Greek Honey Authentication: Botanical Approach
Definition
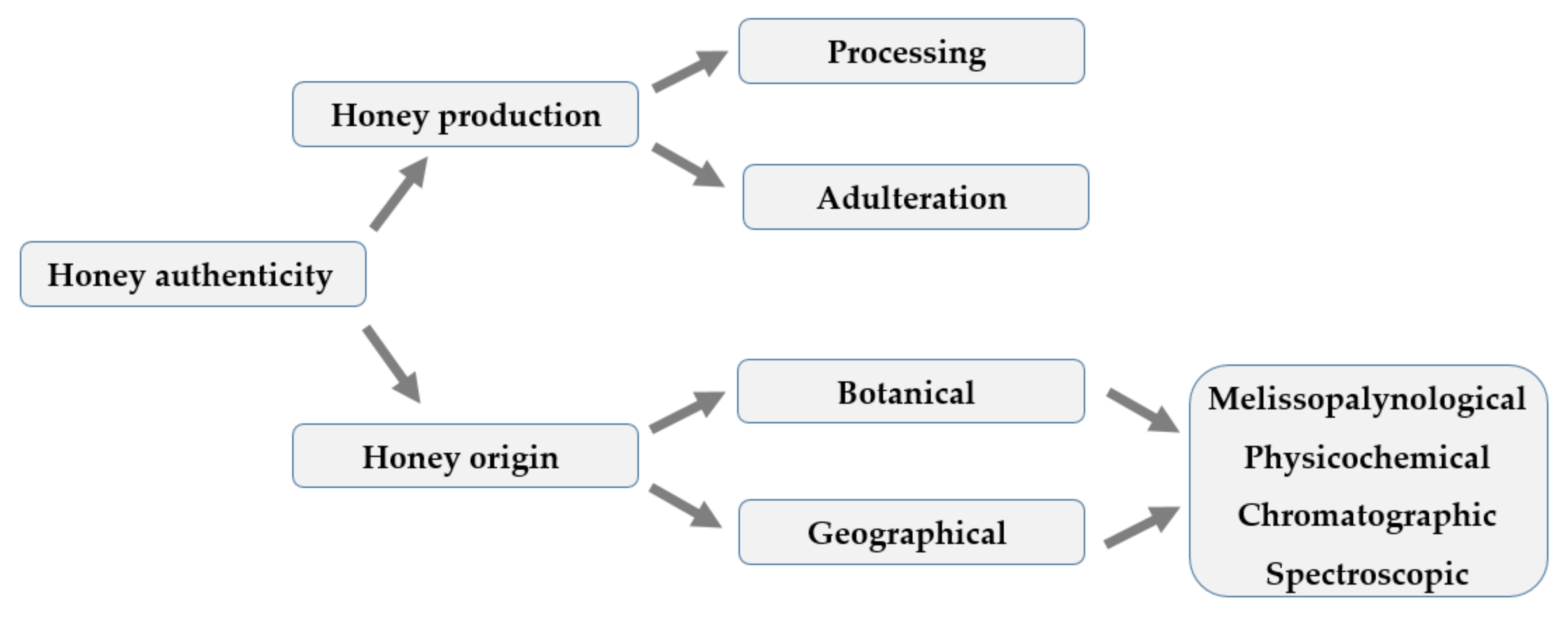
:1. Introduction and Research Field
2. Harvest, Honey Identity, and Authenticity Issues
2.1. Honey Harvesting
2.2. Classical Methods for Honey Authentication
2.3. Analytical Methods for Honey Authentication
2.3.1. Chromatographic Techniques
2.3.2. Spectroscopic Techniques
2.3.3. Other Analytical Techniques
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Entry Link on the Encyclopedia Platform
References
- EU. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. Off. J. Eur. Communities 2002, 10, 47–52. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:010:0047:0052:EN:PDF (accessed on 6 November 2021).
- Herrera, C.M. Gradual replacement of wild bees by honeybees in flowers of the Mediterranean Basin over the last 50 years. Proc. R. Soc. B Biol. Sci. 2020, 287. [Google Scholar] [CrossRef] [PubMed]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer: Berlin/Heidelberg, Germany, 1988; ISBN 978-3-642-72651. [Google Scholar]
- Government Gazette B-239/23-2-2005 Annex II Article 67 of Greek Food Code. 2005. Available online: http://www.minagric.gr/images/stories/docs/agrotis/MeliMelissokomia/KYA_Taytopoiisi_.pdf (accessed on 6 November 2021).
