Arbuscular Mycorrhizal Fungi in Agriculture
Definition
1. Introduction
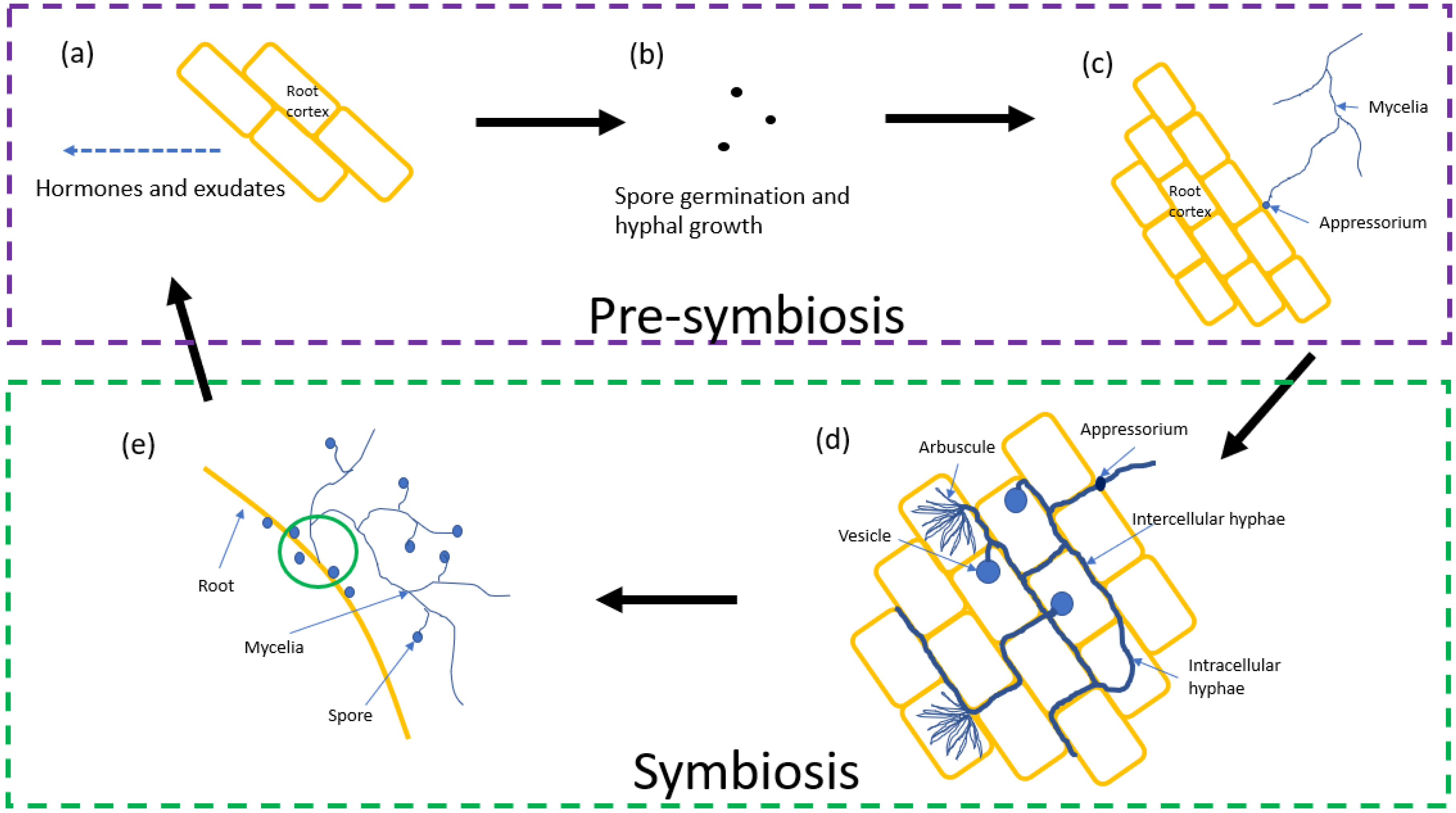
2. Life Cycle
2.1. Pre-Symbiosis
2.2. Symbiosis
3. Mycorrhizal–Host Nutrient Exchange
3.1. Carbon
3.2. Nitrogen
3.3. Potassium
3.4. Phosphorus
3.5. Fatty Acids
3.6. Sulphur
3.7. Micronutrients
4. Arbuscular Mycorrhizal Fungi and Soil Structure
Soil Glomalin
5. Tillage
5.1. Tillage and AM Fungi
5.2. Tillage, Glomalin and Soil Erosion
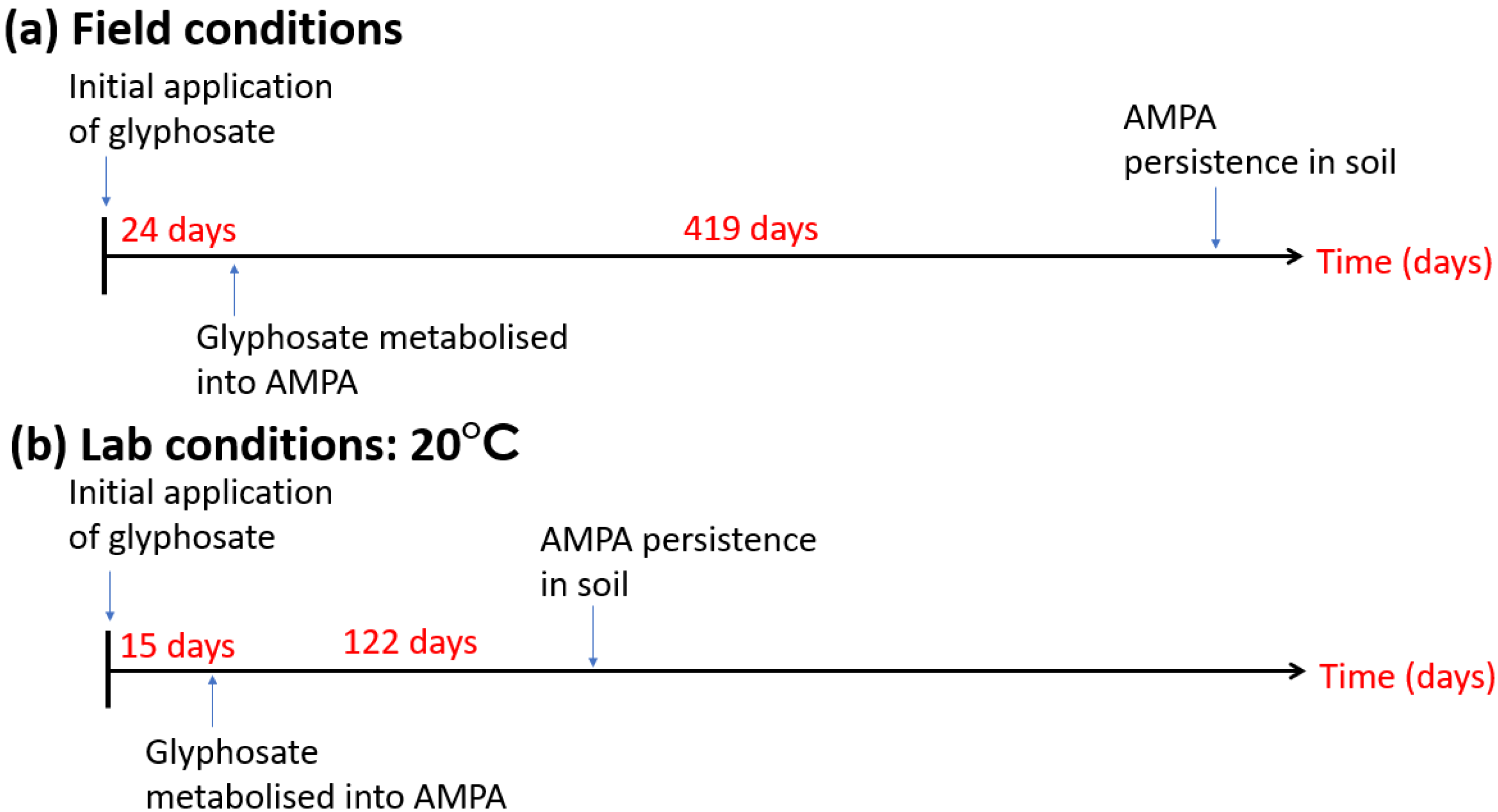
6. AM Fungi and Agrochemicals
7. Abiotic Management of AM Fungi
8. Summary
Funding
Acknowledgments
Conflicts of Interest
Entry Link on the Encyclopedia Platform
References
- Rillig, M.; Wright, S.; Eviner, V. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2002, 2, 325–333. [Google Scholar] [CrossRef]
- Kottke, I.; Nebel, M. The evolution of mycorrhiza-like associations in liverworts: An update. New Phytol. 2005, 167, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Dastogeer, K.M.G.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant Salinity Tolerance Conferred by Arbuscular Mycorrhizal Fungi and Associated Mechanisms: A Meta-Analysis. Front. Plant Sci. 2020, 11, 1927. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1996, 198, 97–107. [Google Scholar] [CrossRef]
- Wright, S.F.; Frankee-Snyder, M.; Morton, J.B. Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil 1996, 181, 193–203. [Google Scholar] [CrossRef]
