The Mysteries of the White Truffle: Its Biology, Ecology and Cultivation
Definition
1. Introduction
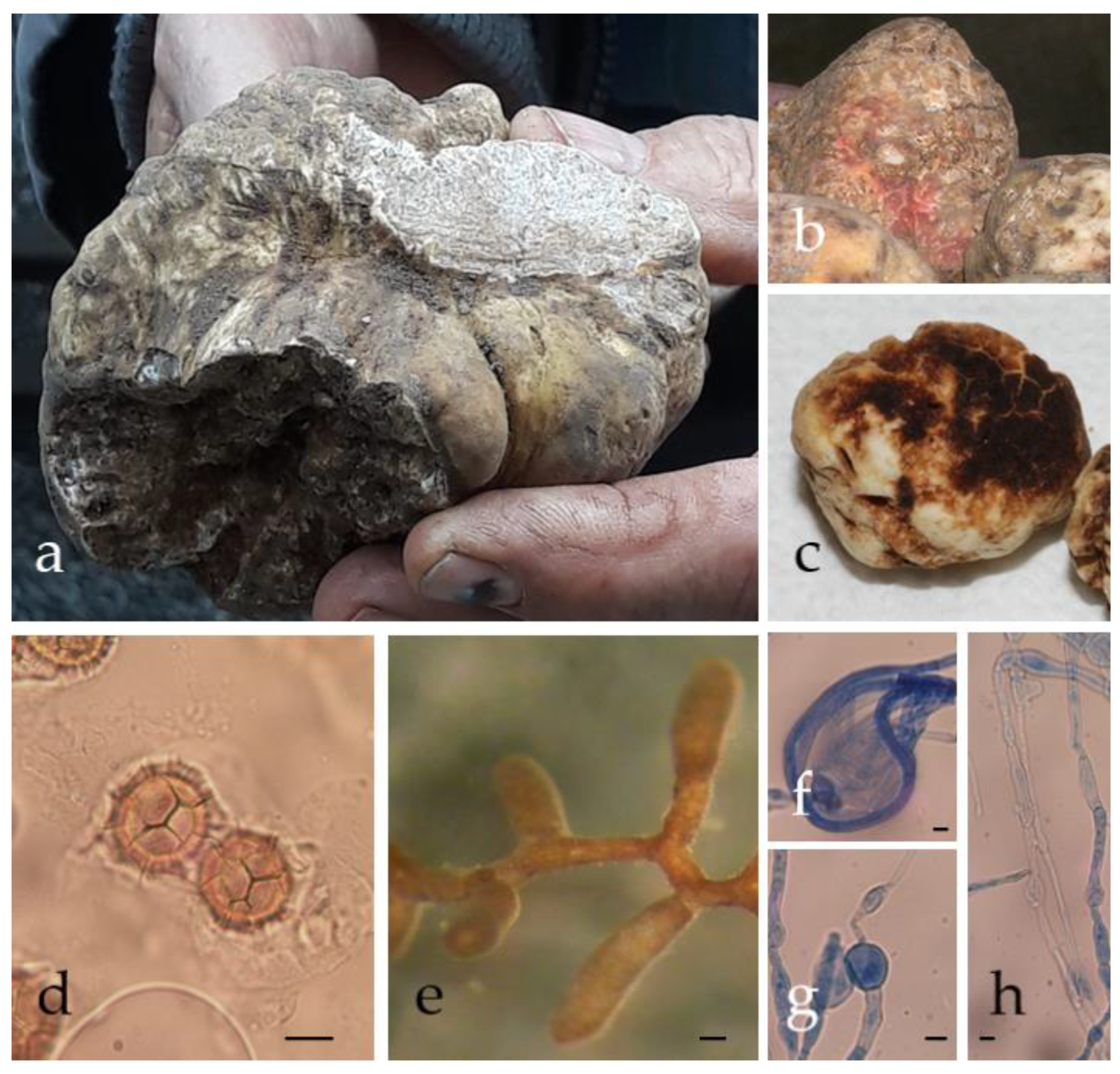
2. Morphology
3. Aroma
4. Biology
5. Distribution and Ecology
6. White Truffle Production and Harvesting in Natural Sites
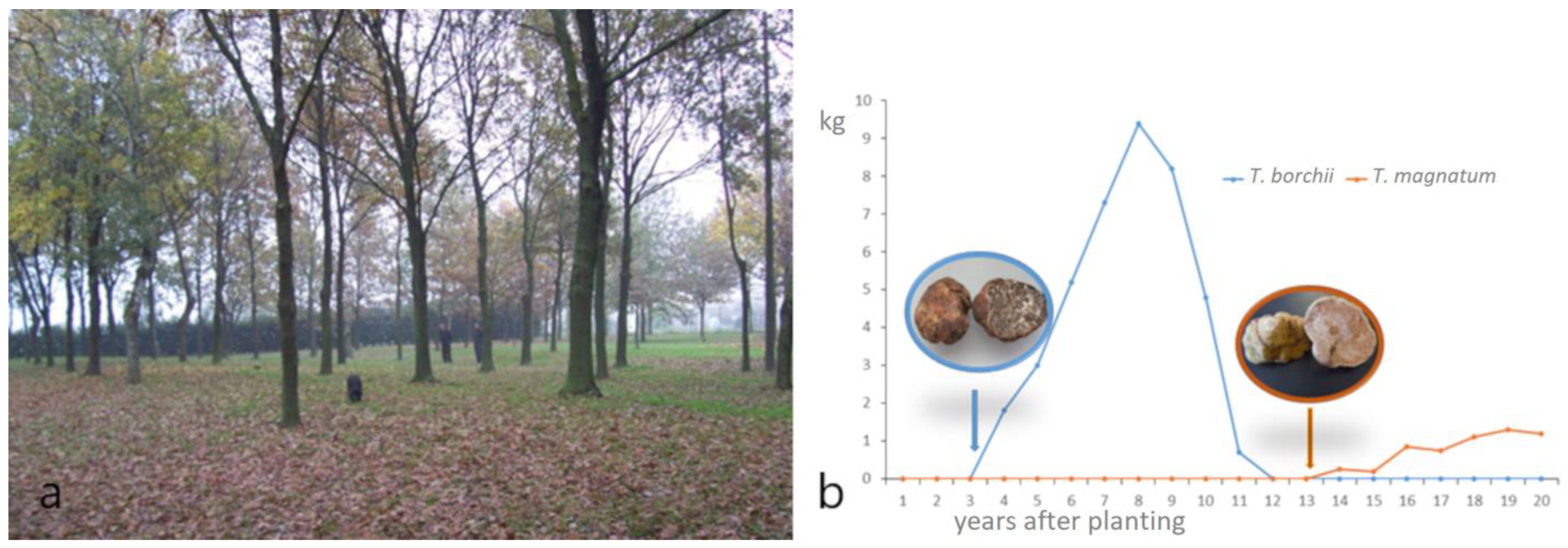
7. Cultivation
8. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mello, A.; Murat, C.; Bonfante, P. Truffles: Much More Than a Prized and Local Fungal Delicacy. FEMS Microbiol. Lett. 2006, 260, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bonito, G.; Smith, M.E.; Nowak, M.; Healy, R.A.; Guevara, G.; Cázares, E.; Kinoshita, A.; Nouhra, E.R.; Domínguez, L.S.; Tedersoo, L.; et al. Historical Biogeography and Diversification of Truffles in the Tuberaceae and Their Newly Identified Southern Hemisphere Sister Lineage. PLoS ONE 2013, 8, e52765. [Google Scholar] [CrossRef]
- Percudani, R.; Trevisi, A.; Zambonelli, A.; Ottonello, S. Molecular Phylogeny of Truffles (Pezizales: Terfeziaceae, Tuberaceae) Derived from Nuclear rDNA Sequence Analysis. Mol. Phylogenet. Evol. 1999, 13, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Jeandroz, S.; Murat, C.; Wang, Y.; Bonfante, P.; le Tacon, F. Molecular Phylogeny and Historical Biogeography of the Genus Tuber, the ‘True Truffles. J. Biogeogr. 2008, 35, 815–829. [Google Scholar] [CrossRef]
- Bonito, G.; Trappe, J.M.; Rawlinson, P.; Vilgalys, R. Improved Resolution of Major Clades within Tuber and Taxonomy of Species within the Tuber gibbosum Complex. Mycologia 2010, 102, 1042–1057. [Google Scholar] [CrossRef]
- The Most Expensive Food in The World: 13 Costly Food Items. Available online: https://luxurycolumnist.com/the-most-expensive-food-in-the-world/ (accessed on 20 September 2022).
- Leonardi, P.; Baroni, R.; Puliga, F.; Iotti, M.; Salerni, E.; Perini, C.; Zambonelli, A. Co-Occurrence of True Truffle Mycelia in Tuber magnatum Fruiting Sites. Mycorrhiza 2021, 31, 389–394. [Google Scholar] [CrossRef]
