The Production of Isophorone
Abstract
1. Introduction
2. Properties and Natural Occurrence
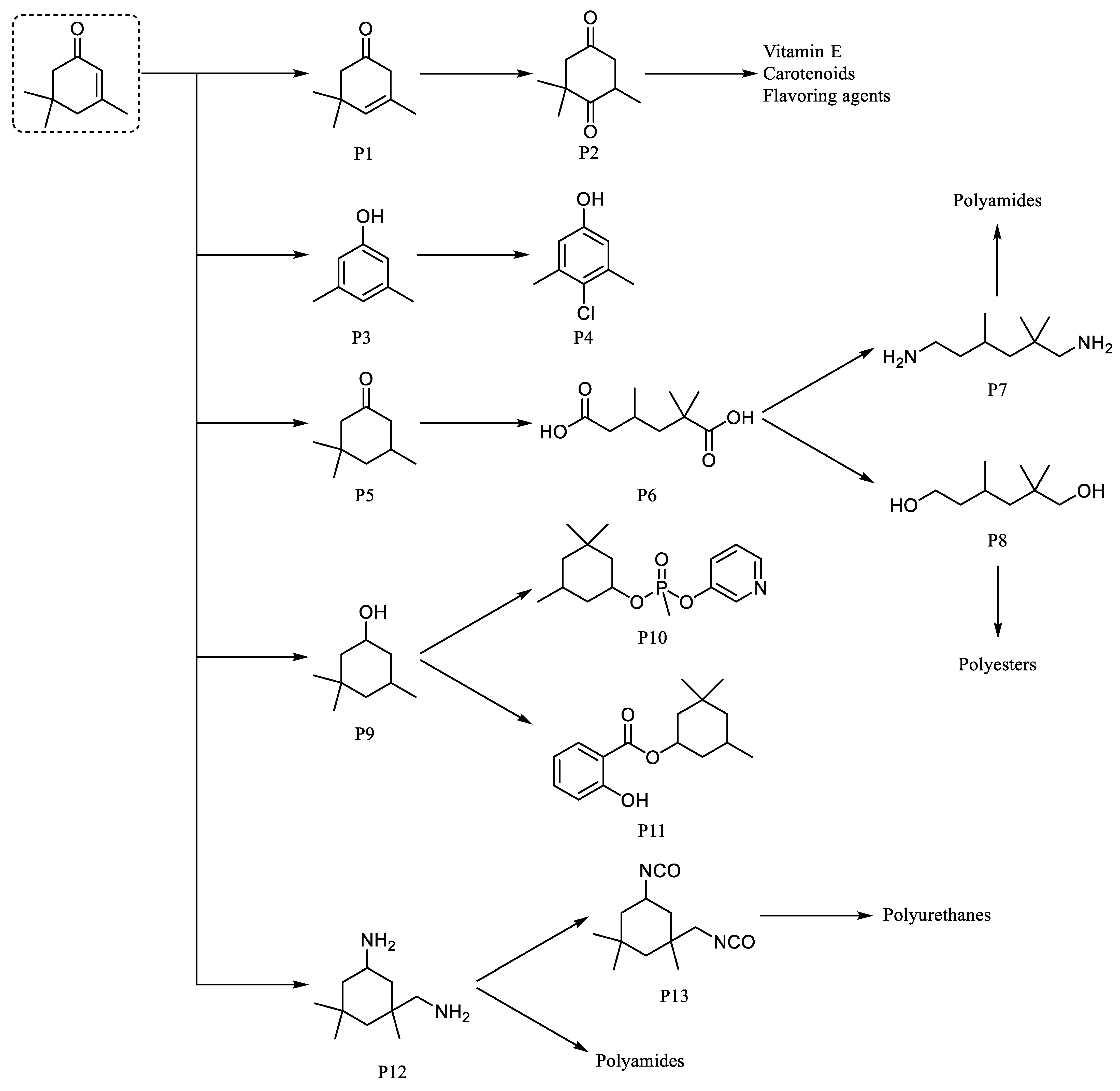
3. Applications and Synthesis
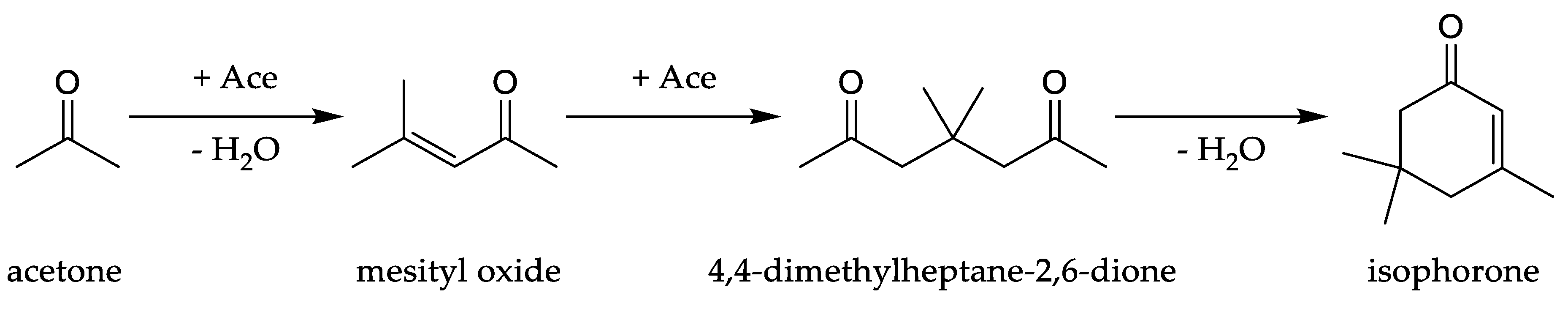
Reaction Mechanism
4. Industrial Manufacturing of Isophorone
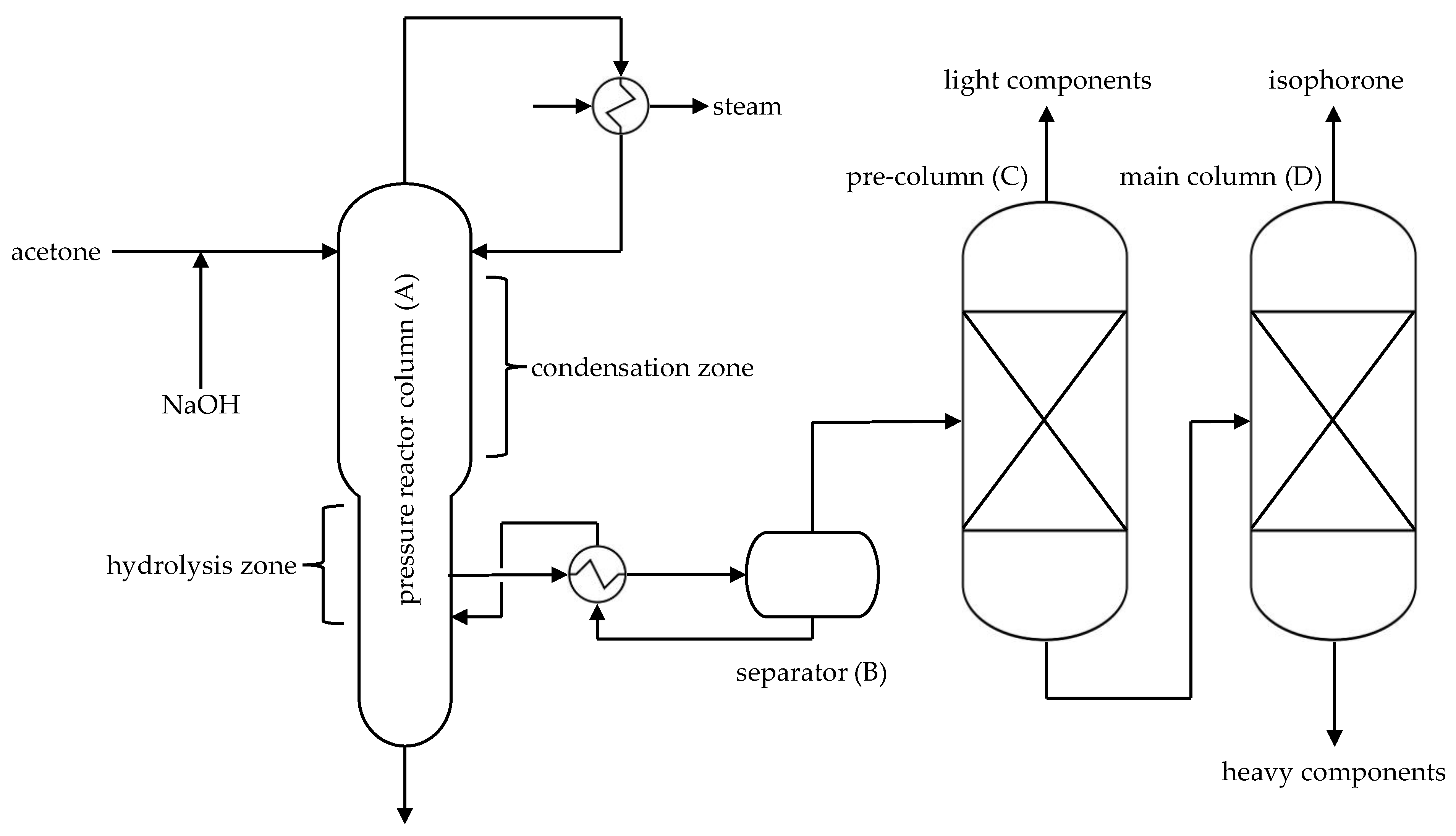
4.1. Liquid-Phase Process
4.2. Vapor-Phase Process
5. Catalysts
5.1. Catalysts for the Liquid-Phase Condensation
5.2. Catalysts for the Vapor-Phase Condensation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Evonik-60 Years Isophorone Chemistry. Available online: https://crosslinkers.evonik.com/media/public/misc/zeitstrahl/index.html (accessed on 30 October 2022).
