The Applications of Microphysiological Systems in Biomedicine: Impact on Urologic and Orthopaedic Research
Definition
:1. Introduction
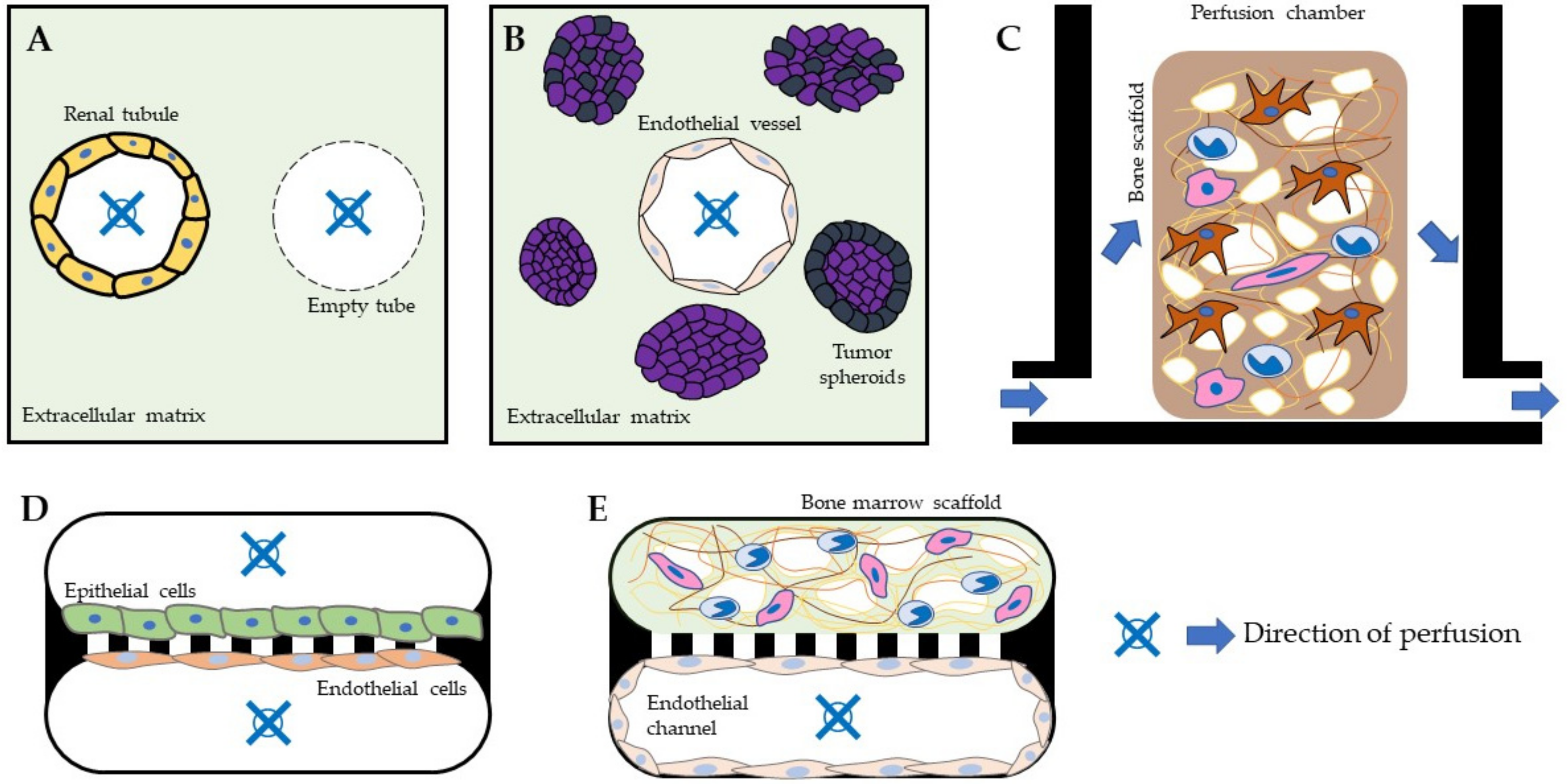
2. Microphysiological Systems and Their Applications
2.1. Perspective on MPS Applications in Drug Development
2.2. Perspective on MPS Applications in Biomedical Research
3. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Arora, M. Cell Culture Media: A Review. Mater. Methods 2013, 3, 175. [Google Scholar] [CrossRef]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.R. HeLa cells 50 years on: The good, the bad and the ugly. Nat. Rev. Cancer 2002, 2, 315–319. [Google Scholar] [CrossRef]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell Dev. Biol. 2021, 9, 1869. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.L.; Marshall, R.N.; Edwards, S.J.; Lord, J.M.; Lavery, G.G.; Breen, L. The effect of young and old ex vivo human serum on cellular protein synthesis and growth in an in vitro model of aging. Am. J. Physiol. Cell Physiol. 2021, 321, C26–C37. [Google Scholar] [CrossRef]
- Verhulst, A.; Sayer, R.; De Broe, M.E.; D’Haese, P.C.; Brown, C.D.A. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol. Pharmacol. 2008, 74, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, S.E.; Chung, G.W.; van Loon, E.; Bakar, N.S.; Dalzell, A.M.; Brown, C.D.A. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflug. Arch. 2012, 464, 601–611. [Google Scholar] [CrossRef]
- Jeske, R.; Yuan, X.; Fu, Q.; Bunnell, B.A.; Logan, T.M.; Li, Y. In Vitro Culture Expansion Shifts the Immune Phenotype of Human Adipose-Derived Mesenchymal Stem Cells. Front. Immunol. 2021, 12, 46. [Google Scholar] [CrossRef]
- Lorian, V.; Satta, G. Differences between in vitro and in vivo studies. Antimicrob. Agents Chemother. 1988, 32, 1600–1601. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Van Ness, K.P.; Chang, S.Y.; Weber, E.J.; Zumpano, D.; Eaton, D.L.; Kelly, E.J. Microphysiological systems to assess nonclinical toxicity. Curr. Protoc. Toxicol. 2017, 2017, 28. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Van Berlo, D.; van de Steeg, E.; Amirabadi, H.E.; Masereeuw, R. The potential of multi-organ-on-chip models for assessment of drug disposition as alternative to animal testing. Curr. Opin. Toxicol. 2021, 27, 8–17. [Google Scholar] [CrossRef]
- Ramsden, D. Leveraging microphysiological systems to address challenges encountered during development of oligonucleotide therapeutics. ALTEX Altern. Anim. Exp. 2021, 39, 273–296. [Google Scholar] [CrossRef]
- Ishida, S. Organs-on-a-chip: Current applications and consideration points for in vitro ADME-Tox studies. Drug Metab. Pharmacokinet. 2018, 33, 49–54. [Google Scholar] [CrossRef]
