1. Introduction
Knowledge of salt composition and suspended matter is a prerequisite for forecasting and developing marine ecosystems. The influence of salinity on the physiological and behavioral parameters of planktonic organisms has been rarely investigated (Lee and Petersen, 2002). Living in a variable salinity mode provides a unique opportunity for individual populations to quickly acclimatize.
During marine research in the Black Sea and Sea of Azov, we conducted experiments to determine the reliability of the measurement results obtained with Conductivity-Temperature-Depth (CTD) and Sound Velocity Profiler (SVP) instruments in the shelf zone. We used the data from these experiments to calculate the salinity and density of seawater.
When studying the results of the joint measurements by the CTD and SVP devices, we identified a number of factors that signified an incorrect interpretation of the obtained data. We compared these data with the values of salinity and density indirectly obtained using well-established algorithms.
We assumed that the use of standard algorithms, which do not consider the effects of solid suspensions in the seawater, results in significant errors in the calculations of salinity and density when processing in situ measurements obtained using the CTD and SVP devices.
We must note that some researchers have been trying to develop methods for correction marine measurement results that would help to determine the true values of absolute salinity and density of seawater with non-standard composition, including samples with solid suspensions.
Some well-known works [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10] have only partially solved this problem. For example, when determining a salinity anomaly
δSA with CTD probes with an electric conductivity measurement channel, the TEOS-10 user manual [
11] suggests to use a chemical conductivity and density model [
1] to assess the correlation between the salinity changes and measured properties of seawater.
This model allows for the determination of the salinity anomaly expressed in (g/kg) through nitrate (NO
3−) and silicate (Si(OH)
4) concentration values in a seawater sample and through the two differences of ΔTA (∆TA = Total Alkalinity (TA) − 0.0023 (
SP/35), mole/kg) and ΔDIC (∆DIC = Dissolved Inorganic Carbon (DIC) − 0.00208 (
SP/35), mole/kg):
where
SP is the practical salinity expressed in practical salinity units (
psu). The ΔTA and ΔDIC differences are determined between the TA and DIC in a sample and the respective best assessments of TA and DIC in standard seawater [
1,
2].
According to [
2], the standard uncertainty of model compliance is 0.08 mg/kg in the oceanic range if the exact amounts of all the substances are known.
Other research works have shown that δSA can be calculated using a simplified empirical equation based on the measurement of SiO2 silicate concentration measurement and the total alkalinity (TA) because these components are measured and well-suited for the assessment of density and salinity changes of the deep water.
This empirical equation is as follows [
3]:
where NTA is the total alkalinity, normalized to salinity 35 (NTA = (TA/
Sp)·35).
According to Millero [
4,
5,
6,
7], ∆[NTA] is the difference between the measured value of normalized total alkalinity (NTA) and the reference value (0.0023 mole/kg) for surface seawater. However, the practical method for determining
δSA is based on the use of density measurements [
8].
As stated in [
3,
9], the method proposed by Millero et al. can be used to calculate
δSA through the following formula:
δρ = 0.75179
δSA.
Unfortunately, the majority of results have been obtained in laboratories because it is difficult to adjust measurement results through bathometer sampling and subsequent seawater density measurements onboard a ship when using, for instance, an Anton Paar DMA 5000 M vibration sensor because the task of keeping of solid suspensions in suspension state in a sample is not easy.
It is difficult to use these models and research works when working in situ in the shelf zone where there are solid suspensions.
2. Materials and Methods
As mentioned above, our research was conducted in the natural conditions of the shelf zone using two devices and one model.
