Abstract
Sustainable solutions to the use of petrochemical products have been increasingly sought after in recent years. While alternatives such as biofuels have been extensively explored and commercialized, major challenges remain in using heterogeneous feedstocks and scaling-up processes. Among biofuels, higher alcohols have recently gained renewed interest, especially in the context of upcycling agri-food residues and other industrial organic wastes. One of the higher alcohols produced via fermentation is butanol, which was developed over a century ago. However, the commercial production of butanol is still not widespread, although diverse feedstocks are readily available. Hydrolysis of the feedstocks and scale-up challenges in the fermentation and purification of butanol are recurring bottlenecks. This review addresses the current state of fermentative butanol production and opportunities to address scale-up challenges, including purification. With the significant interest and promise of precision fermentation, this review also addresses some of the recent advances and potential for enhanced fermentative butanol production.
1. Introduction
Our dependence on fossil fuels is immeasurable, and despite our best efforts to become less dependent, the availability of alternatives is still yet to supplant existing petrochemical-derived products. The staggering increase in primary energy consumption over many decades reinforces our astounding dependence on fossil fuels (Figure 1), which was only made more significant with the dawn of the Industrial Revolution [1,2,3]. Interestingly, in 2019, there was a decrease in primary energy consumption due to the COVID-19 pandemic caused by the SARS-CoV-2 coronavirus, which led to a global lockdown and a reduction in industrial activity and, indeed, human commute in general. However, the pandemic has also offered a glimpse into what a reduction in energy consumption might look like on the overall climate change outlook and humanity’s existence per se. One of the debatable questions was whether COVID-19 could lead to extinction, not because of the COVID-19 disease, but rather due to reduced industrial activity that could lead to temperature increases due to the lack of the dimming effects of aerosols generally associated with industrial activity protecting against such rapid increases in temperatures [4]. While this debate regarding the extinction of life is unlikely to be resolved in the near future, the increasing trend in primary energy consumption derived from fossil fuels since the dip observed in 2019 certainly calls for renewed perspectives into renewable sources of energy for environmental sustainability. Butanol, one of the most promising and widely studied alternatives, is worth revisiting in this context.
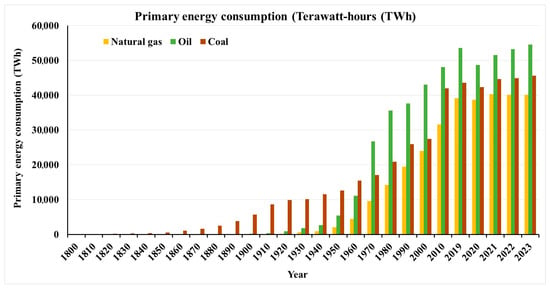
Figure 1.
Primary energy consumption, including natural gas, oil, and coal, over two centuries and into the 21st century (data sources: [2,3]).
While butanol can be chemically synthesized from petrochemical fossil fuels via the hydrolysis of haloalkanes or hydration of alkenes [5], sustainable or renewable sources for energy production have been a long-standing area of focus, particularly with respect to feedstocks and thermochemical or biological approaches. Biofuels, for example, have been routinely used to produce products such as biodiesel and ethanol from feedstocks such as lignocellulosic biomass [6]. The term “biofuels” has been applied to the use of biomass for the production of fuels, be it gas, liquid, or solid, including products such as biomethanol, bioethanol, biohydrogen, biodiesel, biochar, bio-synthetic gas, etc. [7]. Since biomass is a renewable alternative, it was perceived as an ideal substrate for the production of biofuels. Subsequently, the strategy of biofuel production shifted from chemical conversions to biological conversions using microorganisms. The use of microorganisms for the production of value-derived products using biomass is not new and has been in existence for millennia. Beer, for example, has been documented to have been produced and consumed as far back as 7000 years ago in China [8]. Archaeological excavations have revealed that a clay tablet dated back to 6000 BC with likely one of the oldest beer recipes inscribed on it in Mesopotamia [9]. Similarly, the earliest chemical evidence for wine from a Neolithic village’s pottery jar was dated to have been produced around 5400–5000 BC [10]. Whether this is intentional production is still debatable. However, archaeological evidence for an intentionally fermented beverage that was not produced by the well-established Vitis vinifera or its ancestors was found and dated over 7000 BC in a Neolithic area near the Yellow River in China [11]. Since then, the use and exploitation of microorganisms for the production of other value-derived products has slowly gained prominence to the extent that any organic residue can become a source of feedstock for microbial activity.
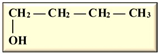
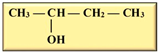
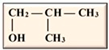
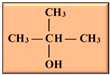
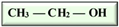
In our current context, the selected feedstocks for the production of the various biofuels have been categorized into generations based on their chronological usage, including the production of biobutanol, which is the focus of this review. Butanol has been seen as ideal due to its higher energy content, lower volatility, lower absorption of water, easier blending with gasoline and, importantly, its compatibility with conventional combustion engines compared to ethanol [12,13] (Table 1). Besides its potential application as an alternative to fossil fuels, butanol has had applications in the chemical industry for the production of methacrylate esters, butyl acrylate, butyl glycol ether, butyl acetate, and plasticizers. Globally, over 50% of the produced butanol is converted to acrylate and methacrylate esters [12,14]. According to Market Research Future, the estimated size of the biobutanol market in 2024 was USD 1.4 billion and is expected to increase from USD 1.18 billion in 2025 to USD 3.5 billion by 2034, with an expected compound annual growth rate (CAGR) of about 13.18% between the forecast period of 2025 to 2034 [15]. The optimism around biobutanol is due to the fact that technological advancements have allowed for reduced cost of production and higher efficiency at scale [15]. Nonetheless, there are still a limited number of industrial biobutanol manufacturing facilities globally [16]. Thus, the objective of this review is to offer some perspectives on biobutanol production, scale-up challenges, and the promise of precision fermentation. While fermentative aspects of biobutanol production have been exhaustively addressed, some of the recurring bottlenecks include inhibitory accumulation of the biobutanol, which precludes higher titers of butanol. Furthermore, many of the studies have focused on laboratory scale process optimization, which is unfortunately not amenable to scale-up. Similarly, metabolic engineering approaches to enhance biobutanol production have been conducted [17], including the use of synthetic biology [18]. Biobutanol production is also likely to receive further impetus due to extensive research efforts toward utilizing feedstocks from agri-food waste streams, which offer new perspectives into upcycling and sustainability approaches in a circular economy context. The development of engineered strains capable of more efficient acetone–butanol–ethanol (ABE) fermentation will certainly aid in the use of agri-food waste streams. However, engineered strains are not available for broader use and have not been reported to be commercially used. Therefore, general ABE fermentation is still dependent on available wild-type strains, which, as mentioned above, are prone to inhibition due to increasing concentrations of butanol during fermentation or other potential inhibitors arising from the side streams.

Table 1.
Isomers of butanol and other alcohols, as well as some of their characteristics (sources: [19,20,21]).
