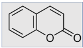
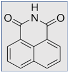
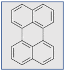
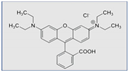
Definition
Fluorescence has been identified as an advantageous feature in smart fabrics, notably for the protection of humans during outdoor athletic activities, as well as for preventing counterfeiting and determining authenticity. Fluorescence in smart fabrics is achieved using dendrimers, rare earth metal compounds, and fluorescent dye. The principal method for producing fluorescent fabrics is to immerse the sample in a solution containing fluorescent agents. However, covalent connections between fluorophores and textile substates should be established to improve the stability and intensity of the fluorescent characteristics. Fabric can be fluorescent throughout, or fluorescent fibers can be woven directly into the textile structures, made of natural (cotton, silk) or synthetic (polyamide- and polyester-based) fibers, into a precise pathway that becomes visible under ultraviolet irradiation.
1. Smart Textiles
Smart textiles are intelligent fabrics or fibers that can respond to external stimuli and adapt to environmental changes [1]. Light, pH, temperature, polar solvents, chemicals, and electricity are some of the physical stimuli with which these materials can interact [2]. Smart textiles are classified into three types: (i) passive smart textiles, which can only sense the environment via sensors; (ii) active smart textiles, which may respond to an external stimulus from the environment by combining an actuator function and a sensor; and (iii) very smart textiles, which can perceive, react, and adjust their behavior in response to the surroundings [3,4]. Smart fibers include [5] (i) shape memory fibers, which can return to their original shape after being deformed by external factors such as pressure and temperature [6]; (ii) photochromic fibers, which are photosensitive fibers that change color under the effect of light [7]; (iii) temperature sensitive fibers, whose characteristics are altered with temperature in a reversible manner [8]; (iv) pH sensitive fibers, which change volume or shape as the pH changes [9]; (v) healthy smart fibers, which safeguard human health by performing antibacterial or deodorant functions, or can be used in health monitoring, personal thermal therapy, and wearable electronics [10].
Shape memory fibers are frequently used to create smart clothing, i.e., deformable clothes that can change shape by changing the temperature, due not only to their good shape memory capabilities, but also to their mechanical strength and elasticity [11]. A luminescent shape memory fiber membrane, made from polyvinyl acetate (PVA) and indocyanine green (ICG), was prepared by electrospinning [12]. When the fibers were immersed in water at 25 °C or heated at 50 °C, shrinkage was achieved. These characteristics were thought to be helpful for medical devices such as gastroesophageal tubes and catheters. Indocyanine green has a near-infrared (NIR) excitation and emission wavelength. It was used as a dye to allow a near-infrared fluorescence (NIRF) imaging system and the detection of the device’s spatial position. Polyurethanes (PU) containing soft segments were discovered to be temperature-sensitive due to soft segment crystal melting [13]. The water vapor permeability of the PU membranes rose considerably as temperature climbed within the crystal melting range of 10 to 40 °C. Over the years, this form of polyurethane was explored by US Army soldiers in order to build an amphibious diving suit that allows comfortable wear both in and out of water [13]. Textiles can be utilized as sensors to determine the pH of the environment by simple visual color observation [14]. For example, because the pH of burn patients’ skin changes during the healing process, the color shift of the bandage made of smart textiles can be utilized to follow the recovery process, allowing the gauze to be removed without damaging the wound [15].
Brief History
During the Elizabethan era (circa 1600), the first conductive gold threads were woven onto textiles for a fashionable touch. The concept of employing metallic strands to adorn linens has been around for millennia [16].
The first wearable computer was created in 1955, and since then, significant effort has been devoted towards embedding electrical functionality into textiles [2]. In 1989, Japan was the first country to coin the concept of smart textiles. The earliest smart textiles were made of silk thread with shape memory characteristics [16].
The design evolution of smart textiles could be divided into three periods [17]. From the 1980s to 1997, the design approach was considered technology-driven, as a lot of studies concentrated on wearable computing and advanced technological applications. A first attempt was made in the experimental lab of the Massachusetts Institute of Technology to link computer hardware to clothing [18]. However, fashion design and commercial inputs were overlooked, and the products were more ‘portable’ than ‘wearable’ [17].
Competence and interest in the fashion and textile industries rose considerably between 1998 and 2000. As a result, the number of initiatives merging the electronic and fashion industries has increased dramatically. Despite the fact that the applications became more wearable, the majority of the results remained prototype clothes due to the immature technologies [17]. To build ready-to-wear electronics, collaborative ventures such as the one between Philips Electronics and Levi Strauss were born. The CD+ jacket included a cell phone, MP3 player, headphones, and a remote control in a jacket [19].
The number of smart textiles on the market expanded considerably from 2001 to 2004. Smart garments became more wearable as a result of a new approach focused on users and consumers. However, modern applications are designed for specific purposes (i.e., health monitoring) rather than adhering to day-to-day activity [17].
From 2006 until the present, the fourth stage has seen rapid advancements in the miniaturization and smart materials spaces, as well as witnessing the maturity of wearables entering the market [20].
The value of the global smart textiles market is estimated around $3.8 billion in 2022 [21]. Looking ahead, the market is expected to attain $15.9 billion by 2028, with a compound annual growth rate (CAGR) of 26.94% from 2022 to 2028 [21]. The expanding trend of device downsizing, together with the increasing combination of smart textiles and wearable devices, became the driving factors in the worldwide smart textiles market. The smart textile business is segmented into North America, Asia Pacific, Europe, Latin America, and the Middle East and Africa, with North America dominating the worldwide market. Adidas AG, AiQ Smart Clothing Inc., Clothing+, Dupont De Nemours Inc., and Gentherm Incorporated are among the leading companies in the global smart textiles market [21].