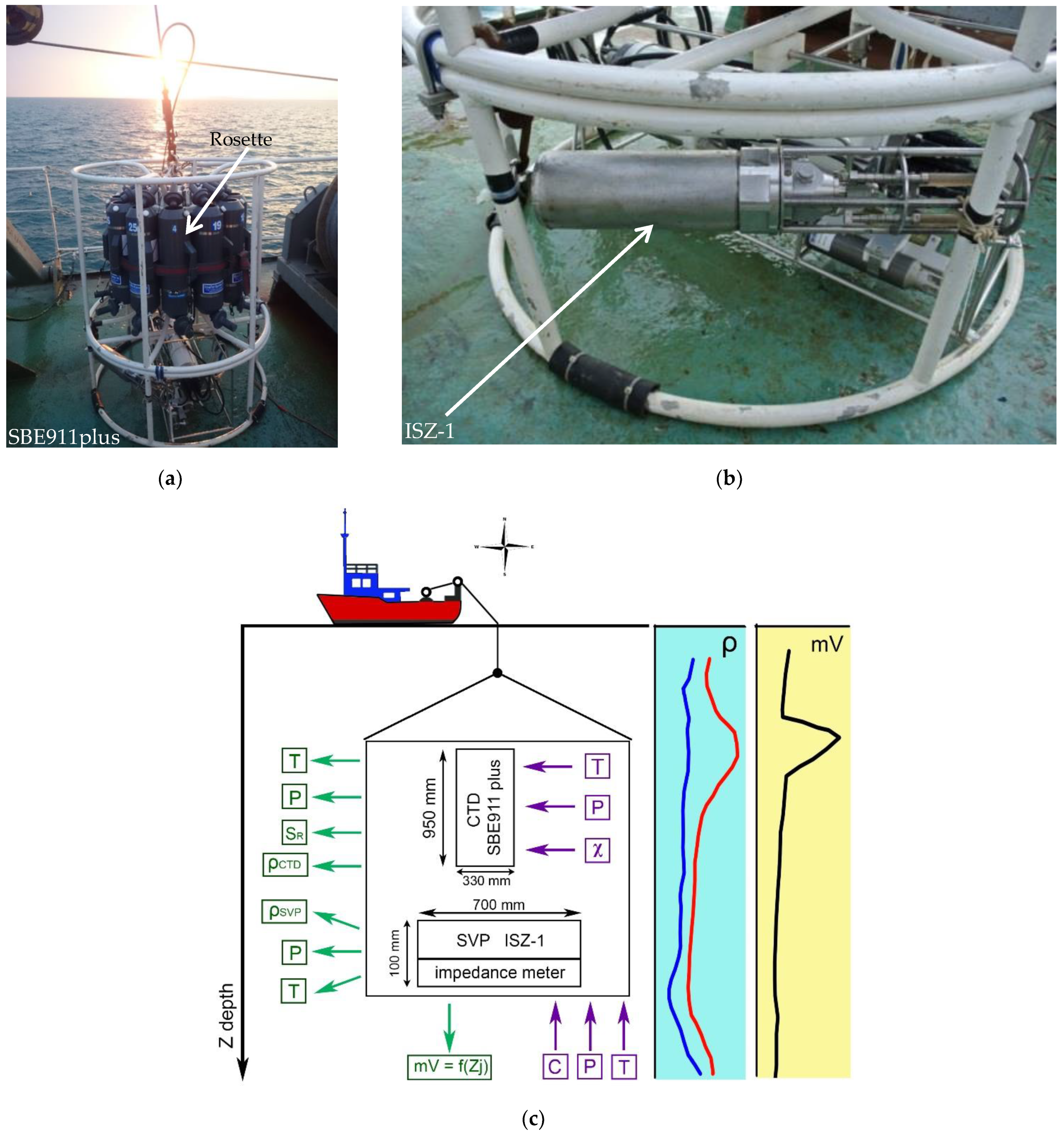
To do this, we complemented a standard SBE911 oceanological probing CTD unit [
12] with an ISZ-1, which is an SVP probe developed at the Institute of Natural and Technical Systems (INTS) [
13] (
Figure 1). The ISZ-1 probe also featured an ultrasonic attenuation measuring channel. Unfortunately, it only worked on a few of the stations and it ran in the testing mode. Data on the metering attenuation duct are provided in [
14]. In the testing mode, an impedance channel model with the 4-electrode cell was used, and its operation is described below.
Figure 1c shows a structural and functional diagram of the entire measuring set of instruments and included devices, as well as their dimensions. The devices were lowered on a cable-rope to a certain depth using a winch installed on the ship. The ship’s coordinates were fixed by navigation systems. The input measured parameters of the water are indicated in purple: T—temperature; P—hydrostatic pressure; c—speed of sound; and
χ—electrical conductivity. The calculated output values are indicated in green: T—temperature; P—hydrostatic pressure;
SR—salinity;
ρCTD—calculated density values from CTD readings;
ρSVP—calculated density values according to SVP readings; and mV—impedance value
Zj. The same figure shows the vertical profiles of the density (
ρSVP and
ρCTD) and impedance distribution expressed in mV.
The specifications for both probes are presented in
Table 1 and
Table 2.