2. Chemical and Biological Synthesis of Butanol
The production of butanol from fossil fuels is certainly feasible [22]. However, such a pathway would not contribute to reduced dependence on fossil fuels, although it has been seen as more cost-effective with significantly higher efficiencies in terms of material and energy [23]. Notwithstanding this current economic discrepancy in chemical synthesis versus biological synthesis, there are other considerations that can be taken as to which particular approach would introduce more long-term sustainable and economical solutions (Figure 2). From a sustainability perspective, the abundance of lignocellulosic biomass augurs well for further investments into enhancing biobutanol production efficiency and lowering the cost of production.
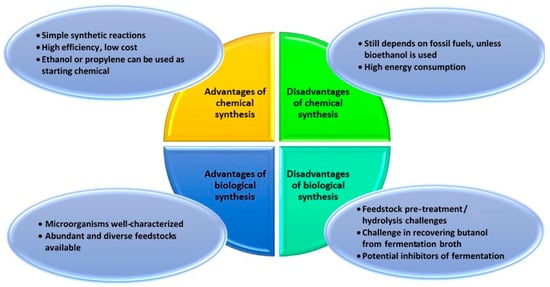
Figure 2.
Some advantages and disadvantages of chemical versus biological synthesis for the production of butanol.
Among the three chemical synthesis processes, viz., crotonaldehyde synthesis, Reppe synthesis, and oxo synthesis (also known as hydroformylation), established for butanol production [21,24] (Figure 3), crotonaldehyde synthesis was one of the earliest approaches up to the 1950s, only to be superseded by oxo synthesis [23]. Crotonaldehyde synthesis occurs via aldol condensation of two acetaldehyde molecules at room temperature and atmospheric pressure to produce 3-hydroxybutyraldehyde, the reaction being catalyzed by an alkali [23]. Subsequently, acidification with acetic acid or phosphoric acid results in a dehydration reaction, leading to the production of crotonaldehyde. Hydrogenation of the latter in the presence of a copper catalyst gives rise to butanol [21,23,24]. In Reppe synthesis, carbonylation of propylene occurs in the presence of carbon monoxide, hydrogen, and a catalyst, resulting in n-butanol and iso-butanol synthesis [24]. The catalyst is generally a tertiary ammonium salt or polynuclear iron carbonyl hydrides, and the reaction proceeds at a temperature of 100 °C and a pressure of 0.5–2 × 106 Pa [24]. Oxo or hydroformylation synthesis is a fundamental step in the production of higher aldehydes and alcohols and has seen major improvements in the use of rhodium-based catalysts [25]. The efficiency and lower cost of production using the Oxo process led to crotonaldehyde synthesis becoming obsolete, in spite of the former being performed at elevated temperatures (80–200 °C) and pressures (20–30 MPa), yielding 75% n-butanol and 25% iso-butanol [21]. However, further improvements have allowed the process to be conducted at lower pressures (1–5 × 106 Pa), yielding 95% n-butanol and 5% iso-butanol [24]. While chemical synthesis is a viable option, it still involves some fossil oil-derived materials such as ethylene, propylene, and triethylaluminium or carbon monoxide and hydrogen [26,27]. Furthermore, most of the chemical synthetic approaches involve the use of catalysts—for example, for the selective synthesis of 1-butanol from ethanol over strontium phosphate hydroxyapatite catalysts [28].
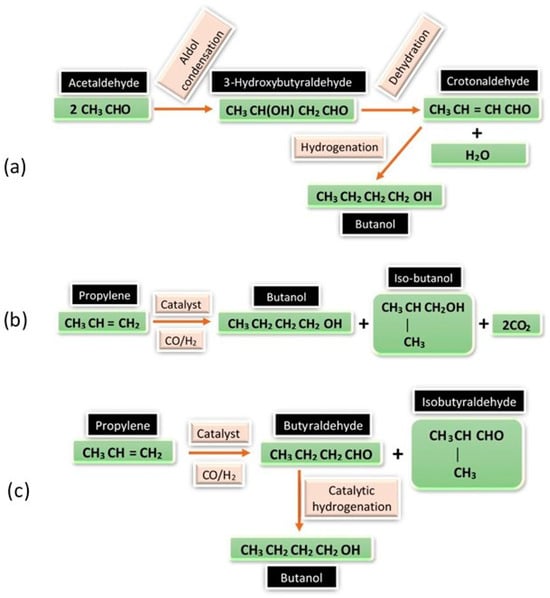
Figure 3.
Chemical synthesis of butanol. (a) Crotonaldehyde synthesis; (b) Reppe synthesis; (c) oxo synthesis. Adapted from [21,23,29].
The biosynthetic pathway for butanol production in microbial systems is generally well understood, with a solventogenic pathway and an acidogenic pathway [30,31] (Figure 4). During exponential growth, mostly organic acids such as acetic acid and butyric acid are produced as a result of sugar metabolism. Due to the production of the acids, the pH decreases, which leads to the triggering of the solventogenic phase, wherein the acids are metabolized to produce ethanol and butanol [32,33]. Essentially, the metabolic pathway for ABE is the glycolytic (Embden–Meyerhof–Parnas) pathway, with the starting fermentable sugar being glucose [34], converting one glucose molecule to two pyruvates, resulting in the production of two molecules of ATP and two molecules of NADH (Figure 4). It should also be noted that besides hexoses, pentoses can also be metabolized, resulting in the synthesis of pentose 5-phosphate, fructose 6-phosphate, and glyceraldehyde 3-phosphate catalyzed by transaldolases, transketolases, pyruvate, and then, in turn, CO2 and Acetyl-CoA [34,35,36]. The latter is then converted to acetone, butanol, butyrate, ethanol, and other intermediates.
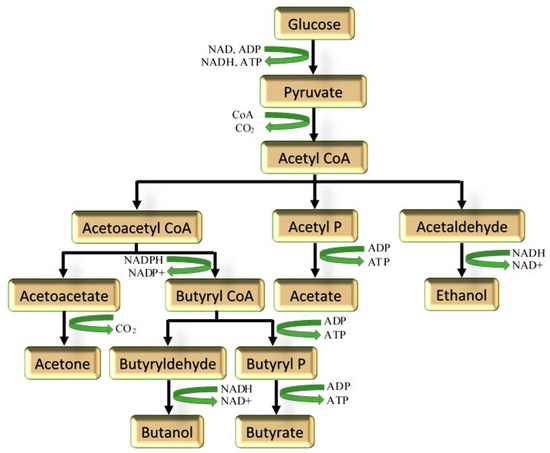
Figure 4.
Schematic of biosynthetic pathway for fermentative butanol. Adapted from [14,22,34,37].
3. Feedstocks and Agri-Food Industry Residues’ Valorization
The fermentative production of butanol has existed for over a century and is commonly referred to as acetone–butanol–ethanol (ABE) fermentation via anaerobic Clostridium species [38]. First described by Weizmann at the beginning of the 20th century [39], it was subsequently referred to as the Weizmann process [40]. Although the microorganism identified by Weizmann was named “BY” and capable of fermenting a variety of starchy substrates to produce butanol and acetone, it was later named Clostridium acetobutylicum [41]. The latter is nowadays one of the most commonly associated microorganisms for fermentative butanol production using various feedstock sources.