- Evonik Industries, A.G. Damit der Lack Nicht ab Ist: Isophoron. Available online: https://history.evonik.com/de/erfindungen/isophoron (accessed on 28 October 2022).
- Schmitt, K. Neuere Acetonchemie. Chem. Ind. 1966, 18, 204. [Google Scholar]
- Matyschok, H. Preparation, properties, and applications of isophorone. Wiad. Chem. 1973, 27, 311. [Google Scholar]
- Salvapati, G.S.; Ramanamurty, K.V.; Janardanarao, M. Selective catalytic self-condensation of acetone. J. Mol. Catal. 1989, 54, 9–30. [Google Scholar] [CrossRef]
- Guan, X.; Liu, X.; Li, G.; Dong, P.; Wang, Z.; Zhao, G. Production, Market and Application prospect of Isophorone. Chem. Technol. Mark. 2005, 5–8. [Google Scholar]
- Drönner, J.; Hausoul, P.; Palkovits, R.; Eisenacher, M. Solid Acid Catalysts for the Hock Cleavage of Hydroperoxides. Catalysts 2022, 12, 91. [Google Scholar] [CrossRef]
- Braithwaite, J.K. Imaging Technology to Lanthanides. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; Wiley: New York, NY, USA, 1995; Volume 14, pp. 485–506. [Google Scholar]
- Isophorone Market by Applications (Paints and Coatings, Printing Inks, Adhesives, Artificial Leather, Agrochemical, Composite), and Region (North America, Europe, Asia Pacific, South America, Middle East & Africa): Global Forecast to 2030. Available online: https://www.marketsandmarkets.com/Market-Reports/isophorone-market-201664726.html (accessed on 19 December 2022).
- Isophorone Market: Isophorone Market Analysis By Product Type (Liquid Condensation & Solid Heterogeneous Catalytic Condensation) By End-Use and By Region–Global Market Insights 2022 to 2032. Analysis of Isophorone Market Covering 30 + Countries Including Analysis of US, Canada, UK, Germany, France, Nordics, GCC Countries, Japan, Korea and Many More. Available online: https://www.factmr.com/report/966/isophorone-market (accessed on 19 December 2022).
- Schmitt, K.; Disteldorf, J.; Baron, W. Verfahren zur Herstellung von Isophoron. German Patent. German Patent 1 095 818, 29 December 1960. [Google Scholar]
- Environmental Health Criteria 174: Isophorone. Available online: https://apps.who.int/iris/bitstream/handle/10665/36888/9241571748-eng.pdf?sequence=1&isAllowed=y (accessed on 30 October 2022).
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Bosa Roca, FL, USA, 2009. [Google Scholar]
- Burdock, G.A. Encyclopedia of Food and Color Additives; CRC Press: Bosa Roca, FL, USA, 1997. [Google Scholar]
- Murphy, E.F.; Bürgi, T.; Baiker, A. Reactive Sites in Isophorone Isomers: H/D-Exchange Studies and Quantum-Chemical Calculations. Helv. Chim. Acta 2001, 84, 2884–2894. [Google Scholar] [CrossRef]
- Shiloff, J.D.; Hunter, N.R. Solvent effects on the photocycloaddition and photoenolisation reactions of isophorone. Can. J. Chem. 1979, 57, 3301–3303. [Google Scholar] [CrossRef]
- Rudolph, A.; Weedon, A.C. Acid catalysis of the photochemical deconjugation reaction of 3-alkyl-2-cyclohexenones. J. Am. Chem. Soc. 1989, 111, 8756–8757. [Google Scholar] [CrossRef]
- Stoye, D.S. Solvents. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2000; pp. 619–688. [Google Scholar] [CrossRef]
- Isophoron. Available online: https://roempp.thieme.de/lexicon/RD-09-01678 (accessed on 1 November 2022).
- 3,5,5-Trimethyl-3-Cyclohexen-1-on. Available online: https://gestis.dguv.de/data?name=490204 (accessed on 30 October 2022).
- Panighel, A.; Maoz, I.; de Rosso, M.; de Marchi, F.; Dalla Vedova, A.; Gardiman, M.; Bavaresco, L.; Flamini, R. Identification of saffron aroma compound β-isophorone (3,5,5-trimethyl-3-cyclohexen-1-one) in some V. vinifera grape varieties. Food Chem. 2014, 145, 186–190. [Google Scholar] [CrossRef]
- Si, W.; Xiong, L.; Zhou, H.; Wu, H.; Liu, Z.; Liu, G.; Liu, Y.; Shen, A.; Liang, X. Comprehensive characterization of ingredients in Crocus sativus L. from different origins based on the combination of targeted and nontargeted strategies. Food Chem. 2022, 397, 133777. [Google Scholar] [CrossRef]
- Zarghami, N.S.; Heinz, D.E. Monoterpene aldehydes and isophorone-related compounds of saffron. Phytochemistry 1971, 10, 2755–2761. [Google Scholar] [CrossRef]
- Kiran, I.; Özşen, Ö.; Çelik, T.; İlhan, S.; Gürsu, B.Y.; Demirci, F. Microbial Transformations of Isophorone by Alternaria alternata and Neurospora crassa. Nat. Prod. Commun. 2013, 8, 59–61. [Google Scholar] [CrossRef]
- Vidal, D.M.; Moreira, M.A.B.; Coracini, M.D.A.; Zarbin, P.H.G. Isophorone derivatives as a new structural motif of aggregation pheromones in Curculionidae. Sci. Rep. 2019, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Francke, W.; Dettner, K. Chemical Signalling in Beetles. In The Chemistry of Pheromones and Other Semiochemicals II.; Topics in Current Chemistry; Schulz, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 85–166. [Google Scholar] [CrossRef]
- Schulz, S.; Francke, W.; Edgar, J.; Schneider, D. Volatile compounds from androconial organs of danaine and ithomiine butterflies. Z. Für Nat. C 1988, 43, 99–104. [Google Scholar] [CrossRef]
- California Air Resources Board. Isophorone. Available online: https://ww2.arb.ca.gov/sites/default/files/classic//toxics/tac/tacil/isophoro.htm (accessed on 11 November 2022).
- Jerković, I.; Kuś, P.M. Terpenes in honey: Occurrence, origin and their role as chemical biomarkers. RSC Adv. 2014, 4, 31710–31728. [Google Scholar] [CrossRef]
- IK, K. A Targeted Metabolomic Procedure for the Identification of Isophorone Related Compounds in Honey. J. Plant Biochem. Physiol. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Ambient Water Quality Criteria for Isophorone. Available online: https://www.epa.gov/sites/default/files/2019-03/documents/ambient-wqc-isophorone-1980.pdf (accessed on 15 April 2022).
- Discover the World of Isophorone Chemistry. Available online: https://crosslinkers.evonik.com/en/about/isophorone-chemistry (accessed on 30 October 2022).
