Distribution of Salts in Milk and Cheese: Critical Methodological Aspects
Abstract
1. Introduction
2. Significance of the Distribution of Salts in Milk
3. Determining the Salt Distribution in Milk
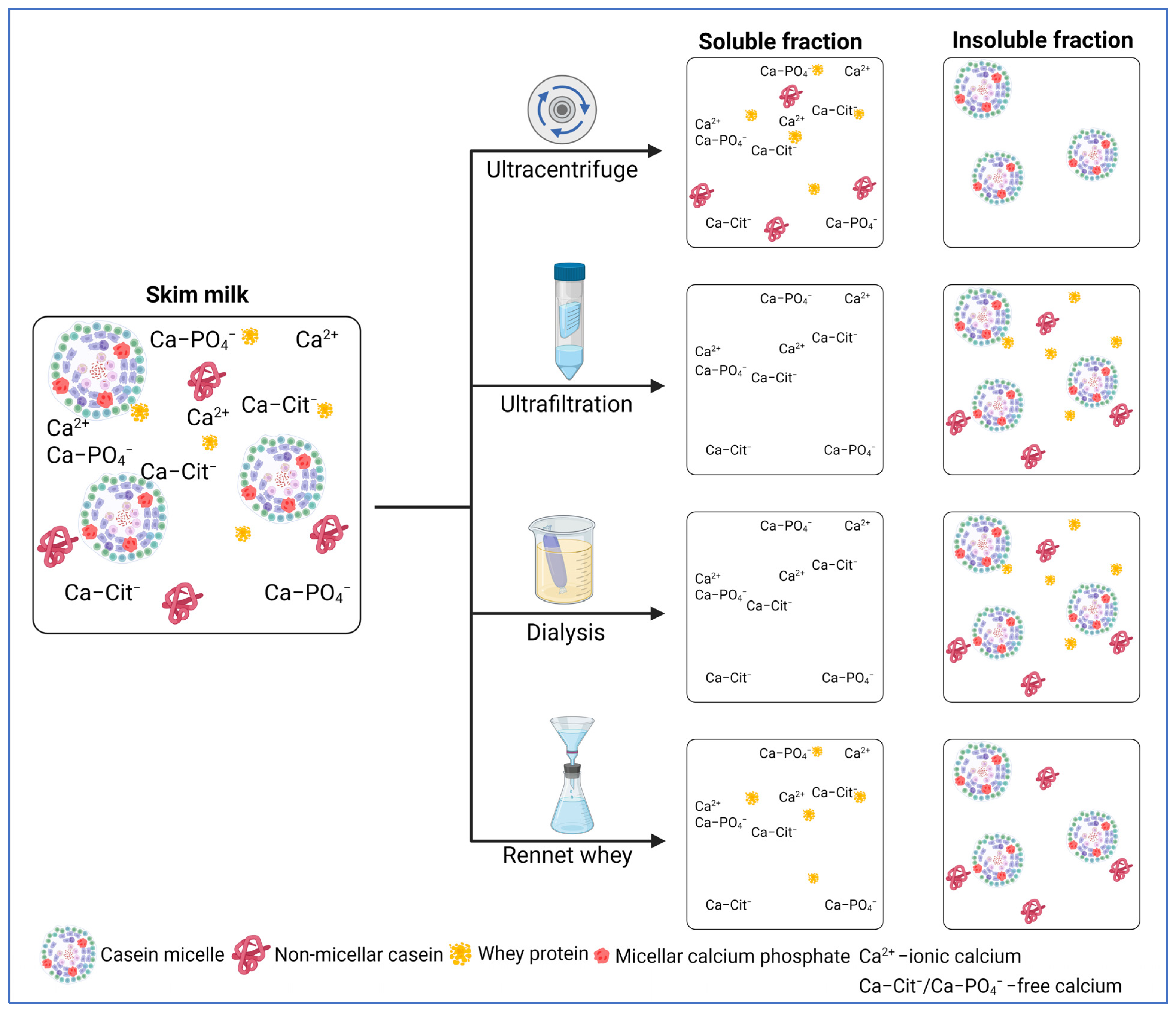
3.1. Methods for Fractionating Milk
3.1.1. (Ultra-)Centrifugation
3.1.2. Ultrafiltration
3.1.3. Dialysis
3.1.4. Rennet-Induced Casein Coagulation
3.1.5. Titration Method
3.2. Method-Dependent Effect on Salt Distribution in Milk
| Milk | Properties | Total Ca (mg/100 g) | % Soluble Ca | Method Used | Reference |
|---|---|---|---|---|---|
| Bovine | Casein = 2.46% | 143 | 42.6 | Centrifugation | [12] |
| Bovine milk | Acidity = 0.163% LA | 126 | 25.1 | Rennet whey | [35] |
| Milk (85 °C for 30 min) | Acidity = 0.161% LA | 134 | 21.2 | Rennet whey | [35] |
| Skim milk | Acidity = 0.15%LA | - | 40 | Dialysis | [27] |
| Skim milk | Acidity = 0.20%LA | - | 42 | Dialysis | [27] |
| Skim milk | Acidity = 0.30%LA | - | 60 | Dialysis | [27] |
| Raw milk | Fat = 1.01%; pH = 6.62 | 111.3 | 40.5 | Ultrafiltration (30 kDa) | [25] |
| Milk (63 °C for 30 min) | Fat =1.01%; pH = 6.62 | 108.7 | 30.9 | Ultrafiltration (30 kDa) | [25] |
| Milk (72 °C for 15 s) | Fat = 1.01%; pH = 6.62 | 110.0 | 27.8 | Ultrafiltration (30 kDa) | [25] |
| Milk (130 °C for 2 s) | Fat = 1.01%; pH = 6.62 | 108.6 | 34.6 | Ultrafiltration (30 kDa) | [25] |
| Whole milk | - | 110.8 | 16 | Dialysis for 16 h | [28] |
| Whole milk | - | 110.8 | 27 | Dialysis for 32 h | [28] |
| Whole milk | - | 110.8 | 42 | Dialysis for 48 h | [28] |
4. Salts’ Distribution in Cheese
4.1. Methods for Salt Distribution in Cheese
4.1.1. Cheese Pressing
4.1.2. Expressible Serum by Centrifugation
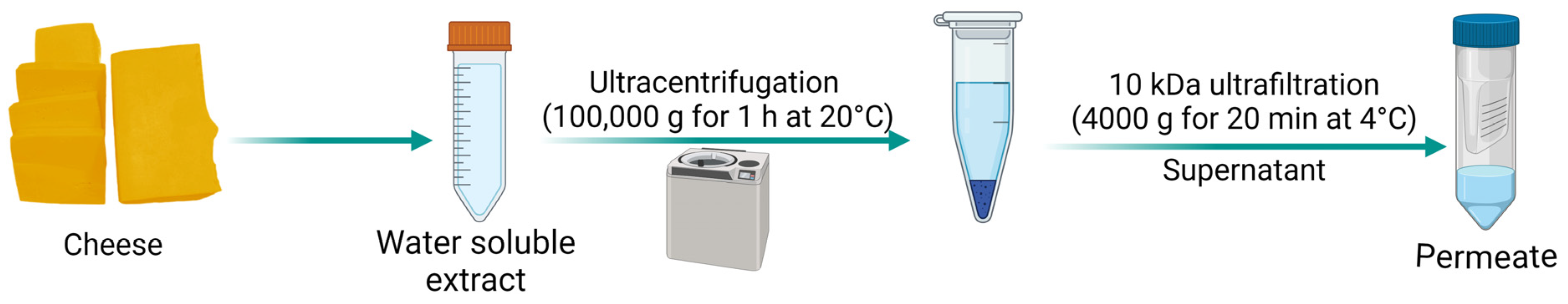
4.1.3. Water-Soluble Extract
4.1.4. Titration Method
4.2. Method-Dependent Effects on Salt Distribution in Cheese
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaucheron, F. Milk salts: Distribution and Analysis. In Encylopedia of Dairy Sciences; Academic Press: Cambridge, MA, USA, 2011; pp. 908–916. [Google Scholar]
- Lucey, J.; Horne, D. Milk salts: Technological significance. In Advanced Dairy Chemistry: Volume 3: Lactose, Water, Salts and Minor Constituents; Springer: Cham, Switzerland, 2009; pp. 351–389. [Google Scholar]
- Walstra, P.; Wouters, J.T.; Geurts, T.J. Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Li, Y.; Corredig, M. Calcium release from milk concentrated by ultrafiltration and diafiltration. J. Dairy Sci. 2014, 97, 5294–5302. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Davies, D. 712. The relation between the chemical composition of milk and the stability of the caseinate complex: I. General introduction, description of samples, methods and chemical composition of samples. J. Dairy Res. 1958, 25, 236–255. [Google Scholar] [CrossRef]