With regard to feedstocks, from the initial simple types termed first generation, additional generations have been recognized based on complexity and the source of feedstocks or microorganisms used [42] (Figure 5).
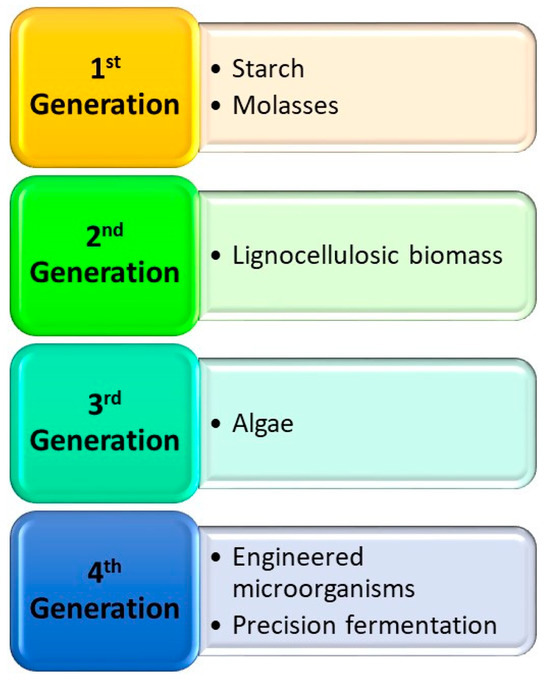
Figure 5.
The four generations of feedstocks used for biobutanol production.
Simple substrates such as molasses from sugarcane or starches from grain crops were considered very accessible and abundant and were, therefore, very popular in the early revolution of the biofuel industries. However, it became apparent that in the urgency to reduce dependence on fossil fuels, there was a significant encroachment into the food chain by siphoning away food for fuel. This valid concern led to a shift toward other sources of feedstocks, such as lignocellulosic materials, which became known as second-generation biofuel, and algae, which became known as third-generation biofuels. Genetically modified microorganisms have been considered fourth-generation biofuels (Table 2). Although the titers and yields are variable and certainly indicate that more improvements need to be made, the availability of genome editing tools is likely to contribute to further improvements.

Table 2.
Butanol production from the selected feedstocks from the four generations. “-“ indicates data not available.
Irrespective of the feedstock or microorganisms, this “siloed” approach to considering the feedstocks is obsolete in the current context. When attempting to use feedstocks from various sources, there is certainly overlap in complexity, and these generational categorizations are no longer necessary. For example, food wastes and agri-food wastes range in complexity from being a mix of simple sugars, starches, proteinaceous, oily, or cellulosic consistencies (Figure 6). The focus, therefore, has to be designing approaches to efficiently process and/or ferment heterogeneous feedstocks.
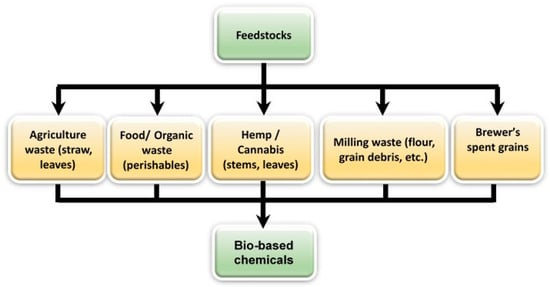
Figure 6.
Some examples of abundant unconventional feedstocks from agri-food industries and other sources.
For example, the demand for plant-based proteins has increased significantly in recent years due to considerations for sustainable production, health, and ethics [53]. Among the options, pulse-derived proteins have emerged as ingredients of choice in the vegetarian/vegan lifestyle diet [54], and the side stream starches (which are of low value) can typically be harnessed to derive additional value by way of fermentative butanol production. Thus, in this scenario, the side stream starch from the grain is not necessarily part of the food chain and is neither first-generation nor any other subsequent generations. This type of side stream residue from the agri-food industry is likely to become a significant source of feedstocks in the near future in addition to other feedstocks. In Canada in 2021, the combined production of dry peas, lentils, and chickpeas was about 4 million tons [55]. One of the challenges for the pulse industry is to continue to add value to side streams like starches. Considering the pulse starch concentration of peas, lentils, and faba bean on a dry weight basis ranging from 40 to 50% [56], it has recently been touted as a feedstock for biobutanol production for value addition.
While pulse starch side streams for biobutanol production are still in the research stage, wheat starch wastewater has been used [57]. Corn stover employing a simultaneous saccharification and fermentation (SSF) approach has been used for fermentative butanol production [58]. The most common approach to ABE fermentation using biomass or starches is through SSF. However, SSF is not without its limitations, such as the presence of inhibitors. However, the inhibitory compounds are more prevalent in complex feedstocks such as lignocellulosic or algal biomass [59]. The choice of any specific approach will depend on the initial experimentation and results. Furthermore, the energy required to pre-treat some of the more complex feedstocks is significant and can add to the cost of processing, although their sugar contents can be as high as 50% [42,60].
4. Scale-Up Challenges and Purification
One of the most commonly overlooked aspects of fermentation is scale-up. All too often, process development and optimization are conducted at small scales with limited foresight into process translation to large scale [61]. Such translation includes an outlook on logistics, finances, timelines, and ultimate objectives [62]. In essence, a techno-economic analysis (TEA) would need to be conducted prior to embarking on a large-scale venture besides the typical process optimization. While there are different approaches to address scale-up challenges, a Process Hazards Analysis (PHA) approach is considered very intuitive for risk management and mitigating risks [61]. Commonly used in the industrial engineering/manufacturing space for risk management and risk mitigation for safety and process operation, it has been sparingly used in the fermentation industry. The food processing and beverage industries employ Good Manufacturing Practices (GMPs), Good Hygiene Practices (GHPs), Quality Management Systems (QMS), and Hazard Analysis and Critical Control Points (HACCPs) to ascertain the safety of the food and beverage for human consumption (for reviews, see [63,64]). However, in industrial fermentation, such practices are limited to ensuring consistent productivity, yield and titers, and, of course, returns. Although biobutanol production at an industrial scale has been established, many of the research and development aspects still neglect to envisage the practicality, feasibility, and economic aspects of the scaled-up process. Thus, a PHA approach would be very useful to guarantee successful scale-up in the identification and assessment of risks and providing mitigating options.
Generally, PHA is defined as the systematic, comprehensive, and analytical review of a process based on a number of standards for the identification and assessment of processes and associated operational hazards and their potential effects [65]. PHA strategies emerged as a result of repeating serious incidents due to operators, personnel, machinery, oversight, etc., with the result that productivity, revenue, public trust, and overall dependability on meeting demands were obvious. Due to this, many of the large manufacturing entities have established policies for PHAs [66] in some form with ranging complexities. In addition to commonly practiced safety procedures and following Standard Operating Procedures (SOPs), PHA incorporates operational safety of processes while also addressing non-safety related potential failures, which lead to failed processes and economic losses. It is important to note that basic operational and general safety procedures would have been implemented prior to proceeding with PHA approaches for desired projects [67]. PHA, in the case of biobutanol production, would start with feedstock selection, pre-treatment conditions and complexity, the fermentation process itself, ABE purification, economic feasibility, financial investments, and finally, available platforms for scaling up production.
