Abstract
About 250 million people affected, 779 million people at risk of infection, and 440 million people with residual morbidity are globally attributable to schistosomiasis. Highly sensitive and specific, simple, and fast to perform diagnostics are required for detecting trace infections, and applications in resource-poor settings and large-scale assessments. Research assessing isothermal diagnoses of S. japonicum, S. haematobium, S. mansoni, mixed infections, and schistosomal hybrids among clinical human specimens was investigated. Loop-mediated isothermal amplification (LAMP), recombinase polymerase amplification (RPA) and combined techniques were identified. Both LAMP and RPA reached species-dependent 100% sensitivity, and detection levels within femtogram and nanogram amounts for pure and hybridale breeds. Cross-reactivity among Schistosoma species and co-endemic pathogens was rare, though research on diagnostic markers and primer optimization should continue. Operating with ready-to-use lyophilized reagents, simplified and inexpensive nucleic acid extraction, tolerability to likely inhibitors, and enzyme stability at ambient temperature is advantageous. RPA performed optimal at 35–39 °C within 5–10 min. while LAMP operated at 61–65 °C for up to 120 min.; properties are preferable over assays requiring expensive laboratory equipment. DNA degradation could be prevented by stabilizing substances. A limitation throughout warranting future research is the small sample size reaching a few hundred participants at the maximum. Isothermal diagnostics are highly valuable in detecting trace infections seen subsequent to chemotherapeutic treatment, and among apparently healthy individuals, both constituting likely sources of ongoing pathogen transmission. Its expansion to the vaccine field for assessing parasitological trial endpoints could be considered.
1. Background
Schistosomiasis as a neglected tropical helminthic disease causes large morbidity and mortality. Among the genus Schistosoma, S. haematobium, S. mansoni, and S. japonicum are the most widespread and clinically relevant species afflicting humans. S. haematobium and S. mansoni are prevalent in Africa and the Middle East; S. mansoni occur also and S. japonicum soley in Latin America and the Caribbean, as well as Asia, respectively [1,2]. The species S. mekongi, S. guineensis, S. intercalatum, and S. malayensis also affect humans, but to a lower extent [1,2]. Of growing concern is cross-species hybridization abated by natural and anthropogenic changes; it leads to a large diversity of novel inter-species and/or inter-lineages through instant acquisition of genetic information [3].
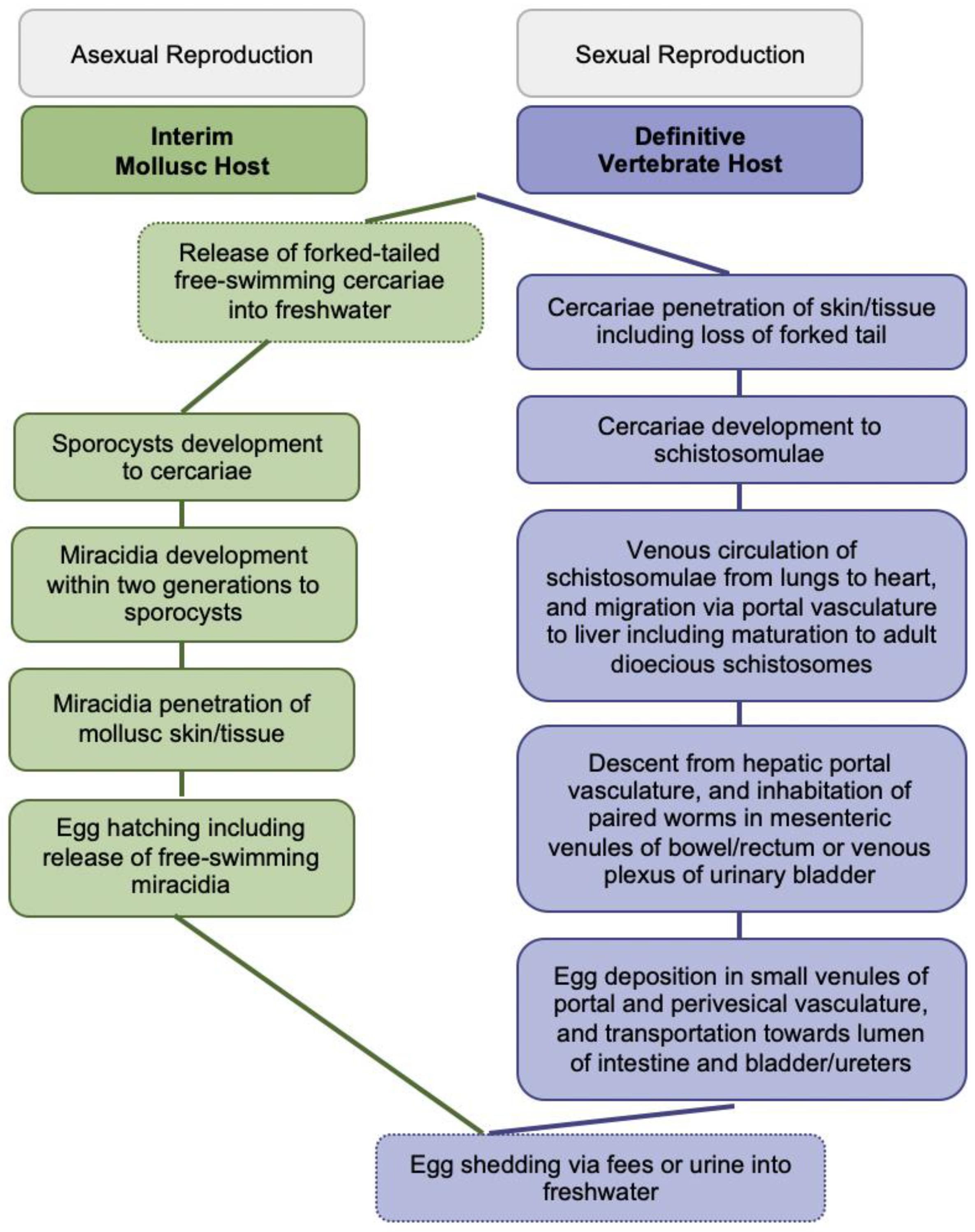
The blood-feeding flukes cause globally more than 250 million people affected, over 779 million people at risk of infection, about 440 million people with residual morbidity, and nearly 500,000 annual deaths [2,4,5]. Infections of vertebrates occur during contact with freshwater infested with skin-penetrating schistosomal cercariae disseminated by species-specific snails; larval cercariae transform into schistosomulae and migrate matured to adult worms to their oviposition sites within the vasculature for mating and sexual reproduction; see Figure 1 for more details. Intact adult worms can persist in immunocompetent definitive hosts for decades. Purposefully, eggs are shed via fecal or urinary routes to continue the transmission from vertebrates to molluscs for asexual reproduction upon hatching of miracidia into freshwater; see Figure 1 for more details [2,4,5].
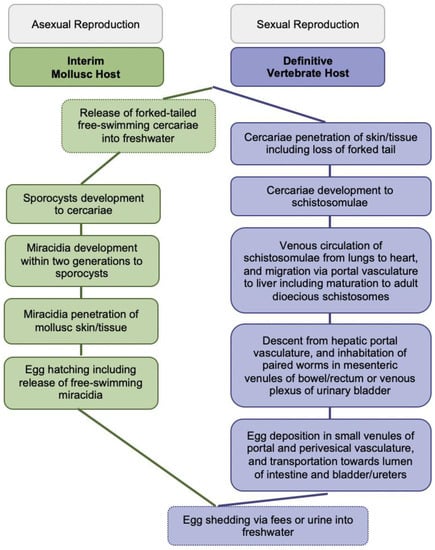
Figure 1.
Generic Schistosoma life cycle.
Acute schistosomiasis or Katayama fever seen among individuals without past parasitic exposure presents as debilitating febrile illness coupled with, e.g., headache, myalgia, fatigue, diarrheal, as well as respiratory symptoms, and hepato- and/or splenomegaly following an incubation period of up to 10–12 weeks. Chronic schistosomiasis manifests as host immunoresponses to unreleased retained eggs, that can lead to long-term complications. Host reactivity presents as bleeding and scarring, chronic inflammations and granulomatous-fibrotic formations around eggs trapped in capillaries damaging species-dependent organs inclusive of liver, intestine, spleen, and the urinary bladder [2,3]. Intestinal chronic schistosomiasis may induce diarrhea or constipation including blood admixture with progression to ulcerations, hyperplasia, polyposis, and fibrosis. S. haematobium causes urogenital pathologies such as dysuria and hematuria and so called female genital schistosomiasis (FGS) if eggs are deposited in the female genitalia [6]. FGS impairs fertility, e.g., ectopic pregnancy and miscarriage, and susceptibility to viruses, e.g., human immunodeficiency virus [7] and papillomavirus, and progressing to malignancies augmented by calcification, e.g., squamous cell carcinomas and sandy patches [8,9,10,11,12]. Ectopic excess egg deposition or erroneous migrating adult worms within the central nervous system can induce cognitive and physical impairments as seen among infested children in endemic settings. Praziquantel (PZQ) is commonly used [7] to treat schistosomiasis and eliminate adult schistosomes by changing irreversibly the permeability and stability of their tegument, but it requires prevailing host immune defense mechanisms for complete efficacy [13]. Control and prevention measures should be complemented by vaccination given the advances in this field to achieve long-term protection against transmission, infection, and disease recurrence [8].