The rapid emergence of the coronavirus disease 19 (COVID-19) pandemic had a significant impact on the textile and garment industries, as well as on smart textiles. The latter can detect body movements, alterations in size of the rib cage during respiration, electrical impulses from organs such as the heart (i.e., electro-cardiography) and skeletal muscles (i.e., electro-myography), or can screen components in biofluids (sweat, saliva, urine, etc.) [22].
2. Fluorescent Textiles
An example of smart textiles is represented by fluorescent fabrics, which emit radiation with a wavelength longer than that of the exciting radiation when exposed to ultraviolet (UV) or blue radiation, resulting in brighter colors than regular textiles.
The primary difference between fluorescent and phosphorescent materials is the permanence of luminescence following removal of the excitation medium. The observed decay in fluorescent substances occurs instantaneously when the excitation source is removed. On the contrary, the decay in phosphorescent mechanisms occurs gradually over time.
2.1. Fluorescence
During the Great Depression years, life was harsh and stunted for everyone, and Bob Switzer, a pre-med student at the University of California, found summer work in a tomato company to support his family financially. After a catastrophic accident, Bob was compelled to stay at home in the absence of light. During this time, Bob and his brother developed an interest in fluorescence, and found several naturally fluorescing organic compounds. The two brothers quickly recognized the potential for fluorescent products in advertising and commercial display and founded their first company (the Fluor-S-Art Co.) in 1934. During World War II, fluorescent products were used by the military for a variety of visual signaling purposes. A milestone came in 1957 with the patent for producing daylight fluorescent pigments combining fluorescent dyes with a new class of polymers for printing technologies [23].
The natural, fascinating phenomenon of fluorescence is based on the concept that certain materials can absorb light of a specific wavelength (ultraviolet) and then release light of another wavelength (visible). In general, luminescence is defined as the radiation released by an atom due to energy absorption and an excited state. Depending on the excitation source (incident radiation, electrons, or particles) different types of phenomena (luminescence) can be distinguished: (i) photoluminescence (fluorescence or phosphorescence) when the source is an electromagnetic radiation; (ii) chemiluminescence when the source is a chemical reaction; (iii) electroluminescence, when the source is an electrical field; (iv) thermoluminescence, in which the luminescence is thermally activated; and (v) mechanoluminescence due to a mechanical action [24,25,26].
Fluorescent materials have attracted significant attention in monitoring, detection, decoration, and anti-counterfeiting applications, and are used in a wide range of sensors such as luminescence probes, optical bioimaging, chemical sensing, fluorescent dyes, and electrochromic displays [27].
Fluorescent materials can be divided into inorganic and organic fluorescent materials [28]. Rare earth ions, i.e., elements of the periodic table compromising light (lanthanum to samarium) and heavy elements (europium to lutetium), are the fundamental components of traditional inorganic fluorescent compounds. The organic molecular luminescent materials have conjugated, heterocyclic rings and are composed of structures that are relatively simple to modify. The nature of the chromophore can be altered by inserting unsaturated groups such as an olefin bond or a benzene ring [28].
Organic fluorescent dyes have simple-to-modify structures when compared to inorganic materials, and they can react with fibers to improve uniformity and sensory comfort [29]. However, compared to quantum dots, they have a smaller fluorescence lifetime and show significant photobleaching [30]. Photobleaching is the chemical destruction of a fluorophore, and the irreversible loss of the original properties. It occurs as a result of many cycles of excitation–relaxation. Photobleaching can decrease the durability and physical appearance of textile, paint, and display products [31]. Even though this phenomenon is not completely understood, it appears that photodegradation can proceed through both oxygen-dependent and oxygen-independent mechanisms. Guha and Basu [32] suggested incorporating fluorescent dye solutions containing rare earth elements such as CeO2 and La2O3 to increase the lifetime of rhodamine dye, fibrin, and collagen. The nanoparticles were demonstrated to be effective at scavenging reactive oxygen species created during the dye irradiation process as well as limiting photobleaching of fluorescent dyes.
2.2. The Importance of Fluorescent Textiles
Fluorescence in textiles can open up new possibilities for innovation (e.g., fabric-based electronic image displays, security barcodes, sensors), human safety in outdoor sports and special services in the armed forces (firefighters, police officers, and soldiers), fashion, and trends [33].
For outdoor sports in low-visibility conditions, technical gear with light-responsive features is crucial for athlete safety (Figure 1). A lack of signaling by users and visibility for automobiles is one of main causes of accidents with pedestrian involvement [34].

Figure 1.
Examples of fluorescent clothes for outdoor sports to improve visibility and prevent accidents. Reproduced from [34].
Many countries have focused on enacting rules requiring warning apparel for the public and vulnerable passengers. In Europe, two standards for visibility clothing (EN ISO 20471:2013 and EN 1150), and one for accessories (EN 13356) have been issued as European Harmonized Standards. Both standards promote the clothing visibility in both daylight (through fluorescent fabric) and low illumination or darkness (via retro-reflective materials) [35].