- Heinonen, I. The Soil Bacteria Associated with Tuber magnatum Productive Sites. Master’s Thesis, University of Helsinki, Helsinki, Finland, 2021. [Google Scholar]
- Urbani, O.; (Truffleland s.r.l, S. Anatolia di Narco, Perugia, Italy). Personal communication, 2022.
- Amicucci, A.; Barbieri, E.; Sparvoli, V.; Gioacchini, A.M.; Calcabrini, C.; Palma, F.; Stocchi, V.; Zambonelli, A. Microbial and Pigment Profile of the Reddish Patch Occurring within Tuber magnatum Ascomata. Fungal Biol 2018, 122, 1134–1141. [Google Scholar] [CrossRef]
- Ratti, C.; Iotti, M.; Zambonelli, A.; Terlizzi, F. Mycoviruses Infecting True Truffles. In True Truffle (Tuber spp.) in the World, 1st ed.; Zambonelli, A., Iotti, M., Murat, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 47, pp. 333–349. [Google Scholar] [CrossRef]
- Monaco, P.; Naclerio, G.; Bucci, A.; Mello, A. Determination of the Peridium Thickness of Tuber magnatum Ascomata from Molise Region. Ital. J. Mycol. 2021, 50, 92–98. [Google Scholar] [CrossRef]
- Hall, I.R.; Brown, G.T.; Zambonelli, A. Taming the Truffle. The History Lore and Science of the Ultimate Mushroom, 1st ed.; Timber Press: Portland, OR, USA, 2007; p. 304. [Google Scholar]
- Fontana, A. Miceli Di Funghi Ipogei in Coltura Pura. In Proceedings of the 1st International Truffle Conference, Spoleto, Italy, 24–25 May 1968; pp. 127–134. [Google Scholar]
- Mischiati, P.; Fontana, A. In Vitro Culture of Tuber magnatum Mycelium Isolated from Mycorrhizas. Mycol. Res. 1993, 97, 40–44. [Google Scholar] [CrossRef]
- Mello, A.; Fontana, A.; Meotto, F.; Comandini, O.; Bonfante, P. Molecular and Morphological Characterization of Tuber magnatum Mycorrhizas in a Long-Term Survey. Microbiol. Res. 2001, 155, 279–284. [Google Scholar] [CrossRef]
- Zambonelli, A.; (University of Bologna, Bologna, Italy). Personal communication, 2022.