- Pohanka, M.; Vlcek, V. Immunoassay of Glomalin by Quartz Crystal Microbalance Biosensor Containing Iron Oxide Nanoparticles. Int. J. Anal. Chem. 2020, 2020, 8844151. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, X.; Lio, Y. Effect of Tillage Treatment on the Diversity of Soil Arbuscular Mycorrhizal Fungal and Soil Aggregate-Associated Carbon Content. Front. Microbiol. 2018, 9, 2986. [Google Scholar] [CrossRef] [PubMed]
- Burri, K.; Groke, C.; Graf, F. Mycorrhizal fungi protect the soil form wind erosion: A wind tunnel study. Land Degrad. Dev. 2011, 24, 292–385. [Google Scholar] [CrossRef]
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. Zero Tillage Systems Conserve Arbuscular Mycorrhizal Fungi, Enhancing Soil Glomalin and Water Stable Aggregates with Implications for Soil Stability. Soil Syst. 2021, 5, 4. [Google Scholar] [CrossRef]
- Jiang, X.; Alan, L.; Wright, X.; Wang, F.; Liang, L. Tillage-induced changes in fungal and bacterial biomass associated with soil aggregates: A long-term field study in a subtropical rice soil in China. Appl. Soil Ecol. 2011, 48, 168–173. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Omacini, M.; Druille, M.; Salminen, J.P.; Saikkonen, K. Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 2018, 642, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Zaller, J.G.; Heigl, F.; Ruess, L.; Grabmaier, A. Glyphosate herbicide affects belowground interactions between earthworms and symbiotic mycorrhizal fungi in a model ecosystem. Sci. Rep. 2014, 4, 5634. [Google Scholar] [CrossRef]
- AHDB. Taking a Look at UK Crop Production 2020/21. 2020. Available online: https://ahdb.org.uk/news/taking-a-look-at-uk-crop-production-2020-21 (accessed on 15 July 2020).