- Karoui, R. Food Authenticity and Fraud. In Chemical Analysis of Food: Techniques and Applications; Academic Press: Cambridge, MA, USA, 2012; pp. 499–517. ISBN 9780123848628. [Google Scholar]
- CEN Workshop Agreement (CWA) 17369:2019—Authenticity and Fraud in the Feed and Food Chain—Concepts, Terms, and Definitions. Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:68640,2273736&cs=1AE0F1E6D2455306ADD8460579462378C (accessed on 6 November 2021).
- Codex Alimentarius—Discussion Paper on Food Integrity and Food Authenticity—Joint FAO/WHO Food Standards Programme. Codex Committee on Food Import and Export Inspection and Certification Systems. Twenty-Fourth Session. Brisbane, Australia, 22–26 October 2018. CX/FICS 18/24/7. Available online: https://www.fao.org/fao-who-codexalimentarius/shproxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-733-24%252FWorking%2BDocuments%252Ffc24_07e.pdf (accessed on 6 November 2021).
- Puścion-Jakubik, A.; Borawska, M.H.; Socha, K. Modern methods for assessing the quality of Bee Honey and botanical origin identification. Foods 2020, 9, 1028. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Visser, F.R.; Allen, J.M.; Shaw, G.J. The effect of heat on the volatile flavour fraction from a unifloral honey. J. Apic. Res. 1988, 27, 175–181. [Google Scholar] [CrossRef]
- Baglio, E. Chemistry and Technology of Honey Production; Springer Briefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-65749-3. [Google Scholar]
- Thrasyvoulou, A.; Manikis, J. Some physicochemical and microscopic characteristics of Greek unifloral honeys. Apidologie 1995, 26, 441–452. [Google Scholar] [CrossRef]
- Karabournioti, S.E.; Tsiripidis, I.; Thrasyvoulou, A.; Eleftheriou, E.P. Melissopalynological attributes of some Greek thyme honeys. J. Apic. Res. 2009, 48, 104–114. [Google Scholar] [CrossRef]
- Dimou, M.; Tananaki, C.; Liolios, V.; Thrasyvoulou, A. Pollen foraging by honey bees (Apis Mellifera L.) in Greece: Botanical and geographical origin. J. Apic. Sci. 2014, 58, 11–23. [Google Scholar] [CrossRef]
- Tsigouri, A.; Passaloglou-Katrali, M.; Sabatakou, O. Palynological characteristics of different unifloral honeys from Greece. Grana 2004, 43, 122–128. [Google Scholar] [CrossRef]
- Xagoraris, M.; Lazarou, E.; Kaparakou, E.H.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Botanical origin discrimination of Greek honeys: Physicochemical parameters versus Raman spectroscopy. J. Sci. Food Agric. 2021, 101, 3319–3327. [Google Scholar] [CrossRef]
- Rodopoulou, M.A.; Tananaki, C.; Dimou, M.; Liolios, V.; Kanelis, D.; Goras, G.; Thrasyvoulou, A. The determination of the botanical origin in honeys with over-represented pollen: Combination of melissopalynological, sensory and physicochemical analysis. J. Sci. Food Agric. 2018, 98, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Tsiknakis, N.; Savvidaki, E.; Kafetzopoulos, S.; Manikis, G.; Vidakis, N.; Marias, K.; Alissandrakis, E. Segmenting 20 types of pollen grains for the cretan pollen dataset v1 (CPD-1). Appl. Sci. 2021, 11, 6657. [Google Scholar] [CrossRef]
- Von Der Ohe, W.; Oddo, L.P.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Rodopoulou, M.A.; Tananaki, C.; Kanelis, D.; Liolios, V.; Dimou, M.; Thrasyvoulou, A. A chemometric approach for the differentiation of 15 monofloral honeys based on physicochemical parameters. J. Sci. Food Agric. 2021, 102, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Badeka, A.V.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Botanical discrimination of Greek unifloral honeys with physico-chemical and chemometric analyses. Food Chem. 2014, 165, 181–190. [Google Scholar] [CrossRef]
- Louppis, A.P.; Karabagias, I.K.; Kontakos, S.; Kontominas, M.G.; Papastephanou, C. Botanical discrimination of Greek unifloral honeys based on mineral content in combination with physicochemical parameter analysis, using a validated chemometric approach. Microchem. J. 2017, 135, 180–189. [Google Scholar] [CrossRef]