Counterfeit products typically catch people’s attention due to the low cost in comparison to original products. However, imitations are of poor quality, and in most cases made from hazardous and harmful materials. The appearance of counterfeit items on the market negatively impacts both customers and producers resulting in a global problem that costs a significant amount of money and items each year. Anti-counterfeiting technology is developed and refined to designate authentic goods that are difficult to be replicated and easy to be distinguished. One of the most valued techniques for protecting goods or documents (i.e., cash, diplomas, packages, and certificates) is to use smart fluorescent materials. Among the various methods for combating textile counterfeiting are the application of microcapsules containing a color former or an activator directly to the yarn, the application of rare earth compounds to a fiber-based polymer via a special spinning process, and the impregnation of fluorescein (FL) into textile support [36].
2.3. Methods to Develop Fluorescent Fabrics
Fluorescent fabrics were made using rare earth metal compounds, pigments, aggregation-induced emission (AIE) molecules, quantum dots, dendrimers, and a fluorescent dye [33]. Dysprosium- and europium-doped strontium aluminate phosphor were combined with adhesive binder and distilled water and then applied directly onto wool fabric using a spray-coating technique [37]. To impart photochromic and fluorescent qualities to the fabrics, an aqueous binder containing inorganic phosphor-based pigments was screen-printed onto knitted cotton fabric [33]. Fluorescein was used as a fluorescent organic dye on the polyester fabric using microwave irradiation technology [38].
A poly(propylenimine) dendrimer was modified with 1,8-naphthalimide units and Zn(II) complexes, and deposited on a cotton fabric by immersing the sample in the dendrimer-containing solution [39]. On cellulose knitted fabrics, a successful fluorescence dyeing process was accomplished using a dye solution containing fluorescein sodium, rhodamine B, and acridine orange [40].
Examples of common fluorescent dyes used to impart fluorescence to textile substrates are shown in Table 1.

Table 1.
Common fluorescent dyes and fabrics substrates.
Several processes are required to create a fabric with a desired functionality [48]. Sizing agents were used to achieve the absorbent capacity, while oxidizing agents were used to obtain the strength, hygroscopicity, dye absorbency, and brightness, and dyes were used to attain certain colorations. These operations were accomplished by traditional pad-dry-cure techniques, requiring huge quantities of energy and water, usually released as effluent [49]. However, a large portion of these species are polluting agents, raising environmental concerns of textile dyeing. Dyes, as well as polymers, are not easy to be degraded and require an increased amount of oxygen to be decomposed by micro-organisms in aerobic decomposition (i.e., elevated biochemical oxygen demand (BOD)) [50]. As a consequence, synthetic colorants, chemicals to aid in the dyeing process that produce beautifully colored pigments, and fluorescent brighteners pose an environmental challenge to the ecosystem’s preservation [50]. A sustainable and clean process exploiting microwave (MW) technology and natural dye (curcumin) was proposed by Popescu et al. [51] to realize fluorescent acrylic fabrics. The final clothes were comfortable, had greater thermal resistance, and were regarded as appealing by young people and children due to their color. Compared to traditional methods, the use of microwave technology led to a reduced involvement of chemicals in the extraction of curcumin from turmeric powder, to a uniform color distribution on the fabric surface, and to a higher fluorescence of dyed fabrics.
In 2001, a series of molecules, i.e., silanoles, with linearly drawn chemical structures that appear to be conjugated, were discovered to be non-luminescent in solution but emissive in aggregated form [52]. This novel phenomenon was named “aggregation-induced emission” (AIE). It was recognized with a significant practical impact since it achieved functional characteristics when aggregates were developed [53]. Tetraphenylethylene (TPE), triphenylamine (TPA), boron-dipyrromethene (BODIPY), pyrene, carbazole, fluorene, or phenothiazine are among the most common compounds exhibiting AIE properties, also known as AIEgens “aggregation-induced emission luminogens” (Figure 2) [54].
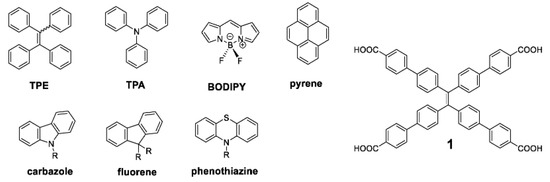
Figure 2.
Structure of some common AIEgens. Reproduced from [54]. “1” denotes the most used compound containing TPE unit in the center and conjugated structures.
Tetraphenylethylene (TPE) is widely used in the field of sensors as a highly efficient AIE fluorescent material, and it is also used to cover smart fabrics. TPE, on the other hand, reduces textile deformability and flexibility in coated textiles [55]. Flexible AIEgen fibers have been realized with tetraphenylethylene (TPE) luminescence and a flexible matrix made of thermoplastic polyurethane (TPU) in a skin-core structure with polyester (PET) fibers. In detail, polyester fibers were used as the core fiber and thermoplastic polyurethane (TPU) particles were used as the matrix in the spinning solution. The electrospinning method was combined with the core-spun process to develop the AIEgen fibers on a large scale. The prepared AIEgen fibers were immediately woven within the textile structures into a variety of patterns such as the letters TGU (acronym for Tiangong University). The letters on the textiles could not be read under normal lighting conditions, but lighted up when exposed to UV light [55].
Fluorescent Polymers in Fluorescent Fabrics
Fluorescent polymeric materials are polymeric molecules endowed with fluorophores attached to the chain [56]. These systems are being used in new applications such as smart polymer machines and sensors [57], organic light-emitting diodes (OLED) [58], molecular thermometers, optoelectronic devices [56], for imaging and therapeutic uses (antibacterial and photothermal) [59], fluorescent probes, and drug delivery carriers [60].