- Iotti, M.; Amicucci, A.; Stocchi, V.; Zambonelli, A. Morphological and Molecular Characterization of Mycelia of some Tuber Species in Pure Culture. New Phytol. 2002, 155, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Rubini, A.; Paolocci, F.; Granetti, B.; Arcioni, S. Morphological Characterization of Molecular-Typed Tuber magnatum Ectomycorrhizae. Mycorrhiza 2001, 11, 179–185. [Google Scholar] [CrossRef]
- Ori, F.; Menotta, M.; Leonardi, M.; Amicucci, A.; Zambonelli, A.; Covès, H.; Selosse, M.A.; Schneider-Maunoury, L.; Pacioni, G.; Iotti, M. Effect of Slug Mycophagy on Tuber aestivum Spores. Fungal Biol. 2021, 125, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.F.; Truong, C.; Jackson, S.; Zúñiga, C.L.; Trappe, J.M.; Vernes, K. Mammalian Mycophagy: A Global Review of Ecosystem Interactions between Mammals and Fungi. FUSE 2022, 9, 99–159. [Google Scholar] [CrossRef]
- Schmidberger, P.C.; Schieberle, P. Characterization of the Key Aroma Compounds in White Alba Truffle (Tuber magnatum Pico) and Burgundy Truffle (Tuber uncinatum) by Means of the Sensomics Approach. J. Agric. Food Chem. 2017, 65, 9287–9296. [Google Scholar] [CrossRef]
- Niimi, J.; Deveau, A.; Splivallo, R. Geographical-Based Variations in White Truffle Tuber magnatum Aroma Is Explained by Quantitative Differences in Key Volatile Compounds. New Phytol. 2021, 230, 1623–1638. [Google Scholar] [CrossRef]
- Splivallo, R.; Ottonello, S.; Mello, A.; Karlovsky, P. Truffle Volatiles: From Chemical Ecology to Aroma Biosynthesis. New Phytol. 2011, 189, 688–699. [Google Scholar] [CrossRef]
- Wernig, F.; Buegger, F.; Pritsch, K.; Splivallo, R. Composition and Authentication of Commercial and Home-Made White Truffle-Flavored Oils. Food Control. 2018, 87, 9–16. [Google Scholar] [CrossRef]
- Phong, W.N.; Gibberd, M.R.; Payne, A.D.; Dykes, G.A.; Coorey, R. Methods Used for Extraction of Plant Volatiles Have Potential to Preserve Truffle Aroma: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1677–1701. [Google Scholar] [CrossRef]
- Gioacchini, A.M.; Menotta, M.; Guescini, M.; Saltarelli, R.; Ceccaroli, P.; Amicucci, A.; Barbieri, E.; Giomaro, G.; Stocchi, V. Geographical Traceability of Italian White Truffle (Tuber magnatum Pico) by the Analysis of Volatile Organic Compounds. Rapid Commun. Mass Spectrom. 2008, 22, 3147–3153. [Google Scholar] [CrossRef]
- Strojnik, L.; Grebenc, T.; Ogrinc, N. Species and Geographic Variability in Truffle Aromas. Food Chem. Toxicol. 2020, 142, 111434. [Google Scholar] [CrossRef] [PubMed]
- Vita, F.; Giuntoli, B.; Bertolini, E.; Taiti, C.; Marone, E.; D’Ambrosio, C.; Trovato, E.; Sciarrone, D.; Zoccali, M.; Balestrini, R.; et al. Tuberomics: A Molecular Profiling for the Adaption of Edible Fungi (Tuber magnatum Pico) to Different Natural Environments. BMC Genom. 2020, 21, 90. [Google Scholar] [CrossRef]
- Buzzini, P.; Gasparetti, C.; Turchetti, B.; Cramarossa, M.R.; Vaughan-Martini, A.; Martini, A.; Pagnoni, U.M.; Forti, L. Production of Volatile Organic Compounds (VOCs) by Yeasts Isolated from the Ascocarps of Black (Tuber melanosporum Vitt.) and White (Tuber magnatum Pico) Truffles. Arch. Microbiol. 2005, 184, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Vahdatzadeh, M.; Deveau, A.; Splivallo, R. The Role of the Microbiome of Truffles in Aroma Formation: A Meta-Analysis Approach. Appl. Environ. Microbiol. 2015, 81, 6946–6952. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Deveau, A.; Valdez, N.; Kirchhoff, N.; Frey-Klett, P.; Karlovsky, P. Bacteria Associated with Truffle-Fruiting Bodies Contribute to Truffle Aroma. Environ. Microbiol. 2015, 17, 2647–2660. [Google Scholar] [CrossRef]
- Niimi, J.; Deveau, A.; Splivallo, R. Aroma and Bacterial Communities Dramatically Change with Storage of Fresh White Truffle Tuber magnatum. LWT 2021, 151, 112125. [Google Scholar] [CrossRef]