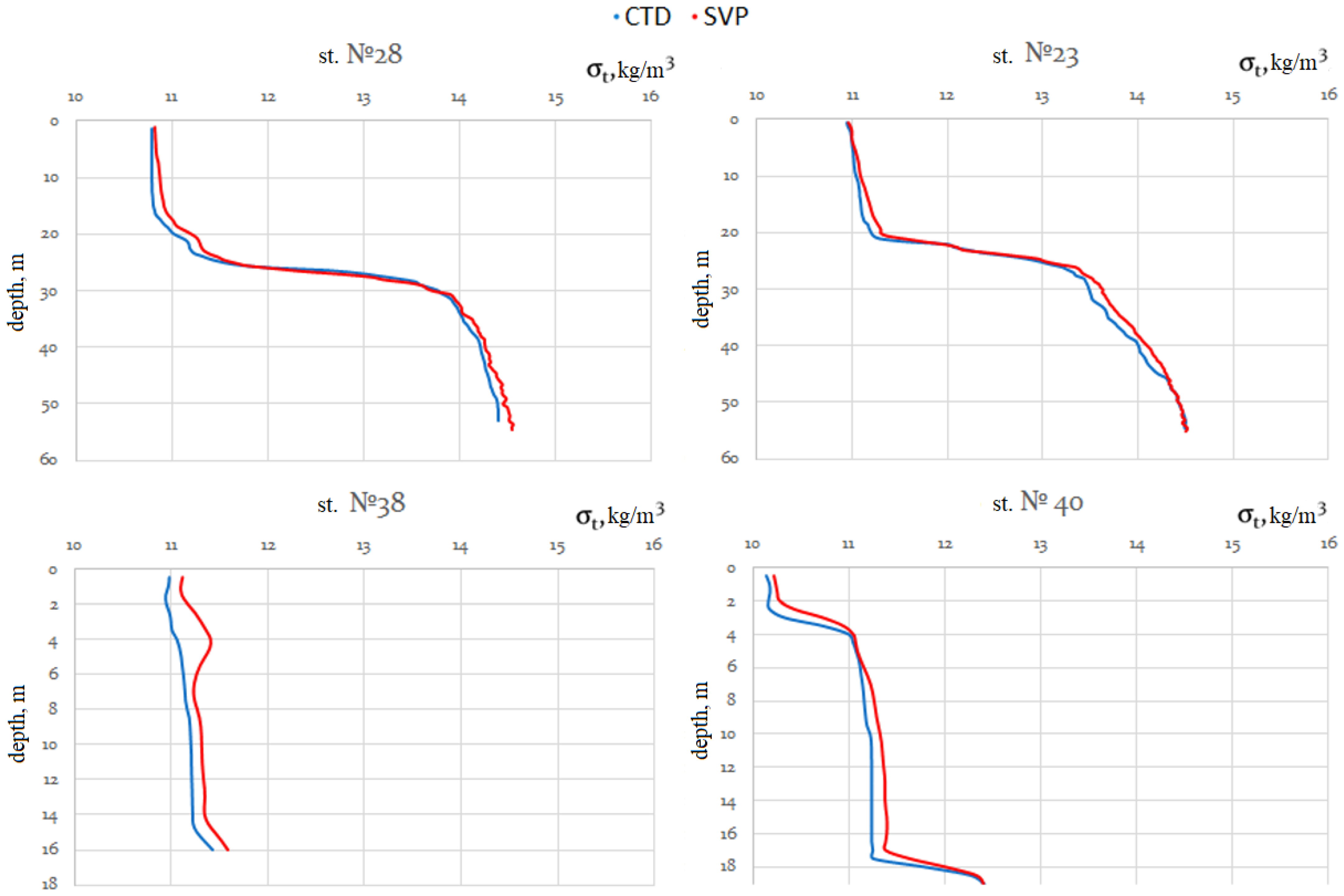
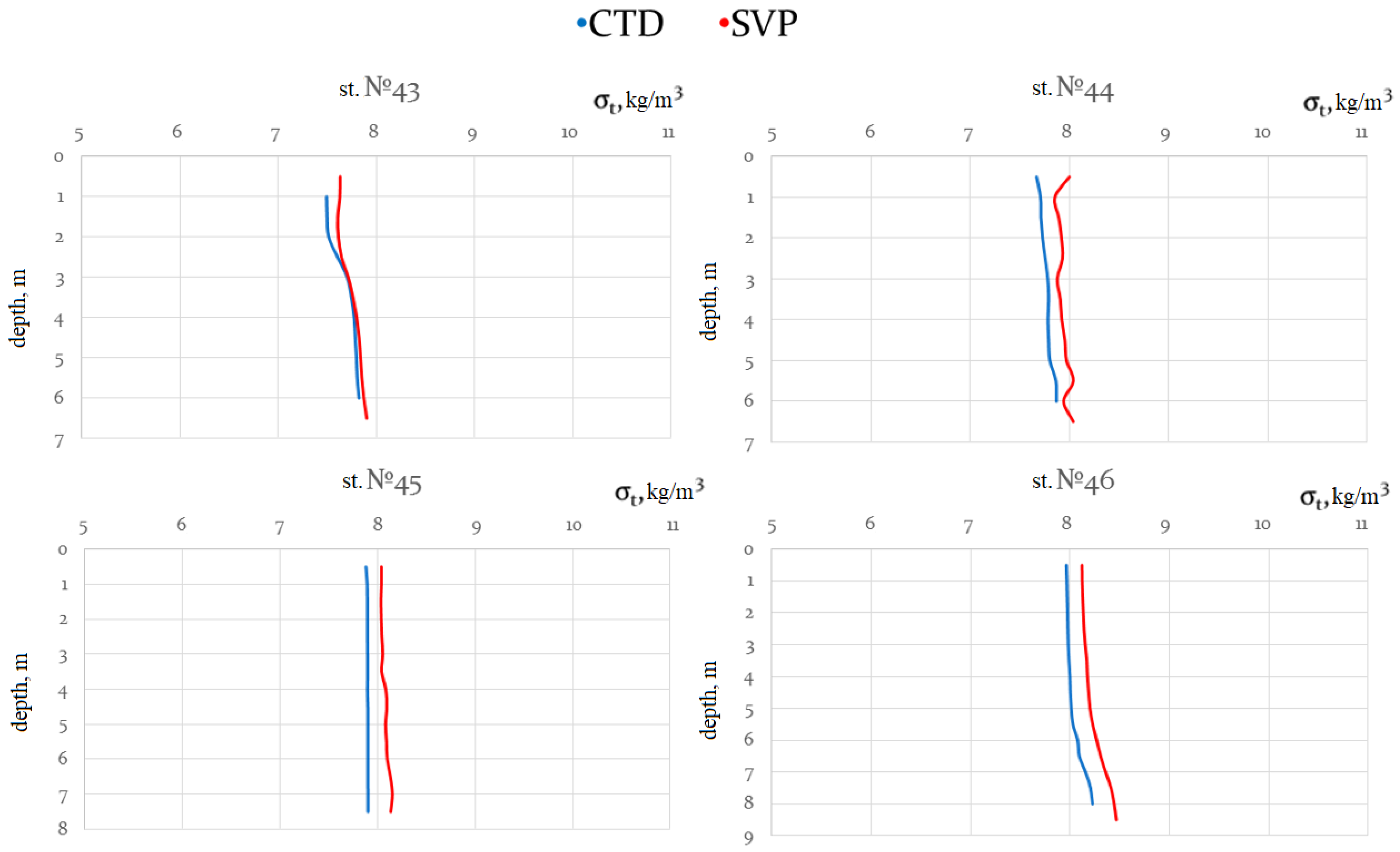
Both devices were mounted on the same basket next to one another, which allowed us to compare measurement results based on two different salinity and density calculation methods using electric conductivity or sound speed. When calculating density and salinity using the data measured by the CTD probe, we used the integrated SeaBird software based on TEOS-10.
When reviewing measurement results obtained using the CTD and SVP devices to determine the causes of discrepancies, we also analyzed the measurement channels for electrical conductivity and sound speed in the seawater. Two other measurement channels (pressure and temperature) were identical in their metrological parameters. To compare the discrepancies in the measurements obtained using the CTD and SVP devices, we compared the values of salinity and density calculated by the algorithms based on TEOS-10 and that have been established for clear seawater without impurities. Next, we review in detail what factors affect the reliability of measurements obtained through in situ devices by analyzing the operations of measurement channels for sound speed and electrical conductivity.
CTD probes widely employ the contact (conductive) method and the contactless (inductive) method to measure the specific electrical conductivity of water. For example, conductive sensors are used in devices such as Neil Brown Instrument Systems (NBIS), Sea Bird Electronics Inc (SBE), Guildline Instruments Ltd. (Guildline), and IDRONAUT S.r.l. Inductive sensors are used in Aanderaa Data Instruments AS, Falmouth Scientific, Inc, and other devices. Despite their advantages (zero contact), inductive sensors have a narrow frequency bandpass, and it is very difficult to extend their functional capacities, e.g., in order to use them for impedance spectroscopy.