In general, fluorescent polymers are naturally fluorescent or the result of functionalization with fluorophores, which typically have conjugated structures [61]. Viable methods to develop fluorescent polymers are polymer synthesis using fluorescent monomers [62], polymer synthesis using fluorescent compounds as initiators [63] or chain transfer agents [64], and chemical bonding between fluorescent molecules and polymers [59,65].
1,8-naphthalimide is a popular dye and fluorophore, extensively applied in analytical, materials, and biological chemistry due to its satisfactory photostability, flexibility, and fluorescent characteristics [66]. As a result of its dyeing and fluorescent qualities, this species offers a wide range of applications also in textiles.
The inclusion of various functional groups influences the chemical structure of 1,8-naphthalimide dyes. 1,8-naphthalimide disperse dyes can be created with a very low rate of water solubility. When applied to synthetic textiles, particularly polyester, the resulting material has good color characteristics as well as high resistance to sublimation. 1,8-naphthalimide dyes can be dissolved in water with positive and negative charges giving rise to acid and cationic dyes, respectively. The latter forms are suitable for coloring wool, polyamide, and polyacrylonitrile fibers. In particular, 1,8-naphthalimide acid dyes have strong wash fastness and can be utilized in the dyeing of anionic fabrics (especially for polyamides) [67].
The presence of an epoxy group in the structure of 1,8-naphthalimide fluorophores allows for the synthesis of molecules with novel qualities by covalent attachment to polyamide and epoxy oligomers. The development of new 1,8-naphthalimide fluorophore containing an active epoxy group to color polyamide textiles was explored in [68]. Using a standard calibration curve, colorimetric analysis revealed that 85–98% of the initial amount of fluorophore was efficiently integrated into the polyamide 6.
Aside from sensor technologies, the combination of fluorescence and conductivity may be useful in the fashion sector, with one application being the manufacturing of security clothing labels. Various methods to synthesize fluorescent conductive wool were presented in [69] to produce a fluorescent coating on the fabric surface: (i) solution polymerization, (ii) encapsulation of fluorescent dyes in the polymer, and (iii) utilization of fluorescent dopants. In the first approach, textiles were pre-dried in fluorescent solutions containing fluorescent dyes such as pyrene, rhodamine B, and fluorescein. Polymerization was induced by spraying the fabrics with pyrrole in ethanol and heating them with a heat gun. Once the pyrrole was spread, the polymerization started instantaneously on the fabric surface. In the second method, the textile was immersed in water containing pyrrole, surfactant, varying concentrations of fluorescent dyes (pyrene or rhodamine B), and iron (III) chloride. The presence of surfactants allowed the pyrrole to form micelles for encapsulation of fluorescent dyes. In the third case, the textile sample was immersed in water containing pyrrole, pyrenesulfonic acid, and iron (III) chloride. Fluorescence and conductivity testing were performed on the coated textiles. The most successful method for textile coating was found to be fluorescent dye encapsulated in polypyrrole (PPy).
The most frequent way to create AIE polymers is to include AIEgens into macromolecular chains, to use AIEgen-based monomers or initiators during polymer synthesis, or to use post-polymerization modification methods [70].
1-(4-Aminophenyl)-1,2,2-triphenylethylene (TPE-NH2) was bonded to the surface of nonwoven polyethylene/polypropylene via radiation-induced graft polymerization (RIGP) and succeeding chemical modification [71]. The method consisted of the following operations: (i) preparation of PE/PP NWF-g-PGMA in which the precursor of poly(glycidyl acrylate) was linked to the fabric surface through covalent bonds; introduction of AIE luminogen via a ring opening reaction between the epoxy group of the PGMA chains and TPE-NH2. Results confirmed an intense green emission and exceptional fluorescence endurance of developed textiles after various washing cycles in strong acid and basic solutions. Silk is a potential natural biopolymer for textile and biomedical applications due to its outstanding flexibility, high biocompatibility, and biodegradability. Many luminous silk threads were prepared through physical interactions with luminescent compounds (through hydrogen bonding or electrostatic attraction) [72]. On the other hand, the physical bonding was frequently unstable resulting in the leakage of fluorescent material into the environment. Furthermore, many organic fluorescent dyes were susceptible to aggregation-caused quenching (ACQ) and required molecular dissolution. As a result, chemical functionalization using solid-state emissive fluorophores appeared to be the ideal method for producing stable fluorescent silk. Five AIEgens were synthesized by functionalizing TPE with propynone groups covering the whole visible region. These molecules were able to react with silk fibers through facile metal-free click bioconjugation. Silk fibers were immersed in AIE solutions at room temperature overnight, yielding fabrics with homogeneous fluorescence. The produced fabrics demonstrated high stability and full-color emission [73].