For example, one of the challenges in ABE production is the selection of feedstocks and process flow improvement that can be translated to large-scale production. After judiciously optimizing the performance and productivity of the selected strain at a small scale using defined media components, not enough resources are allocated to achieve equal or superseding productivity with other feedstocks, especially complex ones. Improving process flow is also important to minimize the duration of fermentation while maximizing yield. Considering the first-generation feedstocks, in the past, saccharification for biofuel production was conducted separately from fermentation in a two-step process. This approach was inefficient and cost-intensive. Improvements have led to the establishment of a single-step process wherein simultaneous saccharification and fermentation (SSF) are conducted in the bioreactor [68]. Other improvements included the use of microbial strains capable of saccharification, followed by fermentative butanol production [69] or conducting both simultaneously as co-cultures [70].
The more complex feedstocks would require more energy to break down and will therefore add to the higher cost of production. Thus, a first-generation feedstock such as starch will require less energy to release the sugars compared to cellulosic feedstocks. While both feedstocks undergo a saccharification step, the cellulosic feedstock has to be broken down into finer particles and hydrolyzed by acid treatment prior to the saccharification step [71,72]. Therefore, in the context of scaling up, these pre-treatments and the process flow to fermentation significantly influence the efficiency of biobutanol production.
Scale-up challenges during fermentation per se have been widely addressed in a number of reviews (e.g., [61,62,73,74]) and for biobutanol production (e.g., [57,69,75]. Suffice it to state that the fermentation processes and designs are well established, with the bottlenecks being in the inhibitory accumulation of butanol and the removal and purification of butanol from fermentation broth. Even in this regard, numerous studies have been conducted, but mostly limited to a small scale or pilot scale and not demonstrated at production or manufacturing scales. Some of the strategies used to address inhibitory butanol concentrations have included the removal of broth and replenishing with fresh media or in-line distillation and removal of accumulated butanol, etc. Commonly, however, batch, fed-batch, continuous, or combination thereof have been used [76]. For example, simply adding butyric acid at a very low concentration was found to promote ABE production by C. beijerinckii strain NCIMB 8052, resulting in a 31% increase in a final butanol titer and a 133% increase in butanol productivity [77]. While this research was performed at a laboratory scale in a benchtop bioreactor, the economic feasibility of butyric acid supplementation at a large scale needs to be further investigated. Besides the inhibitory accumulation of butanol during fermentation, inhibitors derived from lignocellulosic hydrolysis can reduce butanol titers. In a fed-batch strategy using Clostridium beijenrinckii NRRL B-598 and hydrolyzed wheat straw, such inhibitors were effectively eliminated by timing the feed to coincide with the late acidogenesis and early solventogenesis phases, leading to a titer of 7.0 g/L butanol [78]. Employing a detoxification strategy using strong acid cation exchange with wheat straw hydrolysates for fermentation with Clostridium acetobutylicum CICC 8012, a titer of 7.42 g/L butanol was obtained [79]. Toxicity from hydrolysates has also been observed in other biomasses, such as sweet sorghum bagasse, with primary hydrolysis resulting in poor butanol titers, but secondary hydrolysis resulted in 8.9 g/L butanol [80]. In a subsequent study, it was demonstrated that a primary fermentation with the secondary hydrolysate allowed for vigorous cell growth, and when followed by a slow feed of the primary hydrolysate, butanol titers could be increased to 12 g/L [81].
One of the most adaptable strategies for large-scale ABE production is undoubtedly continuous fermentation. This approach allows for the removal of broth during fermentation and replenishment with new media. Thus, the inhibitory effects of butanol can be obviated. The removed broth can be adapted for in-line distillation after the removal of cells and/or cell cycling. The latter approach has been gaining attention due to its higher productivity [82], but scalability is still not clearly defined. In another small-scale study, continuous fermentation in combination with gas stripping was shown to increase productivity by 320% over a 21-day period and was intentionally terminated after this period [83]. Cell cycling, on the other hand, relies on soundly selected ultrafiltration systems, with productivity ranging from 4.1 to 7.55 g/L/h [84,85]. The combination of continuous fermentation and cell recycling via ultrafiltration has steadily improved and has led to a biobutanol productivity of 10.7 g/L/h [86]. However, as with other studies, scale-up feasibility is yet to be demonstrated. Attempts at in situ separation have also been undertaken, including liquid–liquid extraction [87], gas stripping [88], adsorption [89], and pervaporation [90]. It is thus obvious that there are numerous options for increasing the productivity, yield, and titer of biobutanol, but a successful outcome will depend on the adaptability of the process to large scale cost-effectively.
With Clostridial species being predominant in fermentative butanol production [91], it has been observed that these species themselves are inherently affected by metabolic oscillations during fermentation [92,93]. Oscillations are common features of biological systems, the most studied being those associated with circadian rhythms and day and night cycles. The occurrence of metabolic oscillations in Clostridium species can have major implications for scaling up the production of fermentative butanol (reviewed in [94]). For example, during glycerol fermentation by Clostridium pasteurianum, it was observed that there was self-synchronized oscillatory metabolism coinciding with higher glycerol feed unrelated to butanol accumulation [92]. This was deemed a potential problem for scaling up production if glycerol feed concentrations were to be increased for high butanol productivity. The study also reported that lower temperatures stopped the self-synchronized oscillations and could be restored by increasing the temperature [92].
5. The Potential of Precision Fermentation for Improved Biobutanol Production
There have been significant advances in fermentation technology with regard to biobutanol production, including engineered microorganisms. As mentioned earlier, the latter has been considered the fourth generation of biofuels, but as such, it should be considered a system for use with any type of feedstock. Maximizing biobutanol production using any type of feedstock should be the focus if any commercial success is to be attained. With available technologies in genome engineering combined with the availability of heterogeneous feedstocks in the context of upcycling and circular economy, the need for more efficient strains is imperative.
More recently, the advent of precision fermentation has opened new opportunities for metabolic engineering to be more targeted [73]. While mainly focused on specific target molecules, it has the potential to be applied more broadly, including in biobutanol production. It encompasses synthetic biology and a whole repertoire of other tools, such as omics, artificial intelligence, bioinformatics, systems biology, computational biology, and functional characterization. Relying extensively on an understanding of microbial genomes and metabolic functions, it is a perfect approach to enhance biobutanol production in the context of heterogeneous biomass use and to enhance microbial efficiency [95]. Essentially considered an advanced fermentation system, it has the ability to precisely produce specific molecules with very tightly controlled manufacturing practices to enhance the yield of desired products at reduced cost [96]. In the case of biobutanol production, the metabolic engineering of species such as Clostridium would be directed to reduce wasteful pathways and direct synthesis toward butanol [97]. Further advances in technology by way of Internet of Things (IoT)-based sensors have enabled the operation of a low-cost prototype bioreactor for biobutanol production and sample collection and monitoring [98], resulting in improved biobutanol production.