The most successful conductive cells have four electrodes or more. Electrodes are made of platinum and located inside an alumina ceramic or borosilicate glass cylindrical tube. For example, a Sea Bird Electronics Inc (SBE) cell is equipped with 10 mm wide platinized round electrodes and a 190 mm long tube, with an internal diameter of 7 mm.
The design features of the cells and their shielding are well-developed in modern devices; therefore, the influence of external fields is minimized. As a consequence, the effect of interference on the cell is reduced, which contributes to good metrological characteristics. However, the large cell length and a small cross-section of the flow channel make it impossible for water to pass through the cell with the required speed. Thus, forced injection systems (special pumps) are used in this case. The small diameter of the flow channel makes SBE probes very sensitive to impurities.
When electric current passes through a conductive cell, a chain of electric and thermodynamic processes starts in it.
When the current is not supplied to the electrodes, the ions in the solution only take part in thermal motion. When a field of strength E is supplied on a cell, it forces the ions to move towards the electrodes, complementing the thermal motion.
The key problem with conductivity measurement using alternating current is the correct interpretation of the results. This can be complicated by the facts that the equivalent circuit of a cell is usually unknown and that the sample with connected electrodes is basically an electric black box.
When studying the impedance of electrochemical cells, it is necessary to obtain reliable information on electrode processes, i.e., the processes occurring on the surface of contact between the electrode and electrolyte.
Let us review in detail a conductive sensor with 4-electrode cell.
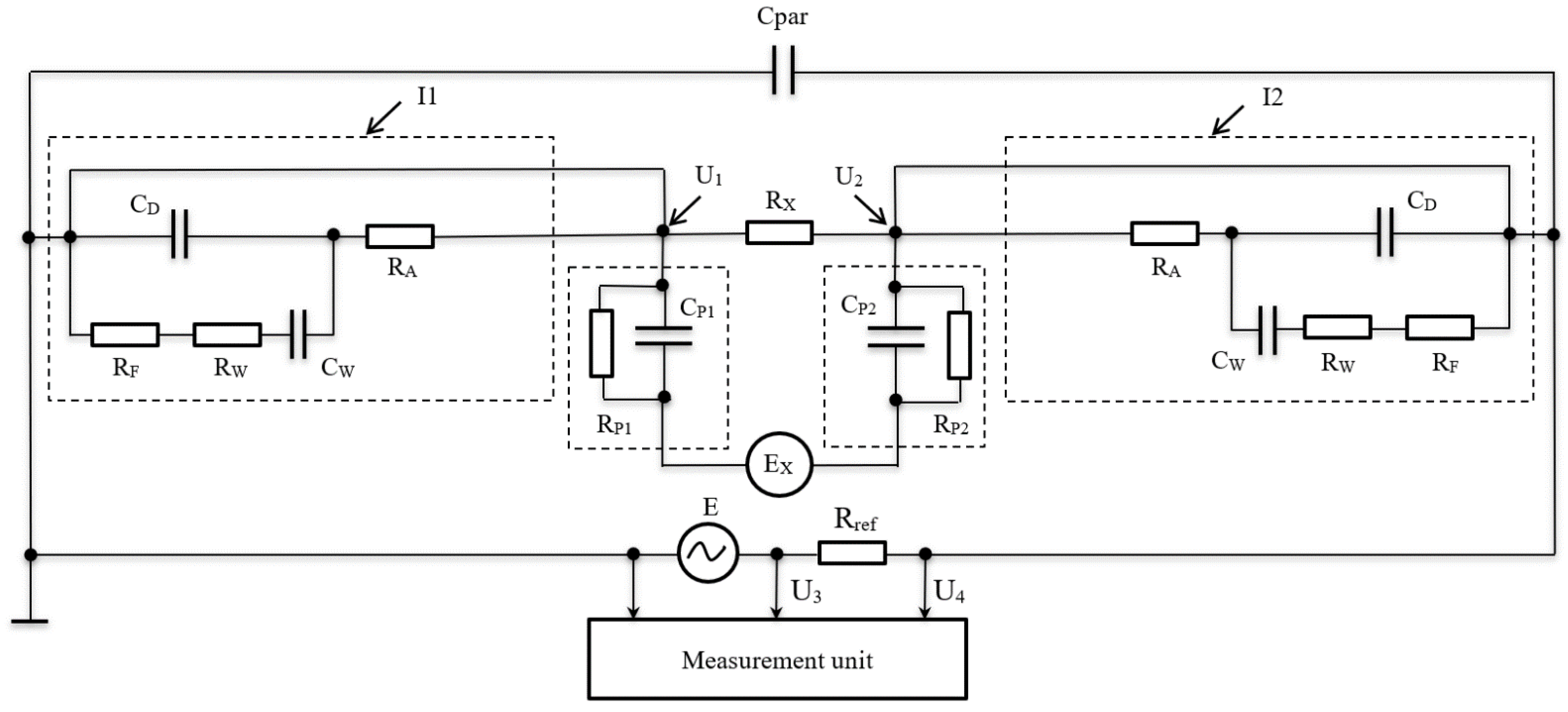
The equivalent circuit of a 4-electrode cell with an auxiliary node to measure impedance is shown in
Figure 2.