- Berruit, A.; Lumini, E.; Baletini, B.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, T.I.; Warner, D.J.; Davies, K.G.; Edmonds-Brown, V. Tillage, Glyphosate and Beneficial Arbuscular Mycorrhizal Fungi: Optimising Crop Management for Plant–Fungal Symbiosis. Agriculture 2020, 10, 520. [Google Scholar] [CrossRef]
- Martins, S.; Medeiros, F.; Lakshamanan, V.; Bias, H. Impact of Seed Exudates on Growth and Biofilm Formation of Bacillus amyloliquefaciens ALB629 in Common Bean. Front. Microbiol. 2018, 8, 2631. [Google Scholar] [CrossRef] [PubMed]
- Smith, S. Q&A: What are strigolactones and why are they important to plants and soil microbes? BMC Biol. 2014, 12, 19. [Google Scholar]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Buee, M.; Rossignol, M.; Jauneau, A.; Ranjeva, R.; Bécard, G. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol. Plant Microbe Interact. 2000, 13, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Thirkell, T.; Pastok, D.; Field, K. Carbon for nutrient exchange between arbuscular mycorrhizal fungi and wheat varieties according to cultivar and changes atmospheric carbon dioxide concentration. Glob. Chang. Biol. 2019, 26, 1725–1738. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Balestrini, R.; Bonfante, P. Cell wall remodeling in mycorrhizal symbiosis: A way towards biotrophism. Front. Plant Sci. 2014, 5, 237. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Harrison, M.J. Accumulation of phosphoinositides in distinct regions of the periarbuscular membrane. New Phytol. 2019, 221, 2213–2227. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Anca, I.-A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 35, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Kariman, K.; Barker, S.J.; Tibbett, M. Structural plasticity in root-fungal symbioses: Diverse interactions lead to improved plant fitness. PeerJ 2018, 6, e6030. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Avio, L.; Fortuna, P.; Pellegrino, E.; Sbrana, C.; Strani, P. At the Root of the Wood Wide Web. Plant Signal. Behav. 2006, 1, 1–5. [Google Scholar] [CrossRef]
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. A comparison of methodologies for the staining and quantification of intracellular components of arbuscular mycorrhizal fungi in the root cortex of two varieties of winter wheat. Access Microbiol. 2020, 1. [Google Scholar] [CrossRef]
- Lerat, S.; Lapointe, L.; Gutjahr, S.; Piche, Y.; Vierheilig, H. Carbon partitioning in a split-root system of arbuscular mycorrhizal plants is fungal and plant species dependent. New Phytol. 2003, 157, 589–595. [Google Scholar] [CrossRef]
- Blanke, V.; Wagner, M.; Renker, C.; Lippert, H.; Michulitz, M.; Kuhn, A.J.; Buscot, F. Arbuscular mycorrhizas in phosphate-polluted soil: Interrelations between root colonization and nitrogen. Plant Soil 2011, 343, 379–392. [Google Scholar] [CrossRef]
- Olsson, P.A.; Rahm, J.; Aliasgharzad, N. Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol. Ecol. 2010, 72, 123–131. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 2008. [Google Scholar]
- Bago, B.; Pfeffer, P.E.; Abubaker, J.; Jun, J.; Allen, J.W.; Brouillette, J.; Douds, D.D.; Lammers, P.J.; Shachar-Hill, Y. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol. 2003, 131, 1496–1507. [Google Scholar] [CrossRef]
- Schubert, A.; Allara, P.; Morte, A. Cleavage of sucrose in roots of soybean (Glycine max) colonized by an arbuscular mycorrhizal fungus. New Phytol. 2004, 1, 495–501. [Google Scholar] [CrossRef]
- Doidy, J.; van Tuinen, D.; Lamotte, O.; Corneillat, M.; Alcaraz, G.; Wipf, D. The Medicago truncatula sucrose transporter family: Characterization and implication of key members in carbon partitioning towards arbuscular mycorrhizal fungi. Mol. Plant 2012, 5, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Becard, G.; Doner, L.W.; Rolin, D.B.; Douds, D.D.; Pfeffer, P.E. Identification and quantification of trehalose in vesicular arbuscular mycorrhizal fungi by in vivo C-13 NMR and HPLC analyses. New Phytol. 1991, 118, 547–552. [Google Scholar] [CrossRef]
- Morgan, J.B.; Connolly, E.L. Plant-Soil Interactions: Nutrient Uptake. Nat. Educ. Knowl. 2013, 4, 2. [Google Scholar]
- Johansen, A.; Jensen, E.S. Transfer of N and P from intact or decomposing roots of pea to barley interconnected by an arbuscular mycorrhizal fungus. Soil Biol. Biochem. 1996, 28, 73–81. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, J.-P.; St-Arnoud, M.; Charest, C. Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartmented system. Can. J. Microbiol. 2004, 50, 251–260. [Google Scholar] [PubMed]
- Tian, J.; Dippold, M.; Pausch, J.; Blagodatskaya, E.; Fan, M.; Li, X.; Kuzyakov, Y. Microbial response to rhizodeposition depending on water regimes in paddy soils. Soil Biol. Biochem. 2013, 65, 195–203. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.; Jin, H.; Abubaker, J.; Douds, D.; Allen, J.; Bucking, H.; Lammers, P.; Schachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, S.; Perez-Tienda, J.; Ellerbeck, M.; Arnould, C.; Chatagnier, O.; Boller, T.; Schussler, A.; Brachmann, A.; Wipf, D.; Ferrol, N. GintAMT3-a low-affinity ammonium transporter of the arbuscular mycorrhizal Rhizophagus irregularis. Front. Plant Sci. 2016, 7, 679. [Google Scholar] [CrossRef]
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009, 181, 199–207. [Google Scholar] [CrossRef]
- Breuillin-Sessoms, F.; Floss, D.S.; Gomez, S.K.; Pumplin, N.; Ding, Y.; Levesque-Tremblay, V.; Noar, R.D.; Daniels, D.A.; Bravo, A.; Eaglesham, J.B. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 2015, 27, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Zimmermann, S. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337. [Google Scholar] [CrossRef] [PubMed]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.A.; Hammer, E.C.; Wallander, H.; Pallon, J. Phosphorus availability influences elemental uptake in the mycorrhizal fungus Glomus intraradices, as revealed by particle-induced X-ray emission analysis. Appl. Environ. Microbiol. 2008, 74, 4144–4148. [Google Scholar] [CrossRef]
