Design and Analysis of Smart Reconstruction Plate for Wireless Monitoring of Bone Regeneration and Fracture Healing in Maxillofacial Reconstruction Applications
Abstract
1. Introduction
2. Material and Methods
2.1. Theory and Principles
2.1.1. Strain Gauge Characteristics
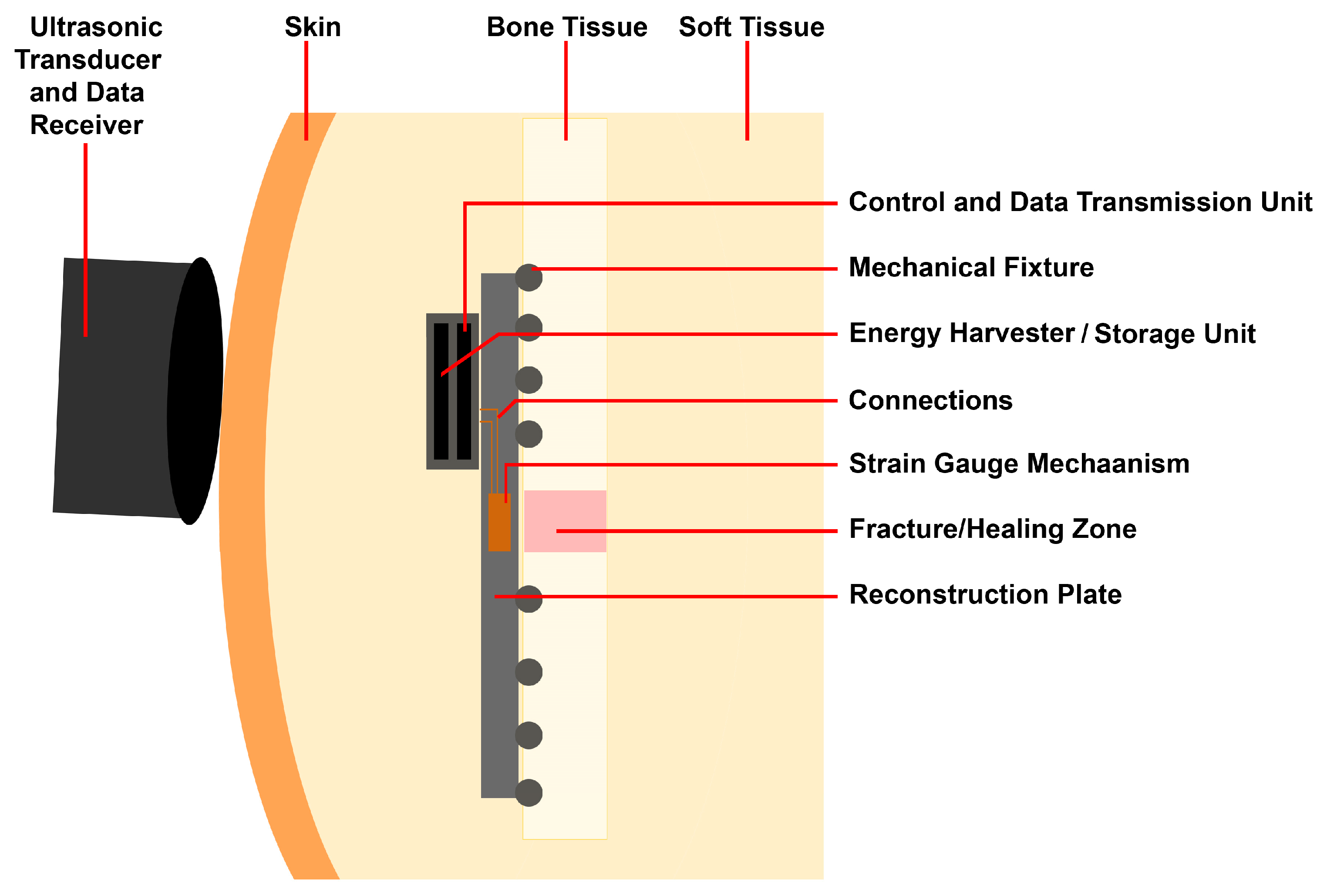
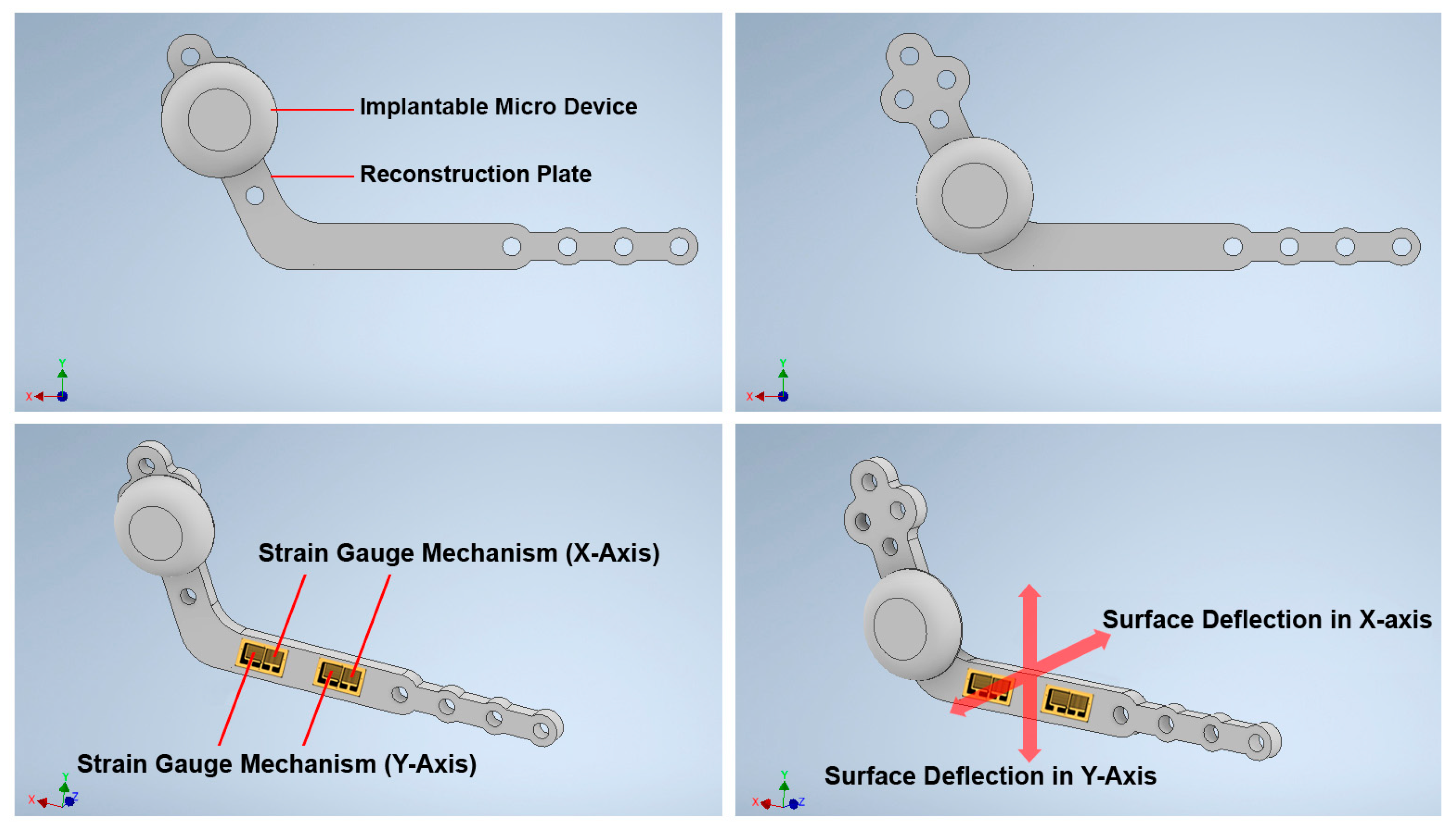
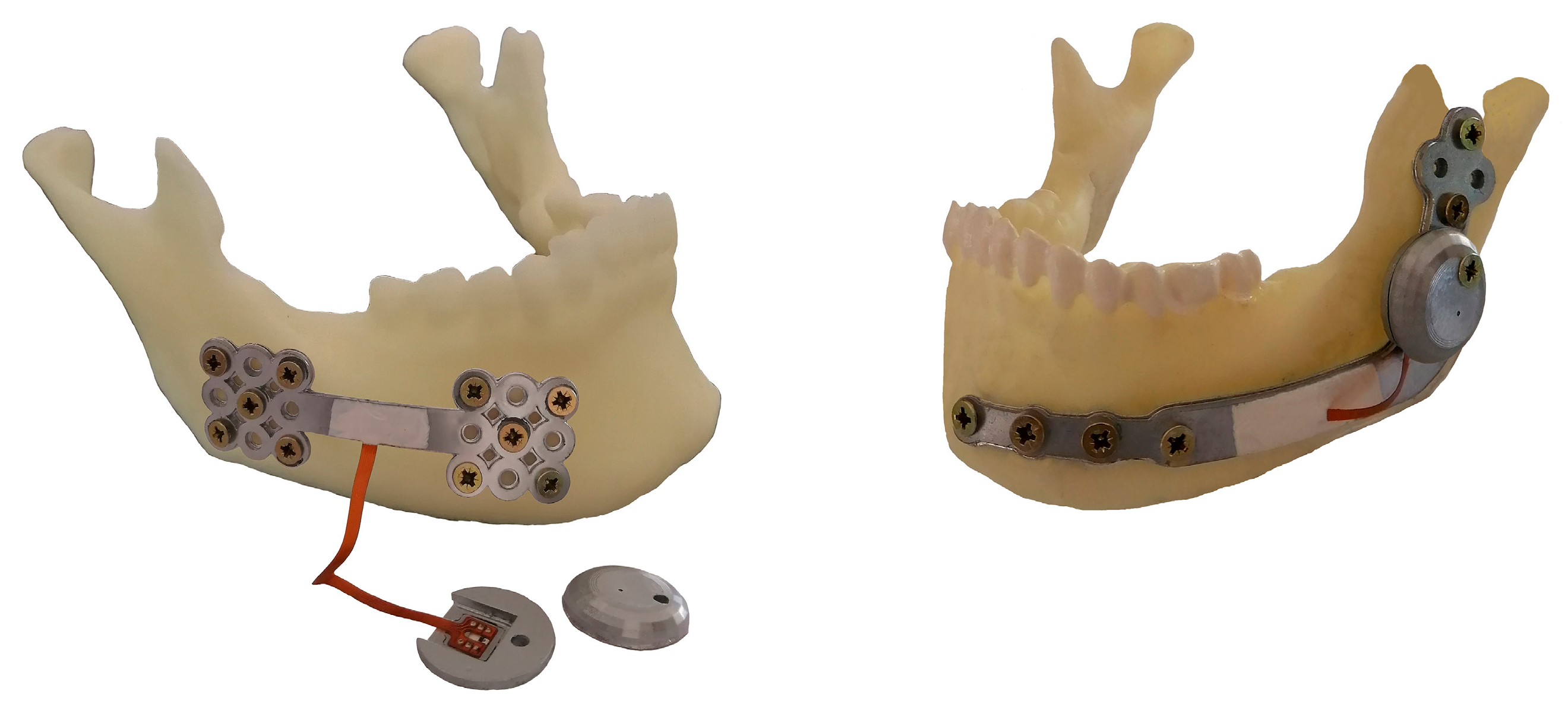
2.1.2. Structure Design of the Customized Reconstruction Plate
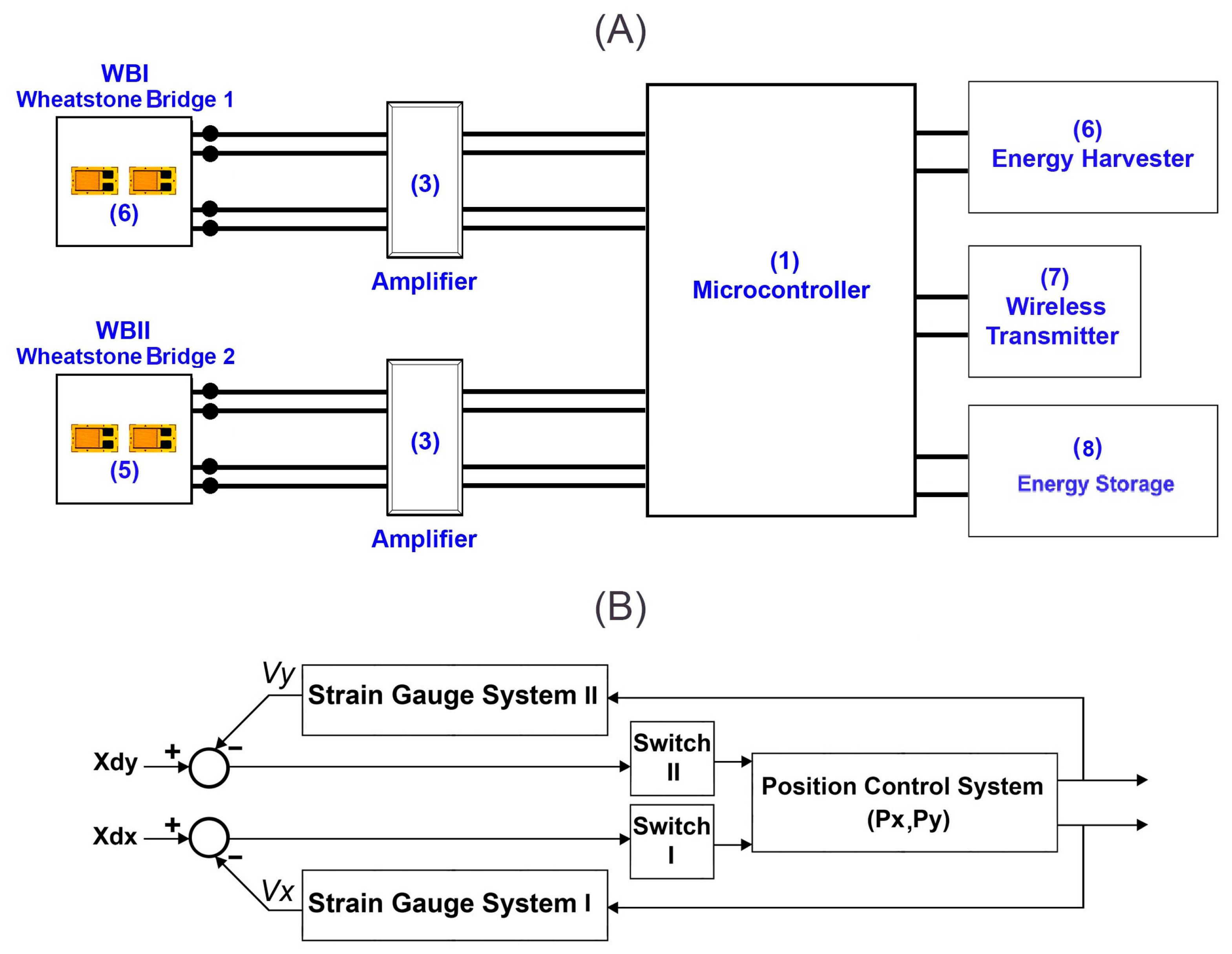
2.2. Control System and Measurement Principles
2.2.1. The Arrangement Strategy of the Strain Gauges
2.2.2. Decoupling the Surface Strain and Respective Applied Forces
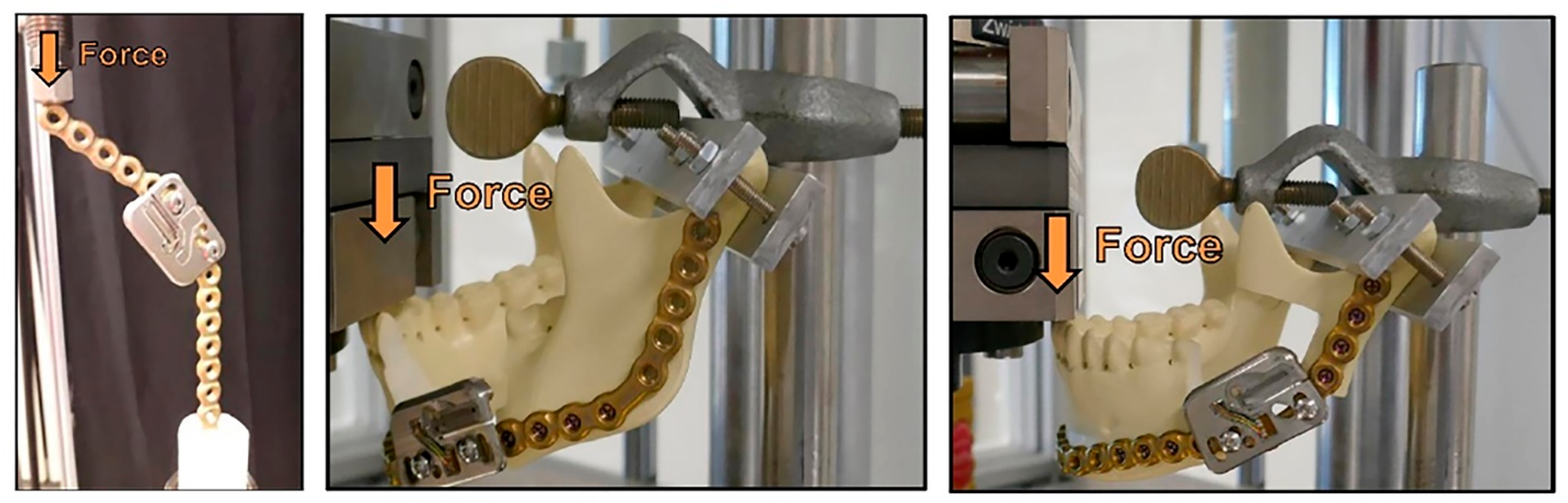
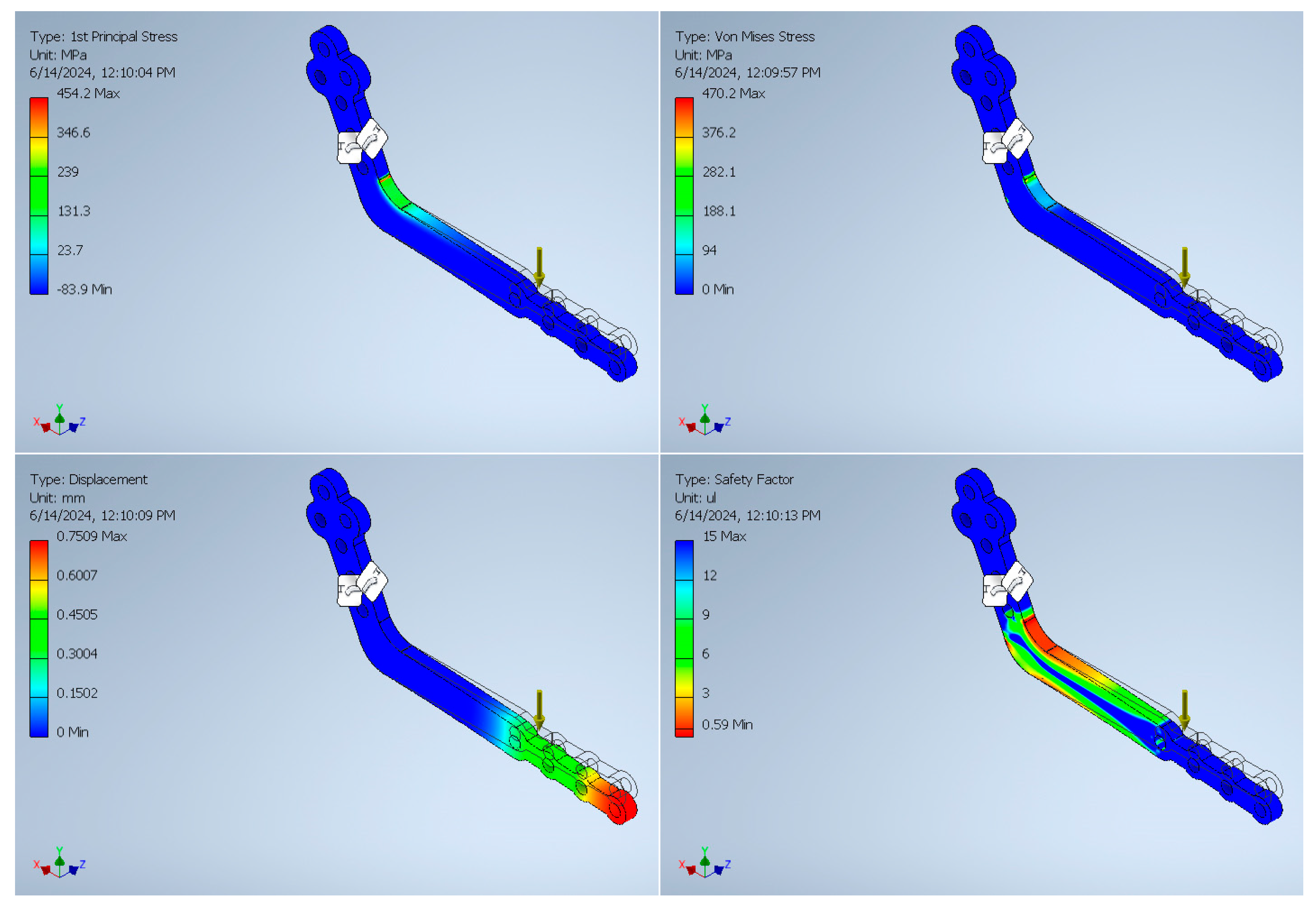
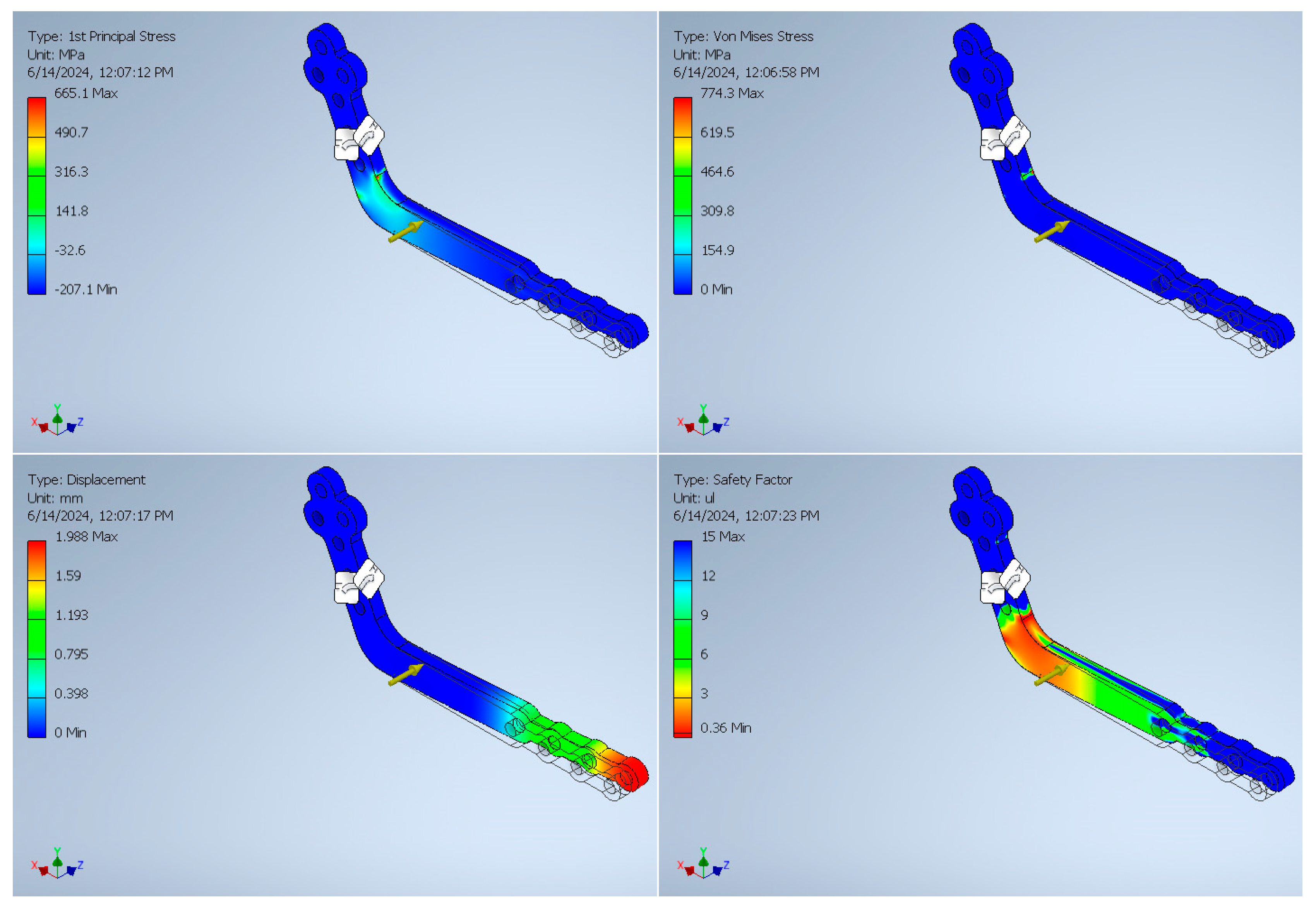
3. Finite Element Analysis Validation
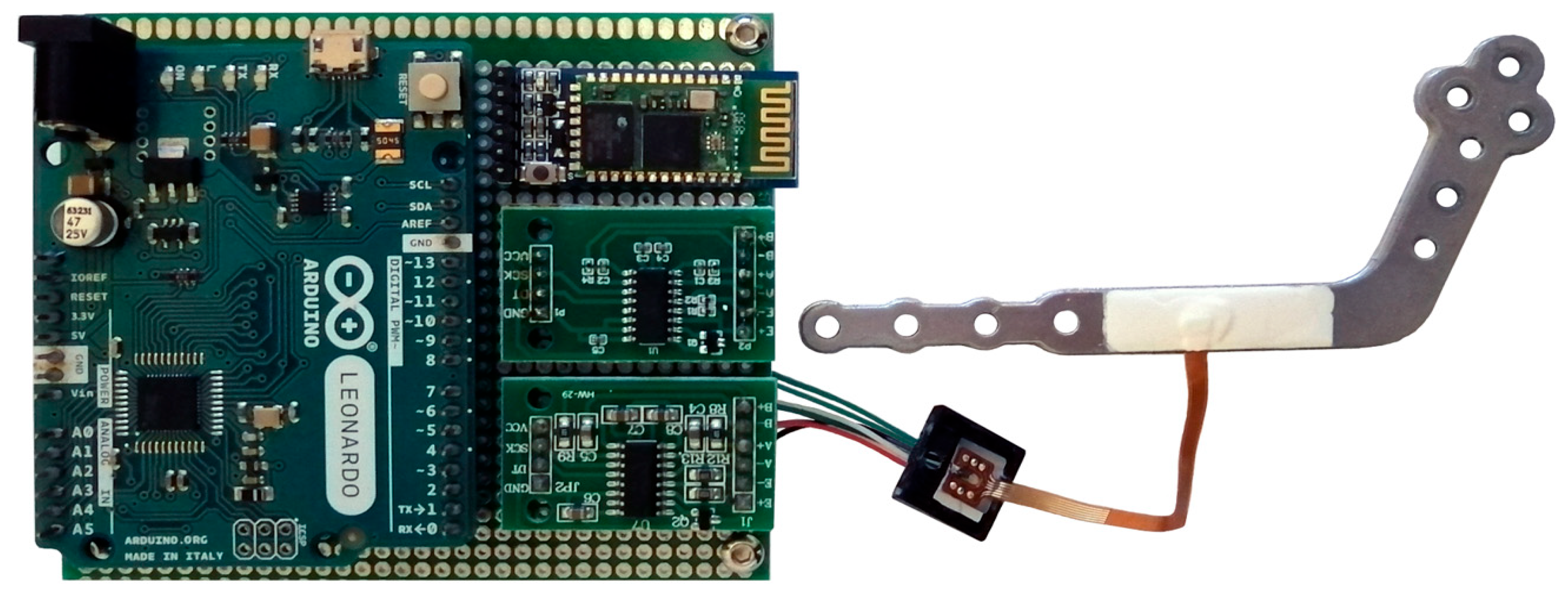
4. Analysis of the Control System and Experimental Results
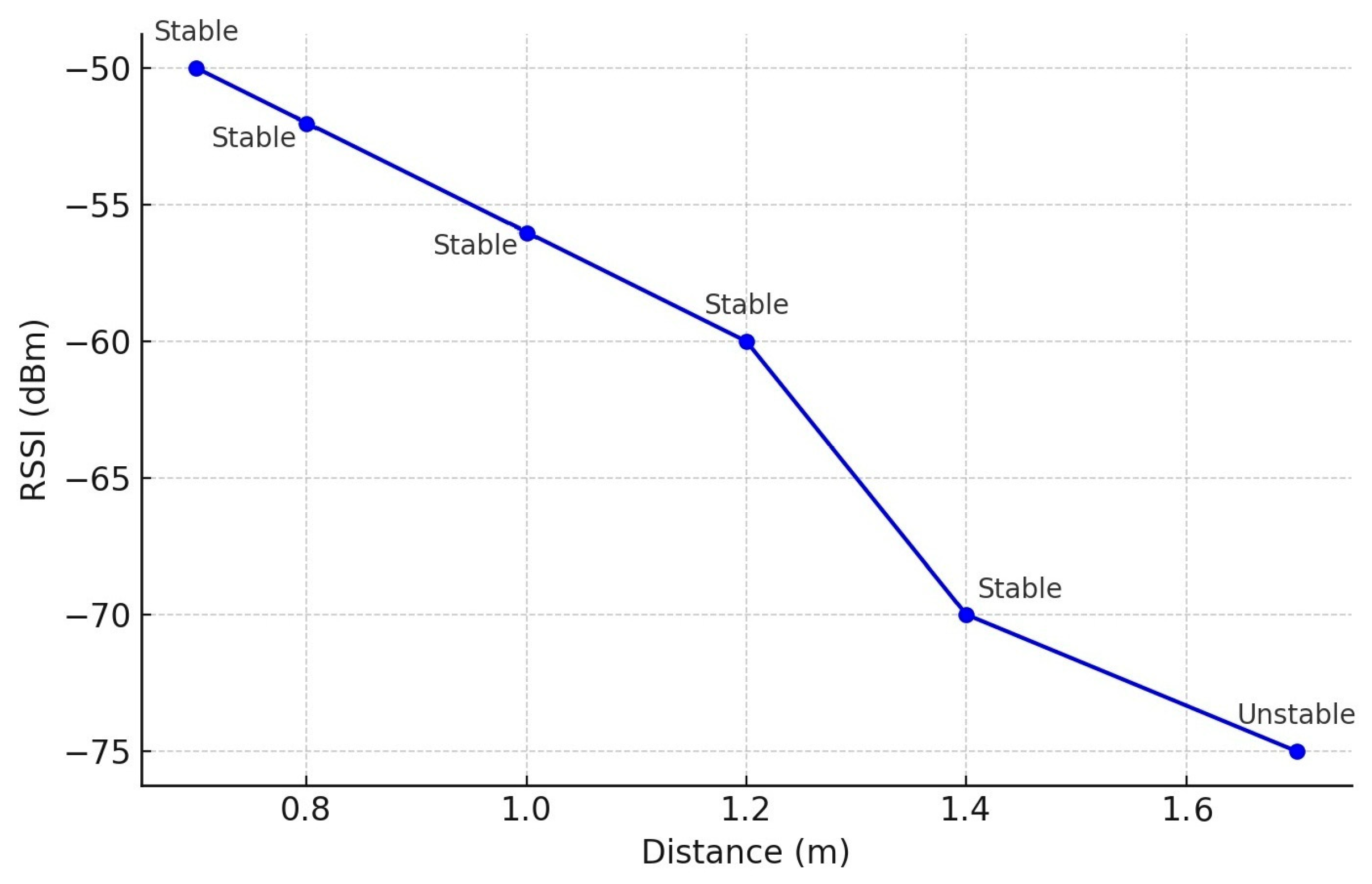
4.1. Signal-to-Noise Ratio (SNR) and Transmission Range Measurement
4.2. Calibration of the Sensor