While precision fermentation is yet to have a significant impact on biobutanol production efficiency enhancement, several conventional strain improvement strategies, such as mutagenesis, directed evolution, genome engineering, and genome editing, have been employed (reviewed in [99]). Interestingly, while the concept of using microorganisms as cell factories in line with precision fermentation for the production of single molecules is a significant topic of discussion, especially in the food industry, elements of it have already been practiced in fermentative butanol pathway modification, albeit unconsciously. For example, while it has been well-established that increased production of butanol inhibits cell growth, thereby limiting high productivity, it became conceivable that the biosynthetic pathway could be modified to produce butyric acid, which could further be processed downstream for conversion to butanol [100]. Indeed, the approach taken in this study included the knockout of genes encoding phosphotransacetylase and acetate kinase in an attempt to shut down the acetate-producing pathway. However, the acetate production was not significantly reduced, although butyric acid production was increased with no inhibitory effects on cell growth [100]. In another study in Clostridium acetobutylicum JB200 using the ClosTron group II intron-based system, a histidine kinase gene, cac3319, was inactivated and led to an increase in butanol production in addition to enhanced tolerance to the increased butanol concentration [101].
Intricately associated with precision fermentation and synthetic biology approaches for engineering microorganisms is the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technology, commonly abbreviated CRISPR/Cas9. CRISPRs [102], or Short Regularly Spaced Repeats, as they were originally termed [103], are unique to prokaryotes (archaea and bacteria), featuring direct repeats of 21–37 bp. Associated with the CRISPR loci were Cas genes [102]. It was subsequently shown that the CRISPR/Cas9 system, as a bacterial immune system mechanism against infecting bacteriophages, could be harnessed for editing genes [104]. With the availability of this new gene editing tool and recent advances, attempts to edit the butanol pathway have been made. With Clostridium species being naturally able to produce butanol, editing the butanol biosynthetic pathway for increased efficiency would have been the target of choice. However, due to the complex physiological nature of Clostridium species and limited genetic manipulation information, other systems were explored for butanol pathway engineering. Thus, using CRISPR/Cas9 editing in Escherichia coli, butanol production was achieved in a micro-aerobic environment [105]. Further knowledge and understanding of the underlying mechanisms of native CRISPR/Cas systems in Clostridium have enabled tailoring the endogenous systems to edit the butanol biosynthetic pathway. Using Clostridium tyrobutyricum, a hyper-butyrate producer, the butyrate:acetate CoA transferase (cat1) gene was replaced with aldehyde/alcohol dehydrogenase genes (adhE1 or adhE2), leading to the near elimination of butyrate production converting the C. tyrobutyricum strain into a hyper-butanol producer [51]. It is likely that with further research into precision fermentation, synthetic biology, and gene editing using the CRISPR/Cas9 system, there will be enhanced biobutanol production for commercially viable manufacturing.
6. Conclusions
The production of biobutanol is certainly not new, having been in existence for over a century. However, the challenges have been in scale-up production and commercial viability. Although the value of biobutanol as a green alternative is well recognized, there has not been enough focus on the establishment of large-scale production research and deep investigation into the inhibitory aspects of butanol accumulation. The opportunities for refinement of the process are currently readily available by way of precision fermentation and advanced manufacturing systems, including AI. Furthermore, the abundance of diverse feedstocks in the context of upcycling and circular economy opens up new opportunities for metabolic engineering of microbial systems to either direct the metabolic pathway for the production of butanol or for metabolizing complex agri-food residues for the release of the fermentable sugars. Of the currently operational commercial biobutanol production facilities, Celtic Renewables (https://www.celtic-renewables.com/, accessed 7 April 2025) in Scotland is producing biobutanol from wastes that include food, drink, and agriculture. Gevo (https://gevo.com/product/isobutanol/, accessed 7 April 2025) in the USA produces iso-butanol from agriculture residues in a side-by-side operation, including ethanol production, thus sharing various streams and minimizing CAPEX and OPEX. However, for further proliferation of commercial biobutanol production facilities globally, the outlined challenges need to be addressed.
Author Contributions
Conceptualization, S.G. and M.Ç.T.; writing—original draft preparation, S.G. and M.Ç.T.; writing—review and editing, S.G. and M.Ç.T.; project administration, S.G.; funding acquisition, S.G. and M.Ç.T. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for biobutanol research in our laboratory from the Saskatchewan Ministry of Agriculture-Agriculture Development Fund (ADF Project # 20220241) is gratefully acknowledged.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Richie, H.; Rosado, P. Fossil Fuels. Available online: https://ourworldindata.org/fossil-fuels (accessed on 26 December 2024).
- Smil, V. Energy Transitions: Global and National Perspectives, 2nd ed.; Praeger: Santa Barbara, CA, USA, 2017. [Google Scholar]
- Energy Institute. Statistical Review of World Energy. Available online: https://www.energyinst.org/statistical-review/ (accessed on 26 December 2024).
- McPherson, G.R. Will COVID-19 Trigger Extinction of All Life on Earth? Earth Environ. Sci. Res. Rev. 2020, 73, 73–74. [Google Scholar] [CrossRef]
- Shapovalov, O.I.; Ashkinazi, L.A. Biobutanol: Biofuel of second generation. Russ. J. Appl. Chem. 2008, 81, 2232–2236. [Google Scholar] [CrossRef]
- Mahapatra, M.; Kumar, A. A Short Review on Biobutanol, a Second Generation Biofuel Production from Lignocellulosic Biomass. J. Clean Energy Technol. 2017, 5, 27–30. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biorefineries for biofuel upgrading: A critical review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Rozelle, S.; Boswell, M. Beer Battles in China: The Struggle over the World’s Largest Beer Market. In The Economics of Beer; Oxford University Press: New York, NY, USA, 2011; pp. 267–286. [Google Scholar] [CrossRef]
- Poelmans, E.; Swinnen, J.F.M. A Brief Economic History of Beer. Econ. Beer 2011, 3–28. [Google Scholar] [CrossRef]
- McGovern, P.E.; Glusker, D.L.; Exner, L.J.; Voigt, M.M. Neolithic resinated wine. Nature 1996, 381, 480–481. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.-S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef]
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef]
- Rosenblatt, D.; Morgan, C.; McConnell, S.; Nuottimäki, J. Particulate Measurements: Ethanol and Isobutanol in Direct Injection Spark Ignited Engines; International Energy Agency-Advanced Motor Fuels: Paris, France, 2015. [Google Scholar]
- Dürre, P. Fermentative Butanol Production. Ann. N. Y. Acad. Sci. 2008, 1125, 353–362. [Google Scholar] [CrossRef]
- Nagrale, P. Biobutanol Market Research Report. Available online: https://www.marketresearchfuture.com/reports/bio-butanol-market-28603 (accessed on 16 March 2025).
