The 1H HR-NMR Methods for the Evaluation of the Stability, Quality, Authenticity, and Shelf Life of Foods
Definition
1. Introduction
2. The 1H HR-NMR Methods
3. Chemical Shift Analysis (CSA) for Food Analysis
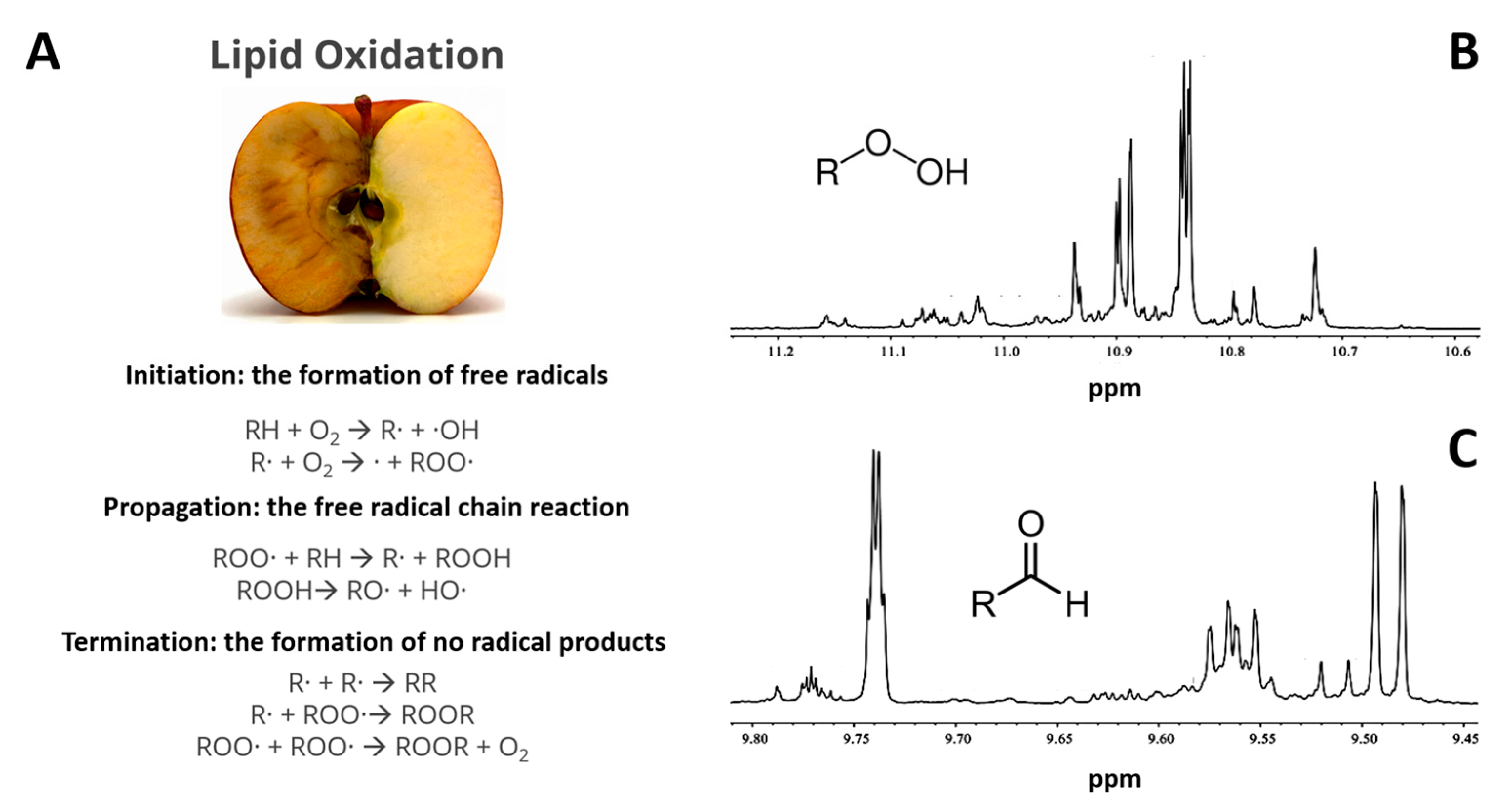
4. Peak Intensity and Area Analysis for Food Quantity Analysis
5. Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Richards, S.A.; Hollerton, J.C. Essential Practical NMR for Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Alderson, T.R.; Kay, L.E. NMR spectroscopy captures the essential role of dynamics in regulating biomolecular function. Cell 2021, 184, 577–595. [Google Scholar] [CrossRef]
- Krivdin, L.B. Computational 1H NMR: Part 3. Biochemical studies. Magn. Reson. Chem. 2020, 58, 15–30. [Google Scholar] [CrossRef]
- Trimigno, A.; Łoniewska, B.; Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Picone, G. The application of High-Resolution Nuclear Magnetic Resonance (HR NMR) in metabolomic analyses of meconium and stool in newborns. A preliminary pilot study of MABEL project: Metabolomics approach for the assessment of Baby-Mother Enteric Microbiota Legacy. PharmaNutrition 2024, 27, 100378. [Google Scholar] [CrossRef]
- Simonetti, G.; Mengucci, C.; Padella, A.; Fonzi, E.; Picone, G.; Delpino, C.; Nanni, J.; De Tommaso, R.; Franchini, E.; Papayannidis, C. Integrated genomic-metabolic classification of acute myeloid leukemia defines a subgroup with NPM1 and cohesin/DNA damage mutations. Leukemia 2021, 35, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.; Pereira, T.C.; Souza, A.R.; Ribeiro, P.R. 1H NMR-based metabolite profiling for biomarker identification. Clin. Chim. Acta 2020, 502, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Szabó, M.; Medina, F.; Gulyás, H. A brief introduction to the basics of NMR spectroscopy and selected examples of its applications to materials characterization. Phys. Sci. Rev. 2021, 6, 20190086. [Google Scholar] [CrossRef]
- Ciampa, A.; Danesi, F.; Picone, G. NMR-Based Metabolomics for a More Holistic and Sustainable Research in Food Quality Assessment: A Narrative Review. Appl. Sci. 2022, 13, 372. [Google Scholar] [CrossRef]
- Hatzakis, E. Nuclear magnetic resonance (NMR) spectroscopy in food science: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Carpena, M.; Garcia-Oliveira, P.; Pereira, A.; Prieto, M.; Simal-Gandara, J. Analytical metabolomics and applications in health, environmental and food science. Crit. Rev. Anal. Chem. 2022, 52, 712–734. [Google Scholar] [CrossRef]