Conventional schistosomal diagnostics include microscopy as the gold standard to visualize fecal and urinary eggs, and the detection of antibodies, antigen(s) and genetic information. However, they vary in sensitivity and specificity depending on, e.g., disease status, endemicity/co-endemicity levels, and chemotherapeutic treatment [7], in particular post-treatment [2,14,15,16]. Oviposition is a common marker to indicate active infection. However, egg shedding starting approximately one month post-infection is impacted by day-to-day variations [7], and the acquisition of, e.g., single sexes, infertile females or senile worms, which make this a rather poor indicator [16,17,18]. Despite, reliable diagnostics are essential for disease monitoring to detect in particular low infection levels promoting pathogen transmission [7], and for evaluating the effectiveness of treatment and control measures [1,17]. Consensus exists that an optimal diagnostic tool must be highly sensitive and specific, simple, and fast to perform and interpret also on different specimen types; favored diagnostics should be capable of detecting acute phase and trace infections supporting early treatment, and cost-effective for use also in resource-limited endemic settings [19].
Nucleic acid amplification is a highly valuable tool for simultaneous detection and species identification at day one post-infection [7] since the introduction of the polymerase chain reaction (PCR) [2,15]. However, PCR-based assays require expensive laboratory and technical equipment besides highly skilled personnel, cold chain for reagent storage, and prolonged reaction times [19,20]. This impacts their large-scale implementation as a genetic high-throughput point-of-care diagnostic measure, especially in resource-constrained settings. Isothermal amplification techniques can overcome aforementioned limitations due to their advancement in speed, simplicity, sensitivity, and specificity [1,21,22]. They detect nucleic acid in an exponential manner without constraints of thermal cycling, and are adaptable to multiplex, quantitative real-time, and reverse transcriptase techniques [23,24]. This is because nucleic acid strands are not heat-denaturated to enable primer binding and initiate amplification reactions since a polymerase of, e.g., Bacillus stearothermophilus (Bst), Bacillus subtilis (Bsu), or Staphylococcus aureus (Sau) with strand-displacement enzymatic activity is used [2,21].
An isothermal amplification technique of great interest is the loop-mediated isothermal amplification (LAMP), first reported by Notomi et al. [25]. LAMP detects few copies of genetic material as seen in low-endemic or newly emerging settings and subsequent to mass drug administration (MDA) [1,17]. Assays exist for various pathogens, e.g., Plasmodium falciparum, Babesia spp., Leishmania spp., Trypanosoma brucei, Ascaris lumbricoides, Ancylostoma spp., Necator spp., Taenia spp., and Toxoplasma gondii [16,19,26,27,28,29]. LAMP produces in a one-step reaction large amounts of lengthy double-stranded genetic information with a mutually complementary sequence and an alternate repeated structure at a constant temperature of 60–65 °C during 60 min reaction time on average [30,31]. Dumbbell-shaped DNA with stem-loops at both ends is formed, which activates steps of polymerization and extension [32]. LAMP performs well with simple isothermal equipment, e.g., heat block or water bath [17]. A pair of highly specific internal primers for strand displacement and synthesis, and external primers are required to detect six distinct sequences among the cognate sites [33]. Amplification can be accelerated and targeted regions expanded through additional loop primers [2,25,34]. Amplified products are visualized by agarose gel electrophoresis, turbidity, and colorimetry based on metal ion indicators and dyes causing color changes that are visible to the naked eye or a fluorometer [1,35,36,37,38]. Reagents are storable at ambient temperature, and test reactions are robust against inhibitory compounds in specimens, and variations in pH and temperature [9,39,40].