The equivalent circuit of the cell in question comprises two current (I1 and I2) and two potential (U1 and U2) electrodes with some of the impedance of the water solution in question between them. Current electrodes I1 and I2 are supplied with alternating current. They can be represented as a capacitance of double layer
CD, parallel with the impedance of electrochemical polarization
and serial-connected to resistance
RW and capacitance
CW related to the concentration polarization (usually referred to as Warburg’s impedance) [
15].
Resistance RA reflects the absorption of atoms, ions, or molecules on the surface of the electrode, and it can be equal to zero under appropriate conditions (e.g., the use of a perfectly polarizable electrode and an inert electrolyte).
In an equivalent circuit, parasitic capacitance
Cpar accounts for a sum of capacitances determined by the dielectric constant of water solution, the distance between the electrodes, the active surface areas of electrodes, and the capacitance between the conductors connected to the electrodes. When considering impedance, it is worth noticing that the parasitic capacitance is especially evident at higher frequencies. For the equivalent circuit shown in
Figure 2, the total impedance of the conductometric cell in question can be expressed as follows, provided that
Cpar = 0:
In this expression, the first component is the true impedance value of solution, the second real component can be denoted as ΔRW is the error caused by polarization phenomena, and the third component is the virtual one that can be determined as capacitive reactance.
This can be reduced by using special circuits integrated into the measuring device and used to obtain additional information on the phase at various frequencies, which can be further used in the research of cell impedance variability. We know that the material and surface condition of electrodes have significant impacts on the polarization impedance value. We assume that the value of polarization impedance is related to the design of the crystalline grid of the electrode material, the absorption properties of its active surface, and the formation of surface oxidic films. Additionally, the frequency of alternating current has a significant impact on the polarization effect. Numerous authors [
16] have claimed that the correlation between
RW and frequency for reversible electrodes made of different materials used in water solutions of various concentrations can be expressed as follows:
where
is a constant. From Formula (4), we can conclude that
RW decreases as the frequency increases and becomes insignificant at frequencies above 1 kHz. On the other hand, the correlation between the polarization capacitance and the frequency is as follows:
The constant
in expressions (4) and (5) reflects the dependence of
RW and
CW, respectively, from concentration, ion diffusion factor, and double-layer potential.
where
;
is the concentration of the potential determining ions of the
i-th kind in the presence of a sufficiently large amount of inert electrolyte, which is introduced to eliminate the migration effect of the potential determining ions;
νi is the number of equivalents of ions arising from the chemical interaction when passing through the dividing surface of one faraday of electricity;
Di is the diffusion coefficient of the potential determining ions; and
E is the double layer potential, corrected for the voltage drop (
E = φ − Δφ).
Unfortunately, the presented linear equations could not produce acceptable results when used for precise calculations in the model. Therefore, we had to use the experimentally obtained coefficients for each specific case.
To eliminate or reduce errors RA when measuring the ohmic resistance of the cell Rx, special conditions (such as a perfectly polarized electrode and inert electrolyte) are sometimes used. As mentioned above, the polarization resistance value ΔRW and the respective error, accounted for in the measured resistance, depend on a large number of various system parameters.
In some cases, the error caused by the impact of polarization resistance ΔRW on the measured resistance Rx may reach 20%. Thus, for high-precision measurements, it is necessary to introduce an allowance for polarization resistance ΔRW. Our experiments confirmed that the minimum error caused by ΔRW for platinum group electrodes at some frequencies is 0.001%.
Concerning design features, many authors [
17] have conducted experiments to prove that the distance between electrodes in a conductometric mesh does not affect the polarization resistance value Δ
RW.