- Picone, G. NMR, Metabonomics and Molecular Profiles: Applications to the Quality Assessment of Foodstuffs; University of Bologna: Bologna, Italy, 2009. [Google Scholar]
- Klassen, A.; Faccio, A.T.; Canuto, G.A.B.; da Cruz, P.L.R.; Ribeiro, H.C.; Tavares, M.F.M.; Sussulini, A. Metabolomics: Definitions and significance in systems biology. Metabolomics Fundam. Clin. Appl. 2017, 3–17. [Google Scholar] [CrossRef]
- Laghi, L.; Picone, G.; Capozzi, F. Nuclear magnetic resonance for foodomics beyond food analysis. TrAC Trends Anal. Chem. 2014, 59, 93–102. [Google Scholar] [CrossRef]
- Brown, M. Processing and food and beverage shelf life. In The Stability and Shelf-Life of Food; Kilcast, D., Subramaniam, P., Eds.; Wood Head Publishing: Cambridge, UK, 2011; pp. 184–236. [Google Scholar]
- Lindon, J.C.; Nicholson, J.K.; Holmes, E.; Everett, J.R. Metabonomics: Metabolic processes studied by NMR spectroscopy of biofluids. Concepts Magn. Reson. Educ. J. 2000, 12, 289–320. [Google Scholar] [CrossRef]
- Ross, A.; Schlotterbeck, G.; Dieterle, F.; Senn, H. NMR spectroscopy techniques for application to metabonomics. In The Handbook of Metabonomics and Metabolomics; Elsevier: Amsterdam, The Netherlands, 2007; Volume 4, p. 55. [Google Scholar]
- Wishart, D.S.; Sykes, B.D. [12] Chemical shifts as a tool for structure determination. Methods Enzymol. 1994, 239, 363–392. [Google Scholar]
- Nerli, S.; McShan, A.C.; Sgourakis, N.G. Chemical shift-based methods in NMR structure determination. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 106–107, 1–25. [Google Scholar] [CrossRef]
- de Oliveira, C.R.; Carneiro, R.L.; Ferreira, A.G. Tracking the degradation of fresh orange juice and discrimination of orange varieties: An example of NMR in coordination with chemometrics analyses. Food Chem. 2014, 164, 446–453. [Google Scholar] [CrossRef]
- Lambert, J.B.; Mazzola, E.P.; Ridge, C.D. Nuclear Magnetic Resonance Spectroscopy: An Introduction to Principles, Applications, and Experimental Methods; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Raja, P.M.V.; Barron, A.R. NMR Spectroscopy. 2022. Available online: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_(Barron)/04%3A_Chemical_Speciation/4.07%3A_NMR_Spectroscopy (accessed on 29 August 2024).