Most AIEgens, on the other hand, incorporate aromatic and conjugated subunits as chromophore sites and have a complex structure, hazardous components, and cytotoxic effects. These factors, together with poor fluorescence and durability, are regarded as the key drawbacks in producing fluorescent fibers. Standard manufacturing procedures such as melting and electrospinning, as well as surface coating, are based on the physical interactions between fibers and luminescent agents. Furthermore, fluorescent polymers possess lower mechanical characteristics, processability, and thermal resistance compared to common polymers such as polypropylene and polyethylene. As a result, developing novel methods for producing fluorescent polymer materials with high fluorescent intensity and endurance, good processability and mechanical properties, and that are free of toxic and aromatic luminogens has become a crucial challenge. Zhang et al. [74] used radiation-induced graft polymerization (RIGP) to covalently attach an unusual nonconjugated chromophore to the surface of a polyethylene/polypropylene nonwoven fabric. Firstly, the poly(glycidyl methacrylate) (PGMA), was covalently bonded on the surface of polyethylene/polypropylene nonwoven fabric via radiation-induced graft polymerization (RIGP). Then, nonconjugated pentaethylenehexamine (PEHA) was introduced through a ring-opening reaction. A vivid blue emission was noticed only after the PEHA bonded to the fabric surface, confirming the essential role of chromophores contained in PEHA in developing the fluorescence. Significant fluorescent stability was achieved in the final textiles, also after severe washing conditions.
3. Conclusions
Smart textiles are intelligent textile structures or fabrics that can detect and respond to environmental stimuli such as light, pH, temperature, polar solvents, chemicals, and electricity. Between 2022 and 2028, the global smart textiles sector is predicted to quadruple. Potential applications of smart textiles are in sensors, probes, medical and biotechnological equipment, and smart clothing. An example of smart textiles are fluorescent fabrics or fibers that absorb light of a given wavelength and then emit light of a different wavelength. Fluorescent fabrics are useful in a variety of applications, including protection, sportswear, fashion clothes, fabric-based electronic image displays, security barcodes, sensor systems, and anti-counterfeiting technology. Fluorescent fabrics can be designed using rare earth metal compounds, pigments, aggregation-induced emission (AIE) molecules, dendrimers, and fluorescent dyes. Common fluorescent dyes are coumarins, naphthalimides, perylenes, and rhodamines. AIEgens are aggregation-induced emission luminogens, i.e., compounds with a conjugated chemical structure that emit light when they aggregate. The most common AIEgens are tetraphenylethylene (TPE), triphenylamine (TPA), BODIPY, pyrene, carbazole, fluorene, and phenothiazine. Fluorescent molecules can be applied both to synthetic and natural textile substrates or fibers such as polyester and polyamide, or wool and cotton. The traditional application method entails submerging fabric samples in solutions containing fluorescent agents for a predetermined amount of time at a temperature above ambient and adjusted pH, followed by washing and drying. Current research interest is devoted to (i) improving textile deformability and flexibility of coated fluorescent textiles, and (ii) improving the durability and stability of fluorescence on textile substrates. The first purpose is met by using more flexible materials and/or core-shell structures, whereas the second is achieved through covalent bonding between fibers and dyes.
Author Contributions
Conceptualization, A.P. and D.A.; writing—original draft preparation, A.P.; writing—review and editing, D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
A.P. wishes to thank the Italian Ministry of Education, Universities and Research (MIUR) in the framework of Action 1.2 “Researcher Mobility” of the Axis I of PON R&I 2014-2020 under the call “AIM: Attrazione e Mobilità Internazionale”.
Conflicts of Interest
Domenico Acierno is an employee of the Regional Center of Competence New Technologies for Productive Activities Scarl.
References
- Koncar, V. Introduction to smart textiles and their applications. In Smart Textiles and Their Applications; Koncar, V., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 1–8. ISBN 9780081005835. [Google Scholar]
- Luiz, H.; Júnior, O.; Neves, R.M.; Monticeli, F.M.; Agnol, L.D. Smart fabric textiles: Recent advances and challenges. Textiles 2022, 2, 582–605. [Google Scholar] [CrossRef]
- Ramlow, H.; Andrade, K.L.; Immich, A.P.S. Smart textiles: An overview of recent progress on chromic textiles. J. Text. Inst. 2020, 112, 152–171. [Google Scholar] [CrossRef]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 053001. [Google Scholar] [CrossRef]