Another isothermal amplification technique of growing interest is the recombinase polymerase amplification (RPA) [21,41]. It performs similar to LAMP, except it forms in the presence of a recombinase protein derived from, e.g., T4-like bacteriophages or Escherichia coli, and a high molecular crowding agent a recombinase-primer complex; both promote primer invasion into double-stranded DNA at cognate sites [42]. The invasion is stabilized by single-stranded binding proteins. Subsequent polymerization and extension of loop-like DNA are induced by a chain-replacement polymerase [42]. RPA operates at 22–45 °C, though best at 37–42 °C, that allows simple cycling equipment, e.g., incubator, heatblock, chemical heater, body heat, or ambient temperature, during a reaction time of less than 30 min [10,11]. RPA operates on many specimens, e.g., cultured organisms, body fluids, surgical biopsies, organ tissues, and animal or plant products, and with lyophilized reagent pellets [10,20,43]. Amplicons are visualized similar to LAMP or by oligo chromatographic lateral flow strips, and fluorescence-labeled probes, i.e., dT-fluorophores coupled with corresponding dT-quenchers [44].
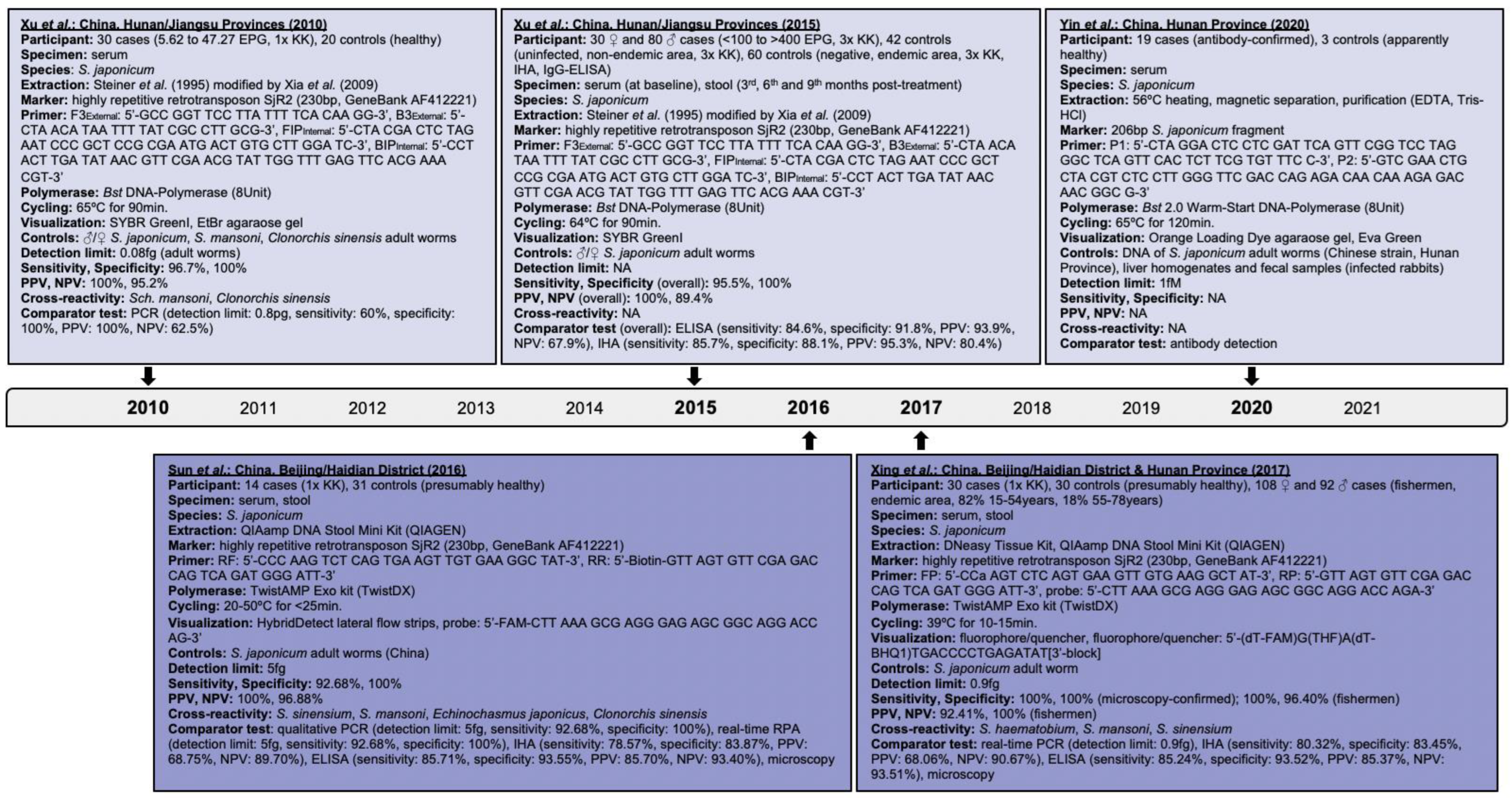
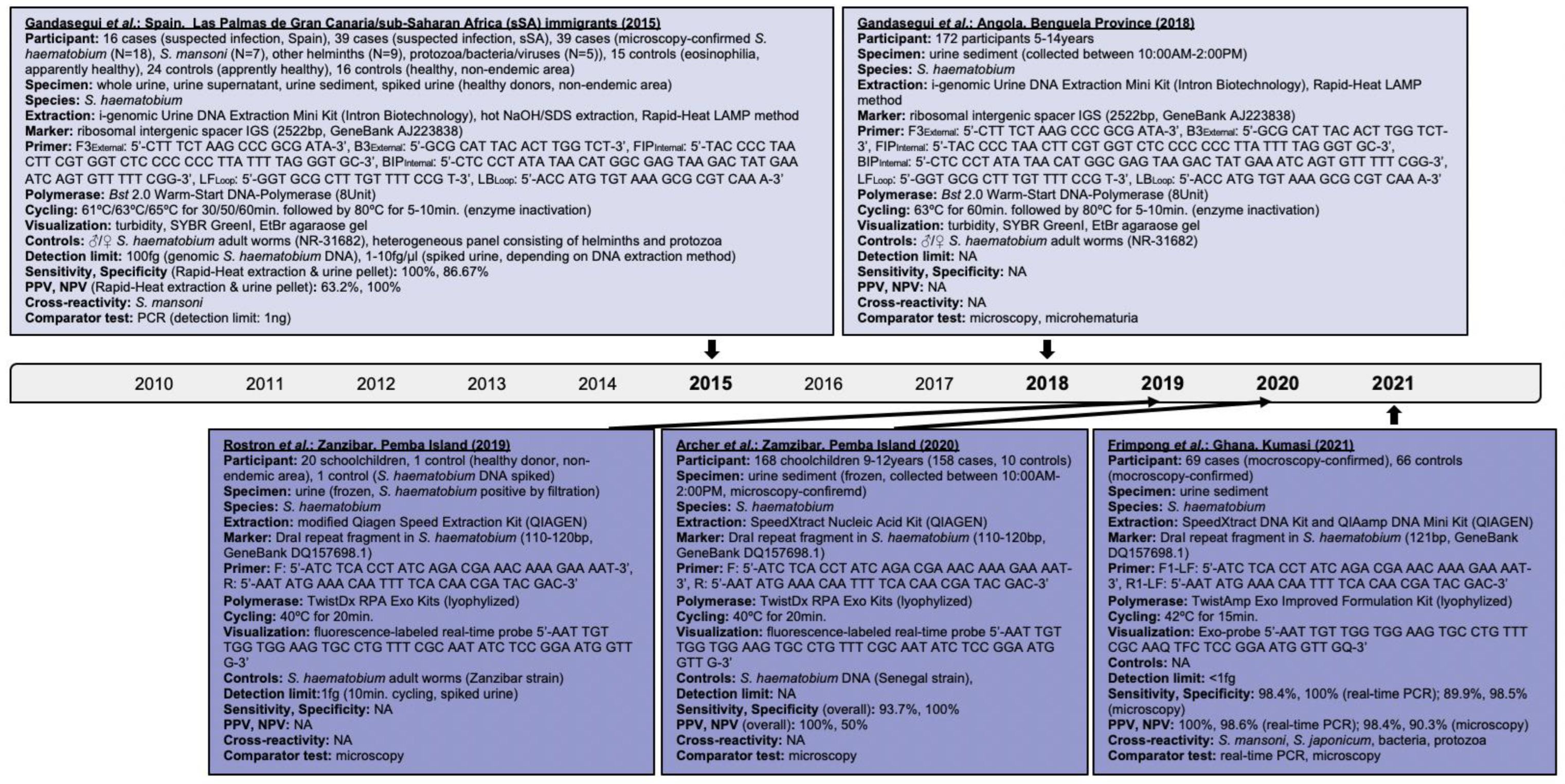
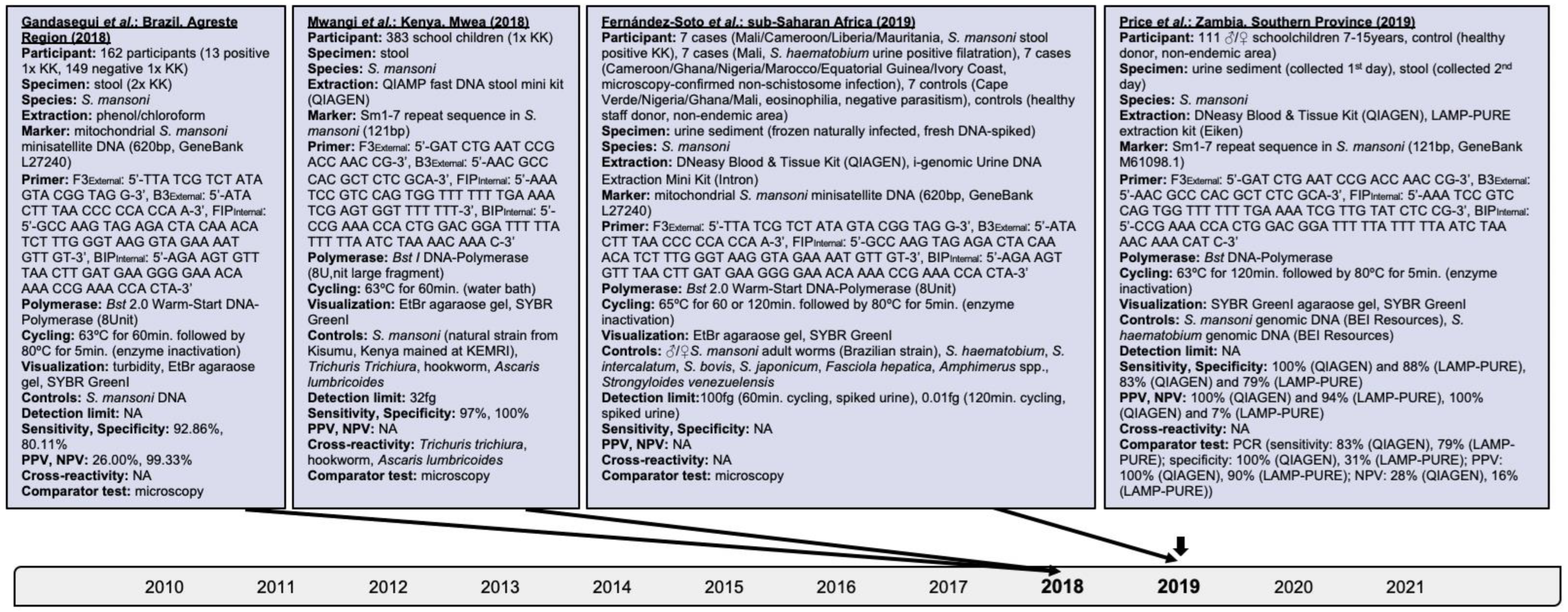
Research performed on the isothermal detection of human schistosomiasis within clinical applications was investigated and findings are presented hereby. The progression of isothermal techniques as a high-throughput point-of-care diagnostic measure for single- and multiple-species identification, including schistosomal hybrids, is also addressed. Searches performed in PubMed, Embase and Web of Science, and details of assays identified for S. japonicum, S. haematobium, S. mansoni, and mixed infections are delineated in Figure 2, Figure 3, Figure 4 and Figure 5, respectively.
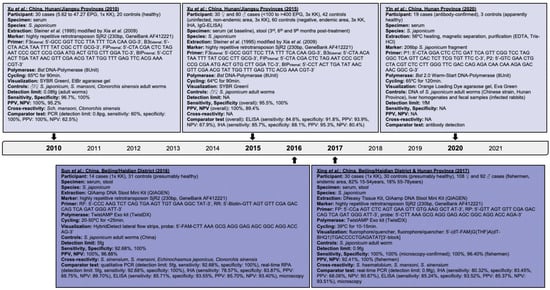
Figure 2.
Articles identified on the isothermal diagnosis of Schistosoma japonicum with details on participants, specimens, assay features, and test evaluation in terms of detection limits, sensitivity, specificity, PPV and NPV versus a comparator test by chronological order of publication date. Articles highlighted in light blue delineate schistosomal diagnosis based on loop-mediated isothermal amplification (LAMP) while articles highlighted in blue delineate schistosomal diagnosis based on recombinase polymerase amplification (RPA). We searched databases of PubMed, Embase and Web of Science for suitable publications on the isothermal diagnosis of schistosomiasis among clinical specimens collected from human subjects by applying the following terms: “schistosomiasis”, “Schistosoma”, “snail fever”, “schistosomiasis MeSH Terms”, ”isothermal”, “diagnosis/diagnostic”, “detection”, and “assay”. The last searches were performed on 31 December 2021. Publications included after removing duplicates, screening titles and abstracts, reading full-texts, and complementing through reference searches were not restricted by time period, but by the availability of full-texts available in English. Experimental animal studies, investigations on intermediate mollusk hosts, reviews and mathematical models were excluded unless considered highly relevant. Abbreviations: S. = Schistosoma, EPG = eggs per gram, KK = Kato-Katz, bp = basepair, min = minutes, EtBr = ethidium bromide, ♂ = male, ♀ = female, PCR = polymerase chain reaction, PPV = positive predictive value, NPV = negative predicitive value, ELISA = enzyme-linked immunosorbent assay, IHA = indirect haemagglutination assay, fg = femtogram, fM = femtomolar, pg = picogram, Bst = Bacillus stearothermophilus, NA = not available, EDTA = ethylenediaminetetraacetic acid, HCl = hydrogen chloride.