- Koutina, G.; Knudsen, J.C.; Andersen, U.; Skibsted, L.H. Influence of colloidal calcium phosphate level on the microstructure and rheological properties of rennet-induced skim milk gels. LWT-Food Sci. Technol. 2015, 63, 654–659. [Google Scholar] [CrossRef]
- AOAC International. Official methods of analysis of the Association of Analytical Chemists International. In Official Methods; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Eshpari, H.; Jimenez-Flores, R.; Tong, P.; Corredig, M. Thermal stability of reconstituted milk protein concentrates: Effect of partial calcium depletion during membrane filtration. Food Res. Int. 2017, 102, 409–418. [Google Scholar] [CrossRef]
- Smith, J. Reviews of the progress of dairy science. J. Dairy Res. 1961, 28, 87. [Google Scholar] [CrossRef]
- Davies, D.; White, J. The use of ultrafiltration and dialysis in isolating the aqueous phase of milk and in determining the partition of milk constituents between the aqueous and disperse phases. J. Dairy Res. 1960, 27, 171–190. [Google Scholar] [CrossRef]
- Van Slyke, L.L.; Bosworth, A.W. Condition of casein and salts in milk. J. Biol. Chem. 1915, 20, 135–152. [Google Scholar] [CrossRef]
- Hassan, A.; Johnson, M.; Lucey, J. Changes in the proportions of soluble and insoluble calcium during the ripening of Cheddar cheese. J. Dairy Sci. 2004, 87, 854–862. [Google Scholar] [CrossRef]
- Lucey, J.; Mishra, R.; Hassan, A.; Johnson, M. Rheological and calcium equilibrium changes during the ripening of Cheddar cheese. Int. Dairy J. 2005, 15, 645–653. [Google Scholar] [CrossRef]
- Barthel, C.; Sandberg, E.; Haglund, E. Research on rennin in cheese. Le Lait 1928, 8, 762–768. [Google Scholar] [CrossRef]
- Deshwal, G.; Fenelon, M.; Gómez-Mascaraque, L.; Huppertz, T. Influence of calcium sequestering salt type and concentration on the characteristics of processed cheese made from Gouda cheese of different ages. Food Res. Int. 2024, 114587. [Google Scholar]
- Lewis, M. Partitioning milk constituents. In Advanced Dairy Chemistry: Volume 3: Lactose, Water, Salts and Minor Constituents; Springer: Berlin/Heidelberg, Germany, 2022; pp. 339–416. [Google Scholar]
- Verma, I.; Sommer, H. Study of the naturally occurring salts in milk. J. Dairy Sci. 1957, 40, 331–335. [Google Scholar] [CrossRef]
- On-Nom, N.; Grandison, A.; Lewis, M. Measurement of ionic calcium, pH, and soluble divalent cations in milk at high temperature. J. Dairy Sci. 2010, 93, 515–523. [Google Scholar] [CrossRef]