- Casieri, L.; Lahmidi, N.A.; Doidy, J.; Veneault-Fourrey, C.; Migeon, A.; Bonneau, L. Biotrophic transportome in mutualistic plant fungal interactions. Mycorrhiza 2013, 23, 597–625. [Google Scholar] [CrossRef]
- Rabie, G.G.; Almadini, A.M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotechnol. 2005, 4, 210–222. [Google Scholar]
- Estrada, B.; Aroca, R.; Maathuis, F.J.M.; Barea, J.M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 2013, 36, 1771–1782. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, S.; Hu, W.; Xiao, L.; Tang, M. Arbuscular mycorrhizal fungus Rhizophagus irregularis increased potassium content and expression of genes encoding potassium channels in Lycium barbarum. Front. Plant Sci. 2017, 8, 440. [Google Scholar] [CrossRef]
- Walder, F.; Boller, T.; Wiemken, A.; Courty, P.E. Regulation of plants’ phosphate uptake in common mycorrhizal networks: Role of intraradical fungal phosphate transporters. Plant Signal. Behav. 2016, 11, e1131372. [Google Scholar] [CrossRef]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef]
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Kobae, Y.; Ohmori, Y.; Saito, C.; Yano, K.; Ohtomo, R.; Fujiwara, T. Phosphate treatment strongly inhibits new arbuscule development but not the maintenance of arbuscule in mycorrhizal rice roots. Plant Physiol. 2016, 171, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Balzergue, C.; Chabaud, M.; Barker, D.G.; Becard, G.; Rochange, S.F. High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front. Plant Sci. 2013, 4, 426. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef]
- Wang, E.; Schornack, S.; Marsh, J.F.; Gobbato, E.; Schwessinger, B.; Eastmond, P.; Schultze, M.; Kamoun, S.; Oldroyd, G.E. A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr. Biol. 2012, 22, 2242–2246. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, X.; Zhu, H.; Yao, Q. Responses of Arbuscular Mycorrhizal Symbiosis to Abiotic Stress: A Lipid-Centric Perspective. Front. Plant Sci. 2020, 11, 578919. [Google Scholar] [CrossRef]
- Wewer, V.; Brands, M.; Dörmann, P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 2014, 79, 398–412. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, X.; Feng, G.; Zhu, H.; Yao, Q. Linking lipid transfer with reduced arbuscule formation in tomato roots colonized by arbuscular mycorrhizal fungus under low pH stress. Environ. Microbiol. 2020, 22, 1036–1051. [Google Scholar] [CrossRef]
- Salvioli, A.; Ghignone, S.; Novero, M.; Navazio, L.; Venice, F.; Bagnaresi, P.; Bonfante, P. Symbiosis with an endobacterium increases the fitness of a mycorrhizal fungus, raising its bioenergetic potential. ISME J. 2016, 10, 130–144. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Fournier, S.; Chervin, C.; Becard, G.; Rochange, S.; Puech-Pages, V. Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886. [Google Scholar] [CrossRef] [PubMed]
- Ropars, J.; Toro, K.S.; Noel, J.; Pelin, A.; Charron, P.; Farinelli, L.; Marton, T.; Krüger, M.; Fuchs, J.; Brachmann, A.; et al. Evidence for the sexual origin of heterokaryosis in arbuscular mycorrhizal fungi. Nat. Microbiol. 2016, 1, 16033. [Google Scholar] [CrossRef] [PubMed]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.; Delaux, P.; Klingl, V.; Röpenack-Lahaye, E.; Wang, T.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. eLife 2017, 6, e29107. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.; Stenke, A.; Peter, T.; Hinckley, E.L.S.; Driscoll, C.T.; Winkel, L.H. Reductions in the deposition of sulfur and selenium to agricultural soils pose risk of future nutrient deficiencies. Commun. Earth Environ. 2021, 2, 101. [Google Scholar] [CrossRef]
- Gahan, J.; Schmalenberger, A. The role of bacteria and mycorrhiza in plant sulfur supply. Front. Plant Sci. 2014, 5, 723. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Mirleau, P. The role of microbes in plant sulphur supply. J. Exp. Bot. 2004, 55, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, P.; Escamez, S.; Trouverie, J.; Avice, J.-C. Sulphur limitation provokes physiological and leaf proteome changes in oilseed rape that lead to perturbation of sulphur, carbon and oxidative metabolisms. BMC Plant Biol. 2013, 13, 23. [Google Scholar] [CrossRef]
- Allen, J.; Shachar-Hill, Y. Sulfur transfer through an arbuscular mycorrhiza. Plant Physiol. 2008, 149, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Buchner, P.; Takahashi, H.; Hawkesford, M. Plant sulphate transporters: Co-ordination of uptake, intracellular and long-distance transport. J. Exp. Bot. 2014, 55, 1765–1773. [Google Scholar] [CrossRef]
- Cregut, M.; Piutti, S.; Slezack-Deschaumes, S.; Benizri, R. Compartmentalization and regulation of arylsulfatase activites in Streptomyces sp., Microbacterium sp. and Rhodococcis sp. soil isolates in response to inorganic sulfate limitation. Microbiol. Res. 2013, 168, 12–21. [Google Scholar] [CrossRef]
- Joner, E.; Briomes, R.; Leyual, C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 2000, 226, 227–234. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Palmer, C.; Guerinot, M.L. A question of balance: Facing the challenges of Cu, Fe, and Zn homeostasis. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, E.; Gomez-Gallego, T.; Azcon-Aguilar, C.; Ferrol, N. Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 2014, 4, 547. [Google Scholar] [CrossRef] [PubMed]
- Lehman, A.; Rillig, M.C. Arbuscular mycorrhizal contributions to uptake of metal cations by cucumber plants at two levels of phosphorus supply. Plant Soil 2015, 278, 361–370. [Google Scholar]
- Schubler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Genera; The Royal Botanic Garden Edinburgh: Edinburgh, Scotland; The Royal Botanic Garden Kew: Richmond, UK; Botanische Staatammlung Munich: München, Germany; Oregon State University: Corvallis, OR, USA, 2010. [Google Scholar]