- Dang, T.; Zhao, M. The application of smart fibers and smart textiles. J. Phys. Conf. Ser. 2021, 1790, 012084. [Google Scholar] [CrossRef]
- Ji, F.; Zhu, Y.; Hu, J.; Liu, Y.; Yeung, L.-Y.; Ye, G.D. Smart polymer fibers with shape memory effect. Smart Mater. Struct. 2006, 15, 1547. [Google Scholar] [CrossRef]
- Parhizkar, M.; Zhao, Y.; Lin, T. Photochromic fibers and fabrics. In Handbook of Smart Textiles; Tao, X., Ed.; Springer: Singapore, 2015; pp. 155–182. ISBN 9789814451451. [Google Scholar]
- Guo, J.; Zhou, B.; Yang, C.; Dai, Q.; Kong, L. Stretchable and temperature-sensitive polymer optical fibers for wearable health monitoring. Adv. Funct. Mater. 2019, 29, 1902898. [Google Scholar] [CrossRef]
- Sahoo, A.; Ramasubramani, K.R.T.; Jassal, M.; Agrawal, A.K. Effect of copolymer architecture on the response of pH sensitive fibers based on acrylonitrile and acrylic acid. Eur. Polym. J. 2007, 43, 1065–1076. [Google Scholar] [CrossRef]
- Xu, D.; Ouyang, Z.; Dong, Y.; Yu, H.Y.; Zheng, S.; Li, S.; Tam, K.C. Robust, breathable and flexible smart textiles as multifunctional sensor and heater for personal health management. Adv. Fiber Mater. 2023, 5, 282–295. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Liu, Y.; Leng, J. Shape memory polymer fibers: Materials, structures, and applications. Adv. Fiber Mater. 2021, 4, 5–23. [Google Scholar] [CrossRef]
- Torbati, A.H.; Mather, R.T.; Reeder, J.E.; Mather, P.T. Fabrication of a light-emitting shape memory polymeric web containing indocyanine green. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.M.; Hu, J.L.; Tao, X.M.; Hu, C.P. Preparation of temperature-sensitive polyurethanes for smart textiles. Text. Res. J. 2006, 76, 406–413. [Google Scholar] [CrossRef]
- Van Der Schueren, L.; de Clerck, K. The use of pH-indicator dyes for pH-sensitive textile materials. Text. Res. J. 2009, 80, 590–603. [Google Scholar] [CrossRef]
- Osti, E. Skin pH variations from the acute phase to re-epithelialization in burn patients treated with new materials (Burnshield®, Semipermeable Adhesive Film, Dermasilk®, and Hyalomatrix®). Non-invasive preliminary experimental clinical trial. Ann. Fires Burn Disaster 2008, 21, 73–77. [Google Scholar]
- Adak, B.; Mukhopadhyay, S. Smart and Functional Textiles; De Gruyter: Berlin, Germany, 2023. [Google Scholar]
- Ariyatum, B.; Holland, R.; Harrison, D.; Kazi, T. The future design direction of Smart Clothing development. J. Text. Inst. 2010, 96, 199–210. [Google Scholar] [CrossRef]
- Singha, K.; Kumar, J.; Pandit, P. Recent advancements in wearable & smart textiles: An overview. Mater. Today Proc. 2019, 16, 1518–1523. [Google Scholar] [CrossRef]
- Latour. Almar Philips and Levi Strauss Team Up to Create Ready-to-Wear Electronics—WSJ. Available online: https://www.wsj.com/articles/SB966795198702775516 (accessed on 24 April 2023).
- Kapfunde, M. Clothing Manufacturers—Exploring Technologies Destined to Change the Clothes We Wear. Available online: https://www.suuchi.com/exploring-technologies-destined-to-change-the-clothes-we-wear/ (accessed on 24 April 2023).
- Smart Textiles Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023–2028. Available online: https://www.researchandmarkets.com/reports/5753466/smart-textiles-market-global-industry-trends (accessed on 25 April 2023).
- Ivanoska-Dacikj, A.; Stachewicz, U. Smart textiles and wearable technologies-opportunities offered in the fight against pandemics in relation to current COVID-19 state. Rev. Adv. Mater. Sci. 2020, 59, 487–505. [Google Scholar] [CrossRef]
- DayGlo Fluorescent Pigments National Historic Chemical Landmark—American Chemical Society. Available online: https://www.acs.org/education/whatischemistry/landmarks/dayglo.html (accessed on 24 April 2023).
- Aditya Khatokar, J.; Vinay, N.; Sanjay, B.; Bhargava, S.; Sudhir Bale, A.; Kolekar, T.R.; Singh, S.; Umarani, S.; Huddar, S.A. Carbon nanodots: Chemiluminescence, fluorescence and photoluminescence properties. Mater. Today Proc. 2021, 43, 3928–3931. [Google Scholar] [CrossRef]
- Drummen, G.P.C. Fluorescent probes and fluorescence (microscopy) techniques—Illuminating biological and biomedical research. Molecules 2012, 17, 14067–14090. [Google Scholar] [CrossRef] [PubMed]
- Chiatti, C.; Kousis, I.; Fabiani, C.; Pisello, A.L. Luminescence for the built environment: From lighting to urban heat island mitigation purposes. In Global Urban Heat Island Mitigation; Khan, A., Akbari, H., Fiorito, F., Mithun, S., Niyogi, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–69. ISBN 9780323855396. [Google Scholar]
- Yu, L.P.; Zhang, X.; Wei, D.X.; Wu, Q.; Jiang, X.R.; Chen, G.Q. Highly efficient fluorescent material based on rare-earth-modified polyhydroxyalkanoates. Biomacromolecules 2019, 20, 3233–3241. [Google Scholar] [CrossRef]
- Wang, H.; Ji, X.; Page, Z.A.; Sessler, J.L. Fluorescent materials-based information storage. Mater. Chem. Front. 2020, 4, 1024–1039. [Google Scholar] [CrossRef]