- Farmer, S.; Kennepohl, D.; Soderberg, T. Integration of 1H NMR Absorptions—Proton Counting. Org. Chem. 2024. [Google Scholar]
- Malet-Martino, M.; Holzgrabe, U. NMR techniques in biomedical and pharmaceutical analysis. J. Pharm. Biomed. Anal. 2011, 55, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Universal quantitative NMR analysis of complex natural samples. Curr. Opin. Biotechnol. 2014, 25, 51–59. [Google Scholar] [CrossRef]
- Cao, R.; Liu, X.; Liu, Y.; Zhai, X.; Cao, T.; Wang, A.; Qiu, J. Applications of nuclear magnetic resonance spectroscopy to the evaluation of complex food constituents. Food Chem. 2021, 342, 128258. [Google Scholar] [CrossRef]
- Lolli, V.; Caligiani, A. How nuclear magnetic resonance contributes to food authentication: Current trends and perspectives. Curr. Opin. Food Sci. 2024, 58, 101200. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Cesare Marincola, F.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353. [Google Scholar] [CrossRef]
- Bingol, K. Recent Advances in Targeted and Untargeted Metabolomics by NMR and MS/NMR Methods. High-Throughput 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Ciampa, A.; Picone, G. Application of HR-NMR for the Metabolic Kinetic Assessment of Red Mullet (Mullus barbatus) and Bogue (Boops boops) Samples during Different Temperature Storage. Metabolites 2023, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Ciampa, A.; Laghi, L.; Picone, G. Validation of a 1 H-NMR Spectroscopy Quantitative Method to Quantify Trimethylamine Content and K-Index Value in Different Species of Fish. J. Food Qual. 2022, 2022, 3612095. [Google Scholar] [CrossRef]
- Spiteri, C.; Lia, F.; Farrugia, C. Determination of the geographical origin of Maltese honey using 1H NMR fingerprinting. Foods 2020, 9, 1455. [Google Scholar] [CrossRef]
- Calò, F.; Girelli, C.R.; Wang, S.C.; Fanizzi, F.P. Geographical origin assessment of extra virgin olive oil via NMR and MS combined with chemometrics as analytical approaches. Foods 2022, 11, 113. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fan, C.; Gao, S.; Zhang, Z.; Bo, L. Classification of the botanical and geographical origins of Chinese honey based on 1H NMR profile with chemometrics. Food Res. Int. 2020, 137, 109714. [Google Scholar] [CrossRef]
- Pacholczyk-Sienicka, B.; Ciepielowski, G.; Albrecht, Ł. The application of NMR spectroscopy and chemometrics in authentication of spices. In Analysis of Food Spices; CRC Press: Boca Raton, FL, USA, 2023; pp. 251–284. [Google Scholar]
- Lau, H.; Laserna, A.K.C.; Li, S.F.Y. 1H NMR-based metabolomics for the discrimination of celery (Apium graveolens L. var. dulce) from different geographical origins. Food Chem. 2020, 332, 127424. [Google Scholar] [CrossRef]
- Mascellani, A.; Hoca, G.; Babisz, M.; Krska, P.; Kloucek, P.; Havlik, J. 1H NMR chemometric models for classification of Czech wine type and variety. Food Chem. 2021, 339, 127852. [Google Scholar] [CrossRef]
- Cagliani, L.; Maestri, G.; Consonni, R. Detection and evaluation of saccharide adulteration in Italian honey by NMR spectroscopy. Food Control 2022, 133, 108574. [Google Scholar] [CrossRef]
- Cerceau, C.I.; Barbosa, L.C.; Alvarenga, E.S.; Maltha, C.R.; Ismail, F.M. 1H-NMR and GC for detection of adulteration in commercial essential oils of Cymbopogon ssp. Phytochem. Anal. 2020, 31, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.; Esslinger, S.; Fauhl-Hassek, C.; Riedl, J. 1H NMR spectroscopy, one-class classification and outlier diagnosis: A powerful combination for adulteration detection in paprika powder. Food Control 2021, 128, 108205. [Google Scholar] [CrossRef]