- Karabagias, I.K. Seeking of reliable markers related to Greek nectar honey geographical and botanical origin identification based on sugar profile by HPLC-RI and electro-chemical parameters using multivariate statistics. Eur. Food Res. Technol. 2019, 245, 805–816. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Vavoura, M.V.; Nikolaou, C.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Floral authentication of Greek unifloral honeys based on the combination of phenolic compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014, 62, 753–760. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.A.; Proestos, C.; Thomaidis, N.S. Honey phenolic compound profiling and authenticity assessment using HRMS targeted and untargeted metabolomics. Molecules 2021, 26, 2769. [Google Scholar] [CrossRef]
- Tsiapara, A.V.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V.; Moutsatsou, P. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: Profile analysis of extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.; Harizanis, P.C. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 2003, 82, 575–582. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Kibaris, A.C.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Flavour compounds of Greek cotton honey. J. Sci. Food Agric. 2005, 85, 1444–1452. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Aroma investigation of unifloral Greek citrus honey using solid-phase microextraction coupled to gas chromatographic-mass spectrometric analysis. Food Chem. 2007, 100, 396–404. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Comparison of the volatile composition in thyme honeys from several origins in Greece. J. Agric. Food Chem. 2007, 55, 8152–8157. [Google Scholar] [CrossRef] [PubMed]
- Tananaki, C.; Thrasyvoulou, A.; Giraudel, J.L.; Montury, M. Determination of volatile characteristics of Greek and Turkish pine honey samples and their classification by using Kohonen self organising maps. Food Chem. 2007, 101, 1687–1693. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Tarantilis, P.A.; Harizanis, P.C.; Alissandrakis, E. Botanical discrimination and classification of honey samples applying gas chromatography/mass spectrometry fingerprinting of headspace volatile compounds. Food Chem. 2010, 121, 856–862. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Nikolaou, C.; Karabagias, V.K. Volatile fingerprints of common and rare honeys produced in Greece: In search of PHVMs with implementation of the honey code. Eur. Food Res. Technol. 2019, 245, 23–39. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Badeka, A.V. The Honey volatile code: A collective study and extended version. Foods 2019, 8, 508. [Google Scholar] [CrossRef]
- Xagoraris, M.; Revelou, P.K.; Dedegkika, S.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. SPME-GC-MS and FTIR-ATR spectroscopic study as a tool for unifloral common greek honeys’ botanical origin identification. Appl. Sci. 2021, 11, 3159. [Google Scholar] [CrossRef]
- Xagoraris, M.; Skouria, A.; Revelou, P.K.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Response surface methodology to optimize the isolation of dominant volatile compounds from monofloral greek thyme honey using SPME-GC-MS. Molecules 2021, 26, 3612. [Google Scholar] [CrossRef]
- Xagoraris, M.; Chrysoulaki, F.; Revelou, P.-K.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Unifloral autumn heather honey from indigenous Greek Erica manipuliflora Salisb.: SPME/GC-MS characterization of the volatile fraction and optimization of the isolation parameters. Foods 2021, 10, 2487. [Google Scholar] [CrossRef]
- Graikou, K.; Andreou, A.; Chinou, I. Chemical profile οf Greek Arbutus unedo honey: Biological properties. J. Apic. Res. 2021. [Google Scholar] [CrossRef]
- Orfanakis, E.; Markoulidakis, M.; Philippidis, A.; Zoumi, A.; Velegrakis, M. Optical spectroscopy methods combined with multivariate statistical analysis for the classification of Cretan thyme, multi-floral and honeydew honey. J. Sci. Food Agric. 2021, 101, 5337–5347. [Google Scholar] [CrossRef] [PubMed]