Figure 3.
Articles identified on the isothermal diagnosis of Schistosoma haematobium with details on participants, specimens, assay features, and test evaluation in terms of detection limits, sensitivity, specificity, PPV and NPV versus a comparator test by chronological order of publication date. Articles highlighted in light blue delineate schistosomal diagnosis based on loop-mediated isothermal amplification (LAMP) while articles highlighted in blue delineate schistosomal diagnosis based on recombinase polymerase amplification (RPA). We searched databases of PubMed, Embase and Web of Science for suitable publications on the isothermal diagnosis of schistosomiasis among clinical specimens collected from human subjects by applying the following terms: “schistosomiasis”, “Schistosoma”, “snail fever”, “schistosomiasis MeSH Terms”, ”isothermal”, “diagnosis/diagnostic”, “detection”, and “assay”. The last searches were performed on 31 December 2021. Publications included after removing duplicates, screening titles and abstracts, reading full-texts, and complementing through reference searches were not restricted by time period, but by the availability of full-texts available in English. Experimental animal studies, investigations on intermediate mollusk hosts, reviews and mathematical models were excluded unless considered highly relevant. Abbreviations: S. = Schistosoma, bp = basepair, min = minutes, EtBr = ethidium bromide, ♂ = male, ♀ = female, PCR = polymerase chain reaction, PPV = positive predictive value, NPV = negative predicitive value, fg = femtogram, ng = nanogram, Bst = Bacillus stearothermophilus, NA = not available, N = sample size, AM = ante merdien, PM = post meridien.

Figure 4.
Articles identified on the isothermal diagnosis of Schistosoma mansoni with details on participants, specimens, assay features, and test evaluation in terms of detection limits, sensitivity, specificity, PPV and NPV versus a comparator test by chronological order of publication date. Articles highlighted in light blue delineate schistosomal diagnosis based on loop-mediated isothermal amplification (LAMP). We searched databases of PubMed, Embase and Web of Science for suitable publications on the isothermal diagnosis of schistosomiasis among clinical specimens collected from human subjects by applying the following terms: “schistosomiasis”, “Schistosoma”, “snail fever”, “schistosomiasis MeSH Terms”, ”isothermal”, “diagnosis/diagnostic”, “detection”, and “assay”. The last searches were performed on 31 December 2021. Publications included after removing duplicates, screening titles and abstracts, reading full-texts, and complementing through reference searches were not restricted by time period, but by the availability of full-texts available in English. Experimental animal studies, investigations on intermediate mollusk hosts, reviews and mathematical models were excluded unless considered highly relevant. Abbreviations: S. = Schistosoma, KK = Kato-Katz, bp = basepair, min = minutes, EtBr = ethidium bromide, ♂ = male, ♀ = female, PCR = polymerase chain reaction, PPV = positive predictive value, NPV = negative predicitive value, fg = femtogram, Bst = Bacillus stearothermophilus, NA = not available.
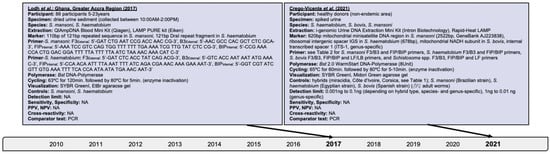
Figure 5.
Articles identified on the isothermal diagnosis of mixed Schistosoma infections with details on participants, specimens, assay features, and test evaluation in terms of detection limits, sensitivity, specificity, PPV and NPV versus a comparator test by chronological order of publication date. Articles highlighted in light blue delineate schistosomal diagnosis based on loop-mediated isothermal amplification (LAMP). We searched databases of PubMed, Embase and Web of Science for suitable publications on the isothermal diagnosis of schistosomiasis among clinical specimens collected from human subjects by applying the following terms: “schistosomiasis”, “Schistosoma”, “snail fever”, “schistosomiasis MeSH Terms”, ”isothermal”, “diagnosis/diagnostic”, “detection”, and “assay”. The last searches were performed on 31 December 2021. Publications included after removing duplicates, screening titles and abstracts, reading full-texts, and complementing through reference searches were not restricted by time period, but by the availability of full-texts available in English. Experimental animal studies, investigations on intermediate mollusk hosts, reviews and mathematical models were excluded unless considered highly relevant. Abbreviations: S. = Schistosoma, bp = basepair, min = minutes, EtBr = ethidium bromide, PCR = polymerase chain reaction, PPV = positive predictive value, NPV = negative predicitive value, ng = nanogram, Bst = Bacillus stearothermophilus, NA = not available, AM = ante merdien, PM = post meridien.
2. Schistosoma japonicum Assays
Searches revealed the application of LAMP on patient sera with microscopy-confirmed S. japonicum infection [26]. The assay builds on the non-long terminal highly repetitive retrotransposon SjR2 marker that identifies schistosomal infestation early, and accounts for 14% of the parasite genome [45,46]. LAMP compared to PCR yielded 96.7% versus 60% sensitivity, respectively. Detection limits of 0.08 femtogram and 0.8 picogram were achieved by LAMP and PCR, respectively; LAMP did not cross-react with S. mansoni and Chlonorchis sinensis [42]. This research builds on investigations of experimentally challenged New Zealand rabbits to evaluate LAMP for assessing the chemotherapeutic efficacy of PZQ (150 mg/kg) administered at week-7 and week-8 post-challenge. Rabbit sera were negative for S. japonicum at week-12 and week-10 post-treatment by LAMP and PCR, respectively, which indicates better sensitivity of LAMP over PCR. Xu et al. could show that LAMP operates better than conventional diagnostics in amplifying genetic information also within a reaction time of 60 min while using non-sophisticated equipment and easy visualization.
LAMP was in other clinical investigations assessed for detecting trace infections of S. japonicum and the chemotherapeutic effectiveness of PZQ [47]. Fecal samples were stratified based on their microscopy-confirmed eggs per gram (EPG) of feces into cohorts of 84.5% (93/110) with <100 EPG, 11.8% (13/110) with 100–400 EPG, and 3.6% (4/110) with >400 EPG [48]. LAMP revealed 95.5% sensitivity and 100% specificity overall, and 95.1% (95% confidence interval (CI): 88.5–100), 97.6% (95%CI: 93.0–100), and 95.1% (95%CI: 88.5–100) sensitivity among cases carrying < 10 EPG, 10–39 EPG, and 40–99 EPG of feces, respectively [47]. The negative conversion rate at three, six, and nine months post-treatment increased from 23.4% (95%CI: 11.3–35.5) to 61.7% (95%: 47.8–75.6), and 83.0% (95%: 72.3–92.7), respectively, while immunoresponses remained at low levels throughout. Notably, 16.7% (10/60; 95%CI: 7.3–26.1) of healthy controls were S. japonicum positive by LAMP.
To further examine trace targets of S. japonicum among microscopy-confirmed fecal samples, Sun et al. combined the lateral flow dipstick technology with an RPA that utilizes the SjR2 marker and ready-prepared lyophilized reagent pellets [24,49]. The detection limit was five femtogram with no cross-reactivity found for the strains assessed (Figure 2); optimal visibility was seen for cycling conditions of 35–45 °C for 5–10 min, which again highlights the assay‘s advantage of operating with simple thermal devices or just body heat. RPA disclosed 92.86% sensitivity and 100% specificity, and good agreement with Kato-Katz by Cohen’s kappa (k = 0.793, Z = 6.36, p-value < 0.0001).
RPA’s performance using the SjR2 marker, lyophilized reagents, and a 6-carboxyfluorescein (FAM) fluorescence to visualize amplicons was evaluated among stool specimens with microscopy-confirmed S. japonicum infestation [44]. Cycling conditions of 39 °C for 10–15 min detected 0.9 femtogram schistosomal DNA, no cross-reaction with other Schistosoma species, and each 100% sensitivity and specificity. RPA also confirmed S. japonicum infections among fecal specimens of highly exposed fishermen with 100% sensitivity and 96.40% (95%CI: 99.32–99.54) specificity, and strong agreement with Kato-Katz by Cohen’s kappa (k = 0.942, 95%CI: 0.89–0.99, p-value < 0.0001).
Applying several primers simultaneously increases chances of primer–primer interactions leading to false positivity [19,38], as seen for the internal transcribed spacer (ITS)-/28S-based S. mansoni RPA developed by Poulton et al. [20]; this hampers the identification of unique target sequences [14]. To address this, a single set of hairpin primers coupled with a real-time LAMP was evaluated [32]. Primers were designed with a target-unrelated stem-loop domain at the 5′-end, and a target-related binding sequence at the 3′-end. Human sera containing S. japonicum antibodies showed 100% (19/19) positivity after seven-time repeated testing, which also indicates LAMP’s robust repeatability strengthened by a relative standard deviation of 3.7%.