- Caetano-Pinto, P.; Janssen, M.J.; Gijzen, L.; Verscheijden, L.; Wilmer, M.J.G.; Masereeuw, R. Fluorescence-Based Transport Assays Revisited in a Human Renal Proximal Tubule Cell Line. Mol. Pharm. 2016, 13, 933–944. [Google Scholar] [CrossRef]
- Van Ness, K.P.; Kelly, E.J.; Cesar, F. Microphysiological systems in adsorption, distribution, metabolism and elimination sciences. Clin. Transl. Sci. 2022, 15, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J.; Chapron, A.; Chapron, B.D.; Voellinger, J.L.; Lidberg, K.A.; Yeung, C.K.; Wang, Z.; Yamaura, Y.; Hailey, D.W.; Neumann, T.; et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016, 90, 627–637. [Google Scholar] [CrossRef]
- Imaoka, T.; Huang, W.; Shum, S.; Hailey, D.W.; Chang, S.-Y.; Chapron, A.; Yeung, C.K.; Himmelfarb, J.; Isoherranen, N.; Kelly, E.J. Bridging the gap between in silico and in vivo by modeling opioid disposition in a kidney proximal tubule microphysiological system. Sci. Rep. 2021, 11, 21356. [Google Scholar] [CrossRef] [PubMed]
- Scotcher, D.; Jones, C.; Rostami-Hodjegan, A.; Galetin, A. Novel minimal physiologically-based model for the prediction of passive tubular reabsorption and renal excretion clearance. Eur. J. Pharm. Sci. 2016, 94, 59–71. [Google Scholar] [CrossRef]
- Van Ness, K.P.; Cesar, F.; Yeung, C.K.; Himmelfarb, J.; Kelly, E.J. Microphysiological Systems in ADME Sciences. Clin. Transl. Sci. 2021, 15, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and challenges in phenotypic drug discovery: An industry perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef]
- Valeur, E.; Guéret, S.M.; Adihou, H.; Gopalakrishnan, R.; Lemurell, M.; Waldmann, H.; Grossmann, T.N.; Plowright, A.T. New Modalities for Challenging Targets in Drug Discovery. Angew. Chemie. Int. Ed. 2017, 56, 10294–10323. [Google Scholar] [CrossRef]
- Isoherranen, N.; Madabushi, R.; Huang, S.M. Emerging Role of Organ-on-a-Chip Technologies in Quantitative Clinical Pharmacology Evaluation. Clin. Transl. Sci. 2019, 12, 113–121. [Google Scholar] [CrossRef]
- Pinto, P.C.; Rönnau, C.; Burchardt, M.; Wolff, I. Kidney Cancer and Chronic Kidney Disease: Too Close for Comfort. Biomedicines 2021, 9, 1761. [Google Scholar] [CrossRef]
- Chapron, A.; Chapron, B.D.; Hailey, D.W.; Chang, S.Y.; Imaoka, T.; Thummel, K.E.; Kelly, E.; Himmelfarb, J.; Shen, D.; Yeung, C.K. An Improved Vascularized, Dual-Channel Microphysiological System Facilitates Modeling of Proximal Tubular Solute Secretion. ACS Pharmacol. Transl. Sci. 2020, 3, 496–508. [Google Scholar] [CrossRef]
- Nieskens, T.T.G.; Magnusson, O.; Andersson, P.; Söderberg, M.; Persson, M.; Sjögren, A.K. Nephrotoxic antisense oligonucleotide SPC5001 induces kidney injury biomarkers in a proximal tubule-on-a-chip. Arch. Toxicol. 2021, 95, 2123–2136. [Google Scholar] [CrossRef]