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brown, J.; Lowe, D.; Kanatas, A.; Schache, A. Mandibular reconstruction with vascularised bone flaps: A systematic review over 25 years. Br. J. Oral Maxillofac. Surg. 2017, 55, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Y.; Jiang, J.; Xu, L.; Chim, H. Distraction lengthening following vascularized second toe transfer for isolated middle finger reconstruction. J. Hand Surg. 2017, 42, e33–e39. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Boonzaier, J.; Vicatos, G.; Hendricks, R. Repair of segmental bone defects in the maxilla by transport disc distraction osteogenesis: Clinical experience with a new device. Ann. Maxillofac. Surg. 2015, 5, 85–88. [Google Scholar] [CrossRef]
- Mathog, R.H.; Boies, L.R., Jr. Nonunion of the mandible. Laryngoscope 1976, 86, 908–920. [Google Scholar] [CrossRef]
- Reiter, M.J.; Schwope, R.B.; Theler, J.M. Postoperative CT of the Mandible Following Trauma: Review of Normal Appearances and Common Complications. Acad. Radiol. 2019, 26, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Rowe, N. Nonunion of the mandible and maxilla. J. Oral Surg. (Am. Dent. Assoc. 1965) 1969, 27, 520–529. [Google Scholar]
- Rai, A.; Jain, A.; Datarkar, A. Comparison of single versus two non-compression miniplates in the management of unfavourable angle fracture of the mandible: A prospective randomized clinical study. Oral Maxillofac. Surg. 2018, 22, 157–161. [Google Scholar] [CrossRef]
- Marcantonio, G.; Vieria, H. Fixation of mandibular fractures with 2.0 m miniplates: Review of 191 cases. J. Oral Maxillofac. Surg. 2003, 61, 430–436. [Google Scholar]
- Chrcanovic, B.R. Fixation of mandibular angle fractures: Clinical studies. Oral Maxillofac. Surg. 2014, 18, 123–152. [Google Scholar] [CrossRef]
- Ellis, E., III. A prospective study of 3 treatment methods for isolated fractures of the mandibular angle. J. Oral Maxillofac. Surg. 2010, 68, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gupta, M.; Bhagol, A. Is a single miniplate at the inferior border adequate in the management of an angle fracture of the mandible? Otolaryngol.—Head Neck Surg. 2011, 145, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone beam computed tomography in implant dentistry: Recommendations for clinical