- Bhattacharyya, A.; Jain, A.; Rajagopalan, G. Biobutanol production from food crops. In Advances and Developments in Biobutanol Production; Segovia-Hernandez, J.G., Behera, S., Sanchez-Ramirez, E., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2023; pp. 245–260. [Google Scholar] [CrossRef]
- Sooch, B.S.; Singh, J.; Verma, D. Insights into metabolic engineering approaches for enhanced biobutanol production. In Advances and Developments in Biobutanol Production; Segovia-Hernandez, J.G., Behera, S., Sanchez-Ramirez, E., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 329–361. [Google Scholar] [CrossRef]
- Nanda, S.; Golemi-Kotra, D.; McDermott, J.C.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Fermentative production of butanol: Perspectives on synthetic biology. New Biotechnol. 2017, 37, 210–221. [Google Scholar] [CrossRef]
- Hongjuan, L.; Genyu, W.; Jianan, Z. The Promising Fuel-Biobutanol. In Liquid, Gaseous and Solid Biofuels; Zhen, F., Ed.; IntechOpen: Rijeka, Croatia, 2013; pp. 175–198. [Google Scholar] [CrossRef]
- AMF. Butanol. Available online: https://www.iea-amf.org/content/fuel_information/butanol/ (accessed on 12 January 2025).
- Panahi, H.K.S.; Dehhaghi, M.; Kinder, J.E.; Ezeji, T.C. A review on green liquid fuels for the transportation sector: A prospect of microbial solutions to climate change. Biofuel Res. J. 2019, 6, 995–1024. [Google Scholar] [CrossRef]
- Ndaba, B.; Chiyanzu, I.; Marx, I. N-Butanol derived from biochemical and chemical routes: A review. Biotechnol. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, M.; Van Hecke, W.; Vanbroekhoven, K. Sustainability metrics of 1-butanol. Catal. Today 2015, 239, 7–10. [Google Scholar] [CrossRef]
- Hahn, H.-D.; Dämbkes, G.; Rupprich, N.; Bahl, H. Butanols. In Ullmann’s Encyclopedia of Industrial Chemistry; VCH Publishers: Deerfield Beach, FL, USA, 2010. [Google Scholar] [CrossRef]
- Lang, Y.D.; Biegler, L.T.; Maier, E.E.; Majewski, R.A. An optimal catalyst management strategy for Oxo processes. Comput. Chem. Eng. 2000, 24, 1549–1554. [Google Scholar] [CrossRef]
- Zverlov, V.V.; Berezina, O.; Velikodvorskaya, G.A.; Schwarz, W.H. Bacterial acetone and butanol production by industrial fermentation in the Soviet Union: Use of hydrolyzed agricultural waste for biorefinery. Appl. Microbiol. Biotechnol. 2006, 71, 587–597. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R.L. Challenges in biobutanol production: How to improve the efficiency? Renew. Sustain. Energy Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Ogo, S.; Onda, A.; Yanagisawa, K. Selective synthesis of 1-butanol from ethanol over strontium phosphate hydroxyapatite catalysts. Appl. Catal. A Gen. 2011, 402, 188–195. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef]
- Ljungdahl, L.G.; Hugenholtz, J.; Wiegel, J. Acetogenic and Acid-Producing Clostridia. In Clostridia; Minton, N.P., Clarke, D.J., Eds.; Springer: Boston, MA, USA, 1989; pp. 145–191. [Google Scholar] [CrossRef]
- Jones, D.T.; Woods, D.R. Solvent Production. In Clostridia; Minton, N.P., Clarke, D.J., Eds.; Springer: Boston, MA, USA, 1989; pp. 105–144. [Google Scholar] [CrossRef]
- Johnson, M.J.; Peterson, W.H.; Fred, E.B. Oxidation and reduction relations between susbtrate and products in the acetonebutyl alcohol fermentation. J. Biol. Chem. 1931, 91, 569–591. [Google Scholar] [CrossRef]
- Bahl, H.; Andersch, W.; Braun, K.; Gottschalk, G. Effect of pH and butyrate concentration on the production of acetone and butanol by Clostridium acetobutylicum grown in continuous culture. Eur. J. Appl. Microbiol. Biotechnol. 1982, 14, 17–20. [Google Scholar] [CrossRef]
- Rogers, P. Genetics and Biochemistry of Clostridium Relevant to Development of Fermentation Processes. In Advances in Applied Microbiology; Laskin, A.I., Ed.; Academic Press: Cambridge, MA, USA, 1986; Volume 31, pp. 1–60. [Google Scholar] [CrossRef]
- Ounine, K.; Petitdemange, H.; Raval, G.; Gay, R. Acetone-butanol production from pentoses by Clostridium acetobutylicum. Biotechnol. Lett. 1983, 5, 605–610. [Google Scholar] [CrossRef]
- Cynkin, M.A.; Delwiche, E.A. Metabolism of pentoses by clostridia. I. Enzymes of ribose dissimilation in extracts of Clostridium perfringens. J. Bacteriol. 1958, 75, 331–334. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Peña, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. mSystems 2017, 2. [Google Scholar] [CrossRef]
- Davies, E.T. Green Biologics Ltd.: Commercialising bio-n-butanol. Green Process. Synth. 2013, 2, 273–276. [Google Scholar] [CrossRef]
- Weizmann, C. Production of Acetone and Alcohols by Bacteriological Processes. U.S. Patent 1,315,585A, 9 September 1919. [Google Scholar]
- Gabriel, C.L. Butanol Fermentation Process. Ind. Eng. Chem. 1928, 20, 1063–1067. [Google Scholar] [CrossRef]
- Jones, D.T.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar] [CrossRef]
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Brück, T. The potential of biofuels from first to fourth generation. PLoS Biol. 2023, 21, e3002063. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Gao, H.-p.; Cao, C.-h. Simultaneous Saccharification and Fermentation for Biobutanol Production from Corn Starch via ABE Fermentation. BioResources 2023, 18, 4935–4942. [Google Scholar] [CrossRef]
- Li, H.-G.; Ofosu, F.K.; Li, K.-T.; Gu, Q.-Y.; Wang, Q.; Yu, X.-B. Acetone, butanol, and ethanol production from gelatinized cassava flour by a new isolates with high butanol tolerance. Bioresour. Technol. 2014, 172, 276–282. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.M.; Wang, Y.; Lee, Y.Y.; Zong, W.; Taylor, S.; McDonald, T.; Wang, Y. Towards comprehensive lignocellulosic biomass utilization for bioenergy production: Efficient biobutanol production from acetic acid pretreated switchgrass with Clostridium saccharoperbutylacetonicum N1-4. Appl. Energy 2019, 236, 551–559. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Cotta, M.A. Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess Biosyst. Eng. 2007, 30, 419–427. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Whang, L.-M.; Chan, K.-C.; Chung, M.-C.; Wu, S.-H.; Liu, C.-P.; Tien, S.-Y.; Chen, S.-Y.; Chang, J.-S.; Lee, W.-J. Biological butanol production from microalgae-based biodiesel residues by Clostridium acetobutylicum. Bioresour. Technol. 2015, 184, 379–385. [Google Scholar] [CrossRef]
- Salaeh, S.; Kongjan, P.; Panphon, S.; Hemmanee, S.; Reungsang, A.; Jariyaboon, R. Feasibility of ABE fermentation from Rhizoclonium spp. hydrolysate with low nutrient supplementation. Biomass Bioenergy 2019, 127, 105269. [Google Scholar] [CrossRef]
- Li, S.-B.; Qian, Y.; Liang, Z.-W.; Guo, Y.; Zhao, M.-M.; Pang, Z.-W. Enhanced butanol production from cassava with Clostridium acetobutylicum by genome shuffling. World J. Microbiol. Biotechnol. 2016, 32, 53. [Google Scholar] [CrossRef]
- Zhang, J.; Zong, W.; Hong, W.; Zhang, Z.-T.; Wang, Y. Exploiting endogenous CRISPR-Cas system for multiplex genome editing in Clostridium tyrobutyricum and engineer the strain for high-level butanol production. Metab. Eng. 2018, 47, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Wasels, F.A.-O.; Chartier, G.; Hocq, R.; Lopes Ferreira, N. A CRISPR/Anti-CRISPR Genome Editing Approach Underlines the Synergy of Butanol Dehydrogenases in Clostridium acetobutylicum DSM 792. Appl. Environ. Microbiol. 2020, 86, e00408-20. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Zannini, E.; Arendt, E.K. Production of pulse protein ingredients and their application in plant-based milk alternatives. Trends Food Sci. Technol. 2021, 110, 364–374. [Google Scholar] [CrossRef]
- Dent, T.; Maleky, F. Pulse protein processing: The effect of processing choices and enzymatic hydrolysis on ingredient functionality. Crit. Rev. Food Sci. Nutr. 2023, 63, 9914–9925. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Table 32-10-0359-01 Estimated Areas, Yield, Production, Average Farm Price and Total Farm Value of Principal Field Crops, in Metric and Imperial Units. Available online: https://doi.org/10.25318/3210035901-eng (accessed on 4 September 2024).