- Pennazza, G.; Fanali, C.; Santonico, M.; Dugo, L.; Cucchiarini, L.; Dachà, M.; D’Amico, A.; Costa, R.; Dugo, P.; Mondello, L. Electronic Nose and GC–MS Analysis of Volatile Compounds in Tuber magnatum Pico: Evaluation of Different Storage Conditions. Food Chem. 2013, 136, 668–674. [Google Scholar] [CrossRef]
- Payen, T.; Murat, C.; Bonito, G. Truffle Phylogenomics: New Insights into Truffle Evolution and Truffle Life Cycle. Adv. Bot. Res. 2014, 70, 211–234. [Google Scholar] [CrossRef]
- Belfiori, B.; Riccioni, C.; Paolocci, F.; Rubini, A. Characterization of the Reproductive Mode and Life Cycle of the Whitish Truffle T. borchii. Mycorrhiza 2016, 26, 515–527. [Google Scholar] [CrossRef]
- Selosse, M.A.; Schneider-Maunoury, L.; Taschen, E.; Rousset, F.; Richard, F. Black Truffle, a Hermaphrodite with Forced Unisexual Behaviour. Trends Microbiol. 2017, 25, 784–787. [Google Scholar] [CrossRef]
- Leonardi, P.; Murat, C.; Puliga, F.; Iotti, M.; Zambonelli, A. Ascoma Genotyping and Mating Type Analyses of Mycorrhizas and Soil Mycelia of Tuber borchii in a Truffle Orchard Established by Mycelial Inoculated Plants. Environ. Microbiol. 2020, 22, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Frizzi, G.; Lalli, G.; Miranda, M.; Pacioni, G. Intraspecific Isozyme Variability in Italian Populations of the White Truffle Tuber magnatum. Mycol. Res. 2001, 105, 365–369. [Google Scholar] [CrossRef]
- Mello, A.; Murat, C.; Vizzini, A.; Gavazza, V.; Bonfante, P. Tuber magnatum Pico, a Species of Limited Geographical Distribution: Its Genetic Diversity inside and Outside a Truffle Ground. Environ. Microbiol. 2005, 7, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Rubini, A.; Paolocci, F.; Riccioni, C.; Vendramin, G.G.; Arcioni, S. Genetic and Phylogeographic Structures of the Symbiotic Fungus Tuber magnatum. Appl. Environ. Microbiol. 2005, 71, 6584–6589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paolocci, F.; Rubini, A.; Riccioni, C.; Arcioni, S. Reevaluation of the Life Cycle of Tuber magnatum. Appl. Environ. Microbiol. 2006, 72, 2390–2393. [Google Scholar] [CrossRef] [PubMed]
- Murat, C.; Payen, T.; Noel, B.; Kuo, A.; Morin, E.; Chen, J.; Kohler, A.; Krizsán, K.; Balestrini, R.; da Silva, C.; et al. Pezizomycetes Genomes Reveal the Molecular Basis of Ectomycorrhizal Truffle Lifestyle. Nat. Ecol. Evol. 2018, 2, 1956–1965. [Google Scholar] [CrossRef]
- Rubini, A.; Belfiori, B.; Riccioni, C.; Arcioni, S.; Martin, F.; Paolocci, F. Tuber melanosporum: Mating Type Distribution in a Natural Plantation and Dynamics of Strains of Different Mating Types on the Roots of Nursery-Inoculated Host Plants. New Phytol. 2011, 189, 723–735. [Google Scholar] [CrossRef]
- Murat, C.; Rubini, A.; Riccioni, C.; de la Varga, H.; Akroume, E.; Belfiori, B.; Guaragno, M.; le Tacon, F.; Robin, C.; Halkett, F.; et al. Fine-Scale Spatial Genetic Structure of the Black Truffle (Tuber melanosporum) Investigated with Neutral Microsatellites and Functional Mating Type Genes. New Phytol. 2013, 199, 176–187. [Google Scholar] [CrossRef]
- Urban, A.; Neuner-Plattner, I.; Krisai-Greilhuber, I.; Haselwandter, K. Molecular Studies on Terricolous Microfungi Reveal Novel Anamorphs of Two Tuber Species. Mycol. Res. 2004, 108, 749–758. [Google Scholar] [CrossRef]
- Healy, R.A.; Smith, M.E.; Bonito, G.M.; Pfister, D.H.; Ge, Z.W.; Guevara, G.G.; Williams, G.; Stafford, K.; Kumar, L.; Lee, T.; et al. High Diversity and Widespread Occurrence of Mitotic Spore Mats in Ectomycorrhizal Pezizales. Mol. Ecol. 2013, 22, 1717–1732. [Google Scholar] [CrossRef]
- Hall, I.R.; (Truffles & Mushrooms (Consulting) Limited, Dunedin, New Zealand). Personal communication, 2022.