- Aranda, C.; Municoy, M.; Guallar, V.; Kiebist, J.; Scheibner, K.; Ullrich, R.; Del Río, J.C.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Selective synthesis of 4-hydroxyisophorone and 4-ketoisophorone by fungal peroxygenases. Catal. Sci. Technol. 2019, 9, 1398–1405. [Google Scholar] [CrossRef]
- Murphy, E.F.; Mallat, T.; Baiker, A.; Schneider, M. Catalytic gas phase oxidation of isophorone to ketoisophorone. Appl. Catal. A Gen. 2000, 197, 295–301. [Google Scholar] [CrossRef]
- Tavanti, M.; Parmeggiani, F.; Castellanos, J.R.G.; Mattevi, A.; Turner, N.J. One-Pot Biocatalytic Double Oxidation of α-Isophorone for the Synthesis of Ketoisophorone. ChemCatChem 2017, 9, 3338–3348. [Google Scholar] [CrossRef]
- Krill, S.; Giray, G.; Hubner, F.; Hahn, R.; Huthmacher, K.; Tanner, H. Method of Producing β-Isophorone by the Isomerization of α-Isophorone. U.S. Patent 5,907,065, 25 May 1999. [Google Scholar]
- Becker, J.J.; Schulte-Elte, K.H.; Strickler, H.; Ohloff, G. 2,2,6-Trimethylcyclohex-5-ene-1,4-Dione. Swiss Patent 585 168, 28 February 1977. [Google Scholar]
- Becker, J.J.; Skorianetz, W.; Hochstrasser, U.P. 2,2,6-Trimethylcyclohex-5-ene-1,4-Dione. Swiss Patent 599 917, 15 June 1978. [Google Scholar]
- Bellut, H. Preparation of 2,6,6-Trimethylcyclohex-2-Ene-1,4-Dione by Oxidation of β-Isophorone. German Patent 38 42 547, 21 June 1990. [Google Scholar]
- Brenner, W. Ketoisophorone. Swiss Patent 605 535, 29 September 1978. [Google Scholar]
- Widmer, E.; Seuret, M. Ketoisophorone. Swiss Patent 611 590, 15 June 1979. [Google Scholar]
- Eggersdorfer, M.; Laudert, D.; Létinois, U.; McClymont, T.; Medlock, J.; Netscher, T.; Bonrath, W. One hundred years of vitamins-a success story of the natural sciences. Angew. Chem. 2012, 51, 12960–12990. [Google Scholar] [CrossRef]
- Müller, M.-A.; Schäfer, C.; Litta, G.; Klünter, A.-M.; Traber, M.G.; Wyss, A.; Ralla, T.; Eggersdorfer, M.; Bonrath, W. 100 Years of Vitamin E: From Discovery to Commercialization. Eur. J. Org. Chem. 2022, 2022, e202201190. [Google Scholar] [CrossRef]
- Demole, E.; Enggist, P. Novel Synthesis of 3,5,5-Trimethyl-4-(2-butenylidene)-cyclohex-2-en-1-one, a Major Constituent ofBurley Tobacco Flavour. Helv. Chim. Acta 1974, 57, 2087–2091. [Google Scholar] [CrossRef]
- Mayer, H.; Montavon, M.; Rüegg, R.; Isler, O. Synthesen in der Carotinoid-Reihe. 22. Mitteilung. Totalsynthese von Rhodoxanthin. Helv. Chim. Acta 1967, 50, 1606–1618. [Google Scholar] [CrossRef]
- Shibagaki, M.; Shibata, S.; Kaneko, H. Syntheses of 8,9-Dehydrotheaspirone and trans- and cis -8,9-Dehydro-4,5-dihydrotheaspirone. Agric. Biol. Chem. 1981, 45, 2911–2913. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Meisel, T.; Mewes, K.; Negahdari, S. Struktur und Geruch, XIII. Synthese und olfaktorische Eigenschaften von Megastigmatrienon-Analoga. Eur. J. Org. Chem. 1991, 1991, 19–25. [Google Scholar] [CrossRef]
- David Raju, B.; Rama Rao, K.; Salvapathi, G.; Sai Prasad, P.; Kanta Rao, P. Aromatization of isophorone to 3,5-xylenol over Cr2O3/SiO2 catalysts. Appl. Catal. A Gen. 2000, 193, 123–128. [Google Scholar] [CrossRef]
- Kirichenko, G.N.; Glazunova, V.I.; Kirichenko, V.Y.; Dzhemilev, U.M. Promising process for synthesis of 3,5-xylenol from isophorone. Pet. Chem. 2006, 46, 434–438. [Google Scholar] [CrossRef]
- van Seters, A.J.C.; Wattimena, F. 3,5-Dimethylphenol. Canadian Patent 1,196,622, 12 November 1985. [Google Scholar]
- Zhang, X.; Wang, R.; Yang, X.; Yu, J. Central composite experimental design applied to the catalytic aromatization of isophorone to 3,5-xylenol. Chemom. Intell. Lab. Syst. 2007, 89, 45–50. [Google Scholar] [CrossRef]
- Wattimena, F. 3,5-Xylenol. U.S. Patent 4,086,282, 25 April 1978. [Google Scholar]
- Huizer, L.; van Namen, D.J.; Oranje, P.J.D. Purification of 3,5-Xylenol. UK Patent 1 560 241, 30 January 1980. [Google Scholar]
- Franck, H.-G.; Stadelhofer, J.W. Industrial Aromatic Chemistry: Raw Materials Processes Products; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar] [CrossRef]
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Arivazhagan, M.; Gayathri, R. NBO, NMR, UV, FT-IR, FT-Raman spectra and molecular structure (monomeric and dimeric structures) investigation of 4-Chloro-3,5-Xylenol: A combined experimental and theoretical study. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2013, 116, 170–182. [Google Scholar] [CrossRef]
- Bonrath, W.; Medlock, J.; Müller, M.-A.; Schütz, J. Catalysis for Fine Chemicals; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021. [Google Scholar] [CrossRef]
- Cotrupe, D.P.; Wellman, W.E.; Burton, P.E. Dihydroisophorone. U.S. Patent 3,446,850, 27 May 1969. [Google Scholar]
- Tungler, A.; Máthé, T.; Petró, J.; Tarnai, T. Enantioselective hydrogenation of isophorone. J. Mol. Catal. 1990, 61, 259–267. [Google Scholar] [CrossRef]
- Xu, L.; Sun, S.; Zhang, X.; Gao, H.; Wang, W. Study on the selective hydrogenation of isophorone. RSC Adv. 2021, 11, 4465–4471. [Google Scholar] [CrossRef]
- Rodríguez-García, L.; Hungerbühler, K.; Baiker, A.; Meemken, F. Enantioselection on Heterogeneous Noble Metal Catalyst: Proline-Induced Asymmetry in the Hydrogenation of Isophorone on Pd Catalyst. J. Am. Chem. Soc. 2015, 137, 12121–12130. [Google Scholar] [CrossRef] [PubMed]
- Ballova, G.D.; Egorova, E.I.; Romantsova, O.N.; Karmakova, V.G.; Prokhorova, L.V.; Zinchenko, V.A.; Emelin, Y.D.; Maladzyanova, L.F. High-Impact Weatherproof Graft Copolymers of Styrene. U.S.S.R. Patent 614118, 5 July 1978. [Google Scholar]
- Camani, A. Beitrag zur Kenntnis der Trimethyladipinsäure und Deren Derivate. PhD Thesis, ETH Zürich, Zürich, Switzerland, 1965. [Google Scholar]
- Siegel, H.; Eggersdorfer, M. Ketones. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2000; pp. 187–205. [Google Scholar] [CrossRef]
- Kleiman, H.L.; Martinat, J.J. Fuel for Cooking, Heating, and Lighting. U.S. Patent 3,894,848, 15 July 1975. [Google Scholar]
- Flitter, D. Purification of Cyclandelate. U.S. Patent 3,663,597, 16 May 1972. [Google Scholar]
- Takahashi, H. 3,3,5-Trimethylcyclohexyl Mandelate. Japanese Patent 48-43115, 17 December 1973. [Google Scholar]
- Yamauchi, K.; Yasui, T.; Fujii, K. 3,3,5-Trimethylcyclohexyl Mandelate. Japanese Patent 54-81240, 28 June 1979. [Google Scholar]
- Kolter, T. EA 1511. Available online: https://roempp.thieme.de/lexicon/RD-05-03539 (accessed on 1 November 2022).
- Dawson, T.P.; Williamson, C.E. 3-Pyridyl Phosphonates. U.S. Patent 3,903,098, 02 September 1975. [Google Scholar]
- Chen, H.; Wang, J.; Ma, Z.; Zhao, J.; Feng, G. Method for Synthesizing Homosalate. Chinese Patent 105541634, 4 November 2014. [Google Scholar]
- Qifa, L.; Rong, W. Method for Preparing Homosalate. Chinese Patent 105085273, 15 March 2017. [Google Scholar]
- Global Isophorone Diamine (IPDA) Market Research Report 2018. Available online: https://www.marketresearch.com/QYResearch-Group-v3531/Global-Isophorone-Diamine-IPDA-Research-11788198/ (accessed on 1 January 2023).
- Global Isophorone Diisocyanate (IPDI) Market Size 2023–2028 Forecast Growth Research, Growth Factor, Key Players, the Impact of COVID-19. Available online: https://www.marketwatch.com/press-release/global-isophorone-diisocyanate-ipdi-market-size-2023-2028-forecast-growth-research-growth-factor-key-players-the-impact-of-covid-19-2022-12-14 (accessed on 1 January 2023).
- Takahoso, H.; Takahashi, N.; Midorikawa, K.; Sato, T. Method for production of 3-cyano-3,5,5-trimethylcyclohexanone. Japanese Patent 4-279559, 5 October 1992. [Google Scholar]
- Sonnenschein, M.F. Polyurethanes; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Huthmacher, K.; Schmitt, H. Process for the Preparation of 1,3,3-Trimethyl-5-oxo-Cyclohexanecarbonitrile. German Patent 39 42 371, 21 May 1992. [Google Scholar]
- Thunberg, J.C.; Begonis, W.B. Process for the Preparation of 3-cyano-3,5,5-Trimethylcyclohexanone by Addition of Hydrogen Cyanide to Isophorone. U.S. Patent 5,011,968, 30 April 1991. [Google Scholar]
- Pontoglio, E.; Parodi, S. Preparation of 3-Cyano-3,5,5′-Trimethyl-1-Cyclohexanone from Isophorone and an Alkaline Cyanide. European Patent 0 425 806, 8 May 1991. [Google Scholar]
- Merger, F.; Priester, C.U.; Witzel, T.; Koppenhoefer, G.; Harder, W. Process for Preparing 3-(Aminomethyl)-3,5,5-Trimethylcyclohexylamine. German Patent 40 10 227, 2 October 1991. [Google Scholar]
- Rautenberg, W.; Mosbach, N.; Erdmann, T. Polyamide Composition Which Is Dyed in Black and Production and Use Thereof. International Patent 2017/144276, 31 August 2017. [Google Scholar]
- Schmitt, K.; Gude, F.; Rindtorff, K., Jr.; Disteldorf, J. Preparation of 1-Isocyanato-3-(Isocyanatomethyl)-3,5,5-Trimethylcyclohexane. German Patent 1 202 785, 14 October 1965. [Google Scholar]
- Evonik Launches First Renewable Isophorone-Based Products. Available online: https://crosslinkers.evonik.com/en/-169696.html (accessed on 6 August 2022).
- Chen, F.; Li, N.; Wang, W.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Catalytic conversion of isophorone to jet-fuel range aromatic hydrocarbons over a MoO(x)/SiO2 catalyst. Chem. Commun. 2015, 51, 11876–11879. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, J.A.; Harvey, B.G. Bio-Based Cycloalkanes: The Missing Link to High-Performance Sustainable Jet Fuels. ChemSusChem 2020, 13, 5777–5807. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.F.; Moore, C.M.; Leal, J.H.; Semelsberger, T.A.; Banh, J.K.; Zhu, J.; McEnally, C.S.; Pfefferle, L.D.; Sutton, A.D. Synthesis of aviation fuel from bio-derived isophorone. Sustain. Energy Fuels 2020, 4, 1088–1092. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Zhang, X.; Han, P.; Xie, J.; Pan, L.; Zou, D.-R.; Liu, S.-H.; Zou, J.-J. Synthesis of high-density and low-freezing-point jet fuel using lignocellulose-derived isophorone and furanic aldehydes. Sustain. Energy Fuels 2018, 2, 1863–1869. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Shi, C.; Pan, L.; Hou, F.; Nie, G.; Xie, J.; Liu, Q.; Zou, J.-J. Self-photosensitized [2 + 2] cycloaddition for synthesis of high-energy-density fuels. Sustain. Energy Fuels 2020, 4, 911–920. [Google Scholar] [CrossRef]
- Tang, H.; Li, N.; Li, S.; Chen, F.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of jet fuel rang cycloalkane from isophorone with glycerol as a renewable hydrogen source. Catal. Today 2017, 298, 16–20. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Zhang, T. Synthesis of renewable high-density fuel with isophorone. Sci. Rep. 2017, 7, 6111. [Google Scholar] [CrossRef]
- Chen, F.; Li, N.; Li, S.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of jet fuel range cycloalkanes with diacetone alcohol from lignocellulose. Green Chem. 2016, 18, 5751–5755. [Google Scholar] [CrossRef]
- Gonçalves, H.; Robinet, G.; Barthelat, M.; Lattes, A. Supramolecularity and Photodimerization of Isophorone: FTIR and Molecular Mechanics Studies. J. Phys. Chem. A 1998, 102, 1279–1287. [Google Scholar] [CrossRef]
- Howard, W.L. Acetone. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 1–15. [Google Scholar] [CrossRef]
- Jahn, U. Phenol. Available online: https://roempp.thieme.de/lexicon/RD-16-01576 (accessed on 4 August 2022).