- Alharbi, H.; Kahfi, J.; Dutta, A.; Jaremko, M.; Emwas, A.-H. The detection of adulteration of olive oil with various vegetable oils–A case study using high-resolution 700 MHz NMR spectroscopy coupled with multivariate data analysis. Food Control 2024, 166, 110679. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Samar, M.; Shami, A.A.; Mumtaz, M.W.; Mukhtar, H.; Tahir, A.; Shahzad-ul-Hussan, S.; Chaudhary, S.U.; Kaka, U. 1H-NMR-based metabolomics: An integrated approach for the detection of the adulteration in chicken, chevon, beef and donkey meat. Molecules 2021, 26, 4643. [Google Scholar] [CrossRef]
- Ogrinc, N.; Košir, I.; Spangenberg, J.; Kidrič, J. The application of NMR and MS methods for detection of adulteration of wine, fruit juices, and olive oil. A review. Anal. Bioanal. Chem. 2003, 376, 424–430. [Google Scholar] [CrossRef]
- Lucarini, M.; Di Cocco, M.E.; Raguso, V.; Milanetti, F.; Durazzo, A.; Lombardi-Boccia, G.; Santini, A.; Delfini, M.; Sciubba, F. NMR-based metabolomic comparison of Brassica oleracea (var. italica): Organic and conventional farming. Foods 2020, 9, 945. [Google Scholar] [CrossRef]
- Hohmann, M.; Christoph, N.; Wachter, H.; Holzgrabe, U. 1H NMR profiling as an approach to differentiate conventionally and organically grown tomatoes. J. Agric. Food Chem. 2014, 62, 8530–8540. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Rosas-Bautista, A.; Rico-Arzate, E.; Cruz-Narvaez, Y.; Zepeda-Vallejo, L.G.; Lalaleo, L.; Hidalgo-Martínez, D.; Becerra-Martínez, E. Study of nutritional quality of pomegranate (Punica granatum L.) juice using 1H NMR-based metabolomic approach: A comparison between conventionally and organically grown fruits. LWT 2020, 134, 110222. [Google Scholar] [CrossRef]
- Picone, G.; Trimigno, A.; Tessarin, P.; Donnini, S.; Rombolà, A.D.; Capozzi, F. 1H NMR foodomics reveals that the biodynamic and the organic cultivation managements produce different grape berries (Vitis vinifera L. cv. Sangiovese). Food Chem. 2016, 213, 187–195. [Google Scholar] [CrossRef]
- Picone, G.; Mezzetti, B.; Babini, E.; Capocasa, F.; Placucci, G.; Capozzi, F. Unsupervised principal component analysis of NMR metabolic profiles for the assessment of substantial equivalence of transgenic grapes (Vitis vinifera). J. Agric. Food Chem. 2011, 59, 9271–9279. [Google Scholar] [CrossRef]
- Mihailova, A.; Kelly, S.D.; Chevallier, O.P.; Elliott, C.T.; Maestroni, B.M.; Cannavan, A. High-resolution mass spectrometry-based metabolomics for the discrimination between organic and conventional crops: A review. Trends Food Sci. Technol. 2021, 110, 142–154. [Google Scholar] [CrossRef]
- Maestrello, V.; Solovyev, P.; Bontempo, L.; Mannina, L.; Camin, F. Nuclear magnetic resonance spectroscopy in extra virgin olive oil authentication. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.P.; Antoniosi Filho, N.R.; Lião, L.M.; Flores, I.S. Evaluation of the Metabolic Profile of Arabica Coffee via NMR in Relation to the Time and Temperature of the Roasting Procedure. J. Braz. Chem. Soc. 2021, 32, 123–136. [Google Scholar] [CrossRef]
