025; 5(4):172. https://doi.org/10.3390/encyclopedia5040172
Chicago/Turabian StyleHolguín-Salas, Alehlí, Carlos Andrés Enríquez-Núñez, Claudia Isabel Sáenz-Marta, and Guadalupe Virginia Nevárez-Moorillón. 2025. "Biosurfactants Produced by Yeasts: Environmental Roles and Biotechnological Applications" Encyclopedia 5, no. 4: 172. https://doi.org/10.3390/encyclopedia5040172
APA StyleHolguín-Salas, A., Enríquez-Núñez, C. A., Sáenz-Marta, C. I., & Nevárez-Moorillón, G. V. (2025). Biosurfactants Produced by Yeasts: Environmental Roles and Biotechnological Applications. Encyclopedia, 5(4), 172. https://doi.org/10.3390/encyclopedia5040172