- Zakoshansky, V. Phenol process celebrates its 60th anniversary: The role of chemical principles in technological breakthroughs. Russ J. Gen. Chem. 2009, 79, 2244–2266. [Google Scholar] [CrossRef]
- Zakoshansky, V.M. The cumene process for phenol-acetone production. Pet. Chem. 2007, 47, 273–284. [Google Scholar] [CrossRef]
- Dürre, P.; Bahl, H.; Gottschalk, G. Die Aceton-Butanol-Gärung: Grundlage für einen modernen biotechnologischen Prozeß? Chem. Ing. Tech. 1992, 64, 491–498. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, Z. Recent progress on industrial fermentative production of acetone–butanol–ethanol by Clostridium acetobutylicum in China. Appl. Microbiol. Biotechnol. 2009, 83, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Eisenacher, M.; Venschott, M.; Dylong, D.; Hoelderich, W.F.; Schütz, J.; Bonrath, W. Upgrading bio-based acetone to diacetone alcohol by aldol reaction using Amberlyst A26-OH as catalyst. React. Kinet. Catal. Lett. 2022, 135, 971–986. [Google Scholar] [CrossRef]
- Verseck, S.; Schaffer, S.; Freitag, W.; Schmidt, F.G.; Orschel, M.; Grund, G.; Schmidt, W.; Bahl, H.J.; Fischer, R.-J.; May, A.; et al. Fermentative Gewinnung von Aceton aus erneuerbaren Rohstoffen mittels neuen Stoffwechselweges. German Patent 10 2007 052 463, 7 May 2009. [Google Scholar]
- Kane, R. Ueber den Essiggeist und einige davon abgeleitete Verbindungen. J. Prakt. Chem. 1838, 15, 129–155. [Google Scholar] [CrossRef]
- Wagner, I.A. The Condensation Products of Acetone. PhD Thesis, Catholic University of America, Washington, DC, USA, 1913. [Google Scholar]
- Kerp, W. Zur Kenntniss des Kampherphorons, des Isophorons und des Mesityloxyds: [Erste Abhandlung.]. Eur. J. Org. Chem. 1896, 290, 123–152. [Google Scholar] [CrossRef]
- Kachler, J. Studien über die Verbindungen aus der Camphergruppe. Justus Liebigs Ann. Chem. 1872, 164, 75–92. [Google Scholar] [CrossRef]
- Knoevenagel, E.; Fischer, C. Synthese und Constitution des Isophorons. Eur. J. Org. Chem. 1897, 297, 185–203. [Google Scholar] [CrossRef]
- Li, Y.; Lü, J.; Jin, Z. Research progress in the synthesis of isophorone by acetone self-condensation. Chem. Ind. Eng. Prog. 2016, 35, 1190–1196. [Google Scholar] [CrossRef]
- Ueda, H.; Takeo, K.; Tsai, P.-L.; Tatsumi, C. Syntheses of Terpenes by the Condensation of Aliphatic Compounds: Part II. Self-Condensation of Mesityloxide and Structures of Isomeric Isoxylitones. Agric. Biol. Chem. 1966, 30, 1004–1014. [Google Scholar] [CrossRef]
- Bianchetti, G.; Pocar, D.; Stradi, R.; Dalla Croce, P.; Vigevani, A. Enamines. XXVI. Autocondensation products of acetone morpholinoenamine. 2-(2-Methyl-1-propenyl)-6,8,8-trimethylbicyclo[4.2.0]oct-2-en-4-one and 3,5,5,7-tetramethylcycloocta-2,7-dien-1-one. Gazz. Chim. Ital. 1967, 97, 872–884. [Google Scholar]
- Mei, J.; Chen, Z.; Yuan, S.; Mao, J.; Li, H.; Yin, H. Kinetics of Isophorone Synthesis via Self-Condensation of Supercritical Acetone. Chem. Eng. Technol. 2016, 39, 1867–1874. [Google Scholar] [CrossRef]
- Cataldo, F. Synthesis of ketonic resins from self-polymerization of acetone, 2. Action of bases on acetone and the synthesis of halogenated and diels-alder adducts. Angew. Makromol. Chem. 1996, 236, 21–33. [Google Scholar] [CrossRef]
- Sokoli, H.U.; Simonsen, M.E.; Nielsen, R.P.; Arturi, K.R.; Søgaard, E.G. Conversion of the matrix in glass fiber reinforced composites into a high heating value oil and other valuable feedstocks. Fuel Process. Technol. 2016, 149, 29–39. [Google Scholar] [CrossRef]
- Synthesis of Isophorone. Available online: https://www.chemicalbook.com/NewsInfo_3703.htm (accessed on 24 August 2022).
- Canning, A.S.; Jackson, S.D.; McLeod, E.; Vass, E.M. Aldol condensation of acetone over CsOH/SiO2: A mechanistic insight using isotopic labelling. Appl. Catal. A Gen. 2005, 289, 59–65. [Google Scholar] [CrossRef]
- Li, G.; Dong, Y.; Fan, Z.; Ma, J.; Yang, L.; Zhang, X.; Teng, Z. Kinetics model of synthesis of isophorone. Lanzhou Ligong Daxue Xuebao 2009, 35, 61–63. [Google Scholar]
- Podrebarac, G.G.; Ng, F.; Rempel, G.L. A kinetic study of the aldol condensation of acetone using an anion exchange resin catalyst. Chem. Eng. Sci. 1997, 52, 2991–3002. [Google Scholar] [CrossRef]
- Kuśtrowski, P.; Sułkowska, D.; Chmielarz, L.; Rafalska-Łasocha, A.; Dudek, B.; Dziembaj, R. Influence of thermal treatment conditions on the activity of hydrotalcite-derived Mg–Al oxides in the aldol condensation of acetone. Microporous Mesoporous Mater. 2005, 78, 11–22. [Google Scholar] [CrossRef]
- Thotla, S.; Agarwal, V.; Mahajani, S.M. Simultaneous production of diacetone alcohol and mesityl oxide from acetone using reactive distillation. Chem. Eng. Sci. 2007, 62, 5567–5574. [Google Scholar] [CrossRef]
- Darda, P.J.; Ranade, V.V. Isophorone reactor: Modelling and performance enhancement. Chem. Eng. J. 2012, 207–208, 349–367. [Google Scholar] [CrossRef]
- Huang, C.; Yang, L.; Ng, F.; Rempel, G.L. Application of catalytic distillation for the aldol condensation of acetone. Chem. Eng. Sci. 1998, 53, 3489–3499. [Google Scholar] [CrossRef]
- Bakker, R.; Hangx, G.; Kwant, G.; Maessen, H.; Markusse, P. Reaction and Catalyst Deactivation Kinetics of the Aldol Condensation of Acetone. Available online: https://www.yumpu.com/en/document/read/13734276/reaction-and-catalyst-deactivation-kinetics-of-the-cpi-research- (accessed on 1 February 2023).