- Utpott, M.; Rodrigues, E.; de Oliveira Rios, A.; Mercali, G.D.; Flôres, S.H. Metabolomics: An analytical technique for food processing evaluation. Food Chem. 2022, 366, 130685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-Y.; Bai, Y.; Wang, C.; Li, C.-B.; Xu, X.-L.; Pan, D.-D.; Cao, J.-X.; Zhou, G.-H. 1H NMR-based metabolomics and sensory evaluation characterize taste substances of Jinhua ham with traditional and modern processing procedures. Food Control 2021, 126, 107873. [Google Scholar] [CrossRef]
- Burton, I.W.; Martinez Farina, C.F.; Ragupathy, S.; Arunachalam, T.; Newmaster, S.; Berrué, F. Quantitative NMR Methodology for the Authentication of Roasted Coffee and Prediction of Blends. J. Agric. Food Chem. 2020, 68, 14643–14651. [Google Scholar] [CrossRef]
- Cui, C.; Xu, Y.; Jin, G.; Zong, J.; Peng, C.; Cai, H.; Hou, R. Machine learning applications for identify the geographical origin, variety and processing of black tea using 1H NMR chemical fingerprinting. Food Control 2023, 148, 109686. [Google Scholar] [CrossRef]
- Tristán, A.I.; Abreu, A.C.; Aguilera-Sáez, L.M.; Peña, A.; Conesa-Bueno, A.; Fernández, I. Evaluation of ORAC, IR and NMR metabolomics for predicting ripening stage and variety in melon (Cucumis melo L.). Food Chem. 2022, 372, 131263. [Google Scholar] [CrossRef]
- Di Matteo, G.; Spano, M.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L.; Ingallina, C.; Sobolev, A.P. Nmr characterization of ten apple cultivars from the piedmont region. Foods 2021, 10, 289. [Google Scholar] [CrossRef]
- Xing, M.; Wang, S.; Lin, J.; Xia, F.; Feng, J.; Shen, G. Composition profiling and authenticity assessment of camellia oil using high field and low field 1H NMR. Molecules 2021, 26, 4738. [Google Scholar] [CrossRef] [PubMed]
- Bambina, P.; Spinella, A.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P.; Cinquanta, L.; Conte, P. 1H-NMR Spectroscopy Coupled with Chemometrics to Classify Wines According to Different Grape Varieties and Different Terroirs. Agriculture 2024, 14, 749. [Google Scholar] [CrossRef]
- Selamat, J.; Rozani, N.A.A.; Murugesu, S. Application of the Metabolomics Approach in Food Authentication. Molecules 2021, 26, 7565. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Cagliani, L.R. The potentiality of NMR-based metabolomics in food science and food authentication assessment. Magn. Reson. Chem. 2019, 57, 558–578. [Google Scholar] [CrossRef] [PubMed]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on metabolomics for food authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Circi, S.; Mannina, L. 6—Advances in Nuclear Magnetic Resonance Spectroscopy for Food Authenticity Testing. In Advances in Food Authenticity Testing; Downey, G., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 147–170. [Google Scholar]
- Pauli, G.F.; Godecke, T.; Jaki, B.U.; Lankin, D.C. Quantitative 1H NMR. Development and potential of an analytical method: An update. J. Nat. Prod. 2012, 75, 834–851. [Google Scholar] [CrossRef]
- Truzzi, E.; Marchetti, L.; Benvenuti, S.; Righi, V.; Rossi, M.C.; Gallo, V.; Bertelli, D. A Novel qNMR Application for the Quantification of Vegetable Oils Used as Adulterants in Essential Oils. Molecules 2021, 26, 5439. [Google Scholar] [CrossRef] [PubMed]
- Triyasmono, L.; Holzgrabe, U. Combination quantitative 1H NMR and chemometric approaches for the assessment of quality control in commercially available products of red fruit (Pandanus conoidues, Lam.) oil. J. Pharm. Biomed. Anal. Open 2023, 1, 100010. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Merkx, D.W.H.; Hong, G.T.S.; Ermacora, A.; van Duynhoven, J.P.M. Rapid Quantitative Profiling of Lipid Oxidation Products in a Food Emulsion by 1H NMR. Anal. Chem. 2018, 90, 4863–4870. [Google Scholar] [CrossRef]