3. Schistosoma haematobium Assays
Initial LAMP experiments for S. haematobium were conducted on urine fractions, i.e., whole urine, supernatant and sediment, of microscopy-confirmed cases [9] using the species-specific ribosomal intergenic spacer (IGS) marker [50]. LAMP performed best with DNA extracted from sediments using the Rapid-Heat LAMP DNA extraction method, i.e., 100% (18/18) positivity. The Bst 2.0 Warm-Start DNA-Polymerase applied operates faster and with higher stability at room temperature compared to the wild-type Bst, which is advantageous for settings lacking cold chain facilities. Positivity was observed among cases infected with other pathogens and also controls, i.e., 11.1% (1/9) other helminths, 20% (1/5) protozoa/bacteria/viruses, 6.7% (1/15) controls with eosinophilia, and 20.8% (5/24) controls without eosinophilia, though unlikely due to cross-reactivity considering the low detection rates. Finding S. haematobium among controls could be explained by low infection levels and day-to-day variability in oviposition. Experiments revealed 100% (95%CI: 81.32–100) sensitivity and 86.67% (95%CI: 75.40–94.05) specificity overall; LAMP’s detection limit was 104 times higher compared to PCR, i.e., 100 femtogram versus one nanogram, and could be optimized to one femtogram or an equivalent of a single cell parasite. LAMP performed similar among 69 urine samples from Egyptian participants, i.e., 100% (95%CI: 88.78–100) sensitivity and 63.16% (95%CI: 48.99–78.19) specificity [12].
Gandasegui et al. evaluated LAMP further among urine sediments of schoolchildren from Angola [51]. Test positivity ranged from 57% (98/172) to 73.8% (127/172) depending on the nucleic acid extraction and visualization methods used (Figure 3). Nevertheless, LAMP revealed tolerability to potential inhibitory substances in biological samples that highlights its practicability for high-throughput large-scale testing [9,51]. Despite SYBR GreenI improved test positivity due to better visibility, approximately 72% positive cases were visible to the naked eye. Interestingly, 78.2% (68/87) to 86.2% (75/87) of microscopy-positive specimens and 48.2% (41/85) to 61.2% (53/85) of microscopy-negative specimens were LAMP-positive. This refers back to LAMP’s capability to detect egg DNA and cell-free circulating DNA of parasitic lysis products [52,53]. Interestingly, 90.3% (56/62) samples positive by microhematuria and microscopy and 57.8% (37/64) samples negative by microhematuria and microscopy were also LAMP-positive. Kabir Patwary et al. found a significant correlation of detecting hematuria and S. haematobium DNA (OR: 6.66; 95%CI: 0.98–99.5; p-value = 0.4142) among cervicovaginal lavages of Zambian women suffering from FGS; 7.11% (16/225) versus 6.2% (14/225) lavages were positive by RPA and PCR, respectively [6].
A real-time RPA targeting the DraI tandem repeat region [54] of S. haematobium was used by Rostron et al. among pediatric urine samples categorized per ova load into very low, low, medium, and high, i.e., 1–10 eggs/10 mL, 11–50 eggs/10mL, 51–400 eggs/10mL, and >400 eggs/10mL, respectively [10,55]. DraI accounts for 15% of the parasite genome [45]. Of specimens with medium and high egg loads, 70% (14/20) revealed strong fluorescence subsequent to just eight minutes of cycling; of samples with very low egg loads, 20% (4/20) were positive, 5% (1/20) inconclusive, and 5% (1/20) negative. Despite RPA’s detection limit was one femtogram, which is below the limit of 10 femtogram by PCR and 100 femtogram by lateral flow assays, test inconclusiveness and negativity could have occurred due to sample degradation, false positivity by microscopy, or inhomogeneous egg distribution in sample aliquots [55]. Rosser and colleagues [10] raised in earlier bench-based experiments of spiked urine the likelihood of cross-amplification with other members of the S. haematobium group possessing the DraI region. Future species-specific assays to identify sub-group species [14] or multiple species [20] could address this [14].
RPA was further applied among pediatric urine samples grouped per ova load and found overall 94% (158/168) positivity, 93.7% (95%CI: 88.7–96.9) sensitivity and 100% (95%CI: 69.1–100) specificity [11]. Interestingly, RPA correctly identified 64 of 70 specimens with ultra-low and 113 of 122 specimens with ultra-low and low S. haematobium egg loads, indicating 91.4% (95%CI: 82.2–96.8) sensitivity and 92.6% (95%CI: 86.5–96.6) specificity; also 93.8% (15/16) specimens containing ≤ 1 egg/10mL urine were positive. This highlights RPA’s capability of detecting trace infection levels.
Frimpong et al. validated a real-time RPA among urine samples of Ghanaian participants [56]. They detected 98.4% (95%CI: 91.6–100) sensitivity and 100% (95%CI: 94.9–100) specificity compared to real-time PCR versus 89.9% (95%CI: 80.2–95.8) sensitivity and 98.5% (95%CI: 91.8–100) specificity compared to microscopy. Degradation of S. haematobium DNA in the urinary milieu could have impacted RPA’s sensitivity. Nevertheless, RPA’s lower detection limit was less than one femtogram. Cross-reactivity with S. mansoni and S. japonicum likely occurred due to sample contamination or DraI being a rather sensitive than specific marker.
4. Schistosoma mansoni Assays
An Sm1–7 sequence-based LAMP was assessed initially among fecal samples of Kenyan schoolchildren; Sm1–7 as a species-specific repeat tandem sequence is present in all life cycle stages [27], and comprises 12% of the S. mansoni genome [45]. Specimens were categorized based on their microscopy-confirmed egg loads [48], i.e., <100 EPG, 100–399 EPG, and >400 EPG, resembling low, moderate, and high infection intensities, respectively. LAMP amplified S. mansoni DNA across all categories while its detection limit was 32 femtogram; its overall positivity was 44.6% (171/383) compared to 46% (176/383) by Kato-Katz, which indicates strong test agreement (k = 0.9). Sensitivity scored 97% and specificity 100%, and no co-endemic helminths, i.e., Ascaris lumbricoides, hookworm and Trichuris trichiura, were detected (Figure 4).
The species-specific repeat tandem mitochondrial S. mansoni minisatellite marker was used in further LAMP experiments [57], i.e., SmMIT-LAMP, among fecal samples of residents from Brazil [28]. SmMIT-LAMP detected parasitic DNA among 8% (13/162) specimens with low eggs counts, i.e., 12–180 EPG confirmed by double Kato-Katz, and 7.4% (12/162) smear-positive and 22.8% (37/162) smear-negative samples; 24.5% (12/49) children aged ≤ 12 years were S. mansoni infected that were missed by microscopy earlier. SmMIT-LAMP demonstrated 92.86% (95%CI: 66.13–99.82) sensitivity and 80.11% (95%CI: 73.64–85.59) specificity overall.
Building on murine experiments [58], Fernández-Soto et al. applied SmMIT-LAMP on urine specimens of infected subjects from sub-Saharan Africa, and urine samples of healthy donors spiked with DNA of S. mansoni, and S. mansoni coupled with S. haematobium, S. intercalatum, S. bovis, S. japonicum, Fasciola hepatica, Amphimerus spp., or Strongyloides venezuelensis [52]. The detection limit of S. mansoni among spiked samples was 100 and 0.01 femtogram following 60 and 120 min cycling, respectively; DNA of other helminths was not amplified. Solely 120 min cycling duration revealed positivity among naturally infected subjects, i.e., 71.4% (5/7) of microscopy-confirmed S. mansoni cases, and 42.9% (3/7) of cases with microscopy-confirmed non-schistosomal infections, i.e., Trichonomas vaginalis, Enterobius vermicularis, and Trichuris trichiura; 57.1% (4/7) controls with eosinophilia as a biomarker for helminthic often prepatent infections lacking parasitism were positive by SmMIT-LAMP while microscopy-confirmed S. haematobium cases remained negative indicating no cross-species reactivity. S. mansoni detection likely was impeded by specimen contamination [19], unfavorable storage conditions including repetitive freezing and thawing, and lack of DNA-stabilizing additive substances avoiding degradation.
SmMIT-LAMP was advanced further to fulfill WHO’s ASSURED criteria, i.e., affordable, sensitive, specific, user-friendly, robust and rapid, equipment-free, and deliverable [21,59]. A novel concentration and desiccation technology utilizing trehalose achieved reagent functionality for up to three weeks and five months at room temperature and 4 °C, respectively [60].
Fernández-Soto et al. applied a real-time species-specific LAMP to skin tissue biopsies of a patient with travel history to Uganda and contact to freshwater of the Bunyonyi Lake few weeks prior to developing thoracic pruritic painless erythematous papules; the patient presented with eosinophilia, but no parasites, cysts and ova in feces [61]. LAMP detected S. mansoni in biopsies; ectopic cutaneous ova and miracidia were found in addition to schistosomal antibodies.