- Lemcoff, N.O.; Cunningham, R.E. Kinetics of diacetone alcohol conversion to mesityl oxide catalyzed by ion exchange resin. J. Catal. 1971, 23, 81–92. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hatfield, J.D. Kinetics and equilibrium data of the dehydration-hydration reaction between diacetone alcohol and mesityl oxide in phosphoric acid. J. Chem. Eng. Data 1985, 30, 149–153. [Google Scholar] [CrossRef]
- Sliepcevich, A.; Moscatelli, D.; Gelosa, S. Kinetic Study of the Polycondensation of Acetone to Produce Isophorone Adopting Alumina and Magnesia as Catalyst. Chem. Eng. Trans. 2007, 11, 605–610. [Google Scholar]
- Bertrand, J.A.; Cheung, D.; Hammerich, A.D.; House, H.O.; Reichle, W.T.; Vanderveer, D.; Zaiko, E.J. Structure of the substance C27H38O formed by the base-catalyzed self-condensation of isophorone. J. Org. Chem. 1977, 42, 1600–1607. [Google Scholar] [CrossRef]
- Mucha, S.G.; Firlej, L.; Bantignies, J.-L.; Żak, A.; Samoć, M.; Matczyszyn, K. Acetone-derived luminescent polymer dots: A facile and low-cost synthesis leads to remarkable photophysical properties. RSC Adv. 2020, 10, 38437–38445. [Google Scholar] [CrossRef]
- Kurzer, F.; Hawkes, J.E.; Cobb, J. Bicyclo[2.2.2]octane-2-spirocyclohexanes, Part 1. Production and Structure of Spirodiisophora-3′,6-dione. Z. Für Nat. B 1991, 46, 1549–1556. [Google Scholar] [CrossRef]
- Kabas, G.; Rutz, H.C. The alkali-catalysed self-condensation of isophorone. Tetrahedron 1966, 22, 1219–1226. [Google Scholar] [CrossRef]
- Franck, H.G.; Turowski, J.; Eurnlu, K.; Storch, G.; Zander, M.; Lemke, R. 3.3.6.8-Tetramethyl-tetralon-(1), ein Kondensationsprodukt des Acetons. Justus Liebigs Ann. Chem. 1969, 724, 94–101. [Google Scholar] [CrossRef]
- Knoevenagel, E.; Beer, H. Ueber die höhermolekularen Condensationsproducte des Acetons. (Saure Condensation des Acetons.). Ber. Dtsch. Chem. Ges. 1906, 39, 3457–3466. [Google Scholar] [CrossRef]
- Knoevenagel, E.; Blach, L. Ueber die höhermolekularen Condensationsproducte des Acetons (alkalische Condensation des Acetons). Ber. Dtsch. Chem. Ges. 1906, 39, 3451–3457. [Google Scholar] [CrossRef]
- Rissafi, B.; El Louzi, A.; Loupy, A.; Petit, A.; Soufiaoui, M.; Fkih Tétouani, S. Solvent-Free Synthesis of Diaryl α-Tetralones via Michael Addition under Microwave Irradiation. Eur. J. Org. Chem. 2002, 2002, 2518. [Google Scholar] [CrossRef]
- Craven, E.C.; Ward, W.R. Phorone and isomeric forms. J. Appl. Chem. 1960, 10, 18–23. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S.G. Organische Chemie, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Faba, L.; Díaz, E.; Ordóñez, S. Base-Catalyzed Reactions in Biomass Conversion: Reaction Mechanisms and Catalyst Deactivation. In Reaction Pathways and Mechanisms in Thermocatalytic Biomass Conversion I; Green Chemistry and Sustainable Technology; Schlaf, M., Zhang, Z.C., Eds.; Springer: Singapore, 2016; pp. 87–122. [Google Scholar] [CrossRef]
- Faba, L.; Criado, Y.A.; Gallegos-Suárez, E.; Pérez-Cadenas, M.; Díaz, E.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Ordóñez, S. Preparation of nitrogen-containing carbon nanotubes and study of their performance as basic catalysts. Appl. Catal. A Gen. 2013, 458, 155–161. [Google Scholar] [CrossRef]
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of Fuels and Chemicals from Biomass: Condensation Reactions and Beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef]
- Seebald, H.J.; Schunack, W. Reaktionen an Aluminiumoxiden. 1. Umsetzungen von Aceton an Alumininumoxiden. Arch. Der Pharm. 1972, 305, 406–417. [Google Scholar] [CrossRef]
- Connolly, E.E. 93. Triacetone dialcohol and its dehydration products. J. Chem. Soc. 1944, 338–339. [Google Scholar] [CrossRef]
- Leopold, R.; Schacke, B. Verfahren zur Herstellung von Phoron und Halbphoron (2 • 6-Dimethylhepten 2-ol 6-on 4). German Patent 483 823, 23 October 1929. [Google Scholar]
- Gillard, R.D.; Heaton, B.T.; Pilbrow, M.P. 4-METHYLPENT-4-EN-2-ONE (iso-MESITYL OXIDE). Org. Prep. Proced. Int. 1974, 6, 131–134. [Google Scholar] [CrossRef]
- Cabani, S.; Ceccanti, N. Equilibria and kinetics of the hydration and cyclisation of semiphorone in acidic media. J. Chem. Soc. B 1966, 77–86. [Google Scholar] [CrossRef]
- Fleischhauer, E.H. Heterogen katalysierte Aldolkondensationen für die Synthese von Duft- und Aromastoffen. PhD Thesis, RWTH Aachen, Aachen, Germany, 2011. [Google Scholar]
- Ivakhnov, A.D.; Skrebets, T.E.; Bogdanov, M.V. Dehydrocondensation of Acetone under Supercritical Conditions. Russ. J. Phys. Chem. B 2019, 13, 1125–1127. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Z.; Liu, Q. One-Pot Synthesis of Methyl-Substituted Benzenes and Methyl-Substituted Naphthalenes from Acetone and Calcium Carbide. Ind. Eng. Chem. Res. 2019, 58, 6226–6234. [Google Scholar] [CrossRef]
- Roger, A.; Godfroid, J.J.; Wiemann, J. Self-condensation of mesityl oxide on magnesia under different pressures. I. Identification of the reaction products. Bull. Soc. Chim. Fr. 1967, 3030. [Google Scholar]
- Franck, H.-G.; Erünlü, R.K.; Lemke, R.; Storch, G.; Turowski, J.; Zander, M. Process for the Production of 3,3,6,8-Tetramethyl-tetralone- (1) from Isophorone Residues. German Patent 1 768 212, 16 December 1971. [Google Scholar]
- Cyrot, E. Mixed condensations of a-ethylenic aldehydes and ketones by vapor-phase heterogeneous catalysis. Synthesis of a-tetralones. Ann. Chim. (Paris) 1971, 6, 413. [Google Scholar]
- Orschel, M.; Jansen, R.; Maier, M.; Grund, G.; Schwarz, M.; Nitz, J.-J.; Hengstermann, A. Verfahren zur Herstellung von Isophoron. German Patent 10 2010 062 587, 14 June 2012. [Google Scholar]
- IARC Working Group on the Identification of Carcinogenic Hazards to Humans. In 1,1,1-Trichloroethane and Four Other Industrial Chemicals; International Agency for Research on Cancer: Lyon, France, 2022; Volume 130.
- Ballard, S.A.; Haury, V.E. Production of Isophorone. U.S. Patent 2,344,226, 14 March 1944. [Google Scholar]
- Cataldo, F. Synthesis of ketonic resins from self-polymerization of acetone, 1. Action of protic and lewis acids on acetone. Angew. Makromol. Chem. 1996, 236, 1–19. [Google Scholar] [CrossRef]
- Vaughn, T.H.; Jackson, D.R. Process for Preparing Isophorone. U.S. Patent 2,183,127, 12 December 1939. [Google Scholar]
- Di Cosimo, J.I.; Apesteguía, C.R. Study of the catalyst deactivation in the base-catalyzed oligomerization of acetone. J. Mol. Catal. A Chem. 1998, 130, 177–185. [Google Scholar] [CrossRef]
- The Global Isophorone Market is Expected to Grow by $ 2.18 bn during 2022-2026 Progressing at a CAGR of 6.05% during the Forecast Period. Available online: https://finance.yahoo.com/news/global-isophorone-market-expected-grow-122700709.html?guccounter=1&guce_referrer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvbS8&guce_referrer_sig=AQAAAFyIrTSyINEWi2V2i1BZEhPGkATucQAQ-JXnEUN3v261y-PtsVauoCON58rrt02LA20PZ0I_z4VGIQT0kec41cRl_MyG-ex5sIuD8ItfqX2Pp-SmnN8y9XNtiPiXCCebKY0xwaEpYcRZs6J12XhgkMknv-mLG6Sddjk2sLREW9yp (accessed on 26 October 2022).
- Isophorone. Available online: https://www.arkema.com/global/en/products/product-finder/product/thiochemicals/solvents/isophorone/ (accessed on 28 October 2022).
- Solvents and Intermediates Based on Isophorone. Available online: https://crosslinkers.evonik.com/en/products/vestasol (accessed on 28 October 2022).
- Baxxodur® EC 201. Available online: https://products.basf.com/global/en/ci/baxxodur-ec-201.html (accessed on 28 October 2022).
- Desmodur® I. Available online: https://solutions.covestro.com/de/products/desmodur/desmodur-i_00480770-12978368?SelectedCountry=US (accessed on 28 October 2022).
- Evonik Celebrates 50 Years of Producing Isophorone Chemicals for Paint and Coatings Market. Available online: https://www.coatingsworld.com/issues/2013-01/view_suppliers-news/evonik-celebrates-50-years-of-producing-isophorone/ (accessed on 28 September 2022).
- Isophorone Market: Isophorone Market: Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2020–2030. Available online: https://www.transparencymarketresearch.com/isophorone-market.html (accessed on 19 December 2022).
- Mao, L.; Li, Q.; Yin, D. The Liquid Phase Condensation of Acetone to Isophorone by One Step. Acta Sci. Nat. Univ. Norm. Hunanensis 2000, 23, 67–71. [Google Scholar]
- Hwang, Y.-L.; Bedard, T.C. Ketones. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Nitz, J.-J.; Kohlstruk, S.; Jansen, R.; Orschel, M.; Merkel, A.; Demming, M.; Mendorf, M.; Doering, J.; Hengstermann, A.; Hoff, A.; et al. Hydrolyse der bei der Produktion von Isophoron anfallenden Rückstände zur Rückgewinnung von Isophoron und Acetonproduction. German Patent 10 2013 215 874, 12 February 2015. [Google Scholar]
- Schmitt, K.; Disteldorf, J.; Baron, W. Verfahren zur Aufarbeitung von Nebenprodukten der Isophoronherstellung. German Patent 1 205 525, 21 November 1965. [Google Scholar]
- Ballard, S.A.; Haury, V.E. Hydrolysis of Acetone Autocondensation Products. U.S. Patent 2,419,051, 15 April 1947. [Google Scholar]
- Winkler, D.L.E.; Raab, W.J.; Ballard, S.A. Hydrolysis of Acetone Condensation Products. U.S. Patent 2,434,631, 13 January 1948. [Google Scholar]
- Societe Industrielle des Derives de L’acetylenene. Isophorone and Its Homologues. UK Patent 733,650, 13 July 1955.