- Gonzalez-Guerrero, M.; Escudero, V.; Saez, A.; Tejada-Jimenez, M. Transition metal transport in pland and associated endosymbionts: Arbuscular mycorrhizal fungi and rhizobia. Front. Plant Sci. 2016, 7, 1088. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Cavagnaro, T.R. Nutrient interactions and arbsuclar mycorrhizas: A meta-analysis of a mycorrhiza-defective mutant and wild-type tomato genotype pair. Plant Soil 2014, 384, 79–92. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Patti, A.F.; Cavagnaro, T.R. Arbuscular mycorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 2013, 371, 299–312. [Google Scholar] [CrossRef]
- Jansa, J.; Mozafar, A.; Frossard, E. Long-distance transport of P and Zn through the hyphae of an arbuscular mycorrhizal fungus in symbiosis with maise. Agronomie 2003, 23, 481–488. [Google Scholar] [CrossRef]
- Driver, J.D.; Holben, W.E.; Rillig, M.C. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 101–106. [Google Scholar] [CrossRef]
- Li, A.-R.; Smith, S.E.; Smith, A.F.; Guan, K.-Y. Inoculation with arbuscular mycorrhizal fungi suppresses initiation of haustoria in the root hemiparasite Pedicularis tricolor. Ann. Bot. 2012, 109, 1075–1080. [Google Scholar] [CrossRef]
- Larson, W.; Pierce, F. The dynamics of soil quality as a measure of sustainable management. In Defining Soil Quality for a Sustainable Environment; Wiley: Madison, WI, USA, 1994; pp. 37–51. [Google Scholar]
- Lovelock, C.E.; Wright, S.F.; Clark, D.A.; Ruess, R.W. Soil stocks of glomalin produced by arbuscular mycorrhizal fungi across a tropical rain forest landscape. J. Ecol. 2004, 92, 278–287. [Google Scholar] [CrossRef]
- Bendini, S.; Pellegrino, E.; Avio, L.; Pellegrino, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1469–1494. [Google Scholar]
- Adeleke, A. Effect of Arbuscular mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria on Glomalin Production. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2010. [Google Scholar]
- Singh, P.; Singh, M.; Tripathi, B. Glomalin: An arbuscular mycorrhizal fungal soil protein. Protoplasma 2012, 250, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Walley, F.; Gilliespie, A.; Adetona, A.; Germinda, J.; Farrell, R. Manipulation of rhizosphere organisms to enhance glomalin production and C sequestration: Pitfalls and promises. Can. J. Plant Sci. 2013, 94, 1025–1032. [Google Scholar] [CrossRef]
- Prassad, M.; Chaudhary, M.; Ramakrishnan, S.; Mahawer, S. Glomalin: A miracle protein for soil sustainability. Indian Farmer 2018, 5, 1092–1100. [Google Scholar]
- Lee, E.-H.; Eo, J.-K.; Ka, K.-H.; Eom, A.-H. Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology 2013, 41, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, L.; Palese, A.M.; Grasso, F.; Duffy, D.H., III; Bati, C.B.; Xiloyannis, C. Mechanical Tillage Diversely Affects Glomalin Content, Water Stable Aggregates and AM Fungal Community in the Soil Profiles of Two Differently Managed Olive Orchards. Biomolecules 2019, 9, 639. [Google Scholar] [CrossRef]
- Sharifi, Z.; Azadi, N.; Rahimi, S.; Certini, G. The response of glomalin-related soil proteins to fire or tillage. Geoderma 2018, 329, 65–72. [Google Scholar] [CrossRef]
- Asmelash, F.; Bekele, T.; Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernandez, M.; Leifheit, E.; Ingraffia, R.; Rillig, M. Subsoil Arbuscular Mycorrhizal Fungi for Sustainability and Climate-Smart Agriculture: A Solution Right under Our Feet? Front. Microbiol. 2019, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Kabir, Z. Tillage or no-tillage: Impact on mycorrhizae. Can. J. Plant Sci. 2005, 85, 23–29. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; van der Heijden, M.; Oehl, F. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Zheng, J.; Luo, Y.; Li, R.; Wang, H.; Qi, H. Effect of long-term tillage on soil aggregates and aggregate-associated carbon in black soil of Northeast China. PLoS ONE 2018, 13, e0199523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Pu, C.; Zhang, X.; Xue, J.; Ren, Y.; Zhao, X.; Chen, F.; Lal, R.; Zhang, H. Crop yields under no-till farming in China: A meta-analysis. Eur. J. Agron. 2017, 84, 67–75. [Google Scholar] [CrossRef]
- Ferrira, C.; da Silva Neta, C.; Pereira, M.; Guede, J.; Rosset, J.; Anjos, L. Dynamics of soil aggregation and organic carbon fractions over 23 years of no-till management. Soil Tillage Res. 2020, 198, 104533. [Google Scholar] [CrossRef]
- Crittenden, S.J.; Poot, N.; Heinen, M.; van Balen, D.J.M.; Pulleman, M.M. Soil physical quality in contrasting tillage systems in organic and conventional farming. Soil Tillage Res. 2015, 154, 136–144. [Google Scholar] [CrossRef]
- Sheehy, J.; Regina, K.; Alakukku, L.; Six, J. Impact of no-till and reduced tillage on aggregation and aggregate-associated carbon in Northern European agroecosystems. Soil Tillage Res. 2015, 150, 107–113. [Google Scholar] [CrossRef]
- Dai, R.J.; Pang, X.G.; Zeng, X.D.; Wang, H.J. Soil carbon density and distribution and influencing factors in Shandong Province. Res. Environ. Sci. 2015, 28, 1449–1458. [Google Scholar]
- Ita, B.N.; Ariga, E.S.; Michieka, R.W.; Muiru, W.M. Comparative Efficiency of Tillage Practices in Maize. Curr. Agric. Res. J. 2014, 2. [Google Scholar] [CrossRef]
- Moussa-Machraoui, S.B.; Errouissi, F.; Ben-Hammouda, M.; Nouira, S. Comparative effects of conventional and no-tillage management on some soil properties under Mediterranean semi-arid conditions in northwestern Tunisia. Soil Tillage Res. 2010, 106, 247–253. [Google Scholar] [CrossRef]
- Moroke, T.S.; Dikinya, O.; Patrick, C. Comparative assessment of water infiltration of soils under different tillage systems in eastern Botswana. Phys. Chem. Earth 2009, 34, 316–323. [Google Scholar] [CrossRef]
- Grange, I.; Prammanee, P.; Prasertsak, P. Comparative Analysis of Different Tillage Systems Used in Sugarcane (Thailand). Aust. Farm Bus. Manag. J. 2005, 2, 46–50. [Google Scholar]