- Miller, C.P.; Tsuchida, C.; Zheng, Y.; Himmelfarb, J.; Akilesh, S. A 3D Human Renal Cell Carcinoma-on-a-Chip for the Study of Tumor Angiogenesis. Neoplasia 2018, 20, 610–620. [Google Scholar] [CrossRef]
- Ramme, A.P.; Koenig, L.; Hasenberg, T.; Schwenk, C.; Magauer, C.; Faust, D.; Lorenz, A.K.; Krebs, A.C.; Drewell, C.; Schirrmann, K.; et al. Autologous induced pluripotent stem cell-derived four-organ-chip. Futur. Sci. OA 2019, 5, FSO413. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Zhou, X.; Geng, X.; Drewell, C.; Hübner, J.; Li, Z.; Zhang, Y.; Xue, M.; Marx, U.; Li, B. Repeated dose multi-drug testing using a microfluidic chip-based coculture of human liver and kidney proximal tubules equivalents. Sci. Rep. 2020, 10, 8879. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.P.; Brandmair, K.; Gerlach, S.; Przibilla, J.; Géniès, C.; Jacques-Jamin, C.; Schepky, A.; Marx, U.; Hewitt, N.J.; Maschmeyer, I.; et al. Demonstration of the first-pass metabolism in the skin of the hair dye, 4-amino-2-hydroxytoluene, using the Chip2 skin–liver microphysiological model. J. Appl. Toxicol. 2021, 41, 1553–1567. [Google Scholar] [CrossRef]
- Kühnl, J.; Tao, T.P.; Brandmair, K.; Gerlach, S.; Rings, T.; Müller-Vieira, U.; Przibilla, J.; Genies, C.; Jaques-Jamin, C.; Schepky, A.; et al. Characterization of application scenario-dependent pharmacokinetics and pharmacodynamic properties of permethrin and hyperforin in a dynamic skin and liver multi-organ-chip model. Toxicology 2021, 448, 152637. [Google Scholar] [CrossRef]
- Tavares, R.S.N.; Phuong-Tao, T.; Maschmeyer, I.; Maria-Engler, S.S.; Schäfer-Korting, M.; Winter, A.; Zoschke, C.; Lauster, R.; Marx, U.; Gaspar, L.R. Toxicity of topically applied drugs beyond skin irritation: Static skin model vs. Two organs-on-a-chip. Int. J. Pharm. 2020, 589, 119788. [Google Scholar] [CrossRef]
- Baert, Y.; Ruetschle, I.; Cools, W.; Oehme, A.; Lorenz, A.; Marx, U.; Goossens, E.; Maschmeyer, I. A multi-organ-chip co-culture of liver and testis equivalents: A first step toward a systemic male reprotoxicity model. Hum. Reprod. 2020, 35, 1029–1044. [Google Scholar] [CrossRef]
- Schimek, K.; Frentzel, S.; Luettich, K.; Bovard, D.; Rütschle, I.; Boden, L.; Rambo, F.; Erfurth, H.; Dehne, E.M.; Winter, A.; et al. Human multi-organ chip co-culture of bronchial lung culture and liver spheroids for substance exposure studies. Sci. Rep. 2020, 10, 7865. [Google Scholar] [CrossRef]