Urine specimens of Zambian schoolchildren four weeks after MDA (40 mg/kg) were investigated by LAMP for the presence of the cell-free repeat Sm1–7 DNA fragment [62]. Positivity by PCR was 77.5% (86/111) versus 93.7% (104/111), and 87.4% (97/111) by LAMP depending on the DNA extraction method. The discrepancies observed by LAMP could be attributable to the extraction of membrane-based versus membrane-free DNA resulting in varying concentrations of inhibitory components. Nevertheless, PCR and LAMP performed better in detecting S. mansoni infections than Kato-Katz, i.e., ≤71.2% (79/111) by PCR and ≤85.6% (95/111) by LAMP. LAMP’s sensitivity among low infection intensities subsequent to MDA ranged from 88% (95%CI: 80–93) to 100% (95%CI: 96–100); its specificity varied from 14% (95%CI: 0.4–58) to 100% (95%CI: 59–100).
5. Assays on Multiple Schistosoma Species including Hybrids
Searches revealed the assessment of LAMP on dried urine sediments from Ghanaian participants for the presence of S. mansoni, S. haematobium, and mixed infections [63,64,65,66]. Depending on the DNA extraction method used, 88.4% (76/86) to 94.2% (81/86), and 86.0% (74/86) S. mansoni cases were found by LAMP and PCR, respectively, in addition to 83.7% (72/86) and 81.4% (70/86) S. haematobium cases, respectively (Figure 5). This confirms the prevalence of concurrent sympatric infections. Notably, LAMP and PCR revealed 5.8% (5/86) and 1.2% (1/86) false negativity for S. mansoni, respectivley.
Further experiments among urine specimens of Zambian schoolchildren aged 8–16 years subsequent to MDA detected 84.6% (110/130) positivity, 96.49% sensitivity, and 100% specificity for S. mansoni, and 61.5% (80/130) positivity, 89.89% sensitivity, and 100% specificity for S. haematobium [67]. Interestingly, all samples were negative by microscopy. LAMP performed differently depending on the DNA extraction method [68].
Song et al. [14] used a two-stage nested-like rapid amplification technique consisting of a first-stage all-target RPA, and a second-stage target-specific LAMP in a microfluidic closed system. Different sample types, i.e., human whole blood, serum, and urine, were investigated without prior nucleic acid extraction during a total reaction time of 40–50 min, i.e., 10–20 min at 37 °C for RPA, and 30 min at 60–65 °C for LAMP, followed by simple visualization with fluorescent or colorimetric dyes. The hybrid RPA/LAMP was highly sensitive and specific, and robust when performed in a single- and multi-plex manner for detecting up to 16 targets inclusive of S. mansoni, S. haematobium, and S. japonicum.
To address the growing concerns of cross-species hybridization leading to large diversity of novel inter-species and/or inter-lineages, LAMP was investigated to detect hybrids originating from S. haematobium and the phylogenetic closely-related livestock species S. bovis [3,69]. Urine specimens from healthy donors were spiked with DNA of hybrids originating from Côte d’Ivoire and Corsica/France; hybrids were confirmed previously based on mitochondrial cytochrome c oxidase (COX) and nuclear ribosomal ITS profiles [3]. Species-specific assays [70] amplified pure S. haematobium, S. mansoni, and S. bovis DNA corroborating LAMP’s high sensitivity and specificity; the latter detected also hybridale DNA of less than 0.001 nanogram though only of strains with S. bovis/S. bovis x S. haematobium profiles. Hybrids likely retrieved from an initial crossing between male S. haematobium and female S. bovis leading to the introgression of S. bovis mitochondrial DNA into the S. haematobium genome [69].
6. Conclusions
Despite the tremendous efforts to prevent and control schistosomiasis, the helminthic disease still causes large morbidity and mortality in developing countries and beyond as seen by the occurrence of inter-species and/or inter-lineages schistosomal hybrids in Europe [3]. Efforts surely are impacted by misdiagnoses and underdiagnoses of infective subjects constituting sources of ongoing parasite transmission due to limitations of conventional diagnostics [2,7,14,15,16]; their performance is influenced by disease status including atypical disease presentation [3,69], endemicity/co-endemicity levels, and chemotherapeutic treatment [1,17]. Isothermal techniques aim to overcome aforementioned limitations.
Investigations on the isothermal detection of schistosomiasis among clinical specimens collected from humans revealed sensitivity reaching 96.7% and 100% for S. japonicum serum- and stool-based assays by LAMP and RPA, respectively; detection levels of 0.08 and 0.9 femtogram by LAMP and RPA were reported, respectively. LAMP and RPA reached up to 100% and 98.4% sensitivity when confirming S. haematobium among urine specimens, respectively; the detection limit was one femtogram for both techniques. LAMP urine- and stool-based assays for detecting S. mansoni varied in sensitivity of 88% to 100%; schistosomal DNA of 0.01 to 32 femtogram was amplified. LAMP detected hybridale DNA below 0.001 nanogram among strains with S. bovis/S. bovis x S. haematobium profiles. A limitation of all investigations warranting future large-scale high-throughput research is the rather small sample size reaching a few hundred participants at the maximum [27].
Cross-reaction with other Schistosoma species and co-endemic helminths, protozoa, bacteria and viruses was rarely seen throughout. Frimpong et al. [56] raised the issue of cross-reactivity between S. mansoni and S. japonicum likely due to DraI being a sensitive rather than specific marker. Likewise, Rosser et al. [10] discussed the possibility of cross-species amplification with species of the S. haematobium group possessing also the DraI region. Further research on species-specific diagnostic markers [14] including primer optimization [19,38] could address this.
LAMP and RPA operated well with ready-to-use lyophilized reagents [9]; desiccated reagents were stable for several weeks at ambient temperature [59,60]. Bst 2.0 Warm-Start DNA-Polymerase within LAMP eases testing due to its stability at room temperature [50]. Such properties are advantageous for settings lacking cold chain facilities. Simplified nucleic acid extraction, such as the Rapid-Heat LAMP DNA extraction method or not extracting nucleic acid at all [63] revealed the tolerability of isothermal diagnostics to potential inhibitory substances in biological samples [9,51]. The concentration of inhibitors could vary depending on levels of membrane-based versus membrane-free DNA [62]. LAMP detects not only ova DNA but also cell-free circulating DNA of parasitic lysis products [52], which is present in plasma during active schistosomiasis, but is cleared following chemotherapeutic treatment [53]. Obtaining good-quality DNA in an inexpensive and practicable manner is highly relevant for resource-poor settings and large-scale epidemiological assessments. RPA performed optimal at 35–39 °C for 5–10 min [44,49] while LAMP required cycling conditions of 61–65 °C for up to 120 min and assay-dependent additional cycling of 5–10 min at 80 °C for enzyme inactivation [50,51]. Such operational factors certainly are advantageous over other assays requiring expensive laboratory and technical equipment. A weakness raised is the possibility of DNA degradation due to unfavorable handling and storage conditions impacting test positivity, which could be prevented by adding stabilizing substances.
Of note is the capability of isothermal diagnostics presented to detect minimal infection levels as reported subsequent to chemotherapeutic treatment, including MDA [47], and among individuals presenting as apparently healthy. This is alarming as such subjects are likely overlooked by conventional diagnostics and constitute an unrecognized source promoting ongoing pathogen spread [9]. LAMP revealed increasing negative conversion rates reaching 83.0% at nine months post-treatment of S. japonicum infections while immunoresponses remained low throughout [47]. Additionally, LAMP’s sensitivity to S. mansoni subsequent to MDA among Zambian schoolchildren reached 100% (95%CI: 59–100) [62]. Likewise, LAMP detected S. mansoni and S. haematobium with 96.49% and 89.89% sensitivity, respectively, subsequent to MDA while microscopy remained negative throughout [67]. Given the recent advances in schistosomal vaccine candidates [8] and the lack of consensus in standardized parameters assessing protection in humans impeded by the lack of diagnostic tools as per WHO’s ASSURED criteria suggests to consider expanding isothermal detection techniques to the field of vaccinology.