- Stanila, S.; Drocas, I.; Molnar, A.; Ranta, O. Studies Regarding Comparative Fuel Consumption at Classical and Conservation Tillage. ProEnviron. Promediu 2013, 6, 199–202. [Google Scholar]
- Saglam, R.; Seven, L.; Kup, F. Comparative analysis of energy input-outputs of different tillage methods in second crop corn production. Not. Sci. Biol. 2020, 12, 356–365. [Google Scholar] [CrossRef]
- Elliot, A.; Daniell, T.; Cameron, D.; Field, K. A commercial arbuscular mycorrhizal inoculm increases root colonization across wheat cultivars but does not increase assimilation of mycorrhiza-acquired nutrients. New Phytol. Found. 2020, 3, 588–599. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; Carvalho, M.; Chatagnier, O.; Tuinen, D. Impact of tillage system on arbuscular mycorrhiza fungal communities in the soil under Mediterranean conditions. Soil Tillage Res. 2012, 121, 63–67. [Google Scholar] [CrossRef]
- Kapoor, R. Arbuscular Mycorrhiza and Reactive Oxygen Species. In Arbuscular Mycorrhiza and Stress Tolerance of Plants; Neeraja, S., Ed.; Springer: Singapore, 2017; pp. 225–243. [Google Scholar]
- Douds, D.D.; Nagahashi, G. Signalling and Recognition Events Prior to Colonisation of Roots by Arbuscular Mycorrhizal Fungi. In Current Advances in Mycorrhizae Research; Podila, G.K., Douds, D.D., Eds.; APS Press: Saint Paul, MN, USA, 2000. [Google Scholar]
- Castillo, C.; Rubio, R.; Rouanet, J.L.; Borie, F. Early effects of tillage and crop rotation on arbuscular mycorrhizal fungal propagules in an Ultisol. Biol. Fertil. Soils 2006, 43, 83–92. [Google Scholar] [CrossRef]
- Bernola, L.; Cange, C.; Way, M.; Gore, J.; Hardke, J.; Stout, M. Natural Colonization of Rice by Arbuscular Mycorrhizal Fungi in Different Production Areas. Rice Sci. 2018, 25, 169–174. [Google Scholar] [CrossRef]
- Galvez, L.; Douds, D.; Wagoner, P. Tillage and farming system affect AM fungus populations, mycorrhizal formation, and nutrient uptake by winter wheat in a high-P soil. Am. J. Altern. Agric. 2001, 16, 152–160. [Google Scholar] [CrossRef]
- Sharma-Poudyal, D.; Schlatter, D.; Yin, C.; Hulbert, S.; Paulitz, T. Long-term no-till: A major driver of fungal communities in dryland wheat cropping systems. PLoS ONE 2017, 12, e0184611. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2008, 435, 824–827. [Google Scholar] [CrossRef]
- Weetle, J.D.; Abril, M.; Blackwell, M. Phylogenetic distribution of fungal sterols. PLoS ONE 2010, 5, e10899. [Google Scholar] [CrossRef]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evol. Int. J. Org. Evol. 2012, 66, 2961–2968. [Google Scholar] [CrossRef]
- Wilson, G.W.T.; Rice, C.; Rillig, M.C.; Springer, A.; Hartnett, D. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef]
- Hontoria, C.; Velásquez, R.; Benito, M.; Almorox, J.; Moliner, A. Bradford-reactive soil proteins and aggregate stability under abandonedversus tilled olive groves in a semi-arid calcisol. Soil Biol. Biochem. 2009, 41, 1583–1585. [Google Scholar] [CrossRef]
- Curaqueo, G.; Barea, J.; Acevedo, E.; Rubio, R.; Cornejo, P.; Borie, F. Effects of different tillage system on arbuscular mycorrhizal fungal propagules and physical properties in a Mediterranean agroecosystem in central Chile. Soil Tillage Res. 2011, 113, 11–18. [Google Scholar] [CrossRef]
- Nautiyal, P.; Rajput, R.; Pandey, D.; Arunachalam, K.; Arunachalam, A. Role of glomalin in soil carbon storage and its variation across land uses in temperate Himalayan regime. Biocatal. Agric. Biotechnol. 2019, 21, 101311. [Google Scholar] [CrossRef]
- Wright, S.F.; Green, V.S.; Cavigelli, M.A. Glomalin in aggregate size classes from three different farming systems. Soil Tillage Res. 2007, 94, 546–549. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2016, 9, 685–692. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessmentsand management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- AHDB (Agriculture and Horticulture Development Board). Nutrient Management Guide (RB209), Section 4 Arable Crops; AHDB: Stoneleigh, UK, 2018. [Google Scholar]
- Battaglin, W.A.; Kolpin, D.W.; Scribner, E.A.; Kuivila, K.M.; Sandstrom, M.W. Glyphosate, other herbicides, and transformation products in Midwestwern streams. J. Am. Water Resour. Assoc. 2005, 41, 323–332. [Google Scholar] [CrossRef]
- Xu, J.J.; Fang, X.; Li, C.Y.; Yang, L.; Chen, X.Y. General and specialized tyrosine metabolism pathways in plants. aBIOTECH 2019, 1, 97–105. [Google Scholar] [CrossRef]
- Palme, K.; Nagy, F. A new gene for auxin synthesis. Cell 2008, 133, 31–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neumann, E.; Kohls, S.; Landsberg, E.; Stock-Olivera, K.; Yamada, T.; Romheld, V. Relevance of glyphosate transfer to non-target plants via the rhizosphere. J. Plant Dis. Prot. 2006, 20, 963. [Google Scholar]
- Druille, M.; Omacini, M.; Golluscio, R.A. Glyphosate reduces spore viability and root colonization of arbuscular mycorrhizal fungi. Appl. Soil Ecol. 2013, 64, 99–103. [Google Scholar] [CrossRef]
- Bott, S.; Tesfamariam, T.; Kania, A.; Eman, B.; Aslan, N.; Romheld, V.; Neumann, G. Phytotoxicity of glyphosate soil residues re-mobilised by phosphate fertilization. Plant Soil 2011, 342, 249–263. [Google Scholar] [CrossRef]
- Druille, M.; Omacini, M.; Golluscio, R.A.; Cabello, M.N. Arbuscular mycorrhizal fungi are directly and indirectly affected by glyphosate application. Appl. Soil Ecol. 2013, 72, 143–149. [Google Scholar] [CrossRef]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar]
- Schonbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.S.; Kabsch, W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthasein atomic detail. Proc. Natl. Acad. Sci. USA 2001, 98, 1376–1380. [Google Scholar] [CrossRef]
- Montgomery, H.J.; Monreal, C.; Young, J.C.; Seifert, K. Determination of soil fungal biomass from ergosterolanalyses. Soil Biol. Biochem. 2000, 32, 1207–1217. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes Vertical Distribution of Soil Bacterial and Fungal Communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Marin, M.; Ybara, M.; Fe, A.; Garcia-Ferriz, L. Effects of arbsulr mycorrhizal fungi and pesticides on Cyara cardunculus growth. Agric. Food Sci. Finl. 2002, 11, 245–251. [Google Scholar] [CrossRef]