- Xagoraris, M.; Revelou, P.K.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. The use of right angle fluorescence spectroscopy to distinguish the botanical origin of Greek common honey varieties. Appl. Sci. 2021, 11, 4047. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Vlasiou, M.; Kontakos, S.; Drouza, C.; Kontominas, M.G.; Keramidas, A.D. Geographical discrimination of pine and fir honeys using multivariate analyses of major and minor honey components identified by 1H NMR and HPLC along with physicochemical data. Eur. Food Res. Technol. 2018, 244, 1249–1259. [Google Scholar] [CrossRef]
- Kazalaki, A.; Misiak, M.; Spyros, A.; Dais, P. Identification and quantitative determination of carbohydrate molecules in Greek honey by employing 13C NMR spectroscopy. Anal. Methods 2015, 7, 5962–5972. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017, 217, 445–455. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and classification of commercial thyme honeys produced in specific Mediterranean countries according to geographical origin, using physicochemical parameter values and mineral content in combination with chemometrics. Eur. Food Res. Technol. 2017, 243, 889–900. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Kontakos, S.; Drouza, C.; Papastephanou, C. Characterization and Botanical Differentiation of Monofloral and Multifloral Honeys Produced in Cyprus, Greece, and Egypt Using Physicochemical Parameter Analysis and Mineral Content in Conjunction with Supervised Statistical Techniques. J. Anal. Methods Chem. 2018, 1–10. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.P.; Halagarda, M.; Popek, S.; Georgiou, C.A. Geographical origin and botanical type honey authentication through elemental metabolomics via chemometrics. Food Chem. 2021, 338, 127936. [Google Scholar] [CrossRef] [PubMed]
- Louppis, A.P.; Karabagias, I.K.; Papastephanou, C.; Badeka, A. Two-way characterization of beekeepers’ honey according to botanical origin on the basis of mineral content analysis using ICP-OES implemented with multiple chemometric tools. Foods 2019, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Stefas, D.; Gyftokostas, N.; Couris, S. Laser induced breakdown spectroscopy for elemental analysis and discrimination of honey samples. Spectrochim. Acta-Part B At. Spectrosc. 2020, 172, 105969. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Casiello, G.; Kontakos, S.; Louppis, A.P.; Longobardi, F.; Kontominas, M.G. Investigating the impact of botanical origin and harvesting period on carbon stable isotope ratio values (13C/12C) and different parameter analysis of Greek unifloral honeys: A chemometric approach for correct botanical discrimination. Int. J. Food Sci. Technol. 2016, 51, 2460–2467. [Google Scholar] [CrossRef]

| Scientific Name | Flowering Period | Nectar | Pollen | Honeydew | Honey Name | Commercially Widespread |
|---|---|---|---|---|---|---|
| Blossom Honeys | ||||||
| Arbutus unedo L. | November–December | 3 * | 2 | - | Strawberry tree | + ** |
| Castanea sativa Miller | June | 2–3 | 3 | 1–2 | Chestnut | ++ |
| Ceratonia siliqua L. | September–October | 3 | 3 | 2 | Carob | + |
| Citrus spp. | March–April | 3 | 2 | - | Citrus, orange etc. | ++ |
| Erica arborea L. | October–November | 2–3 | 2–3 | - | Spring Heather | ++ |
| Erica manipuliflora Salisb. | March | 3 | 2–3 | - | Autumn Heather | ++ |
| Eucalyptus spp. | May–July | 2–3 | 2–3 | - | Eucalyptus | + |
| Gossypium hirsutum L. | July–September | - | - | Cotton | ++ | |
| Helianthis annuus L. | June–August | 2–3 | 2–3 | - | Sunflower | + |
| Paliurus spina-christi Miller | May–June | 2–3 | 2 | - | Jerusalem thorn | + |
| Phlomis spp. | 2–3 | - | - | Jerusalem sage | + | |
| Pimpinella anisum L. | 1–2 | 1–2 | - | Anise | + | |
| Polygonum aviculare L. | July–August | 2 | 2 | - | Common knotweed | + |
| Salvia officinalis L. | 2–3 | 2 | - | Sage | + | |