- Anema, S.G. Effect of milk solids concentration on the pH, soluble calcium and soluble phosphate levels of milk during heating. Dairy Sci. Technol. 2009, 89, 501–510. [Google Scholar] [CrossRef]
- Tanguy, G.; Tuler-Perrone, I.; Dolivet, A.; Santellani, A.-C.; Leduc, A.; Jeantet, R.; Schuck, P.; Gaucheron, F. Calcium citrate insolubilization drives the fouling of falling film evaporators during the concentration of hydrochloric acid whey. Food Res. Int. 2019, 116, 175–183. [Google Scholar]
- Ferrer, M.A.; Alexander, M.; Corredig, M. Does ultrafiltration have a lasting effect on the physico-chemical properties of the casein micelles? Dairy Sci. Technol. 2011, 91, 151–170. [Google Scholar] [CrossRef]
- Clark, L., Jr. An easily constructed apparatus for preparing ultrafiltrates. J. Lab. Clin. Med. 1951, 37, 481–484. [Google Scholar]
- Pouliot, Y.; Boulet, M.; Paquin, P. An experimental technique for the study of milk salt balance. J. Dairy Sci. 1989, 72, 36–40. [Google Scholar]
- Wang, Q.; Holt, C.; Nylander, T.; Ma, Y. Salt partition, ion equilibria, and the structure, composition, and solubility of micellar calcium phosphate in bovine milk with added calcium salts. J. Dairy Sci. 2020, 103, 9893–9905. [Google Scholar]
- Yoo, S.-H.; Kang, S.-B.; Park, J.-H.; Lee, K.-S.; Kim, J.-M.; Yoon, S.-S. Effect of heat-treat methods on the soluble calcium levels in the commercial milk products. Food Sci. Anim. Resour. 2013, 33, 369–376. [Google Scholar] [CrossRef]
- Deshwal, G.; Fenelon, M.; Gómez-Mascaraque, L.; Huppertz, T. Influence of citrate-and phosphate-based calcium sequestering salts on the disruption of casein micelles. Food Hydrocoll. 2024, 153, 109970. [Google Scholar] [CrossRef]
- Lampitt, L.H.; Bushill, J.H.; Filmer, D.F. Dialysis of milk: Salt equilibrium with special reference to calcium, magnesium and phosphorus. Biochem. J. 1937, 31, 1861. [Google Scholar] [CrossRef] [PubMed]
- Magee, H.E.; Harvey, D. Studies on the Effect of Heat on Milk: Some Physico-Chemical Changes induced in Milk by Heat. Biochem. J. 1926, 20, 873. [Google Scholar] [CrossRef]
- Ling, E.R. 134. The Titration of Milk and Whey as a Means of estimating the Colloidal Calcium Phosphate of Milk. J. Dairy Res. 1936, 7, 145–155. [Google Scholar] [CrossRef]
- Britten, M.; Giroux, H.J. Rennet coagulation of heated milk: A review. Int. Dairy J. 2022, 124, 105179. [Google Scholar] [CrossRef]