- Jin, H.; Germida, J.; Walley, F. Suppressive effects of seed-applied fungicides on arbuscular mycorrhizal fungi (AMF) differ with fungicide mode of action and AMF species. Appl. Soil Ecol. 2013, 72, 22–30. [Google Scholar] [CrossRef]
- Burrows, R.L.; Ahmed, I. Fungicide seed treatments minimally affect arbuscular-mycorrhizal fungal (AMF) colonization of selected vegetable crops. J. Biol. Sci. 2007, 7, 417–420. [Google Scholar] [CrossRef][Green Version]
- Channabasava, H.; Jorquera, M. Effects of fungicides on association of arbuscular mycorrhiza fungus Rhizophagus fasciculatus and growth of Proso millet (Panicum miliaceum L.). J. Soil Sci. Plant Nutr. 2015, 15, 35–45. [Google Scholar] [CrossRef]
- Kjoller, R.; Rosendahl, S. Effects of fungicides on arbuscular mycorrhizal fungi: Differential responses in alkaline phosphatase activity of external and internal hyphae. Biol. Fertil. Soils 2000, 31, 361–365. [Google Scholar] [CrossRef]
- Rose, D.J.; Santra, D.K. Proso millet (Panicum miliaceum L.) fermentation for fuel ethanol production. Ind. Crops Prod. 2013, 43, 602–605. [Google Scholar] [CrossRef]
- Thoeming, G.; Draeger, G.; Poehling, H.M. Soil application of azadirachtin and 3-tigloyl-azadirachtol to control western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae) translocation and persistence in bean plants. Pest Manag. Sci. 2006, 62, 759–767. [Google Scholar] [CrossRef]
- Ipsilants, I.; Samourelis, C.; Karpouzas, D. The impact of biological pesticides on arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2012, 45, 147–155. [Google Scholar] [CrossRef]
- Rosendahl, S. Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol. 2008, 178, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Gopal, M.; Gupta, A.; Arunachalam, V.; Magu, S.P. Impact of azadirachtin, an insecticidal allelochemical from neem on soil microflora, enzyme and respiratory activities. Bioresour. Technol. 2007, 98, 3154–3158. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tong, R.; Shi, Z.; Xu, X.; He, X. Inoculations with arbuscular mycorrhizal fungi increase vegetable yields and decrease phoxim concentrations in carrot and green onion and their soils. PLoS ONE 2011, 6, e16949. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.; Raza, S.; Khan, M.; Ashraf, M.; Ahmed, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Devi, T.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Status of the Worlds’s Soil Resources (SWSR)—Main Report, United Nations; Food and Agriculture Organization: Rome, Italy, 2015. [Google Scholar]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562. [Google Scholar] [PubMed]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef]
- Liu, C.; Ravnskov, S.; Liu, F.; Rubæk, G.H.; Andersen, M.N. Arbuscular mycorrhizal fungi alleviate abiotic stresses in potato plants caused by low phosphorus and deficit irrigation/partial root-zone drying. J. Agric. Sci. 2018, 156, 46–58. [Google Scholar] [CrossRef]
- Bona, E.; Scarafoni, A.; Marsano, F.; Boatti, L.; Copetta, A.; Massa, N.; Gamalero, E.; D’Agostino, G.; Cesaro, P.; Cavaletto, M.; et al. Arbuscular mycorrhizal symbiosis affects the grain proteome of Zea mays: A field study. Sci. Rep. 2016, 6, 26439. [Google Scholar] [CrossRef] [PubMed]
- Ingraffia, R.; Amato, G.; Sosa-Hernández, M.; Frenda, A.; Rillig, M.; Giambalvo, D. Nitrogen Type and Availability Drive Mycorrhizal Effects on Wheat Performance, Nitrogen Uptake and Recovery, and Production Sustainability. Front. Plant Sci. 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Vannini, C.; Ortolani, F.; Garcia-Seco, D.; Chiapello, M.; Novero, M.; Domingo, G.; Terzi, V.; Morcia, C.; Bagnaresi, P.; et al. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 9625. [Google Scholar] [CrossRef]
- Cabral, C.; Ravnskov, S.; Tringovska, I.; Wollenweber, B. Arbuscular mycorrhizal fungi modify nutrient allocation and composition in wheat (Triticum aestivum L.) subjected to heat-stress. Plant Soil 2016, 408, 385–399. [Google Scholar] [CrossRef]
- Abdi, N.; van Biljon, A.; Steyn, C.; Labuschagne, M.T. Bread Wheat (Triticum aestivum) Responses to Arbuscular Mycorrhizae Inoculation under Drought Stress Conditions. Plants 2021, 10, 1756. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-L.; Lin, X.-G.; Wang, J.-H.; Shen, W.-S.; Wu, S.; Peng, S.-P.; Mao, T.-T. Arbuscular Mycorrhizal Fungal Inoculation Enhances Suppression of Cucumber Fusarium Wilt in Greenhouse Soils. Pedosphere 2010, 20, 586–593. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- García de León, D.; Vahter, T.; Zobel, M.; Koppel, M.; Edesi, L. Different wheat cultivars exhibit variable responses to inoculation with arbuscular mycorrhizal fungi from organic and conventional farms. PLoS ONE 2020, 15, e0233878. [Google Scholar] [CrossRef]
- Reva, M.; Cano, C.; Herrera, M.; Bago, A. Arbuscular Mycorrhizal Inoculation Enhances Endurance to Severe Heat Stress in Three Horticultural Crops. HortScience 2021, 56, 396–406. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Gill, S.S.; Fujita, M. Physiological role of nitric oxide in plants grown under adverse environmental conditions. In Plant Acclimation to Environmental Stress; Tuteja, N., Gill, S.S., Eds.; Springer Science + Business Media: New York, NY, USA, 2013; pp. 269–322. [Google Scholar]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Sui, X.; Fan, X.; Jia, T.; Song, F. Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) Grasses via Altering Antioxidant Enzyme Activities and Photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. The Tripartite Rhizobacteria-AM Fungal-Host Plant Relationship in Winter Wheat: Impact of Multi-Species Inoculation, Tillage Regime and Naturally Occurring Rhizobacteria Species. Plants 2021, 10, 1357. [Google Scholar] [CrossRef]