- Nakano, S.; Obase, K.; Nakamura, N.; Kinoshita, A.; Kuroda, K.; Yamanaka, T. Mitospore Formation on Pure Cultures of Tuber japonicum (Tuberaceae, Pezizales) in Vitro. Mycorrhiza 2022, 32, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Taschen, E.; Rousset, F.; Sauve, M.; Benoit, L.; Dubois, M.P.; Richard, F.; Selosse, M.A. How the Truffle Got Its Mate: Insights from Genetic Structure in Spontaneous and Planted Mediterranean Populations of Tuber melanosporum. Mol. Ecol. 2016, 25, 5611–5627. [Google Scholar] [CrossRef] [PubMed]
- de la Varga, H.; le Tacon, F.; Lagoguet, M.; Todesco, F.; Varga, T.; Miquel, I.; Barry-Etienne, D.; Robin, C.; Halkett, F.; Martin, F.; et al. Five Years Investigation of Female and Male Genotypes in Périgord Black Truffle (Tuber melanosporum Vittad.) Revealed Contrasted Reproduction Strategies. Environ. Microbiol 2017, 19, 2604–2615. [Google Scholar] [CrossRef] [PubMed]
- Staubli, F.; Imola, L.; Dauphin, B.; Molinier, V.; Pfister, S.; Piñuela, Y.; Schürz, L.; Sproll, L.; Steidinger, B.S.; Stobbe, U.; et al. Hidden Fairy Rings and Males—Genetic Patterns of Natural Burgundy Truffle (Tuber aestivum Vittad.) Populations Reveal New Insights into Its Life Cycle. Environ. Microbiol 2022, 1, 1–16. [Google Scholar] [CrossRef]
- le Tacon, F.; Rubini, A.; Murat, C.; Riccioni, C.; Robin, C.; Belfiori, B.; Zeller, B.; de la Varga, H.; Akroume, E.; Deveau, A.; et al. Certainties and Uncertainties about the Life Cycle of the Périgord Black Truffle (Tuber melanosporum Vittad.). Ann. For. Sci. 2016, 73, 105–117. [Google Scholar] [CrossRef]
- Schneider-Maunoury, L.; Deveau, A.; Moreno, M.; Todesco, F.; Belmondo, S.; Murat, C.; Courty, P.E.; Jąkalski, M.; Selosse, M.A. Two Ectomycorrhizal Truffles, Tuber melanosporum and T. aestivum, Endophytically Colonise Roots of Non-Ectomycorrhizal Plants in Natural Environments. New Phytol. 2020, 225, 2542–2556. [Google Scholar] [CrossRef]
- Büntgen, U.; Lendorff, H.; Lendorff, A.; Leuchtmann, A.; Peter, M.; Bagi, I.; Egli, S. Truffles on the Move. Front. Ecol. Environ. 2019, 17, 200–202. [Google Scholar] [CrossRef]
- Bratek, Z.; Gógán, A.; Halász, K.; Bagi, I.; Erdei, V.; Bujàki, G. The Northernmost Habitats of Tuber magnatum Known from Hungary. In Proceedings of the 1st Hypogean Mushroom Conference, Rabat, Malta, 6–8 April 2004; pp. 6–8. [Google Scholar]
- Suwannarach, N.; Kumla, J.; Meerak, J.; Lumyong, S. Tuber magnatum in Thailand, a First Report from Asia. Mycotaxon 2017, 132, 635–642. [Google Scholar] [CrossRef]
- Gregori, G.L. Problems and Expectations with the Cultivation of Tuber magnatum. In Proceedings of the 2nd International Conference on Edible Mycorrhizal Mushrooms, Cristchurch, New Zealand, 3–6 July 2001; pp. 1–9. [Google Scholar]
- Marjanović, Z.; Glišić, A.; Mutavdžić, D.; Saljnikov, E.; Bragato, G. Ecosystems Supporting Tuber magnatum Pico Production in Serbia Experience Specific Soil Environment Seasonality That May Facilitate Truffle Lifecycle Completion. Appl. Soil Ecol. 2015, 95, 179–190. [Google Scholar] [CrossRef]
- Iotti, M.; Leonardi, P.; Vitali, G.; Zambonelli, A. Effect of Summer Soil Moisture and Temperature on the Vertical Distribution of Tuber magnatum Mycelium in Soil. Biol. Fertil. Soils 2018, 54, 707–716. [Google Scholar] [CrossRef]
- Bragato, G.; Marjanović, Ž.S. Soil Characteristics for Tuber magnatum. In True Truffle (Tuber spp.) in the World, 1st ed.; Zambonelli, A., Iotti, M., Murat, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 47, pp. 191–209. [Google Scholar] [CrossRef]
- Bencivenga, M.; Granetti, B. Flora, Vegetazione e Natura Dei Terreni Di Alcune Tartufaie Naturali Di Tuber magnatum Pico Dell’Italia Centrale. In Proceedings of the 2nd International Truffle Conference, Spoleto, Italy, 24–27 November 1988; pp. 415–431. [Google Scholar]
- Julou, T.; Burghardt, B.; Gebauer, G.; Berveiller, D.; Damesin, C.; Selosse, M.A. Mixotrophy in Orchids: Insights from a Comparative Study of Green Individuals and Nonphotosynthetic Individuals of Cephalanthera damasonium. New Phytol. 2005, 166, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Ouanphanivanh, N.; Merényi, Z.; Orczán, K.; Bratek, Z.; Szigeti, Z.; Illyés, Z. Could Orchids Indicate Truffle Habitats?: Mycorrhizal Association between Orchids and Truffles. Acta Biol. Szeged. 2008, 52, 229–232. Available online: https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2630 (accessed on 22 September 2022).