- Cane, C.; Yeomans, B. Verfahren zur Herstellung von Isophoron. German Patent 26 45 281, 21 April 1977. [Google Scholar]
- Chen, Z.; Li, H.; Mao, J.; Yin, H.; Hu, B. Method of Hydrolysis of Acetone Polymer in Supercritical Water Medium. Chinese Patent 103145541, 4 February 2015. [Google Scholar]
- Cook, J.B. Industrial application of reduce-reuse-recycle strategies in the production of isophorone. Master’s Thesis, University of South Alabama, Mobile, AL, USA, 2007. [Google Scholar]
- Cane, C.; Yeomans, B. Process for the Production of Isophorone. U.S. Patent 4,059,632, 22 November 1977. [Google Scholar]
- Fewlass, M.W. Purification of Isophorone. UK Patent 833,099, 21 April 1960. [Google Scholar]
- Craven, E.C.; Fewlass, M.W. Purification of Isophorone. UK Patent 832,124, 6 April 1960. [Google Scholar]
- Dannenberg, H. Stabilization of Unsaturated Ketones. U.S. Patent 2,566,792, 4 September 1951. [Google Scholar]
- Dannenberg, H. Stabilization of Unsaturated Ketones. U.S. Patent 2,444,006, 22 June 1948. [Google Scholar]
- Bailey, H.C.; Bartlett, J.S. Isophorone Compositions with Long Storage Life. UK Patent 2 029 415, 19 March 1980. [Google Scholar]
- Papa, A.J. Low Color Refined Isophorone. U.S. Patent 4,434,301, 28 February 1984. [Google Scholar]
- Cheminal, B.; Kiener, P. Colorless and Stable Isophorone. U.S. Patent 4,248,673, 3 February 1981. [Google Scholar]
- Jia, T.; Zhang, J.; Liu, Z. Preparation and property of Y-Mg-Al-layered double oxides. Adv. Mater. Res. 2012, 396-398, 764–767. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Z.; Lv, J. Isophorone Catalyst. Chinese Patent 106423125, 20 November 2018. [Google Scholar]
- Liu, Y.; Sun, K.; Ma, H.; Xu, X.; Wang, X. Cr, Zr-incorporated hydrotalcites and their application in the synthesis of isophorone. Catal. Commun. 2010, 11, 880–883. [Google Scholar] [CrossRef]
- Faba, L.; Díaz, E.; Ordóñez, S. Gas phase acetone self-condensation over unsupported and supported Mg–Zr mixed-oxides catalysts. Appl. Catal. B Environ. 2013, 142–143, 387–395. [Google Scholar] [CrossRef]
- Bagabas, A.A.; Mokhtar, M.; Akhmedov, V.M.; Narasimharao, K.; Basahel, S.N.; Al-Rabiah, A. Ru–C–ZnO Composite Catalysts for the Synthesis of Methyl Isobutyl Ketone via Single Step Gas Phase Acetone Self-Condensation. Catal. Lett. 2014, 144, 1278–1288. [Google Scholar] [CrossRef]
- Li, K.; Chen, X.; Zhou, W.; Ma, Z.; Li, Z.; Zhao, Y.; Hou, L.; Wang, H.; Jiang, H. Heterogeneous Catalytic Method for Synthesis of Isophorone from Acetone. Chinese Patent 101875602, 24 April 2013. [Google Scholar]
- Wu, Y.; Tan, J.; Liang, D.; Liu, J. Isophorone prepared with acetone by catalysis of composite oxide solid alkali. Petrochem. Technol. 2012, 19, 7–11. [Google Scholar]
- Zhang, X.L. Research on Solid-base Catalysts for Synthesis Isophorone. Master’s Thesis, China University of Petroleum, Beijing, China, 2009. [Google Scholar]
- Chen, F. Preparation of Isophorone by Condensation of Acetone Catalyzed by Magnesium-Aluminum Composite Oxide. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2014. [Google Scholar]
- Liu, Z.; Li, H. Catalyst for Preparing Isophorone by Acetone Condensation Method. Chinese Patent 101698147, 25 January 2012. [Google Scholar]
- Kane, S.M.; Timonen, R.S.; Leu, M.-T. Heterogeneous Chemistry of Acetone in Sulfuric Acid Solutions: Implications for the Upper Troposphere. J. Phys. Chem. A 1999, 103, 9259–9265. [Google Scholar] [CrossRef]
- Taco-Vasquez, S. Transformation of Acetone and Isopropanol to Hydrocarbons using HZSM-5 Catalyst. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2009. [Google Scholar]
- Faba, L.; Gancedo, J.; Quesada, J.; Diaz, E.; Ordóñez, S. One-Pot Conversion of Acetone into Mesitylene over Combinations of Acid and Basic Catalysts. ACS Catal. 2021, 11, 11650–11662. [Google Scholar] [CrossRef]
- Jahn, U. Mesitylen. Available online: https://roempp.thieme.de/lexicon/RD-13-01361 (accessed on 1 November 2022).
- Quesada, J.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of catalyst morphology and hydrogen co-feeding on the acid-catalysed transformation of acetone into mesitylene. Catal. Sci. Technol. 2020, 10, 1356–1367. [Google Scholar] [CrossRef]
- Reif, P.; Rosenthal, H.; Rose, M. Biomass-Derived Aromatics by Solid Acid-Catalyzed Aldol Condensation of Alkyl Methyl Ketones. Adv. Sustain. Syst. 2020, 4, 1900150. [Google Scholar] [CrossRef]
- Kawai, W. Polymerization of Acetone. BCSJ 1962, 35, 516. [Google Scholar] [CrossRef]
- Ramanamurty, K.V.; Salvapati, G.S. Catalytic Cyclocondensation of acetone to isophorone. Indian J. Chem. Sect. B Org. Med. Chem. 1999, 38, 24–28. [Google Scholar]
- Orschel, M.; Grund, G.; Schneider, R. Verfahren zur Elektrochemischen Herstellung von Isophoron. German Patent 10 2010 029 272, 1 December 2011. [Google Scholar]
- Ballard, S.A.; Haury, V.E. Production of Isophorone and Related Products. U.S. Patent 2,399,976, 7 May 1946. [Google Scholar]
- Walton, J.R.; Yeomans, B. Isophorone Production Using a Potassium Hydroxide Catalyst. U.S. Patent 3,981,918, 21 September 1976. [Google Scholar]
- Chen, Z.; Li, H.; Lin, H.; Xu, Y.; Wang, C. Method for Preparing Alpha-Isophorone. Chinese Patent 101633610, 17 October 2012. [Google Scholar]
- He, Y.; Yuan, M.; Li, H.; Ding, J.; Li, Y.; Du, Y.; Luo, W.; Zhao, W.; Zhang, Z.; Yu, X. Method for Preparing Isophorone by Acetone Liquid Condensation with Alkali Catalyst. Chinese Patent 102516051, 1 January 2014. [Google Scholar]
- Zhou, Z.; Xu, J.; Fei, A.; Tan, C.; Huang, Z.; Xiao, S.; Yan, Y.N. A Kind of Preparation Method of the Isophorone Using Organic imidazoles System Quaternary Ammonium Strong Base Catalyst. Chinese Patent 106892807, 3 May 2019. [Google Scholar]
- Zhou, Z.; Xu, J.; Fei, A.; Tan, C.; Huang, Z.; Xiao, S.; Yan, Y.N. A Kind of Preparation Method of the Isophorone Using Organic Methenamine System Quaternary Ammonium Strong Base Catalyst. Chinese Patent 106831377, 3 May 2019. [Google Scholar]
- Zhou, Z.; Xu, J.; Fei, A.; Tan, C.; Huang, Z.; Xiao, S.; Yan, Y.N. A Kind of Preparation Method of the Isophorone Using Organic Pyridine System Quaternary Ammonium Strong Base Catalyst. Chinese Patent 106905128, 3 May 2019. [Google Scholar]
- Li, Y.; Meng, H.; Lu, Y.; Li, C. Efficient Catalysis of Calcium Carbide for the Synthesis of Isophorone from Acetone. Ind. Eng. Chem. Res. 2016, 55, 5257–5262. [Google Scholar] [CrossRef]
- Nanjing Kaixuan Chemical Technology Co., Ltd., China. Preparation Method of Isophorone. Chinese Patent 112239400, 19 January 2021. [Google Scholar]
- Qiao, S.; Zhang, Y.; Mao, J.; Yan, H.; Pan, H. Preparation Method of Alpha-Isophorone. Chinese Patent 110885286, 20 May 2022. [Google Scholar]
- Teissier, R.; Kervennal, J. Process for Obtaining Isophorone. U.S. Patent 5,849,957, 15 December 1998. [Google Scholar]
- Xu, X.; Meng, H.; Lu, Y.; Li, C. Aldol condensation of refluxing acetone on CaC2 achieves efficient coproduction of diacetone alcohol, mesityl oxide and isophorone. RSC Adv. 2018, 8, 30610–30615. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Vikenteva, Y.A.; Ananikov, V.P. Calcium-Based Sustainable Chemical Technologies for Total Carbon Recycling. ChemSusChem 2019, 12, 1483–1516. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.H.; Bailey, W.A. Separation of by-Products from Isophorone. U.S. Patent 2,351,352, 13 June 1944. [Google Scholar]
- Schmitt, K.; Disteldorf, J.; Baron, W. Verfahren zur Gewinnung von Reinisophoron. German Patent 1 144 269, 28 February 1963. [Google Scholar]
- Schmitt, K.; Disteldorf, J.; Baron, W. Verfahren und Vorrichtung zur Herstellung von Reinem Isophoron. German Patent 1 165 018, 12 March 1964. [Google Scholar]