- Kozjek, K.; Kundel, D.; Kushwaha, S.; Olsson, P.; Ahrén, D.; Fliessbach, A.; Birkhofer, K.; Hedlund, K. Long-term agricultural management impacts arbuscular mycorrhizal fungi more than short-term experimental drought. Appl. Soil Ecol. 2021, 168, 104140. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Sanchez-Romera, B.; Aroca, R.; Asins, M.; Declerk, S.; Dodd, I.; Martinez-Andujar, C.; Albacete, A.; Ruiz-Lozano, J. Exploring the use of recombinant inbred lines in conjunction with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016, 131, 47–57. [Google Scholar] [CrossRef]
- Mitra, D.; Navendra, U.; Panneerselvam, U.; Ansuman, S.; Ganeshamurthy, A.N.; Divya, J. Role of mycorrhiza and its associated bacteria on plant growth promotion and nutrient management in sustainable agriculture. Int. J. Life Sci. Appl. Sci. 2019, 1, 1–10. [Google Scholar]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef] [PubMed]
- Furze, J.; Martin, A.; Nasielski, J.; Thevathasan, N.; Gordon, A.; Isaac, M. Resistance and resilience of root fungal communities to water limitation in a temperate agroecosystem. Ecol. Evol. 2017, 7, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Rodriguez, P.; Morales, M.; Dell’Amico, J.; Torrecillas, A.; Alarcon, J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Min, L.; Li, Y.; Hu, Q.; Zhu, L.; Gao, W.; Ding, Y.; Liu, S.; Yang, X.; Zhang, X. Sugar and auxin signaling pathways respond to high-temperate stress during another development as revealed by transcript profiling analysis in cotton. Plant Physiol. 2014, 164, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensiative maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerance cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed]

| Tillage Type | Equipment Employed | Tillage Characteristics | Impact on Soil and Arbuscular Mycorrhizal Fungi |
|---|---|---|---|
| Conventional | Mouldboard plough |
|
|
| Reduced | Rotary disc |
|
|
| Strip | Specialist equipment
|
|
|
| Zero | Direct seed drill |
|
|
| Method of Application | Product | Active Component | Crop Type | Effect on AM Fungal Abundance | Effect on Sporulation | Effect on Soil Glomalin Concentration |
|---|---|---|---|---|---|---|
| Seed treatment | Agrox™ | Captan | Pea (Pisum sativum), Chickpea (Cicer arietinum) | Neutral | No change | No change |
| Allegiance™ | Metalaxyl | Pea, Chickpea | Negative | No change | No change | |
| Apron Maxx RTA™ | Fludioxonil and metalaxyl | Pea, Chickpea | Negative | No change | No change | |
| Trilex AL™ | Trifloxystrobin and metalaxyl | Pea, Chickpea | Negative | No change | No change | |
| Vitaflo 280™ | Carbathiin and thiram | Pea, Chickpea | Negative | Inhibited | Reduced | |
| Crown™ | Carbathiin and thiabendazole | Pea, Chickpea | Negative | Inhibited | Reduced | |
| Thiram 75wp™ | Thiram | Pea, Chickpea | Neutral | No change | No change | |
| Plant application | Benomyl | 1-[(butyamino)carboyl-1H-benzimidazole-2yl] carbonate | Proso millet (Panicum miliaceum) | Negative | No change | No change |
| Bavistin | Methylbenzimidazol-2-yl carbonate | Proso millet | Negative | No change | No change | |
| Agrox™ | Captan | Proso millet | Positive | No change | No change | |
| Mancozeb | Manganese ethylenebis (dithiocarbomate) (polymatrix) complex zinc salt | Proso millet | Negative | No change | No change | |
| Soil drench | Benomyl | 1-[(butyamino)carboyl-1H-benzimidazole-2yl] carbonate | Cucumber (Cucumis sativus) | Negative | Inhibited | Reduced |
| Fenpropimorph | Rac-(2R,6S)-4-[(2E)-3-(-4-tert-butylphenyl)-2-methylpropyl]-2,6-dimethylmorpholine | Cucumber | Negative | Inhibited | Reduced | |
| Propiconazole | 1-((2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-20yl)methyl)-1H-1,2,4-triazole | Cucumber | Negative | Inhibited | Reduced | |
| Propiconazole and fenpropimorph | As above | Cucumber | Negative | Inhibited | Reduced |
| Plant Stressor | Crop | AM Fungal Inoculum | Crop Response |
|---|---|---|---|
| Salinity | Cucmis sativus L. | Glomus intraradices, Glomus mossaea | Increased chlorophyll content in leaves and overall biomass |
| Solanum lycopersicum L. | Glomus intraradices | Increased ion absorption and leaf chlorophyll | |
| Leymus chinensis | Glomus mosseae | Increased AM fungal colonisation, water content, and phosphorus and nitrogen uptake | |
| Triticum aestivum L. | Rhizophagus intraradices | Maintianed overall biomass, increased water uptake | |
| Heat | Triticum aestivum L. | Rhizophagus irregularis Rhizophagus intraradices | Increased nutrient content and uptake, increase to overall biomass and water content |
| Zea mays L. | Rhizophagus intradices | Increased crop biomass and leaf chlorophyll | |
| Drought | Triticum aestivum L. | Glomus mosseae Glomus fasciculatum Rhizophagus irregularis Rhizophagus intraradices | Increased crop biomass, ascorbic acid content, and leaf chlorophyll |
| Triticum aestivum | Glomus masseae | Increased crop biomass, ascorbic acid content, nitrogen and phosphorus metabolism, and leaf chlorophyll | |
| Triticum durum | Rhizophagus intraradices | Increased metal ions (copper, zinc, manganese) | |
| Zea mays | Rhizophagus intraradices | Increased absorption of phosphorus, potassium, nitrogen and magnesium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkes, T.I. Arbuscular Mycorrhizal Fungi in Agriculture. Encyclopedia 2021, 1, 1132-1154. https://doi.org/10.3390/encyclopedia1040085
Wilkes TI. Arbuscular Mycorrhizal Fungi in Agriculture. Encyclopedia. 2021; 1(4):1132-1154. https://doi.org/10.3390/encyclopedia1040085
Chicago/Turabian StyleWilkes, Thomas I. 2021. "Arbuscular Mycorrhizal Fungi in Agriculture" Encyclopedia 1, no. 4: 1132-1154. https://doi.org/10.3390/encyclopedia1040085
APA StyleWilkes, T. I. (2021). Arbuscular Mycorrhizal Fungi in Agriculture. Encyclopedia, 1(4), 1132-1154. https://doi.org/10.3390/encyclopedia1040085