use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Avery, C.; Clifford, N.; Niamat, J.; Vaidhyanath, R. Early detection of bone union with transcutaneous ultrasound in the management of non-union of the mandible. Br. J. Oral Maxillofac. Surg. 2011, 49, 661–663. [Google Scholar] [CrossRef]
- Haug, R.H.; Fattahi, T.T.; Goltz, M. A biomechanical evaluation of mandibular angle fracture plating techniques. J. Oral Maxillofac. Surg. 2001, 59, 1199–1210. [Google Scholar] [CrossRef]
- Schupp, W.; Arzdorf, M.; Linke, B.; Gutwald, R. Biomechanical testing of different osteosynthesis systems for segmental resection of the mandible. J. Oral Maxillofac. Surg. 2007, 65, 924–930. [Google Scholar] [CrossRef]
- Rothweiler, R.M.; Zankovic, S.; Brandenburg, L.S.; Fuessinger, M.-A.; Gross, C.; Voss, P.J.; Metzger, M.-C. Feasibility of Implant Strain Measurement for Assessing Mandible Bone Regeneration. Micromachines 2022, 13, 1602. [Google Scholar] [CrossRef]
- Burny, F.; Donkerwolcke, M.; Moulart, F.; Bourgois, R.; Puers, R.; Van Schuylenbergh, K.; Barbosa, M.; Paiva, O.; Rodes, F.; Bégueret, J.B. Concept, design and fabrication of smart orthopedic implants. Med. Eng. Phys. 2000, 22, 469–479. [Google Scholar] [CrossRef]