- Nie, W.; Wu, J.; Yang, J.; Hu, L. Fabrication of sustainable hydrophobic cotton fabrics with fluorescence-emitting performance using novel 1,8-naphthalimide functional molecules. ACS Sustain. Chem. Eng. 2023, 11, 3873–3881. [Google Scholar] [CrossRef]
- Cai, S.; Hu, S.; Wu, J.; Huang, A.; Geng, L.; Peng, X. Interfacial polyelectrolyte complexation spinning of cellulose nanofibers/CdTe quantum dots for anti-counterfeiting fluorescent textiles. Fibers Polym. 2022, 23, 1235–1243. [Google Scholar] [CrossRef]
- Demchenko, A.P. Photobleaching of organic fluorophores: Quantitative characterization, mechanisms, protection. Methods Appl. Fluoresc. 2020, 8, 022001. [Google Scholar] [CrossRef]
- Guha, A.; Basu, A. Role of rare earth oxide nanoparticles (CeO2 and La2O3) in suppressing the photobleaching of fluorescent organic dyes. J. Fluoresc. 2014, 24, 683–687. [Google Scholar] [CrossRef]
- Khattab, T.A.; Rehan, M.; Hamouda, T. Smart textile framework: Photochromic and fluorescent cellulosic fabric printed by strontium aluminate pigment. Carbohydr. Polym. 2018, 195, 143–152. [Google Scholar] [CrossRef]
- Santos, G.; Marques, R.; Silva, S.; Oliveira, J.; Castro, P.; Pereira, C.; Pinheiro, M. Innovative high-visibility protective clothing development. Textiles 2021, 1, 405–418. [Google Scholar] [CrossRef]
- Intertek. High Visibility Clothing & Accessories Requirements for Europe; Intertek: Oak Brook, IL, USA, 2009. [Google Scholar]
- Baatout, K.; Saad, F.; Baffoun, A.; Mahltig, B.; Kreher, D.; Jaballah, N.; Majdoub, M. Luminescent cotton fibers coated with fluorescein dye for anti-counterfeiting applications. Mater. Chem. Phys. 2019, 234, 304–310. [Google Scholar] [CrossRef]
- Khattab, T.A.; Rehan, M.; Hamdy, Y.; Shaheen, T.I. Facile Development of photoluminescent textile fabric via spray coating of Eu(II)-doped strontium aluminate. Ind. Eng. Chem. Res. 2018, 57, 11483–11492. [Google Scholar] [CrossRef]
- Saad, F.; Baffoun, A.; Mahltig, B.; Hamdaoui, M. Polyester fabric with fluorescent properties using microwave technology for anti-counterfeiting applications. J. Fluoresc. 2022, 32, 327–345. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D.; Vasileva-Tonkova, E.; Alexandrova, R. Surface functionalization of cotton fabric with fluorescent dendrimers, spectral characterization, cytotoxicity, antimicrobial and antitumor activity. Chemosensors 2019, 7, 17. [Google Scholar] [CrossRef]
- Liu, H.; Lu, M.; Pan, F.; Ning, X.; Ming, J. Influence of fluorescent dyes for dyeing of regenerated cellulose fabric. Text. Res. J. 2020, 90, 1385–1395. [Google Scholar] [CrossRef]
- Czajkowski, W.; Kaźmierska, M. Coumarin-derived fluorescent dyes. Przem. Chem. 2002, 81, 177–180. [Google Scholar]
- Elgemeie, G.H.; Ahmed, K.A.; Ahmed, E.A.; Helal, M.H.; Masoud, D.M. A simple approach for the synthesis of coumarin fluorescent dyes under microwave irradiation and their application in textile printing. Pigment Resin Technol. 2016, 45, 217–224. [Google Scholar] [CrossRef]
- Millington, K.R.; Maurdev, G. The generation of superoxide and hydrogen peroxide by exposure of flourescent whitening agents to UVA radiation and its relevance to the rapid photoyellowing of whitened wool. J. Photochem. Photobiol. A Chem. 2004, 165, 177–185. [Google Scholar] [CrossRef]
- Ameuru, U.S.; Yakubu, M.K.; Bello, K.A.; Nkeonye, P.O.; Halimehjani, A.Z. Synthesis of disperse dyes derived from 4-amino-N-decyl-1, 8-naphthalimide and their dyeing properties on polyester fabrics. Dye. Pigment. 2018, 157, 190–197. [Google Scholar] [CrossRef]
- Bojinov, V.; Konstantinova, T. Synthesis of polymerizable 1,8-naphthalimide dyes containing hindered amine fragment. Dye. Pigment. 2002, 54, 239–245. [Google Scholar] [CrossRef]
- Forootan, H.; Gharanjig, K.; Ghasemi, E.; Mazhar, M.; Gharanjik, A.; Jahankaran, S. Investigation of synthesis, application and fluorescent properties of novel acid dyes based on perylene on polyamide fabrics. Fibers Polym. 2023, 24, 627–639. [Google Scholar] [CrossRef]
- Park, Y.K.; Oh, B.M.; Jo, A.R.; Han, J.H.; Lim, J.Y.; Oh, H.J.; Lim, S.J.; Kim, J.H.; Lee, W.S. Fabrication of colorimetric textile sensor based on rhodamine dye for acidic gas detection. Polymers 2020, 12, 431. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-sustainability of the textile production: Waste recovery and current recycling in the composites world. Polymers 2020, 13, 134. [Google Scholar] [CrossRef]
- Patti, A.; Acierno, D. Towards the sustainability of the plastic industry through biopolymers: Properties and potential applications to the textiles world. Polymers 2022, 14, 692. [Google Scholar] [CrossRef]
- Uddin, F. Environmental hazard in textile dyeing wastewater from local textile industry. Cellulose 2021, 28, 10715–10739. [Google Scholar] [CrossRef]