- Yoshida, Y. Production of Isophorone. Japanese Patent 8-245486, 24 September 1996. [Google Scholar]
- Yoshida, Y. Production of Isophorone. Japanese Patent 8-245485, 24 September 1996. [Google Scholar]
- Orschel, M.; Jansen, R.; Maier, M.; Nitz, J.-J. Verfahren zur Herstellung von Isophoron in Gegenwart Mindestens Eines Entschäumers in der Abwasserkolonne im Aufarbeitungsteil. German Patent 10 2011 075 777, 15 November 2012. [Google Scholar]
- Fukada, I.; Matsuba, K. Production of Acetone Dehydrocondensate. Japanese Patent 9-59204, 4 March 1997. [Google Scholar]
- Fukada, I.; Matsuba, K. Production of Acetone Dehydrative Condensation Product. Japanese Patent 9-151153, 10 June 1997. [Google Scholar]
- Fukada, I.; Matsuba, K. Production of Isophorone. Japanese Patent 9-157208, 17 June 1997. [Google Scholar]
- Fukada, I.; Matsuba, K. Production of Isophorone. Japanese Patent 9-157207, 17 June 1997. [Google Scholar]
- Fukada, I.; Matsuba, K. Production of Acetone Dehydrative Condensation Product. Japanese Patent 9-151152, 10 June 1997. [Google Scholar]
- Fukada, I.; Matsuba, K. Production of Isophorone. Japanese Patent 9-169687, 30 June 1997. [Google Scholar]
- Fukada, I.; Matsuba, K. Production of Isophorone. Japanese Patent 9-169688, 30 June 1997. [Google Scholar]
- Song, C.; Tain, B.; Tang, G.; Xiang, L. Composite oxide and preparation method and application thereof. Chinese Patent 114425318, 3 May 2022. [Google Scholar]
- Schutz, A.A.; Cullo, L.A. Method of Making Isophorne. U.S. Patent 5,202,496, 13 April 1993. [Google Scholar]
- Sun, L.; Hou, Y.; Chai, Y.; Wang, F.; Liu, C. Equipment and Method for Preparing Isophorone by Acetone Gas Phase Condensation. Chinese Patent 107573227, 17 October 2017. [Google Scholar]
- Zhao, J.; Zhang, Y.; Li, C. Method for Preparing Catalyst Used in Synthesizing Isophorone through Acetone Multiphase Method. Chinese Patent 102258994, 20 June 2012. [Google Scholar]
- León, M.; Faba, L.; Díaz, E.; Bennici, S.; Vega, A.; Ordóñez, S.; Auroux, A. Consequences of MgO activation procedures on its catalytic performance for acetone self-condensation. Appl. Catal. B Environ. 2014, 147, 796–804. [Google Scholar] [CrossRef]
- Ordóñez, S.; Díaz, E.; León, M.; Faba, L. Hydrotalcite-derived mixed oxides as catalysts for different C–C bond formation reactions from bioorganic materials. Catal. Today 2011, 167, 71–76. [Google Scholar] [CrossRef]
- Kelkar, C.; Schutz, A. Efficient hydrotalcite-based catalyst for acetone condensation to α-isophorone—Scale up aspects and process development. Appl. Clay Sci. 1998, 13, 417–432. [Google Scholar] [CrossRef]
- Gao, P. Study on Catalyst and Technology for Synthesis of Isophorone from Acetone in Gas-Solid Multiphase Process. Master’s Thesis, China University of Petroleum, Beijing, China, 2008. [Google Scholar]
- Ma, C.-X.; Liu, G.; Zhu, W.-C.; Wang, L.-X.; Li, Y.-F.; Zhang, W.-X.; Jia, M.-J. Gas-phase Aldol Condensation of Acetone over Mg-Al Oxides Prepared by Co-precipitation Methods. Chem. J. Chin. Univ. 2009, 30, 2429–2433. [Google Scholar]
- Liu, Z.; Liu, Y.; Lu, B. Hydrotalcite synthesis and the effect on acetone catalytic condensation to isophorone. J. Guangzhou Univ. Nat. Sci. Ed. 2011, 4, 14–19. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, Z.L.; Qin, Z.Z.; Liu, Y.; Wang, Q.Y.; Zou, H.B.; Chen, S.Z.; Liang, H. Preparation of MgAlY-LDO Solid Base Catalysts and their Catalytic Performance on the Synthesis of Isophorone via Acetone Condensation. AMR 2012, 550–553, 424–428. [Google Scholar] [CrossRef]
- Krivtsov, I.; Faba, L.; Díaz, E.; Ordóñez, S.; Avdin, V.; Khainakov, S.; Garcia, J.R. A new peroxo-route for the synthesis of Mg–Zr mixed oxides catalysts: Application in the gas phase acetone self-condensation. Appl. Catal. A Gen. 2014, 477, 26–33. [Google Scholar] [CrossRef]
- Manríquez, M.E.; Hernández-Cortez, J.G.; Wang, J.A.; Chen, L.F.; Zuñiga-Moreno, A.; Gómez, R. Synthesis of transition metal doped lamellar double hydroxides as base catalysts for acetone aldol condensation. Appl. Clay Sci. 2015, 118, 188–194. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, B.; Ren, H. Synthesis of Isophorone from Acetone over CsX Molecular Sieves. Chem. Ind. Eng. 2018, 35, 12–18. [Google Scholar]
- Stevens, M.G.; Chen, D.; Foley, H.C. Oxidized caesium/nanoporous carbon materials: Solid-base catalysts with highly-dispersed active sites. Chem. Commun. 1999, 275–276. [Google Scholar] [CrossRef]
- Zamora, M.; López, T.; Gómez, R.; Asomoza, M.; Melendrez, R. Oligomerization of acetone over titania-doped catalysts (Li, Na, K and Cs): Effect of the alkaline metal in activity and selectivity. Catal. Today 2005, 107–108, 289–293. [Google Scholar] [CrossRef]
- Thomas, L.; Tanner, R.; Gill, P.; Wells, R.; Bailie, J.E.; Kelly, G.; Jackson, S.D.; Hutchings, G. Aldol condensation reactions of acetone over alkali-modified vanadium phosphate catalysts. Phys. Chem. Chem. Phys. 2002, 4, 4555–4560. [Google Scholar] [CrossRef]
- Qian, X.; Qian, J.; Cui, Y.; Chen, J. Method for Preparing Isophorone at Normal Pressure. Chinese Patent 102633612, 25 June 2014. [Google Scholar]
- Wang, Y.-H.; Wang, G.-J.; Xiao, J.-H.; Ma, J. The Condensation of Acetone over Solid Base Catalyst. Chem. J. Chin. Univ. 1993, 14, 1448. [Google Scholar]
- Al-Ghamdi, K. An investigation of Heterogeneous Base Catalysed Acetone Conversion. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2011. [Google Scholar]
- Crossley, A.W.; Gilling, C. Preparation of Trimethylcyclohexenone (Isophorone) from Ethyl Malonate and Chlorodimethylcyclohexenone. Proc. Chem. Soc. London 1910, 25, 96. [Google Scholar]
- Crossley, A.W.; Gilling, C. IV.—Hydroaromatic ketones. Part I. Synthesis of trimethylcyclohexenone (isophorone) and some homologues. J. Chem. Soc. Trans. 1909, 95, 19–29. [Google Scholar] [CrossRef]
- Flego, C.; Perego, C. Acetone condensation as a model reaction for the catalytic behavior of acidic molecular sieves: A UV–Vis study. Appl. Catal. A Gen. 2000, 192, 317–329. [Google Scholar] [CrossRef]
- Kozlowski, J.T. Carbon-Carbon Bond Forming Reactions over Metal Oxide Catalysts. PhD Thesis, University of Virginia, Charlottesville, VA, USA, 2013. [Google Scholar]
- Kuśtrowski, P.; Sułkowska, D.; Pytlowany, R.; Dziembaj, R. Kinetics of self-condensation of acetone over heterogeneous Ba(OH)2 and Sr(OH)2 catalysts. React. Kinet. Catal. Lett. 2004, 81, 3–11. [Google Scholar] [CrossRef]
- Liu, R. Study of Acetone Condensation to Isophorone over Solid Catalyst. Master’s Thesis, Hebei University of Technology, Shijiazhuang, China, 2008. [Google Scholar]
- Lu, J. Condensation process and kinetic characteristics of acetone in magnesia-alumina catalyst. Shihua Jishu Yu Yingyong 2002, 20, 15–18. [Google Scholar]
- Orel, V.B.; Vitkovskaya, N.M.; Bobkov, A.S.; Semenova, N.V.; Schmidt, E.Y.; Trofimov, B.A. Aldol Condensation Versus Superbase-Catalyzed Addition of Ketones to Acetylenes: A Quantum-Chemical and Experimental Study. J. Org. Chem. 2021, 86, 7439–7449. [Google Scholar] [CrossRef]
- Peng, W.; Li, J.; Chen, B.; Wang, N.; Luo, G.; Wei, F. Mesoporous MgO synthesized by a homogeneous-hydrothermal method and its catalytic performance on gas-phase acetone condensation at low temperatures. Catal. Commun. 2016, 74, 39–42. [Google Scholar] [CrossRef]
- Shylesh, S.; Kim, D.; Gokhale, A.A.; Canlas, C.G.; Struppe, J.O.; Ho, C.R.; Jadhav, D.; Yeh, A.; Bell, A.T. Effects of Composition and Structure of Mg/Al Oxides on Their Activity and Selectivity for the Condensation of Methyl Ketones. Ind. Eng. Chem. Res. 2016, 55, 10635–10644. [Google Scholar] [CrossRef]