- Fountain, S.; Windolf, M.; Henkel, J.; Tavakoli, A.; Schuetz, M.A.; Hutmacher, D.W.; Epari, D.R. Monitoring healing progression and characterizing the mechanical environment in preclinical models for bone tissue engineering. Tissue Eng. Part B Rev. 2016, 22, 47–57. [Google Scholar] [CrossRef]
- Windolf, M.; Varjas, V.; Gehweiler, D.; Schwyn, R.; Arens, D.; Constant, C.; Zeiter, S.; Richards, R.G.; Ernst, M. Continuous Implant Load Monitoring to Assess Bone Healing Status—Evidence from Animal Testing. Medicina 2022, 58, 858. [Google Scholar] [CrossRef]
- Gutwald, R.; Jaeger, R.; Lambers, F.M. Customized mandibular reconstruction plates improve mechanical performance in a mandibular reconstruction model. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, K.; Steffen, C.; Koerdt, S.; Doll, C.; Ebker, T.; Nahles, S.; Flügge, T.; Heiland, M.; Beck-Broichsitter, B.; Rendenbach, C. Patient-specific 3D-printed miniplates for free flap fixation at the mandible: A feasibility study. Front. Surg. 2022, 9, 778371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Fan, Y.-Y.; Jiang, X.-F.; Baur, D.A. A customized fixation plate with novel structure designed by topological optimization for mandibular angle fracture based on finite element analysis. Biomed. Eng. Online 2017, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Koper, D.C.; Leung, C.A.; Smeets, L.C.; Laeven, P.F.; Tuijthof, G.J.; Kessler, P.A. Topology optimization of a mandibular reconstruction plate and biomechanical validation. J. Mech. Behav. Biomed. Mater. 2021, 113, 104157. [Google Scholar] [CrossRef]
- Lisiak-Myszke, M.; Marciniak, D.; Bieliński, M.; Sobczak, H.; Garbacewicz, Ł.; Drogoszewska, B. Application of finite element analysis in oral and maxillofacial surgery—A literature review. Materials 2020, 13, 3063. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Lai, Q.; Xue, R.; Zhu, K.; Deng, Y. Finite element analysis of 3D-printed personalized titanium plates for mandibular angle fracture. Comput. Methods Biomech. Biomed. Eng. 2022, 26, 78–89. [Google Scholar] [CrossRef]

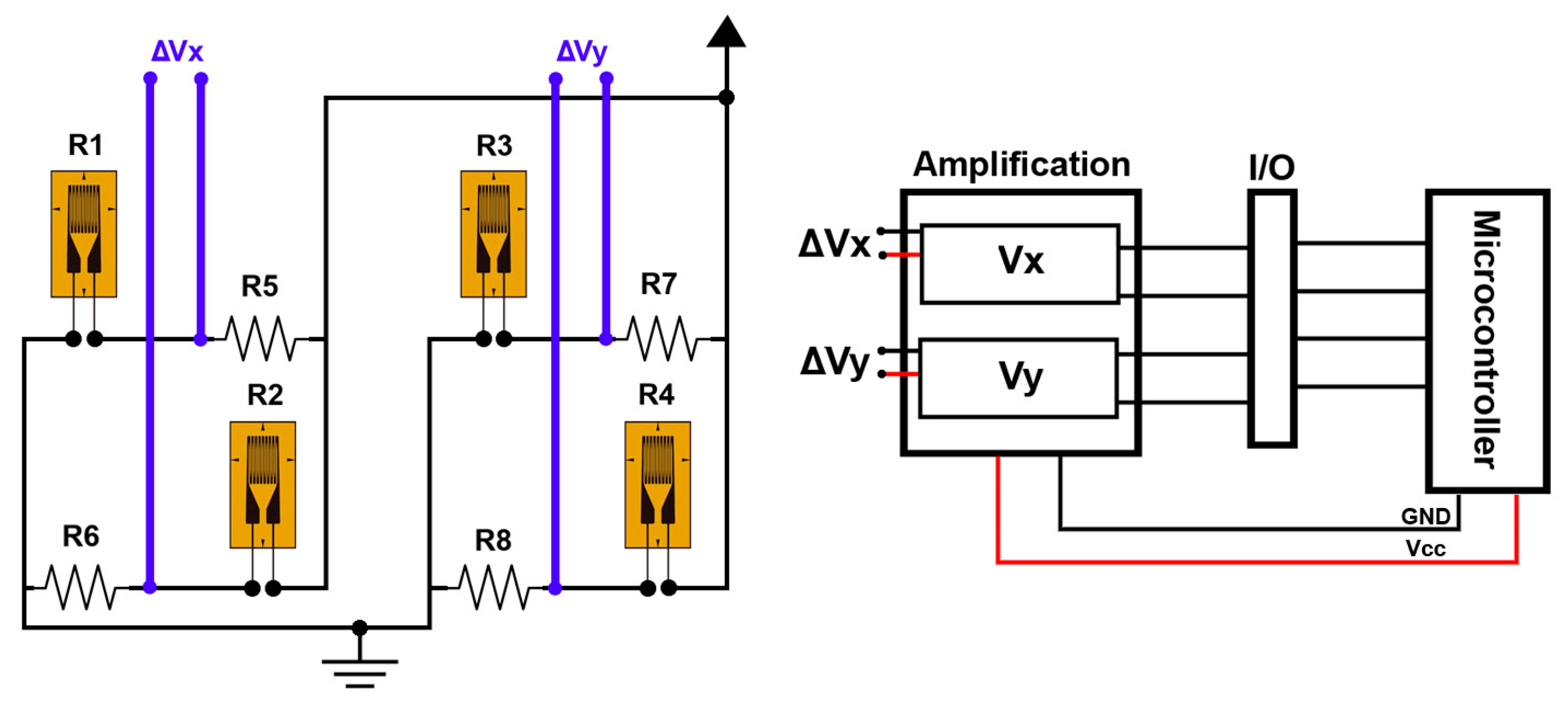

| Measurement System | Strain Gauge | Applied Force in X-Axis (Fx) | Applied Force in Y-Axis (Fy) |