- Sieber, S.; Wirth, L.; Cavak, N.; Koenigsmark, M.; Marx, U.; Lauster, R.; Rosowski, M. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med. 2018, 12, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Schoon, J.; Hesse, B.; Rakow, A.; Ort, M.J.; Lagrange, A.; Jacobi, D.; Winter, A.; Huesker, K.; Reinke, S.; Cotte, M.; et al. Metal-Specific Biomaterial Accumulation in Human Peri-Implant Bone and Bone Marrow. Adv. Sci. 2020, 7, 2000412. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Saxena, Y.; Routh, S.; Mukhopadhaya, A. Immunoporosis: Role of Innate Immune Cells in Osteoporosis. Front. Immunol. 2021, 12, 3168. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef]
- Perino, G.; De Martino, I.; Zhang, L.; Xia, Z.; Gallo, J.; Natu, S.; Langton, D.; Huber, M.; Rakow, A.; Schoon, J.; et al. The contribution of the histopathological examination to the diagnosis of adverse local tissue reactions in arthroplasty. EFORT Open Rev. 2021, 6, 399–419. [Google Scholar] [CrossRef]
- Scheinpflug, J.; Pfeiffenberger, M.; Damerau, A.; Schwarz, F.; Textor, M.; Lang, A.; Schulze, F. Journey into bone models: A review. Genes 2018, 9, 247. [Google Scholar] [CrossRef]
- Matziolis, D. Osteogenic Predifferentiation of Human Bone Marrow-Derived Stem Cells by Short-Term Mechanical Stimulation. Open Orthop. J. 2011, 5, 1–6. [Google Scholar] [CrossRef]
- Matziolis, G.; Tuischer, J.; Kasper, G.; Thompson, M.; Bartmeyer, B.; Krocker, D.; Perka, C.; Duda, G. Simulation of cell differentiation in fracture healing: Mechanically loaded composite scaffolds in a novel bioreactor system. Tissue Eng. 2006, 12, 201–208. [Google Scholar] [CrossRef]
- Petersen, A.; Joly, P.; Bergmann, C.; Korus, G.; Duda, G.N. The impact of substrate stiffness and mechanical loading on fibroblast-induced scaffold remodeling. Tissue Eng. Part A 2012, 18, 1804–1817. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Feliciano, S.; Martin, I.; de Wild, M.; Wendt, D. Novel Perfused Compression Bioreactor System as an in vitro Model to Investigate Fracture Healing. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef]
- Richards, R.G.; Simpson, A.E.; Jaehn, K.; Furlong, P.I.; Stoddart, M.J. Establishing a 3D ex vivo culture system for investigations of bone metabolism and biomaterial interactions. ALTEX Altern. Tierexp. 2007, 24, 56–59. [Google Scholar]
- Kodzius, R.; Schulze, F.; Gao, X.; Schneider, M.R. Organ-on-chip technology: Current state and future developments. Genes 2017, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sim, W.Y.; Min, B.H.; Yang, S.S.; Khademhosseini, A.; Kaplan, D.L. Chip-Based Comparison of the Osteogenesis of Human Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stem Cells under Mechanical Stimulation. PLoS ONE 2012, 7, e46689. [Google Scholar] [CrossRef]