- Veshchitsky, G.A.; Smirnov, A.V.; Mashchenko, N.V.; Koklin, A.E.; Bogdan, V.I. Self-Condensation of Acetone of Strontium Stannate under Supercritical Conditions. Russ. J. Phys. Chem. B 2021, 15, 1299–1302. [Google Scholar] [CrossRef]
- Diez, V.; Apesteguia, C.; Di Cosimo, J. Effect of the acid-base properties of Mg-Al mixed oxides on the catalyst deactivation during aldol condensation reactions. Lat. Am. Appl. Res. 2003, 33, 79–86. [Google Scholar]
- Kuśtrowski, P.; Sułkowska, D.; Chmielarz, L.; Cap, S. Production of isophorone by the vapour-phase self-condensation of acetone over the hydrotalcite-derived catalysts. Pol. J. Chem. Technol. 2004, 6, 41–46. [Google Scholar]
- Liang, D. Study on Solid Bases: Synthesis and Their Catalytic Performance to Isoporone. Master’s Thesis, Dalian University of Technology, Dalian, China, 2011. [Google Scholar]
- Yuan, L. Synthesis of isophoroneon acid-base bifunctional catalysts by machine mixing method. Chem. Ind. Eng. Prog. 2015, 34, 3060–3064. [Google Scholar]
- Zamora, M.; López, T.; Gómez, R.; Asomoza, M.; Meléndrez, R. Acetone gas phase condensation on alkaline metals doped TiO2 sol–gel catalysts. Appl. Surf. Sci. 2005, 252, 828–832. [Google Scholar] [CrossRef]
- Bruson, H.A. Preparation of Isophorones. U.S. Patent 2,148,103, 21 February 1939. [Google Scholar]
- Grebinoski, M.C.; Glassman, D.; Elias, C.L.; Schutz, A.A. Isophorone process. U.S. Patent 5,352, 839, 4 October 1994. [Google Scholar]
- He, Y.; Tian, B.; Yun, M.; Bian, L.; Dong, L.; Zhou, R.; Sun, Q.; Shi, D.; Liu, Z. Method and Chemical Apparatus for Preparing Isophorone from Acetone. Chinese Patent 112441896, 5 March 2021. [Google Scholar]
- He, Y.; Li, Y.; Tian, B.; Bian, L.L.; Zhou, R.; Guan, M.; Li, J.; Sun, Y. Method for Preparing Isophorone by Using Plug Flow Rectification Column Plate and Acetone Liquid Phase Condensation. Chinese Patent 110038317, 10 April 2020. [Google Scholar]
- Jianming, C.; Cui, Y.; Yao, Z.; Zhu, H.; Yan, Y.; Jianjun, Z. Preparation Method of Isophorone. Chinese Patent 103467263, 17 June 2015. [Google Scholar]
- Papa, A.J.; Kaiser, S.W. Preparation of Isophorone and Mesityl Oxide from Acetone. U.S. Patent 4,535,187, 13 August 1985. [Google Scholar]
- Park, J.W.; Row, K.; Park, J.W. Molded Catalyst from Catalyst Power and Binder for Producing Cyclic Ketone. Korean Patent 10-2022-055782, 4 May 2022. [Google Scholar]
- Xue, Y.; Qiao, X.; Post, M.; Jia, Z.; Li, J.; Sun, Y.; Jia, H. Preparation Method and Device of Isophorone. Chinese Patent 110903180, 8 November 2022. [Google Scholar]
- Yoshihiko, H.; Katsuhiko, H. Production of Dimer and/or Trimer OF Acetone. Japanese Patent 1-175952, 12 July 1989. [Google Scholar]
- Zhang, J. Catalyst for Synthesizing Isophorone. Chinese Patent 103691416, 2 April 2014. [Google Scholar]



| Name | α-Isophorone | β-Isophorone | γ-Isophorone |
|---|---|---|---|
| structure | 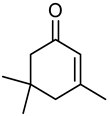 |  |  |
| CAS-number | 78-59-1 | 471-01-2 | 72212-29-4 |
| Molar mass | 138.21 g/mol | 138.21 g/mol | 138.21 g/mol |
| Density (20 °C) | 0.92 g/cm3 | 0.91 g/cm3 | n/a |
| Melting point | −8.1 °C | −40.7 °C | n/a |
| Boiling point | 215 °C | Decomposition | n/a |
| Catalyst a | T (°C) | Reactor | Time b | X (%) | S (%) | Y (%) | Refs. |
|---|---|---|---|---|---|---|---|
| 0.7% NaOH | 250 | batch | 4 h | 68 | 84 | n/a | [161] |
| ≈4% NaOH | 220 | batch | 4 h | n/a | 81 | 53 | [114] |
| 10% NaOH | 320 | continuous | 2 min | n/a | 93 | 93 | [200] |
| 20% NaOH | 150 | batch | 3 h | n/a | 39 | 7 | [150] |
| 25% NaOH | 170 | continuous | 37 min | n/a | n/a | 4 c | [198] |
| 0.7% KOH | 250 | batch | 4 h | 68 | 93 | n/a | [161] |
| 0.75% KOH | ≈205 | continuous | n/a | n/a | 86 | 7 | [199] |
| 1.8% KOH | 280 | continuous | 5 min | >20 | >90 | n/a | [201] |
| 10% KOH | 300 | continuous | 1 min | n/a | 92 | 91 | [200] |
| 30.3% KOH | 169 | continuous | 17.5 min | n/a | n/a | 6 | [198] |
| Methylmetheneamine hydroxide | 180 | batch | 5 h | 55 | 63 | n/a | [203] |
| Benzylmentheneamine hydroxide | 150 | batch | 6 h | 59 | 65 | n/a | [203] |
| tert-Butylmethenamine hydroxide | 160 | batch | 7 h | 60 | 66 | n/a | [203] |
| 1,3-Dimethylimidazole hydroxide | 180 | batch | 5 h | 62 | 71 | n/a | [202] |
| 1,3-Di-tert-butylimidazole hydroxide | 150 | batch | 6 h | 67 | 69 | n/a | [202] |
| 1,3-Di-n-butylimidazole hydroxide | 160 | batch | 7 h | 68 | 71 | n/a | [202] |
| N,3 Dimethylpyridine hydroxide | 150 | batch | 6 h | 55 | 63 | n/a | [204] |
| N-tert-butyl-3-methylpyridine hydroxide | 160 | batch | 7 h | 57 | 62 | n/a | [204] |
| CaC2 | 150 | batch | 2 h | 81 | n/a | 21 | [205] |
| Al2O3, basic resin | 210 | batch | 7–8 h | n/a | 89 | 71 | [206] |
| Mg1−xAlxO1+x | 200 | batch | 1 h | 38 | 51 | n/a | [208] |
| Sr0.1Mg0.6Al0.15Pr0.05O | 270 | continuous | 1 min | 41 | 91 | n/a | [207] |
| Catalyst | T (°C) | Reactor | Residence Time | X (%) | S (%) | Y (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Magnesium oxide | 450 | continuous | n/a | 37 | 33 | n/a | [228] |
| Mg–Al mixed oxide | 250 | continuous | n/a | 7 | 23 | n/a | [229] |
| Mg–Al mixed oxide | 250 | continuous | 30 min | 25 | 75 | n/a | [230] |
| Mg–Al mixed oxide | 250 | continuous | 1 h | 20 | 65 | n/a | [231] |
| Mg–Al mixed oxide | 250 | continuous | 1 h | n/a | 86 | 73 | [232] |
| Mg–Al mixed oxide | 290 | continuous | n/a | 36 | 95 | n/a | [184] |
| Mg–Al mixed oxide | 300 | continuous | n/a | 20 | 56 | n/a | [233] |
| Mg–Al–Y-layered double-oxide | 300 | continuous | 9.5 min | 38 | 59 | 22 | [234] |
| Cr–Mg–Al mixed oxide | 240 | continuous | n/a | 25 | 74 | n/a | [181] |
| Zr–Mg–Al mixed oxide | 240 | continuous | n/a | 37 | 73 | n/a | [181] |
| La–Mg–Al mixed oxide | 250 | continuous | 1 h | 29 | 64 | 19 | [186] |
| La–Mg–Al mixed oxide | 250 | continuous | 37.5 min | 41 | 72 | 29 | [187] |
| Ca–Mg–Al mixed oxide | 250 | continuous | 37.5 min | 28 | 80 | 23 | [187] |
| Ca–Mg–Al mixed oxide | 250 | continuous | 1 h | 55 | 65 | 36 | [185] |
| Ca–Zr–Mg–Al mixed oxide | 300 | continuous | 30 min | 35 | 97 | n/a | [227] |
| Mg–Zr mixed oxide | 450 | continuous | n/a | 43 | 9 | n/a | [235] |
| Mg-Al-Ca–Ba–Zr–Ce mixed oxide | 300 | continuous | n/a | 86 | n/a | 76 | [188] |
| molecular sieve | 350 | continuous | 30 min | 26 | 52 | n/a | [237] |
| Cs/nanoporous carbon | 225 | continuous | n/a | 12 | 61 | n/a | [238] |
| CsOH/SiO2 | 400 | continuous | n/a | 3 | 19 | n/a | [113] |
| Na+/TiO2 | 300 | continuous | n/a | 20 | 4 | n/a | [239] |
| Na+-(VO)2P2O7 | 400 | continuous | n/a | 7 | >95 | n/a | [240] |
| NaOH | 220–350 | continuous | n/a | 32 | 60 | n/a | [241] |
| Ca(OH)2-CaO | 550 | continuous | n/a | 47 | >80 | n/a | [242] |
| Ca(OH)2 | 350 | continuous | n/a | n/a | n/a | 25 | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruther, T.; Müller, M.-A.; Bonrath, W.; Eisenacher, M. The Production of Isophorone. Encyclopedia 2023, 3, 224-244. https://doi.org/10.3390/encyclopedia3010015
Ruther T, Müller M-A, Bonrath W, Eisenacher M. The Production of Isophorone. Encyclopedia. 2023; 3(1):224-244. https://doi.org/10.3390/encyclopedia3010015
Chicago/Turabian StyleRuther, Timm, Marc-André Müller, Werner Bonrath, and Matthias Eisenacher. 2023. "The Production of Isophorone" Encyclopedia 3, no. 1: 224-244. https://doi.org/10.3390/encyclopedia3010015
APA StyleRuther, T., Müller, M.-A., Bonrath, W., & Eisenacher, M. (2023). The Production of Isophorone. Encyclopedia, 3(1), 224-244. https://doi.org/10.3390/encyclopedia3010015