- Van Slyke, D.D. On the measurement of buffer values and on the relationship of buffer value to the dissociation constant of the buffer and the concentration and reaction of the buffer solution. J. Biol. Chem. 1922, 52, 525–570. [Google Scholar] [CrossRef]
- Lucey, J.; Hauth, B.; Gorry, C.; Fox, P. The acid-base buffering properties of milk. Milchwissenschaft 1993, 48, 268–272. [Google Scholar]
- Miwa, N.; Yokoyama, K.; Wakabayashi, H.; Nio, N. Effect of deamidation by protein-glutaminase on physicochemical and functional properties of skim milk. Int. Dairy J. 2010, 20, 393–399. [Google Scholar] [CrossRef]
- Regnault, S.; Dumay, E.; Cheftel, J.C. Pressurisation of raw skim milk and of a dispersion of phosphocaseinate at 9 C or 20 C: Effects on the distribution of minerals and proteins between colloidal and soluble phases. J. Dairy Res. 2006, 73, 91–100. [Google Scholar] [CrossRef]
- Mattick, E.C.; Hallett, H.S. The effect of heat on milk:(A) On the coagulability by rennet.(B) On the nitrogen, phosphorus and calcium content. J. Agric. Sci. 1929, 19, 452–462. [Google Scholar] [CrossRef]
- Deshwal, G.; Gómez-Mascaraque, L.; Fenelon, M.; Huppertz, T. Soluble salts in processed cheese prepared with citrate- and phosphate-based calcium sequestering salts. Molecules 2024, 29, 3631. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.A.; Holt, C.; Brooker, B.E.; Banks, J.M.; Manson, W. Inorganic constituents of cheese: Analysis of juice from a one-month-old Cheddar cheese and the use of light and electron microscopy to characterize the crystalline phases. J. Dairy Res. 1988, 55, 255–268. [Google Scholar] [CrossRef]
- Roy, D.; Pitre, M.; Blanchette, L.; Savoie, L.; Bélanger, G.; Ward, P.; Maubois, J. Monitoring proteolysis and cheese juice composition during ripening of Cheddar cheese made from microfiltered milk. Le Lait 1997, 77, 521–541. [Google Scholar] [CrossRef]
- Guinee, T.P.; Harrington, D.; Corcoran, M.O.; Mulholland, E.O.; Mujllins, C. The compositional and functional properties of commercial Mozzarella, Cheddar and analogue pizza cheeses. Int. J. Dairy Technol. 2000, 53, 51–56. [Google Scholar] [CrossRef]
- Monib, A.M.M.F. The Calcium-Paracaseinate-Phosphate-Complex Under Conditions Similar to Those in Cheese. Doctoral Dissertation, Landbouwhogeschool Wageningen, Veenman en Zonen N.V., Wageningen, The Netherlands, 1962. [Google Scholar]
- Lee, M.-R.; Johnson, M.; Govindasamy-Lucey, S.; Jaeggi, J.; Lucey, J. Insoluble calcium content and rheological properties of Colby cheese during ripening. J. Dairy Sci. 2010, 93, 1844–1853. [Google Scholar] [CrossRef]
- Guo, M.R.; Kindstedt, P.S. Age-related changes in the water phase of Mozzarella cheese. J. Dairy Sci. 1995, 78, 2099–2107. [Google Scholar] [CrossRef]