| Thymbra capitata L. | June–July | 2–3 | 2 | - | Thyme | +++ |
| Honeydew Honeys | ||||||
| Abies cephalonica Link. | May–July | - | - | 3 | Fir | ++ |
| Pinus spp. | March–April, June–August, September–October | - | - | 3 | Pine | +++ |
| Quercus spp. | - | 3 | 3 | Oak | + | |
| Pine | Fir | Chestnut | Heather | Thyme | Citrus | Cotton | Sunflower | |
|---|---|---|---|---|---|---|---|---|
| Moisture (%) | - | ≤18.5 | - | - | - | - | - | - |
| Electrical conductivity (Ms cm−1) | ≥0.9 | ≥1.0 | ≥1.1 | - | ≤0.6 | ≤0.45 | - | - |
| Main pollen (%) of pollen of nectar plants | - | - | ≥87 | ≥45 | ≥18 * | ≥3 | ≥3 | ≥20 |
| HDE/P ** | varies | varies | - | - | - | - | - | - |
| TPG/10g *** | varies | varies | ≥100,000 | - | <90,000 | <70,000 | <90,000 | <55,000 |
| major presence of characteristic honeydew elements | minor presence of characteristic honeydew elements | - | - | - | - | - | - |
| Analytical Technique | Abbreviation | Main Analytes and Parameters |
|---|---|---|
| Melissopalynological and Physicochemical techniques | ||
| Optical microscopy | OM | Pollen analysis |
| Scanning Electron Microscope | SEM | |
| Conductimetry | Electrical conductivity | |
| Refractometer | Moisture | |
| Colorimetry-Photometry | Diastase (Heat abuse) | |
| Hydroxymethylfurfural (HMF) (Heat abuse) | ||
| Potentiometry | Acidity | |
| International commission on Illumination | CIE | Lightness, color, hue |
| Viscometer | Rheological properties | |
| pH-meter | pH | |
| Chromatographic techniques | ||
| High-Performance Liquid Chromatography Diode-Array Detector | HPLC-DAD | Hydroxymethylfurfural (HMF) |
| Phenolics | ||
| High-Performance Liquid Chromatography Refractive Index Detector | HPLC-RID | Sugars |
| High-Performance Liquid Chromatography Fluorescence Detector | HPLC-FS | Amino acids |
| Phenolics | ||
| High-Performance Liquid Chromatography Pulsed Amperometric Detector | HPLC-PAD | Sugars |
| High-Performance Thin-Layer Chromatography | HPTLC | Phenolics |
| Non-volatile components | ||
| Sugars and/or fructose/glucose ratio | ||
| Hydroxymethylfurfural (HMF) | ||
| Liquid Chromatography Mass Spectrometry | LC-MS | Hydroxymethylfurfural (HMF) |
| Phenolics | ||
| Gas Chromatography Mass Spectrometry | GC-MS | Volatiles |
| Semi-volatiles | ||
| Spectroscopic techniques | ||
| Ultraviolet–Visible Spectroscopy | UV–Vis | Spectrum of phenolics |
| Raman Spectroscopy | Raman | Sugars spectra and minor components |
| Fourier-Transform Mid-Infrared Spectroscopy | FT-MIR | Sugars spectra and minor components |
| Fourier-Transform Near-Infrared Spectroscopy | FT-NIR | Sugars spectra and minor components |
| Fluorescence Spectroscopy | FS | Spectra of amino acids, phenolics, Maillard reaction by-products |
| Nuclear Magnetic Resonance | NMR | Sugars, untargeted and targeted screening |
| Other techniques | ||
| Isotope-Ration Mass Spectrometry | IRMS | Isotope ration of H, C, N, S, and/or 13C ratios |
| Inductively Coupled Plasma Mass Spectrometry | ICP-MS | Chemical elements |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xagoraris, M.; Revelou, P.-K.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Greek Honey Authentication: Botanical Approach. Encyclopedia 2021, 1, 1322-1333. https://doi.org/10.3390/encyclopedia1040099
Xagoraris M, Revelou P-K, Alissandrakis E, Tarantilis PA, Pappas CS. Greek Honey Authentication: Botanical Approach. Encyclopedia. 2021; 1(4):1322-1333. https://doi.org/10.3390/encyclopedia1040099
Chicago/Turabian StyleXagoraris, Marinos, Panagiota-Kyriaki Revelou, Eleftherios Alissandrakis, Petros A. Tarantilis, and Christos S. Pappas. 2021. "Greek Honey Authentication: Botanical Approach" Encyclopedia 1, no. 4: 1322-1333. https://doi.org/10.3390/encyclopedia1040099
APA StyleXagoraris, M., Revelou, P.-K., Alissandrakis, E., Tarantilis, P. A., & Pappas, C. S. (2021). Greek Honey Authentication: Botanical Approach. Encyclopedia, 1(4), 1322-1333. https://doi.org/10.3390/encyclopedia1040099