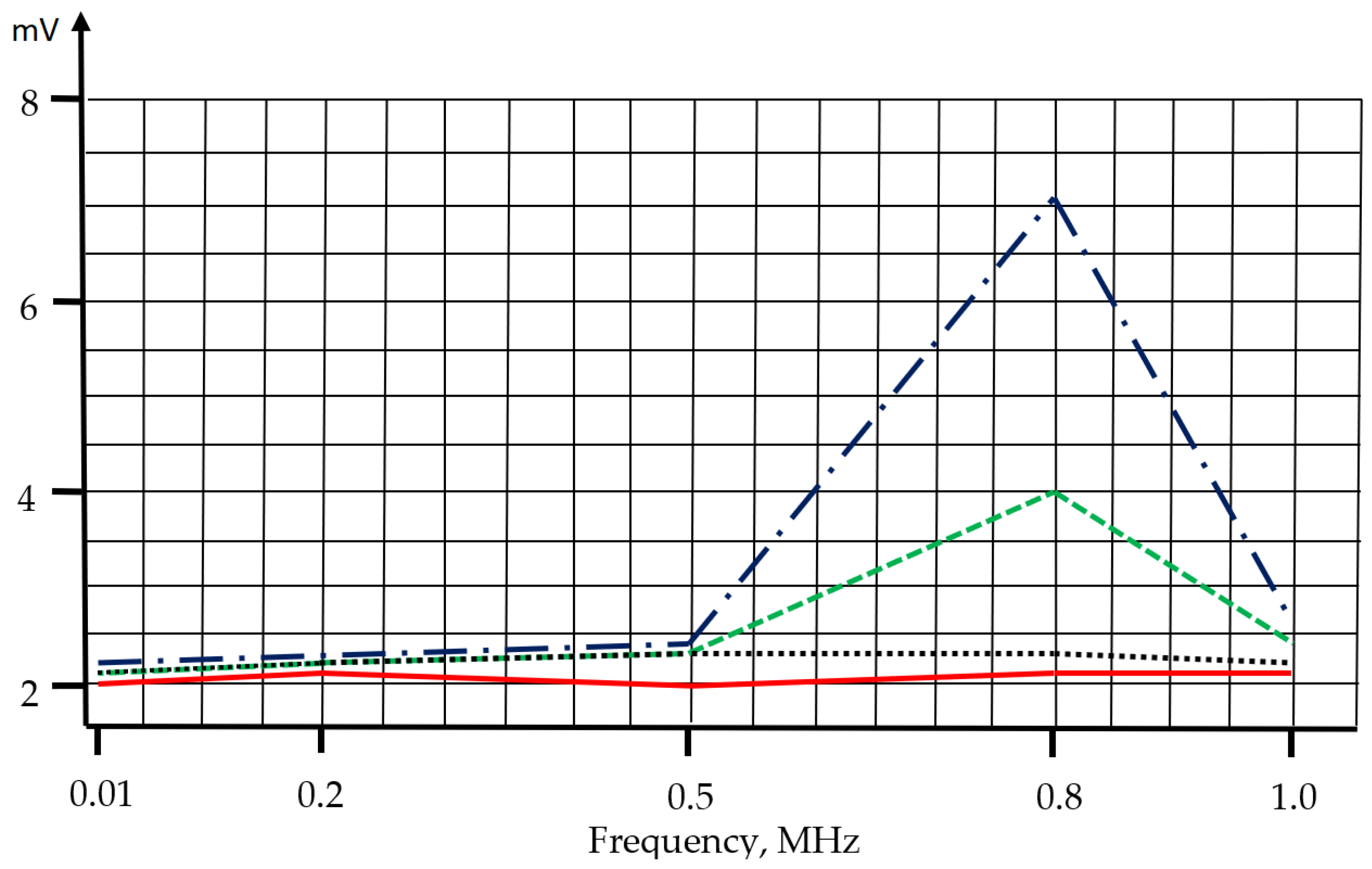
We used the abovementioned recommendations to develop a simplified model of the electrical conductivity sensor with four platinum electrodes placed inside a quartz glass tube with an inner diameter of 8 mm and a length of 60 mm; this tube was then placed in a sealed case and tested under high pressure. Like the SBE911 model described above that features a water pump, this sensor design is not perfect. The sensor model was also equipped with an impedance measurement module operating in situ at constant frequencies within the range of 0.01–1.0 MHz. The sensor model was tested during the marine trip together with the CTD and SVP devices. It produced additional data about suspended impurities in the seawater. The impedance sensor model helped us record suspended impurities corresponding to the impurities recorded by the ultrasonic SVP device and missing in the electric conductivity channel of the CTD probe. In this case, the operating frequency was about 0.8 MHz.