- Pulse Canada. Pulse Starches. Available online: https://pulsecanada.com/processing/pulse-starch (accessed on 24 February 2025).
- Luo, W.; Zhao, Z.; Pan, H.; Zhao, L.; Xu, C.; Yu, X. Feasibility of butanol production from wheat starch wastewater by Clostridium acetobutylicum. Energy 2018, 154, 240–248. [Google Scholar] [CrossRef]
- Dong, J.-J.; Ding, J.-C.; Zhang, Y.; Ma, L.; Xu, G.-C.; Han, R.-Z.; Ni, Y. Simultaneous saccharification and fermentation of dilute alkaline-pretreated corn stover for enhanced butanol production by Clostridium saccharobutylicum DSM 13864. FEMS Microbiol. Lett. 2016, 363, fnw003. [Google Scholar] [CrossRef]
- Muthan, K.; Poomani, M.S.; Mariappan, I.; Subramanian, V. Biobutanol for Biofuel: Technologies and Commercial Approach. In Biomass, Bioenergy & Bioeconomy; Kothari, R., Singh, A., Arora, N.K., Eds.; Springer Nature: Singapore, 2022; pp. 141–159. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Wang, Q.; Ma, H. Production of butanol from biomass: Recent advances and future prospects. Environ. Sci. Pollut. Res. 2019, 26, 20164–20182. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, S.; Kim, S.H.; Vujanovic, V. Scaling-up production of plant endophytes in bioreactors: Concepts, challenges and perspectives. Bioresour. Bioprocess. 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Crater, J.S.; Lievense, J.C. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 2018, 365, fny138. [Google Scholar] [CrossRef]
- Kotsanopoulos, K.V.; Arvanitoyannis, I.S. The Role of Auditing, Food Safety, and Food Quality Standards in the Food Industry: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 760–775. [Google Scholar] [CrossRef]
- Panghal, A.; Chhikara, N.; Sindhu, N.; Jaglan, S. Role of Food Safety Management Systems in safe food production: A review. J. Food Saf. 2018, 38, e12464. [Google Scholar] [CrossRef]
- Nolan, D.P. Application of HAZOP and What-If Safety Reviews to the Petroleum, Petrochemical and Chemical Industries; Noyes Publications: Park Ridge, NJ, USA, 1994; p. 134. [Google Scholar]
- Chastain, J.W.; Delanoy, P.; Devlin, C.; Mueller, T.; Study, K. Beyond HAZOP and LOPA: Four different company approaches. Process Saf. Prog. 2017, 36, 38–53. [Google Scholar] [CrossRef]
- Cameron, I.; Mannan, S.; Nemeth, E.; Park, S.; Pasman, H.; Rogers, W.; Seligmann, B. Process hazard analysis, hazard identification and scenario definition: Are the conventional tools sufficient, or should and can we do much better? Process Saf. Environ. Prot. 2017, 110, 53–70. [Google Scholar] [CrossRef]
- Gottumukkala, L.D.; Haigh, K.; Görgens, J. Trends and advances in conversion of lignocellulosic biomass to biobutanol: Microbes, bioprocesses and industrial viability. Renew. Sustain. Energy Rev. 2017, 76, 963–973. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Amiri, H.; Asadollahi, M.A. Enhanced aerobic conversion of starch to butanol by a symbiotic system of Clostridium acetobutylicum and Nesterenkonia. Biochem. Eng. J. 2020, 164, 107752. [Google Scholar] [CrossRef]
- Tran, H.T.M.; Cheirsilp, B.; Hodgson, B.; Umsakul, K. Potential use of Bacillus subtilis in a co-culture with Clostridium butylicum for acetone–butanol–ethanol production from cassava starch. Biochem. Eng. J. 2010, 48, 260–267. [Google Scholar] [CrossRef]
- Vivek, N.; Nair, L.M.; Mohan, B.; Nair, S.C.; Sindhu, R.; Pandey, A.; Shurpali, N.; Binod, P. Bio-butanol production from rice straw—Recent trends, possibilities, and challenges. Bioresour. Technol. Rep. 2019, 7, 100224. [Google Scholar] [CrossRef]
- Gottumukkala, L.D.; Parameswaran, B.; Valappil, S.K.; Mathiyazhakan, K.; Pandey, A.; Sukumaran, R.K. Biobutanol production from rice straw by a non acetone producing Clostridium sporogenes BE01. Bioresour. Technol. 2013, 145, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Bioengineered Enzymes and Precision Fermentation in the Food Industry. Int. J. Mol. Sci. 2023, 24, 10156. [Google Scholar] [CrossRef]
- Burke, F. Scale Up and Scale Down of Fermentation Processes. In Practical Fermentation Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008; pp. 231–269. [Google Scholar] [CrossRef]
- Birgen, C.; Dürre, P.; Preisig, H.A.; Wentzel, A. Butanol production from lignocellulosic biomass: Revisiting fermentation performance indicators with exploratory data analysis. Biotechnol. Biofuels 2019, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cong, W.; Zhang, J.A. Biobutanol Production from Acetone–Butanol–Ethanol Fermentation: Developments and Prospects. Fermentation 2023, 9, 847. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Blaschek, H.P. Effects of supplementary butyrate on butanol production and the metabolic switch in Clostridium beijerinckii NCIMB 8052: Genome-wide transcriptional analysis with RNA-Seq. Biotechnol. Biofuels 2013, 6, 138. [Google Scholar] [CrossRef]
- Branska, B.; Koppova, K.; Husakova, M.; Patakova, P. Application of fed-batch strategy to fully eliminate the negative effect of lignocellulose-derived inhibitors in ABE fermentation. Biotechnol. Biofuels Bioprod. 2024, 17, 87. [Google Scholar] [CrossRef]
- Liu, G.; Yi, Z.; Li, J.; Yang, L.; Fang, Y.; Du, A.; He, K.; Zhao, H.; Jin, Y. Detoxification with resin promotes the shift from acidogenesis to solventogenesis and prevents acid crash during butanol fermentation from wheat straw. Biomass Convers. Biorefin. 2024, 14, 16857–16866. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Klasson, K.T.; Liu, S. Butanol production from sweet sorghum bagasse with high solids content: Part I—Comparison of liquid hot water pretreatment with dilute sulfuric acid. Biotechnol. Prog. 2018, 34, 960–966. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Klasson, K.T.; Liu, S. High solid fed-batch butanol fermentation with simultaneous product recovery: Part II—Process integration. Biotechnol. Prog. 2018, 34, 967–972. [Google Scholar] [CrossRef]