- Illyés, Z.; Ouanphanivanh, N.; Rudnóy, S.; Orczán, Á.K.; Bratek, Z. The Most Recent Results on Orchid Mycorrhizal Fungi in Hungary. Acta Biol. Hung. 2011, 61, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Teššitelová, T.; Teššitel, J.; Jersáková, J.; Říhová, G.; Selosse, M.A. Symbiotic Germination Capability of Four Epipactis Species (Orchidaceae) Is Broader than Expected from Adult Ecology. Am. J. Bot. 2012, 99, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, S.; Leonardi, P.; Zambonelli, A. Symbiotic Interations between Orchids and Tuber borchii. Ital. J. Mycol. 2022, 51, 58–65. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Yu, F. Non-Host Plants: Are They Mycorrhizal Networks Players? Plant. Divers. 2022, 44, 127–134. [Google Scholar] [CrossRef]
- Murat, C.; Vizzini, A.; Bonfante, P.; Mello, A. Morphological and Molecular Typing of the Below-Ground Fungal Community in a Natural Tuber magnatum Truffle-Ground. FEMS Microbiol. Lett. 2005, 245, 307–313. [Google Scholar] [CrossRef]
- Leonardi, M.; Iotti, M.; Oddis, M.; Lalli, G.; Pacioni, G.; Leonardi, P.; Maccherini, S.; Perini, C.; Salerni, E.; Zambonelli, A. Assessment of Ectomycorrhizal Fungal Communities in the Natural Habitats of Tuber magnatum (Ascomycota, Pezizales). Mycorrhiza 2013, 23, 349–358. [Google Scholar] [CrossRef]
- Iotti, M.; Leonardi, M.; Oddis, M.; Salerni, E.; Baraldi, E.; Zambonelli, A. Development and Validation of a Real-Time PCR Assay for Detection and Quantification of Tuber magnatum in Soil. BMC Microbiol 2012, 12, 93. [Google Scholar] [CrossRef]
- Zampieri, E.; Murat, C.; Cagnasso, M.; Bonfante, P.; Mello, A. Soil Analysis Reveals the Presence of an Extended Mycelial Network in a Tuber magnatum Truffle-ground. FEMS Microbiol. Ecol. 2010, 71, 43–49. [Google Scholar] [CrossRef]
- Ceruti, A.; Fontana, A.; Nosenzo, C. European Species of the Genus Tuber. An. Historical Revision., 1st ed.; Museo Regionale di Scienze Naturali: Torino, Italy, 2003; p. 467. [Google Scholar]
- Le Tacon, F. Influence of Climate on Natural Distribution of Species and Truffle Production. In True Truffle (Tuber spp.) in the World, 1st ed.; Zambonelli, A., Iotti, M., Murat, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 47, pp. 153–167. [Google Scholar] [CrossRef]
- Iotti, M.; Leonardi, M.; Lancellotti, E.; Salerni, E.; Oddis, M.; Leonardi, P.; Perini, C.; Pacioni, G.; Zambonelli, A. Spatio-Temporal Dynamic of Tuber magnatum Mycelium in Natural Truffle Grounds. PLoS ONE 2014, 9, e115921. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Björk, R.G.; Epron, D.; Kieliszewska-Rokicka, B.; Kjøller, R.; et al. The Production and Turnover of Extramatrical Mycelium of Ectomycorrhizal Fungi in Forest Soils: Role in Carbon Cycling. Plant Soil 2013, 366, 1–27. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The Mycorrhiza Helper Bacteria Revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Monaco, P.; Naclerio, G.; Mello, A.; Bucci, A. Role and Potentialities of Bacteria Associated with Tuber magnatum: A Mini-Review. Front. Microbiol. 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Citterio, B.; Cardoni, P.; Potenza, L.; Amicucci, A.; Stocchi, V.; Gola, G.; Nuti, M. Isolation of Bacteria from Sporocarps of Tuber magnatum Pico, Tuber borchii Vitt. and Tuber maculatum Vitt. In Biotechnology of Ectomycorrhizae; Springer: Berlin/Heidelberg, Germany, 1995; pp. 241–248. [Google Scholar] [CrossRef]
- Barbieri, E.; Guidi, C.; Bertaux, J.; Frey-Klett, P.; Garbaye, J.; Ceccaroli, P.; Saltarelli, R.; Zambonelli, A.; Stocchi, V. Occur-rence and Diversity of Bacterial Communities in Tuber magnatum during Truffle Maturation. Environ. Microbiol. 2007, 9, 2234–2246. [Google Scholar] [CrossRef]
- Barbieri, E.; Ceccaroli, P.; Saltarelli, R.; Guidi, C.; Potenza, L.; Basaglia, M.; Fontana, F.; Baldan, E.; Casella, S.; Ryahi, O.; et al. New Evidence for Nitrogen Fixation within the Italian White Truffle Tuber magnatum. Fungal Biol. 2010, 114, 936–942. [Google Scholar] [CrossRef]
- Monaco, P.; Bucci, A.; Naclerio, G.; Mello, A. Heterogeneity of the White Truffle Tuber magnatum in a Limited Geographic Area of Central-Southern Italy. Environ. Microbiol. Rep. 2021, 13, 591–599. [Google Scholar] [CrossRef]
- Il Tartufo: Una Simbiosi Multipla. Available online: https://www.georgofili.it/ (accessed on 21 September 2022).