- Guo, M.R.; Gilmore, J.A.; Kindstedt, P.S. Effect of sodium chloride on the serum phase of Mozzarella cheese. J. Dairy Sci. 1997, 80, 3092–3098. [Google Scholar]
- Upreti, P.; Metzger, L. Influence of calcium and phosphorus, lactose, and salt-to-moisture ratio on Cheddar cheese quality: pH changes during ripening. J. Dairy Sci. 2007, 90, 1–12. [Google Scholar]
- Fathollahi, I.; Hesari, J.; Azadmard, S.; Oustan, S. Influence of proteolysis and soluble calcium levels on textural changes in the interior and exterior of Iranian UF white cheese during ripening. Int. J. Nutr. Food Eng. 2010, 4, 399–404. [Google Scholar]
- Guinee, T.; O’Kennedy, B.T. The effect of calcium content of Cheddar-style cheese on the biochemical and rheological properties of processed cheese. Dairy Sci. Technol. 2009, 89, 317–333. [Google Scholar]
- Boutrou, R.; Gaucheron, F.; Piot, M.; Michel, F.; Maubois, J.-L.; Léonil, J. Changes in the composition of juice expressed from Camembert cheese during ripening. Le lait 1999, 79, 503–513. [Google Scholar] [CrossRef]
- Lucey, J.; Gorry, C.; Fox, P. Changes in the acid-base buffering curves during the ripening of Emmental cheese. Milchwissenschaft 1993, 48, 183–186. [Google Scholar]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering capacity of dairy products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Upreti, P.; Bühlmann, P.; Metzger, L. Influence of calcium and phosphorus, lactose, and salt-to-moisture ratio on Cheddar cheese quality: pH buffering properties of cheese. J. Dairy Sci. 2006, 89, 938–950. [Google Scholar] [CrossRef]

| Constituents | Concentration in Milk (g/kg) | Micellar (% of Total) | Soluble (% of Total) |
|---|---|---|---|
| Lactose | 49–51 | - | 100 |
| Caseins | 28.5 | ||
| αS1-casein | 11.6 | 93.9 | 6.1 |
| αS2-casein | 3.1 | 96.8 | 3.2 |
| β-casein | 10.3 | 87.4 | 12.6 |
| κ-casein | 3.4 | 85.3 | 14.7 |
| Cationic Salts | |||
| Ca | 1.04–1.28 | 69 | 31 |
| Mg | 0.10–0.15 | 47 | 53 |
| K | 1.21–1.68 | ≤5 | ≥95 |
| Na | 0.35–0.60 | ≤2 | ≥98 |
| Anionic Salts | |||
| Chloride | 0.78–1.20 | ≤5 | ≥95 |
| Total P | 0.93–1.0 | 46 | 54 |
| Inorganic P (as PO4) | 0.65 | 53 | 47 |
| Citrate | 1.32–2.08 | 14 | 86 |
| Cheese | % Moisture in Cheese | Total Ca (g/kg Cheese) | % Soluble Ca | Cheese Fraction Analyzed | Reference |
|---|---|---|---|---|---|
| Cheddar | 35.7 | 6.88 | 57.0 | Cheese juice | [37] |
| Cheddar | 32.8 | 6.50 | 4.6 g/kg juice | Cheese juice | [38] |
| Cheddar | 37.2 | 7.12 | 38.2% | Cheese juice | [39] |
| Gouda/Edam | 30–50 | 9–50 | Cheese juice | [40] | |