- Survase, S.A.; van Heiningen, A.; Granström, T. Continuous bio-catalytic conversion of sugar mixture to acetone–butanol–ethanol by immobilized Clostridium acetobutylicum DSM 792. Appl. Microbiol. Biotechnol. 2012, 93, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Microbial production of a biofuel (acetone–butanol–ethanol) in a continuous bioreactor: Impact of bleed and simultaneous product removal. Bioprocess Biosyst. Eng. 2013, 36, 109–116. [Google Scholar] [CrossRef]
- Schlote, D.; Gottschalk, G. Effect of cell recycle on continuous butanol-acetone fermentation with Clostridium acetobutylicum under phosphate limitation. Appl. Microbiol. Biotechnol. 1986, 24, 1–5. [Google Scholar] [CrossRef]
- Tashiro, Y.; Takeda, K.; Kobayashi, G.; Sonomoto, K. High production of acetone–butanol–ethanol with high cell density culture by cell-recycling and bleeding. J. Biotechnol. 2005, 120, 197–206. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Malaviya, A.; Lee, S.Y. Acetone–butanol–ethanol production with high productivity using Clostridium acetobutylicum BKM19. Biotechnol. Bioeng. 2013, 110, 1646–1653. [Google Scholar] [CrossRef]
- Khedkar, M.A.; Nimbalkar, P.R.; Gaikwad, S.G.; Chavan, P.V.; Bankar, S.B. Solvent extraction of butanol from synthetic solution and fermentation broth: Batch and continuous studies. Sep. Purif. Technol. 2020, 249, 117058. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, H.; Yan, X.; Zhou, Y.; Cheng, K.; Zhang, J.A. High-efficiency acetone-butanol-ethanol production and recovery in non-strict anaerobic gas-stripping fed-batch fermentation. Appl. Microbiol. Biotechnol. 2017, 101, 8029–8039. [Google Scholar] [CrossRef] [PubMed]
- Raganati, F.; Procentese, A.; Olivieri, G.; Russo, M.E.; Salatino, P.; Marzocchella, A. Bio-butanol separation by adsorption on various materials: Assessment of isotherms and effects of other ABE-fermentation compounds. Sep. Purif. Technol. 2018, 191, 328–339. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Wang, N.; Chen, X.; Li, B.; Shi, J.; Li, X. Process optimization of acetone-butanol-ethanol fermentation integrated with pervaporation for enhanced butanol production. Biochem. Eng. J. 2021, 173, 108070. [Google Scholar] [CrossRef]
- Jones, D.T.; Schulz, F.; Roux, S.; Brown, S.D. Solvent-Producing Clostridia Revisited. Microorganisms 2023, 11, 2253. [Google Scholar] [CrossRef]
- Johnson, E.E.; Rehmann, L. Self-Synchronized Oscillatory Metabolism of Clostridium pasteurianum in Continuous Culture. Processes 2020, 8, 137. [Google Scholar] [CrossRef]
- Mahamkali, V.; Valgepea, K.; de Souza Pinto Lemgruber, R.; Plan, M.; Tappel, R.; Köpke, M.; Simpson, S.D.; Nielsen, L.K.; Marcellin, E. Redox controls metabolic robustness in the gas-fermenting acetogen Clostridium autoethanogenum. Proc. Natl. Acad. Sci. USA 2020, 117, 13168–13175. [Google Scholar] [CrossRef]
- Tyszak, A.; Rehmann, L. Metabolic Oscillation Phenomena in Clostridia Species—A Review. Fermentation 2024, 10, 156. [Google Scholar] [CrossRef]
- Hassoun, A.; Bekhit, A.E.; Jambrak, A.R.; Regenstein, J.M.; Chemat, F.; Morton, J.D.; Gudjónsdóttir, M.; Carpena, M.; Prieto, M.A.; Varela, P.; et al. The fourth industrial revolution in the food industry-part II: Emerging food trends. Crit. Rev. Food Sci. Nutr. 2022, 64, 407–437. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for future food systems. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Lee Jin, Y.; Lee, J.; Park Jin, H.; Im Jung, A.; Eom, M.-H.; Lee, J.; Lee, S.-H.; Song, H.; Cho, J.-H.; et al. Enhanced Butanol Production Obtained by Reinforcing the Direct Butanol-Forming Route in Clostridium acetobutylicum. mBio 2012, 3. [Google Scholar] [CrossRef]
- Klaithin, E.; Matimapa-Kay, V.; Daosud, W.; Laoonguthai, Y. Effective IoT-Based Arduino Design for Automated Bioreactor Control and Sample Collection in Biobutanol Production. IEEE Sens. J. 2024, 24, 37997–38004. [Google Scholar] [CrossRef]
- Li, S.; Huang, L.; Ke, C.; Pang, Z.; Liu, L. Pathway dissection, regulation, engineering and application: Lessons learned from biobutanol production by solventogenic clostridia. Biotechnol. Biofuels 2020, 13, 39. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Yang, S.-T. Butyric acid and hydrogen production by Clostridium tyrobutyricum ATCC 25755 and mutants. Enzym. Microb. Technol. 2006, 38, 521–528. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, J.; Yu, L.; Tang, I.C.; Xue, C.; Yang, S.-T. Engineering Clostridium acetobutylicum with a histidine kinase knockout for enhanced n-butanol tolerance and production. Appl. Microbiol. Biotechnol. 2015, 99, 1011–1022. [Google Scholar] [CrossRef]
- Jansen, R.; Embden, J.D.A.v.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Mojica, F.J.M.; Díez-Villaseñor, C.; Soria, E.; Juez, G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol. Microbiol. 2000, 36, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Heo, M.-J.; Jung, H.-M.; Um, J.; Lee, S.-W.; Oh, M.-K. Controlling Citrate Synthase Expression by CRISPR/Cas9 Genome Editing for n-Butanol Production in Escherichia coli. ACS Synth. Biol. 2017, 6, 182–189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).