In summary, LAMP and RPA reached species-dependent sensitivity up to 100% and detection in the femtogram range within clinical applications for diagnosing human schistosomiasis; hybridale DNA was found in nanogram amounts. This is advantageous for revealing trace infection levels subsequent to chemotherapeutic treatment and among apparently healthy individuals. The possibility of inter-species and intra-clade schistosomal cross-reactivity warrant further research for optimized diagnostic markers and primers. The test reagents’ stability at ambient temperature, and tolerability to potential inhibitors permitting simplified nucleic acid extraction are favorable in particular for resource-poor settings and large-scale investigations; added stabilizing substances may avoid DNA degradation ameliorating test positivity. Cycling conditions of 5–10 min at 35–39 °C for RPA, and up to 120 min at 61–65 °C for LAMP with additional assay-dependent 5–10 min at 80 °C for enzyme inactivation allow simple cycling equipment; amplicons are easily visible also with just the naked eye. It is worthwhile to consider expanding isothermal techniques beyond just diagnostic purposes and the effectiveness of chemotherapeutics to the field of vaccinology given the recent advances for assessing parasitological trial endpoints indicating protection from the blood-feeding flukes.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Avendaño, C.; Patarroyo, M. Loop-Mediated Isothermal Amplification as Point-of-Care Diagnosis for Neglected Parasitic Infections. Int. J. Mol. Sci. 2020, 21, 7981. [Google Scholar] [CrossRef]
- Diego, J.G.-B.; Fernández-Soto, P.; Febrer-Sendra, B.; Crego-Vicente, B.; Muro, A. Loop-Mediated Isothermal Amplification in Schistosomiasis. J. Clin. Med. 2021, 10, 511. [Google Scholar] [CrossRef]
- Panzner, U.; Boissier, J. Natural intra- and intercalde human hybrid schostosomes in Africa with considerations on prevention through vaccination Microorganisms. Microorganism 2021, 9, 1465. [Google Scholar] [CrossRef]
- Nelwan, M.L. Schistosomiasis: Life Cycle, Diagnosis, and Control. Curr. Ther. Res. 2019, 91, 5–9. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Patwary, F.K.; Archer, J.; Sturt, A.S.; Webb, E.L. Female Genital Schistosomiasis: Diagnostic Validation for Recombinant DNA-Polymerase-Amplification Assay using Cervicovaginal Lavage. Int. J. Obstet. Gynaecol. 2021, 128 (Suppl. S2), 248. [Google Scholar]
- Le, L.; Hsieh, M.H. Diagnosing Urogenital Schistosomiasis: Dealing with Diminishing Returns. Trends Parasitol. 2017, 33, 378–387. [Google Scholar] [CrossRef]
- Panzner, U.; Excler, J.L.; Kim, H.J. Recent advances and methodological considerations on vaccine candidates for human schistosomiasis Front. Trop. Dis. 2021, 2, 719369. [Google Scholar]
- Gandasegui, J.; Fernández-Soto, P.; Carranza-Rodríguez, C.; Perez-Arellano, J.-L.; Vicente, B.; López-Abán, J.; Muro, A. The Rapid-Heat LAMPellet Method: A Potential Diagnostic Method for Human Urogenital Schistosomiasis. PLoS Negl. Trop. Dis. 2015, 9, e0003963. [Google Scholar] [CrossRef]
- Rosser, A.; Rollinson, D.; Forrest, M.S.; Webster, B.L. Isothermal Recombinase Polymerase amplification (RPA) of Schistosoma haematobium DNA and oligochromatographic lateral flow detection. Parasites Vectors 2015, 8, 446. [Google Scholar] [CrossRef]
- Archer, J.; Barksby, R.; Pennance, T.; Rostron, P.; Bakar, F.; Knopp, S.; Allan, F.; Kabole, F.; Ali, S.M.; Ame, S.M.; et al. Analytical and Clinical Assessment of a Portable, Isothermal Recombinase Polymerase Amplification (RPA) Assay for the Molecular Diagnosis of Urogenital Schistosomiasis. Molecules 2020, 25, 4175. [Google Scholar] [CrossRef]
- Bayoumi, A.; Al-Refai, S.A.; Badir, M.S.; El-Aal, A.A.A.; El Akkad, D.M.H.; Saad, N.; Elesaily, K.M.; Aziz, I.Z.A. Loop-Mediated Isothermal Amplification (Lamp): Sensitive and Rapid Detection of Schistosoma Haematobium DNA in Urine Samples of Egyptian Suspected Cases. J. Egypt Soc. Parasitol. 2016, 46, 299–308. [Google Scholar]
- Eyoh, E.; McCallum, P.; Killick, J.; Amanfo, S.; Mutapi, F.; Astier, A.L. The anthelmintic drug praziquantel promotes human Tr1 differentiation. Immunol. Cell Biol. 2019, 97, 512–518. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Mauk, M.G.; Rankin, S.C.; Lok, J.B.; Greenberg, R.M.; Bau, H.H. Two-Stage Isothermal Enzymatic Amplification for Concurrent Multiplex Molecular Detection. Clin. Chem. 2017, 63, 714–722. [Google Scholar] [CrossRef]
- Zhao, G.-H.; Li, J.; Blair, D.; Li, X.-Y.; Elsheikha, H.M.; Lin, R.-Q.; Zou, F.-C.; Zhu, X.-Q. Biotechnological advances in the diagnosis, species differentiation and phylogenetic analysis of Schistosoma spp. Biotechnol. Adv. 2012, 30, 1381–1389. [Google Scholar] [CrossRef]
- Mosquera-Romero, M.; Zuluaga-Idárraga, L.; Tobón-Castaño, A. Challenges for the diagnosis and treatment of malaria in low transmission settings in San Lorenzo, Esmeraldas, Ecuador. Malar. J. 2018, 17, 440. [Google Scholar] [CrossRef]
- Cavalcanti, M.G.; Cunha, A.F.A.; Peralta, J.M. The Advances in Molecular and New Point-of-Care (POC) Diagnosis of Schistosomiasis Pre- and Post-praziquantel Use: In the Pursuit of More Reliable Approaches for Low Endemic and Non-endemic Areas. Front. Immunol. 2019, 10, 858. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Yin, X.; Hua, W.; Hou, M.; Ji, M.; Yu, C.; Wu, G. Application of DNA-based diagnostics in detection of schistosomal DNA in early infection and after drug treatment. Parasites Vectors 2011, 4, 164. [Google Scholar] [CrossRef]
- Deng, M.-H.; Zhong, L.-Y.; Kamolnetr, O.; Limpanont, Y.; Lv, Z.-Y. Detection of helminths by loop-mediated isothermal amplification assay: A review of updated technology and future outlook. Infect. Dis. Poverty 2019, 8, 20. [Google Scholar] [CrossRef]
- Poulton, K.; Webster, B. Development of a lateral flow recombinase polymerase assay for the diagnosis of Schistosoma mansoni infections. Anal. Biochem. 2018, 546, 65–71. [Google Scholar] [CrossRef]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. TrAC Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Li, H.-M.; Qin, Z.-Q.; Bergquist, R.; Qian, M.-B.; Xia, S.; Lv, S.; Xiao, N.; Utzinger, J.; Zhou, X.-N. Nucleic acid amplification techniques for the detection of Schistosoma mansoni infection in humans and the intermediate snail host: A structured review and meta-analysis of diagnostic accuracy. Int. J. Infect. Dis. 2021, 112, 152–164. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Bais, S.; Mauk, M.G.; Bau, H.H.; Greenberg, R.M. Molecular Detection of Schistosome Infections with a Disposable Microfluidic Cassette. PLoS Negl. Trop. Dis. 2015, 9, e0004318. [Google Scholar] [CrossRef]
- Wong, Y.-P.; Othman, S.; Lau, Y.-L.; Radu, S.; Chee, H.-Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Xu, J.; Rong, R.; Zhang, H.; Shi, C.; Zhu, X.; Xia, C. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int. J. Parasitol. 2010, 40, 327–331. [Google Scholar] [CrossRef]
- Mwangi, I.N.; Agola, E.L.; Mugambi, R.M.; Shiraho, E.A.; Mkoji, G.M. Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for Diagnosis of Schistosoma mansoni Infection in Faecal Samples. J. Parasitol. Res. 2018, 2018, 1267826. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; Muro, A.; Barbosa, C.S.; De Melo, F.L.; Loyo, R.; Gomes, E.C.D.S. A field survey using LAMP assay for detection of Schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: Assessment in human and snail samples. PLoS Negl. Trop. Dis. 2018, 12, e0006314. [Google Scholar] [CrossRef]
- Yansouni, C.P.; Bottieau, E.; Lutumba, P.; Winkler, A.S.; Lynen, L.; Buscher, P.; Jacobs, J.; Gillet, P.; Lejon, V.; Alirol, E.; et al. Rapid diagnostic tests for neurological infections in central Africa. Lancet Infect. Dis. 2013, 13, 546–558. [Google Scholar] [CrossRef]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, Z.; Li, G.; Huang, J.; Guo, Q.; Meng, X. A DNA molecular diagnostic technology with LAMP-like sensitivity based on one pair of hairpin primers-mediated isothermal polymerization amplification. Anal. Chim. Acta 2020, 1134, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kaiglová, A.; Beňo, P.; Changoma, M.J.S. Detection of schistosomiasis applicable for primary health care facilities in endemic regions of Africa. Biologia 2017, 72, 1113–1120. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Zhang, X.; Lowe, S.B.; Gooding, J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 2014, 61, 491–499. [Google Scholar] [CrossRef]
- Weerakoon, K.; Gobert, G.N.; Cai, P.; McManus, D.P. Advances in the Diagnosis of Human Schistosomiasis. Clin. Microbiol. Rev. 2015, 28, 939–967. [Google Scholar] [CrossRef]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.-J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, Q.; Fu, Z.; Liu, J.; Lin, J.; Xiao, K.; Sun, P.; Cong, X.; Liu, R.; Hong, Y. Reviews and advances in diagnostic research on Schistosoma japonicum. Acta Trop. 2020, 213, 105743. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Teramura, T.; Adachi, K.; Tsuneda, S.; Kurata, S.; Nakamura, K.; Kanagawa, T.; Noda, N. Technique for Quantitative Detection of Specific DNA Sequences Using Alternately Binding Quenching Probe Competitive Assay Combined with Loop-Mediated Isothermal Amplification. Anal. Chem. 2007, 79, 5608–5613. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Yu, X.; Feng, J.; Sun, K.; Fu, W.; Wang, Y.; Zou, M.; Xia, W.; Luo, Z.; He, H.; et al. Field evaluation of a recombinase polymerase amplification assay for the diagnosis of Schistosoma japonicum infection in Hunan province of China. BMC Infect. Dis. 2017, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Song, L.-G.; Xie, H.; Liang, J.-Y.; Yuan, D.-Y.; Wu, Z.-D.; Lv, Z.-Y. Nucleic acid detection in the diagnosis and prevention of schistosomiasis. Infect. Dis. Poverty 2016, 5, 25. [Google Scholar] [CrossRef]
- Guo, J.-J.; Zheng, H.-J.; Xu, J.; Zhu, X.-Q.; Wang, S.-Y.; Xia, C.-M. Sensitive and Specific Target Sequences Selected from Retrotransposons of Schistosoma japonicum for the Diagnosis of Schistosomiasis. PLoS Negl. Trop. Dis. 2012, 6, e1579. [Google Scholar] [CrossRef]
- Xu, J.; Guan, Z.-X.; Zhao, B.; Wang, Y.-Y.; Cao, Y.; Zhang, H.-Q.; Zhu, X.-Q.; He, Y.-K.; Xia, C.-M. DNA Detection of Schistosoma japonicum: Diagnostic Validity of a LAMP Assay for Low-Intensity Infection and Effects of Chemotherapy in Humans. PLoS Negl. Trop. Dis. 2015, 9, e0003668. [Google Scholar] [CrossRef]
- WHO Expert Committee. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis; World Health Organization: Geneva, Switzerland, 2002; pp. 1–57.