| Low-moisture mozzarella | 46.4 | 7.09 | 22.7% | Cheese juice | [39] |
| Analog pizza cheese | 48.9 | 6.32 | 47.0% | Cheese juice | [39] |
| Cheddar | 38.6 | 8.33 | 30.0% | Cheese juice | [12] |
| Colby | 38.7 | 6.84 | 75.2% | Cheese juice | [41] |
| Colby | 36.6 | 5.69 | 68.1% | Cheese juice | [41] |
| Cheddar | 38.6 | 8.33 | 24.0% | Titration | [12] |
| Mozzarella | 45.6 | 7.58 | 3.0 g/kg juice | Expressible serum | [42] |
| Mozzarella | 48.6 | 6.57 | 3.2 g/kg juice | Expressible serum | [42] |
| Brine-salted mozzarella | 49.4 | 6.49 | 3.2 g/kg juice | Expressible serum | [43] |
| Unsalted mozzarella | 50.9 | 6.68 | 3.6 g/kg juice | Expressible serum | [43] |
| Cheddar | 32.1 | 6.90 | 37.3% | Water-soluble extract | [44] |
| Iranian UF white cheese | 64.1 | - | 45.0% | Water-soluble extract | [45] |
| Processed cheese | 47.3 | 3.75 | 20.2% | Water-soluble extract | [46] |
| Processed cheese | 46.9 | 6.15 | 18.1% | Water-soluble extract | [46] |
| Attributes | Cheese Juice | Water Soluble Extract | Expressible Serum | Titration |
|---|---|---|---|---|
| Sample size | >100 g | 10–20 g | 10–20 g | <10 g |
| Time for one sample | >1.5 h | >1.5 h | 1.25 h | 1.5 h |
| Method variables | Pressure, ratio of cheese and sand | Water to cheese ratio, centrifugation speed and time | Centrifugation speed and time | Amount of cheese and water, speed of titration |
| Ease of use | Labor intensive | Less labor intensive than cheese juice method | Less labor intensive than cheese juice method | Can be performed automatically by high-end titrator |
| Requirements | Cheese press, sand, and muslin cloth | Stomacher, centrifuge, and water bath | Centrifuge | Titrator with dosing units and pH electrodes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshwal, G.K.; van der Meulen, L.; Huppertz, T. Distribution of Salts in Milk and Cheese: Critical Methodological Aspects. Encyclopedia 2024, 4, 1629-1643. https://doi.org/10.3390/encyclopedia4040107
Deshwal GK, van der Meulen L, Huppertz T. Distribution of Salts in Milk and Cheese: Critical Methodological Aspects. Encyclopedia. 2024; 4(4):1629-1643. https://doi.org/10.3390/encyclopedia4040107
Chicago/Turabian StyleDeshwal, Gaurav Kr, Liesbeth van der Meulen, and Thom Huppertz. 2024. "Distribution of Salts in Milk and Cheese: Critical Methodological Aspects" Encyclopedia 4, no. 4: 1629-1643. https://doi.org/10.3390/encyclopedia4040107
APA StyleDeshwal, G. K., van der Meulen, L., & Huppertz, T. (2024). Distribution of Salts in Milk and Cheese: Critical Methodological Aspects. Encyclopedia, 4(4), 1629-1643. https://doi.org/10.3390/encyclopedia4040107