- Barbieri, E.; Ceccaroli, P.; Palma, F.; Agostini, D.; Stocchi, V. Ectomycorrhizal Helper Bacteria: The Third Partner in the Symbiosis. In Edible Ectomycorrhizal Mushrooms, 1st ed.; Zambonelli, A., Bonito, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 34, pp. 125–141. [Google Scholar] [CrossRef]
- Antony-Babu, S.; Deveau, A.; van Nostrand, J.D.; Zhou, J.; le Tacon, F.; Robin, C.; Frey-Klett, P.; Uroz, S. Black Truffle-Associated Bacterial Communities during the Development and Maturation of Tuber melanosporum Ascocarps and Putative Functional Roles. Environ. Microbiol. 2014, 16, 2831–2847. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Bonito, G.M. The Truffle Microbiome: Species and Geography Effects on Bacteria Associated with Fruiting Bodies of Hypogeous Pezizales. Microb. Ecol. 2016, 72, 4–8. [Google Scholar] [CrossRef]
- Sillo, F.; Vergine, M.; Luvisi, A.; Calvo, A.; Petruzzelli, G.; Balestrini, R.; Mancuso, S.; de Bellis, L.; Vita, F. Bacterial Communities in the Fruiting Bodies and Background Soils of the White Truffle Tuber magnatum. Front. Microbiol. 2022, 13, 1288. [Google Scholar] [CrossRef]
- Pavić, A.; Stanković, S.; Saljnikov, E.; Krüger, D.; Buscot, F.; Tarkka, M.; Marjanović, Ž. Actinobacteria May Influence White Truffle (Tuber magnatum Pico) Nutrition, Ascocarp Degradation and Interactions with Other Soil Fungi. Fungal Ecol. 2013, 6, 527–538. [Google Scholar] [CrossRef]
- Giorgio, M.; Niccolò, B.G.M.; Benedetta, T.; Luisa, M.; Leonardo, B.F.; Gregory, B.; Pietro, B.; Alberto, A.; Domizia, D.; Emidio, A. Fungal and Bacterial Diversity in the Tuber magnatum Ecosystem and Microbiome. Microb. Ecol. 2022, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lalli, G.; Leonardi, M.; Oddis, M.; Pacioni, G.; Salerni, E.; Iotti, M.; Zambonelli, A. Expanding the Understanding of a Forest Ectomycorrhizal Community by Combining Root Tips and Fruiting Bodies: A Case Study of Tuber magnatum Stands. Turk. J. Botany 2015, 39, 527–534. [Google Scholar] [CrossRef]
- Mello, A.; Miozzi, L.; Vizzini, A.; Napoli, C.; Kowalchuk, G.; Bonfante, P. Bacterial and Fungal Communities Associated with Tuber magnatum-productive Niches. Plant. Biosyst. 2010, 144, 323–332. [Google Scholar] [CrossRef]
- Zampieri, E.; Mello, A.; Bonfante, P.; Murat, C. PCR Primers Specific for the Genus Tuber reveal the Presence of Several Truffle Species in a Truffle-Ground. FEMS Microbiol. Lett. 2009, 297, 67–72. [Google Scholar] [CrossRef]
- Truffle Hunting and Extraction in Italy, Traditional Knowledge and Practice-Intangible Heritage-Culture Sector–UNESCO. Available online: https://ich.unesco.org/en/RL/truffle-hunting-and-extraction-in-italy-traditional-knowledge-and-practice-01395 (accessed on 22 September 2022).
- Mipaaf-Tavolo Tecnico Settore Tartufo. Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/11100 (accessed on 22 September 2022).
- Truffle Turism. I Sapori Della Collina Di Torino. Available online: https://isaporidellacollinaditorino.com/en/truffle-turism-2/ (accessed on 22 September 2022).