- Sun, K.; Xing, W.; Yuanyuan, W.; Fu, W.; Wang, Y.; Zou, M.; Luo, Z.; Xu, D. Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum. Parasites Vectors 2016, 9, 476. [Google Scholar] [CrossRef]
- Kane, R.A.; Rollinson, D. Comparison of the intergenic spacers and 3′ end regions of the large subunit (28S) ribosomal RNA gene from three species of Schistosoma. Parasitology 1998, 117, 235–242. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; DaCal, E.; Rodríguez, E.; Saugar, J.M.; Yepes, E.; Aznar, M.L.; Espasa, M.; Ninda, A.; Bocanegra, C.; et al. Field and laboratory comparative evaluation of a LAMP assay for the diagnosis of urogenital schistosomiasis in Cubal, Central Angola. Trop. Med. Int. Health 2018, 23, 992–1001. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Gandasegui, J.; Rodríguez, C.C.; Pérez-Arellano, J.L.; Vicente, B.C.; Diego, J.G.-B.; López-Abán, J.; Vicente, B.; Muro, A. Detection of Schistosoma mansoni-derived DNA in human urine samples by loop-mediated isothermal amplification (LAMP). PLoS ONE 2019, 14, e0214125. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, D.; Panning, M.; Quack, T.; Kramme, S.; Burchard, G.-D.; Grevelding, C.; Drosten, C. Diagnosing Schistosomiasis by Detection of Cell-Free Parasite DNA in Human Plasma. PLoS Negl. Trop. Dis. 2009, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Ibironke, O.A.; Shiff, C.; Garba, A.; Phillips, A.E.; Lamine, S.M. Diagnosis of Schistosoma haematobium by Detection of Specific DNA Fragments from Filtered Urine Samples. Am. J. Trop. Med. Hyg. 2011, 84, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Rostron, P.; Pennance, T.; Bakar, F.; Rollinson, D.; Knopp, S.; Allan, F.; Kabole, F.; Ali, S.M.; Ame, S.M.; Webster, B.L. Development of a recombinase polymerase amplification (RPA) fluorescence assay for the detection of Schistosoma haematobium. Parasites Vectors 2019, 12, 514. [Google Scholar] [CrossRef]
- Frimpong, M.; Kyei-Tuffuor, L.; Fondjo, L.A.; Ahor, H.S.; Adjei-Kusi, P.; Maiga-Ascofare, O.; Phillips, R.O. Evaluation of a real-time recombinase polymerase amplification assay for rapid detection of Schistosoma haematobium infection in resource-limited setting. Acta Trop. 2021, 216, 105847. [Google Scholar] [CrossRef]
- Jannotti-Passos, L.K.; Vidigal, T.H.D.A.; Dias-Neto, E.; Pena, S.D.J.; Simpson, A.J.G.; Dutra, W.O.; Souza, C.P.; Carvalho-Parra, J.F. PCR Amplification of the Mitochondrial DNA Minisatellite Region to Detect Schistosoma mansoni Infection in Biomphalaria glabrata Snails. J. Parasitol. 1997, 83, 395–399. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Arahuetes, J.G.; Hernández, A.S.; López-Abán, J.; Santiago, B.V.; Muro, A. A Loop-Mediated Isothermal Amplification (LAMP) Assay for Early Detection of Schistosoma mansoni in Stool Samples: A Diagnostic Approach in a Murine Model. PLoS Negl. Trop. Dis. 2014, 8, e3126. [Google Scholar] [CrossRef]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Crego-Vicente, B.; Alonso-Castrillejo, S.; Febrer-Sendra, B.; Gómez-Sánchez, A.; Vicente, B.; López-Abán, J.; Muro, A. Progress in loop-mediated isothermal amplification assay for detection of Schistosoma mansoni DNA: Towards a ready-to-use test. Sci. Rep. 2019, 9, 14744. [Google Scholar] [CrossRef]
- Garcia-Bernalt, D.; Fernandez-Soto, P.; Alonso-Castrillejo, S.; Crego-Vicente, B.; Febrer, B.; Gomez, A.; Vicente, B.; Lopez-Aban, J.; Muro, A. Stabilization of SmMIT-LAMP reagents for application inn point-of-care diagnostic of schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 2019, 113 (Suppl. S1), S163. [Google Scholar]
- Camprubí, D.; Mendez, A.R.; Rodríguez, N.; Valls, M.E.; Garcia-Herrera, A.; Estrach, T.; Fustà, X.; Diego, J.G.-B.; Fernández, I.; Muñoz, J. A 38-year-old woman with zosteriform skin lesions. PLoS Negl. Trop. Dis. 2017, 11, e0005906. [Google Scholar] [CrossRef]
- Price, M.; Cyrs, A.; Sikasunge, C.S.; Mwansa, J.; Lodh, N. Testing the Infection Prevalence of Schistosoma mansoni after Mass Drug Administration by Comparing Sensitivity and Specificity of Species-Specific Repeat Fragment Amplification by PCR and Loop-Mediated Isothermal Amplification. Am. J. Trop. Med. Hyg. 2019, 101, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Lodh, N.; Mikita, K.; Bosompem, K.M.; Anyan, W.K.; Quartey, J.K.; Otchere, J.; Shiff, C.J. Point of care diagnosis of multiple schistosome parasites: Species-specific DNA detection in urine by loop-mediated isothermal amplification (LAMP). Acta Trop. 2017, 173, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lodh, N.; Mikita, K.; Bosompem, K.M.; Anyan, W.K.; Quartey, J.K.; Otchere, J.; Shiff, C.J. Point of care diagnosis for multiple schistosome parasites: Species-specific DNA detection from single urine sample by lamp and PCR. Am. J. Trop. Med. Hyg. 2017, 97 (Suppl. S1), 233. [Google Scholar]
- Lodh, N.; Mikita, K.; Shiff, C. Lamp: Point-of -care diagnosis for Schistosoma mansoni and S. haematobium. Am. J. Trop. Med. Hyg. 2015, 93, 158–159. [Google Scholar]
- Lodh, N.; Mikita, K.; Naples, J.M.; Bosompem, K.M.; Quartey, J.; Shiff, C.J. Detecting multi schistosome species dna in single urine samples by lamp: A novel diagnostic test for schistosomiasis. Am. J. Trop. Med. Hyg. 2014, 91 (Suppl. S1), 348. [Google Scholar]
- Lodh, N.; Pulkkila, B.; Price, M.; Alhakimi, S.; Sikasunge, C.; Mwansa, J. Prevalence of due-schistosome infection among school children after MDA in Zambia: Comparison of six different diagnostics. Trop. Med. Int. Health 2021, 26 (Suppl. S1), 76–77. [Google Scholar]
- Pulkkila, B.; Sikasunge, C.S.; Mwansa, J.; Lodh, N. POC-LAMP for human schistosomes comparative cost and time analysis for variable arrangements. Am. J. Trop. Med. Hyg. 2019, 101, 213. [Google Scholar]
- Crego-Vicente, B.; Fernández-Soto, P.; Febrer-Sendra, B.; Diego, J.G.-B.; Boissier, J.; Angora, E.; Oleaga, A.; Muro, A. Application of a Genus-Specific LAMP Assay for Schistosome Species to Detect Schistosoma haematobium x Schistosoma bovis Hybrids. J. Clin. Med. 2021, 10, 1308. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Avendaño, C.; Sala-Vizcaíno, A.; Crego-Vicente, B.; Febrer-Sendra, B.; Diego, J.G.-B.; Oleaga, A.; López-Abán, J.; Vicente, B.; Patarroyo, M.A.; et al. Molecular Markers for Detecting Schistosoma Species by Loop-Mediated Isothermal Amplification. Dis. Markers 2020, 2020, 8042705. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).