- Siciliano, C.; Belsito, E.; De Marco, R.; Di Gioia, M.L.; Leggio, A.; Liguori, A. Quantitative determination of fatty acid chain composition in pork meat products by high resolution 1H NMR spectroscopy. Food Chem. 2013, 136, 546–554. [Google Scholar] [CrossRef]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the western diet: How we got here. Mo. Med. 2020, 117, 536. [Google Scholar] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global impacts of western diet and its effects on metabolism and health: A narrative review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Newsome, R.; Yang, Y.; Jobin, C. Western diet influences on microbiome and carcinogenesis. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2023; p. 101756. [Google Scholar]
- Scettri, A.; Schievano, E. Quantification of polyols in sugar-free foodstuffs by qNMR. Food Chem. 2022, 390, 133125. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Chey, W.D. A systematic review of the effects of polyols on gastrointestinal health and irritable bowel syndrome. Adv. Nutr. 2017, 8, 587–596. [Google Scholar] [CrossRef]
- Shumilina, E.; Slizyte, R.; Mozuraityte, R.; Dykyy, A.; Stein, T.A.; Dikiy, A. Quality changes of salmon by-products during storage: Assessment and quantification by NMR. Food Chem. 2016, 211, 803–811. [Google Scholar] [CrossRef]
- Ciampa, A.; Picone, G.; Laghi, L.; Nikzad, H.; Capozzi, F. Changes in the amino acid composition of Bogue (Boops boops) fish during storage at different temperatures by 1H-NMR spectroscopy. Nutrients 2012, 4, 542–553. [Google Scholar] [CrossRef]
- LeMieux, M.J.; Aljawadi, A.; Moustaid-Moussa, N. Nutrimetabolomics. Adv. Nutr. 2014, 5, 792–794. [Google Scholar] [CrossRef]
- Srivastava, U.; Kanchan, S.; Kesheri, M.; Singh, S. Nutrimetabolomics: Metabolomics in Nutrition Research. In Metabolomics: Recent Advances and Future Applications; Soni, V., Hartman, T.E., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 241–268. [Google Scholar]
- Ulaszewska, M.M.; Weinert, C.H.; Trimigno, A.; Portmann, R.; Andres Lacueva, C.; Badertscher, R.; Brennan, L.; Brunius, C.; Bub, A.; Capozzi, F.; et al. Nutrimetabolomics: An Integrative Action for Metabolomic Analyses in Human Nutritional Studies. Mol. Nutr. Food Res. 2019, 63, 1800384. [Google Scholar] [CrossRef]
- Münger, L.H.; Trimigno, A.; Picone, G.; Freiburghaus, C.; Pimentel, G.g.; Burton, K.J.; Pralong, F.o.P.; Vionnet, N.; Capozzi, F.; Badertscher, R. Identification of urinary food intake biomarkers for milk, cheese, and soy-based drink by untargeted GC-MS and NMR in healthy humans. J. Proteome Res. 2017, 16, 3321–3335. [Google Scholar] [CrossRef]
- Trimigno, A.; Picone, G.; Capozzi, F. A 1H NMR-Based Metabolomics Approach on Dietary Biomarker Research in Human Urine. Magn. Reson. Food Sci. Defin. Food Magn. Reson. 2015, 143. [Google Scholar] [CrossRef]
- Chowdhury, C.R.; Kavitake, D.; Jaiswal, K.K.; Jaiswal, K.S.; Reddy, G.B.; Agarwal, V.; Shetty, P.H. NMR-based metabolomics as a significant tool for human nutritional research and health applications. Food Biosci. 2023, 53, 102538. [Google Scholar] [CrossRef]