- Águeda, B.; Zambonelli, A.; Molina, R. Tuber 2013: Scientific Advances in Sustainable Truffle Culture. Mycorrhiza 2014, 24, 1–4. [Google Scholar] [CrossRef]
- Salerni, E.; Iotti, M.; Leonardi, P.; Gardin, L.; D’Aguanno, M.; Perini, C.; Pacioni, P.; Zambonelli, A. Effects of Soil Tillage on Tuber magnatum Development in Natural Truffières. Mycorrhiza 2014, 24, 79–87. [Google Scholar] [CrossRef]
- Laruccia, N.; Marletto, V.; Leonardi, P.; Puliga, F.; Zambonelli, A. Map of Suitability for the Spontaneous Growth of Tuber magnatum in Emilia-Romagna (Italy). Ital. J. Mycol. 2020, 49, 38–53. [Google Scholar] [CrossRef]
- Reyna, S.; Garcia-Barreda, S. Black Truffle Cultivation: A Global Reality. For. Syst. 2014, 23, 317–328. [Google Scholar] [CrossRef]
- Giovannetti, G. Prima Produzione Di Carpofori Di Tuber magnatum Pico Da Piante Fornite Da Vivai Specializzati. In Proceedings of the 2nd International Truffle Conference, Spoleto, Italy, 24–27 November 1988; pp. 297–302. [Google Scholar]
- Gregori, G.L.; Donnini, D.; Bencivenga, M. Tuber magnatum: Alcuni Esempi Produttivi Di Tartufaie Coltivate in Italia. In Proceedings of the 3rd International Truffle Conference, Spoleto, Italy, 25–28 November 2008; pp. 741–749. [Google Scholar]
- Bullini, L.; Biocca, E.; Chevalier, G.; Dupré, C.; Ferrara, A.M.; Palenzona, M.; Urbanelli, S. Use of Genetic Markers in the Study of Mycorrhizae and Mycelia of the Genus Tuber. In Proceedings of the International Symposium on Biotechnology of Ectomycorrhizae: Molecular approaches, Urbino, Italy, 9–11 November 1994; pp. 9–11. [Google Scholar]
- Iotti, M.; Piattoni, F.; Zambonelli, A. Techniques for Host Plant Inoculation with Truffles and Other Edible Ectomycorrhizal Mushrooms. In Edible Ectomycorrhizal Mushrooms, 1st ed.; Zambonelli, A., Bonito, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–161. [Google Scholar] [CrossRef]
- Donnini, D.; Bencivenga, M. Micorrize Inquinanti Frequenti Nelle Piante Tartufigene. Micol. Ital. 1995, 2, 185–207. [Google Scholar]
- Amicucci, A.; Zambonelli, A.; Guidi, C.; Stocchi, V. Morphological and Molecular Characterisation of Pulvinula constellatio Ectomycorrhizae. FEMS Microbiol. Lett. 2001, 194, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Donnini, D.; di Massimo, G.; Baciarelli Falini, L.; Bencivenga, M. Le (ECM) Delle Tartufaie Naturali e Coltivate in Umbria. In Proceedings of the 100th Congress of Italian Botany Society, Rome, Italy, 20–23 September 2005; pp. 846–847. [Google Scholar]
- Donnini, D.; Bencivenga, M.; Baciarelli Falini, L. Risultati Di Esperienze Pluriennali Nella Coltivazione Di Tuber magnatum Pico in Umbria. Micol. Ital. 2000, 29, 33–39. [Google Scholar]
- Previati, A.; (Ferrara, Italy). Personal communication, 2022.
- Zambonelli, A.; Lotti, M.; Giomaro, G.; Hall, I.; Stocchi, V.T. borchii Cultivation: An Interesting Perspective. In Proceedings of the 2nd International Conference on Edible Mycorrhizal Mushrooms, Crop & Food Research, Christcurch, New Zealand, 3–6 July 2002; pp. 1–7. [Google Scholar]
- Fleming, N. Fungal Findings Excite Truffle Researchers and Gastronomes. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Amicucci, A.; Zambonelli, A.; Giomaro, G.; Potenza, L. Identification of Ectomycorrhizal Fungi of the Genus Tuber by Species-specific ITS Primers. Mol. Ecol. 1998, 7, 273–277. [Google Scholar] [CrossRef]
- Bach, C.; Beacco, P.; Cammaletti, P.; Babel-Chen, Z.; Levesque, E.; Todesco, F.; Cotton, C.; Robin, B.; Murat, C. First Production of Italian White Truffle (Tuber magnatum Pico) Ascocarps in an Orchard Outside Its Natural Range Distribution in France. Mycorrhiza 2021, 31, 383–388. [Google Scholar] [CrossRef]
- Olivier, J.M.; Savignac, J.C.; Sourzat, P. Truffe et Trufficulture, 3rd ed.; Fanlac: Perigueux, France, 2018; p. 352. [Google Scholar]
- Todesco, F.; Belmondo, S.; Guignet, Y.; Laurent, L.; Fizzala, S.; le Tacon, F.; Murat, C. Soil Temperature and Hydric Potential Influences the Monthly Variations of Soil Tuber aestivum DNA in a Highly Productive Orchard. Sci. Rep. 2019, 9, 12964. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziosi, S.; Hall, I.R.; Zambonelli, A. The Mysteries of the White Truffle: Its Biology, Ecology and Cultivation. Encyclopedia 2022, 2, 1959-1971. https://doi.org/10.3390/encyclopedia2040135
Graziosi S, Hall IR, Zambonelli A. The Mysteries of the White Truffle: Its Biology, Ecology and Cultivation. Encyclopedia. 2022; 2(4):1959-1971. https://doi.org/10.3390/encyclopedia2040135
Chicago/Turabian StyleGraziosi, Simone, Ian Robert Hall, and Alessandra Zambonelli. 2022. "The Mysteries of the White Truffle: Its Biology, Ecology and Cultivation" Encyclopedia 2, no. 4: 1959-1971. https://doi.org/10.3390/encyclopedia2040135
APA StyleGraziosi, S., Hall, I. R., & Zambonelli, A. (2022). The Mysteries of the White Truffle: Its Biology, Ecology and Cultivation. Encyclopedia, 2(4), 1959-1971. https://doi.org/10.3390/encyclopedia2040135