- Popescu, V.; Astanei, D.G.; Burlica, R.; Popescu, A.; Munteanu, C.; Ciolacu, F.; Ursache, M.; Ciobanu, L.; Cocean, A. Sustainable and cleaner microwave-assisted dyeing process for obtaining eco-friendly and fluorescent acrylic knitted fabrics. J. Clean. Prod. 2019, 232, 451–461. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Sánchez-Ruiz, A.; Sousa-Herves, A.; Tolosa, J.; Navarro, A.; García-Martínez, J.C. Aggregation-induced emission properties in fully π-conjugated polymers, dendrimers, and oligomers. Polymers 2021, 13, 213. [Google Scholar] [CrossRef]
- Jiang, Q.; Yuan, H.; Dong, K.; Lin, J.H.; Wu, L.; Tang, Y. Continuous and scalable manufacture of aggregation induced emission luminogen fibers for anti-counterfeiting and hazardous gas detecting smart textiles. Mater. Des. 2021, 205, 109761. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Pakhira, M.; Nandi, A.K. Fluorescence in “nonfluorescent” polymers. ACS Omega 2020, 5, 30747–30766. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, S.; Shan, J.; Peng, J.; Wei, L.; Xu, X. Formation and photoluminescence of fluorescent polymers. Int. J. Polym. Sci. 2010, 2010, 526348. [Google Scholar] [CrossRef]
- Wei, Q.; Ge, Z.; Voit, B. Thermally activated delayed fluorescent polymers: Structures, properties, and applications in OLED devices. Macromol. Rapid Commun. 2019, 40, 1800570. [Google Scholar] [CrossRef]
- Thomas, A.; Appidi, T.; Jogdand, A.B.; Ghar, S.; Subramaniyam, K.; Prabusankar, G.; Mohanty, J.R.; Rengan, A.K. Facile synthesis of fluorescent polymer encapsulated metal (PoeM) nanoparticles for imaging and therapeutic applications. ACS Appl. Polym. Mater. 2020, 2, 1388–1397. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, X.; Zhu, J.; Zhu, X. Novel AIEgen-functionalized Diselenide-crosslinked polymer gels as fluorescent probes and drug release carriers. Polymers 2020, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zheng, B.; Pan, D.; Yang, R.; Xu, Y.; Wang, L.; Yang, M. Unexpected fluorescence from polymers containing dithio/amino-succinimides. Polym. Chem. 2015, 6, 6133–6139. [Google Scholar] [CrossRef]
- Breul, A.M.; Hager, M.D.; Schubert, U.S. Fluorescent monomers as building blocks for dye labeled polymers: Synthesis and application in energy conversion, biolabeling and sensors. Chem. Soc. Rev. 2013, 42, 5366–5407. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, P.; Bai, T.; Kong, J. N,N-dimethyl-substituted boron ketoiminates for multicolor fluorescent initiators and polymers. Macromolecules 2020, 53, 3339–3348. [Google Scholar] [CrossRef]
- Sha, Y.; Zhu, Q.; Wan, Y.; Li, L.; Wang, X.; Xue, G.; Zhou, D. Synthesis of polymer with defined fluorescent end groups via reversible addition fragmentation transfer polymerization for characterizing the conformations of polymer chains in solutions. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2413–2420. [Google Scholar] [CrossRef]
- Zhaoqiang, W.; Lingzhi, M. Progress in fluorescent polymers. Prog. Chem. 2021, 33, 914–925. [Google Scholar] [CrossRef]
- Dong, H.Q.; Wei, T.B.; Ma, X.Q.; Yang, Q.Y.; Zhang, Y.F.; Sun, Y.J.; Shi, B.B.; Yao, H.; Zhang, Y.M.; Lin, Q. 1,8-naphthalimide-based fluorescent chemosensors: Recent advances and perspectives. J. Mater. Chem. C 2020, 8, 13501–13529. [Google Scholar] [CrossRef]
- Dodangeh, M.; Grabchev, I.; Staneva, D.; Gharanjig, K. 1,8-naphthalimide derivatives as dyes for textile and polymeric materials: A review. Fibers Polym. 2021, 22, 2368–2379. [Google Scholar] [CrossRef]
- Philipova, T.; Petkov, I. Synthesis, spectral properties, and application of 1,8-naphthalimide fluorophores for modified polymers. J. Appl. Polym. Sci. 2001, 80, 1863–1869. [Google Scholar] [CrossRef]
- Kaynak, A.; Foitzik, R.C.; Pfeffer, F.M. Fluorescence and conductivity studies on wool. Mater. Chem. Phys. 2009, 113, 480–484. [Google Scholar] [CrossRef]
- Hu, R.; Kang, Y.; Tang, B.Z. Recent advances in AIE polymers. Polym. J. 2016, 48, 359–370. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Wang, M.; Li, R.; Yuan, M.; Feng, X.; He, Y.; Xing, Z.; Hu, J.; Wu, G. Electron beam-induced preparation of AIE non-woven fabric with excellent fluorescence durability. Appl. Surf. Sci. 2021, 541, 148382. [Google Scholar] [CrossRef]
- Lin, N.; Hu, F.; Sun, Y.; Wu, C.; Xu, H.; Liu, X.Y. Construction of white-light-emitting silk protein hybrid films by molecular recognized assembly among hierarchical structures. Adv. Funct. Mater. 2014, 24, 5284–5290. [Google Scholar] [CrossRef]
- Liu, C.; Bai, H.; He, B.; He, X.; Zhang, J.; Chen, C.; Qiu, Y.; Hu, R.; Zhao, F.; Zhang, Y.; et al. Functionalization of silk by AIEgens through facile bioconjugation: Full-color fluorescence and long-term bioimaging. Angew. Chem. Int. Ed. 2021, 60, 12424–12430. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Zhang, M.; Li, R.; Wang, M.; Qiu, L.; Yuan, M.; Feng, X.; Xing, Z.; Hu, J.; et al. Radiation-induced in situ-printed nonconjugated fluorescent nonwoven fabric with superior fluorescent properties. ACS Appl. Mater. Interfaces 2020, 12, 49258–49264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).