- Sun, Q.; Choudhary, S.; Mannion, C.; Kissin, Y.; Zilberberg, J.; Lee, W.Y. Ex vivo replication of phenotypic functions of osteocytes through biomimetic 3D bone tissue construction. Bone 2018, 106, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Nieskens, T.T.G.; Persson, M.; Kelly, E.J.; Sjögren, A.K. A multicompartment human kidney proximal tubule-on-a-chip replicates cell polarization–dependent cisplatin toxicity. Drug Metab. Dispos. 2020, 48, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Jiang, L.; Ivich, F.; Tahsin, S.; Tran, M.; Frank, S.B.; Miranti, C.K.; Zohar, Y. Human stroma and epithelium co-culture in a microfluidic model of a human prostate gland. Biomicrofluidics 2019, 13, 064116. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Dhar, N.; Thacker, V.V.; Simonet, T.M.; Signorino-Gelo, F.; Knott, G.; McKinney, J.D. Dynamic persistence of intracellular bacterial communities of uropathogenic escherichia coli in a human bladder-chip model of urinary tract infections. Elife 2021, 10, e66481. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lozito, T.P.; Alexander, P.G.; Gottardi, R.; Tuan, R.S. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1Β. Mol. Pharm. 2014, 11, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Iordachescu, A.; Hughes, E.A.B.; Joseph, S.; Hill, E.J.; Grover, L.M.; Metcalfe, A.D. Trabecular bone organoids: A micron-scale ‘humanised’ prototype designed to study the effects of microgravity and degeneration. npj Microgravity 2021, 7, 17. [Google Scholar] [CrossRef]
- Chou, D.B.; Frismantas, V.; Milton, Y.; David, R.; Pop-Damkov, P.; Ferguson, D.; MacDonald, A.; Vargel Bölükbaşı, Ö.; Joyce, C.E.; Moreira Teixeira, L.S.; et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 2020, 4, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Aung, A.; Varghese, S. Skeletal muscle-on-a-chip: An in vitro model to evaluate tissue formation and injury. Lab Chip 2017, 17, 3447–3461. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fernández-Garibay, X.; Castaño, A.G.; De Chiara, F.; Hernández-Albors, A.; Balaguer-Trias, J.; Ramón-Azcón, J. Muscle-on-a-chip with an on-site multiplexed biosensing system for: In situ monitoring of secreted IL-6 and TNF-α. Lab Chip 2019, 19, 2568–2580. [Google Scholar] [CrossRef]
- Peel, S.; Jackman, M. Imaging microphysiological systems: A review. Am. J. Physiol. Cell Physiol. 2021, 320, C669–C680. [Google Scholar] [CrossRef]
- Caetano-Pinto, P.; Stahl, S.H. Perspective on the application of microphysiological systems to drug transporter studies. Drug Metab. Dispos. 2018, 46, 1647–1657. [Google Scholar] [CrossRef]
- Ingber, D.E. Is it Time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies? Adv. Sci. 2020, 7, 2002030. [Google Scholar] [CrossRef]
| Organ/Tissue | Cell Type | MPS Model | Stimulus | Purpose | Ref |
|---|---|---|---|---|---|
| Kidney | Human renal epithelial proximal tubule cells | Nortis a ParVivo Dual-chip | Continuous flow | Improve renal drug secretion studies in vitro | [23,31,59] |