- Hernandez-Baixauli, J.; Quesada-Vázquez, S.; Mariné-Casadó, R.; Gil Cardoso, K.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Baselga-Escudero, L. Detection of early disease risk factors associated with metabolic syndrome: A new era with the NMR metabolomics assessment. Nutrients 2020, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Winkvist, A.; Rådjursöga, M.; Ellegård, L.; Pedersen, A.; Lindqvist, H.M. Identification of single and combined serum metabolites associated with food intake. Metabolites 2022, 12, 908. [Google Scholar] [CrossRef] [PubMed]

| Application | Example | References | |
|---|---|---|---|
| Geographical Origin and Traceability | The metabolic profile of a food product is often influenced by its geographical origin. NMR metabolomics can be used to verify the claimed origin of products like wine, olive oil, coffee, honey, and tea. | Wines from different regions have distinct metabolite profiles due to variations in soil, climate, and grape variety. NMR can detect these differences, confirming whether a wine is truly from a specified region. | [32,33,34,35,36,37] |
| Detection of Adulteration | NMR metabolomics is highly effective in detecting adulteration, where a product is diluted or mixed with lower-quality ingredients. | MR can identify the addition of cheaper oils in high-quality olive oil by detecting unusual metabolites that should not be present in pure samples. | [38,39,40,41,42,43] |
| Identification of Organic vs. Conventional Farming | The metabolic profiles of products grown through organic farming can differ from those grown using conventional farming methods. NMR metabolomics can help authenticate claims of organic production. | Organic vegetables might have different levels of certain metabolites compared to conventionally grown ones due to the absence of synthetic fertilizers and pesticides. | [44,45,46,47,48,49] |
| Authentication of Processing Methods | The way a product is processed can significantly alter its metabolic profile. NMR metabolomics can be used to authenticate products based on specific processing methods. | In coffee, the metabolite profile differs significantly between washed and dry-processed beans. NMR can be used to confirm whether a batch of coffee has been processed as claimed. | [25,50,51,52,53,54] |
| Varietal Identification | Different varieties of the same product, such as apples, grapes, or tomatoes, have unique metabolic signatures. NMR can be used to verify whether the claimed variety matches the actual variety of the product. | In tea, different varieties of Camellia sinensis (the plant used to produce tea) can be distinguished by their specific metabolite profiles, which NMR can accurately identify. | [55,56,57,58,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picone, G. The 1H HR-NMR Methods for the Evaluation of the Stability, Quality, Authenticity, and Shelf Life of Foods. Encyclopedia 2024, 4, 1617-1628. https://doi.org/10.3390/encyclopedia4040106
Picone G. The 1H HR-NMR Methods for the Evaluation of the Stability, Quality, Authenticity, and Shelf Life of Foods. Encyclopedia. 2024; 4(4):1617-1628. https://doi.org/10.3390/encyclopedia4040106
Chicago/Turabian StylePicone, Gianfranco. 2024. "The 1H HR-NMR Methods for the Evaluation of the Stability, Quality, Authenticity, and Shelf Life of Foods" Encyclopedia 4, no. 4: 1617-1628. https://doi.org/10.3390/encyclopedia4040106
APA StylePicone, G. (2024). The 1H HR-NMR Methods for the Evaluation of the Stability, Quality, Authenticity, and Shelf Life of Foods. Encyclopedia, 4(4), 1617-1628. https://doi.org/10.3390/encyclopedia4040106