|---|---|---|---|
| Wheatstone half-bridge I | R1 | + | 0 |
| R2 | − | 0 | |
| Wheatstone half-bridge II | R3 | 0 | + |
| R4 | 0 | − |
| Test Condition | RSSI (dBm) | Noise Floor (dBm) | SNR (dB) |
|---|---|---|---|
| Best Case (RSSI −65 dBm) | −65 | −90 | 25 |
| Worst Case (RSSI −68 dB) | −68 | −90 | 22 |
| Typical Case (RSSI −66 dB) | −66 | −90 | 24 |
| Average (RSSI −67 dBm) | −67 | −90 | 23 |
| Feature | Proposed System | Reference [17] |
|---|---|---|
| Power Source | Ultrasonic energy harvester (wireless) | Li-ion battery (limited lifespan, bulky) |
| Implant size | Miniaturized, integrated on RP surface | Larger, external instrumentation |
| Biocompatibility | Waterproof, biocompatible device | Not detailed |
| Suitability for MRA | Compact, custom-fit for MRA | Not suitable due to size/power limits |
| Energy Sustainability | Passive operation via ultrasonic recharge | Limited by battery depletion |
| Customizability | Designed for 3D-printed Ti-6Al-4V RPs | Not customizable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatefi, S.; Smith, F.; Auld, K.; Van Aardt, S. Design and Analysis of Smart Reconstruction Plate for Wireless Monitoring of Bone Regeneration and Fracture Healing in Maxillofacial Reconstruction Applications. Metrology 2025, 5, 32. https://doi.org/10.3390/metrology5020032
Hatefi S, Smith F, Auld K, Van Aardt S. Design and Analysis of Smart Reconstruction Plate for Wireless Monitoring of Bone Regeneration and Fracture Healing in Maxillofacial Reconstruction Applications. Metrology. 2025; 5(2):32. https://doi.org/10.3390/metrology5020032
Chicago/Turabian StyleHatefi, Shahrokh, Farouk Smith, Kayla Auld, and Stefan Van Aardt. 2025. "Design and Analysis of Smart Reconstruction Plate for Wireless Monitoring of Bone Regeneration and Fracture Healing in Maxillofacial Reconstruction Applications" Metrology 5, no. 2: 32. https://doi.org/10.3390/metrology5020032
APA StyleHatefi, S., Smith, F., Auld, K., & Van Aardt, S. (2025). Design and Analysis of Smart Reconstruction Plate for Wireless Monitoring of Bone Regeneration and Fracture Healing in Maxillofacial Reconstruction Applications. Metrology, 5(2), 32. https://doi.org/10.3390/metrology5020032