| Human renal epithelial proximal tubule cells | Emulate a Organ-Chip | Continuous flow | Improve nephrotoxicity evaluation in vitro | [60] | |
| Prostate | Human benign epithelial prostate cells | Bespoke b | Continuous flow | Evaluate prostate paracrine secretion in vitro | [61] |
| Kidney Cancer | Human renal cell carcinoma/endothelial cells | Nortis a ParVivo Single-chip | Continuous flow | Recapitulate kidney cancer derived angiogenesis in vitro | [33] |
| Bladder | Human bladder epithelial and endothelial cells/E. coli | Emulate a Organ-Chip | Continuous flow/cyclic compression | Recreate bladder infections conditions and recapitulate immune response in vitro | [62] |
| Bone | Human mesenchymal stromal cells/osteoblasts/bone marrow mononuclear cells | TissUse a Humimic-Chip 2 | Pulsating cyclic flow | Study heavy metal nanotoxicity derived from medical bone implants | [43] |
| Human bone marrow mononuclear cells | Bespoke b | Continuous flow | Recapitulate osteoarthritis in vitro | [63] | |
| Human bone marrow mononuclear cells | Bespoke b | Cyclic compression | Improve the study of the bone healing process in vitro | [54] | |
| Human osteoblasts/osteoclasts | NASA-Synthecon b | Rotating centrifugal force (artificial gravity) | Study bone loss in a microgravity environment | [64] | |
| Bone marrow | Human bone marrow mononuclear cells/hematopoietic stem cells | Emulate a | Continuous flow | Improve the study of hematopoietic defects | [65] |
| Skeletal muscle | Mouse myoblast cell line C2C12 | Bespoke b | Tension | Recreate muscle injury in vitro | [66] |
| Mouse myoblast cell line C2C12 | Bespoke b | Continuous flow | Improve muscle metabolic studies in vitro | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caetano-Pinto, P.; Schoon, J. The Applications of Microphysiological Systems in Biomedicine: Impact on Urologic and Orthopaedic Research. Encyclopedia 2022, 2, 1128-1137. https://doi.org/10.3390/encyclopedia2020075
Caetano-Pinto P, Schoon J. The Applications of Microphysiological Systems in Biomedicine: Impact on Urologic and Orthopaedic Research. Encyclopedia. 2022; 2(2):1128-1137. https://doi.org/10.3390/encyclopedia2020075
Chicago/Turabian StyleCaetano-Pinto, Pedro, and Janosch Schoon. 2022. "The Applications of Microphysiological Systems in Biomedicine: Impact on Urologic and Orthopaedic Research" Encyclopedia 2, no. 2: 1128-1137. https://doi.org/10.3390/encyclopedia2020075
APA StyleCaetano-Pinto, P., & Schoon, J. (2022). The Applications of Microphysiological Systems in Biomedicine: Impact on Urologic and Orthopaedic Research. Encyclopedia, 2(2), 1128-1137. https://doi.org/10.3390/encyclopedia2020075