A simplified function diagram of the impedance measurement module together with the equivalent 4-electrode conductometric cell is shown above in
Figure 2. The impedance measurement module consisted of the reference resistor Rref, from which the amplitude of a sinusoidal signal of a certain frequency was fed to the measuring unit. The impedance was determined by the equation
Zj = U4·Rref/(U3 − U4). The impedance measurement module operated at 5 constant frequencies—0.01, 0.2, 0.5, 0.8, and 1.0 MHz—with a homogenization duration of 1 s an each of them. The entire measurement cycle lasted for 5 s.
If we extend the functional capabilities of metering channels for electrical conductivity and sound speed of the CTD and SVP devices by using impedance and acoustic attenuation, we could obtain quantitative and qualitative parameters of suspended materials in seawater without using any additional tools.
The key measured parameters related to absolute salinity (SA) and density (ρ) include relative electrical conductance (χ), sound speed in water (c), temperature (T), and hydrostatic pressure (P). To calculate absolute salinity and density using the parameters measured in situ, we used the following algorithms:
(1) SA = SA1(T, P, χ).
(2) SA = SA2(T, P, c).
(3) SA = SA3(T, P, ρ).
(4) ρ = ρ1(T, P, c).
(5) ρ = ρ2(T, P, SA1).
(6) ρ = ρ3(T, P, SA2).
The connection between absolute salinity and practical salinity of seawater is set by the following relationship in TEOS-10 manual [
11]:
Here, δSA(x, y, P), g/kg is the “absolute salinity anomaly”, which should consider the disturbances in the constancy of the salt composition of seawater. Thus, to the problems associated with finding reliable values of the practical salinity (SP) of seawater (which is calculated by considering conductivity and should characterize the influence of the ionic component of the mass of dissolved substances), if the water passes through a 0.2 μm filter, there are added the problems of zoning of disturbances of the seawater salt composition constancy and definition estimates of the corresponding values of the anomaly of absolute salinity with reference to geographic coordinates (x, y) and depth (or pressure, P) with considering the presence of solid suspensions.
To calculate the density on the SVP device data, we used the equation of state [
18]:
To construct this equation, we used the international Equation of State of Seawater TEOS-10 [
11]. The initial data array {
ρm, cm, Tm, Pm, SAm},
m = 1,
…, M was generated with using density and sound speed equations from the TEOS-10 system [
11]. The couples of the value densities
ρ (
Tm,
Pm, and
SAm) and sound speeds
c (
Tm,
Pm, and
SAm) were calculated between melting curve and 40 °C, between 0 and 120 MPa of hydrostatic pressure, and between 0 and 42 g/kg of salinity. The ranges of changes in the speed of sound and density were approximately 1300–1800 m/s and 990–1090 kg/m
3, respectively.
The seawater density interpolation Equation (8) has the following polynomial form:
where
γ =
(ρ − ρ0)/
ρ*;
τ = (
T − T0)/
T*;
π = (
Pabs − P0)/
P*; ω = (
c − c0)/
c*;
ρ0 = 990 kg/m
3;
ρ* = 100 kg/m
3;
T0 = −10 °C;
T* = 50 °C;
P0 = 0.101325 MPa;
P* = 120 MPa;
c0 = 1300 m/s; and
c* = 500 m/s.
Equation (9) explicitly expresses the functional dependency between the density of seawater and parameters T, P, and c and can be useful in marine research. The advantage of this equation is that salinity is not explicitly present in it, although, of course both the density and the sound speed of seawater are functionally related to its salinity. An equation like this helps to eliminate the indirect (via conductivity) salinity measurements by replacing them with direct sound speed measurements.
CTD and SVP probes are used for the synchronous in situ measurements of two different groups of seawater parameters (see
Table 3).
The two groups of parameters measured by the CTD and SVP probes can be further used to calculate of two usually different density values of seawater.
The differences in the density values obtained from CTD (ρCTD) and SVP (ρSVP) data are due to the fact that there are fundamental and significant differences between the physical processes of the passage of electric current and the propagation of sound in seawater. It should be noted that the value of the measured CTD relative electrical conductivity is only affected by the total ionic component of substances dissolved in seawater, i.e., only electrolyte solutions in seawater. At the same time, the speed of sound propagation measured by SVP is affected by the total mass fraction of all substances dissolved and suspended in seawater.
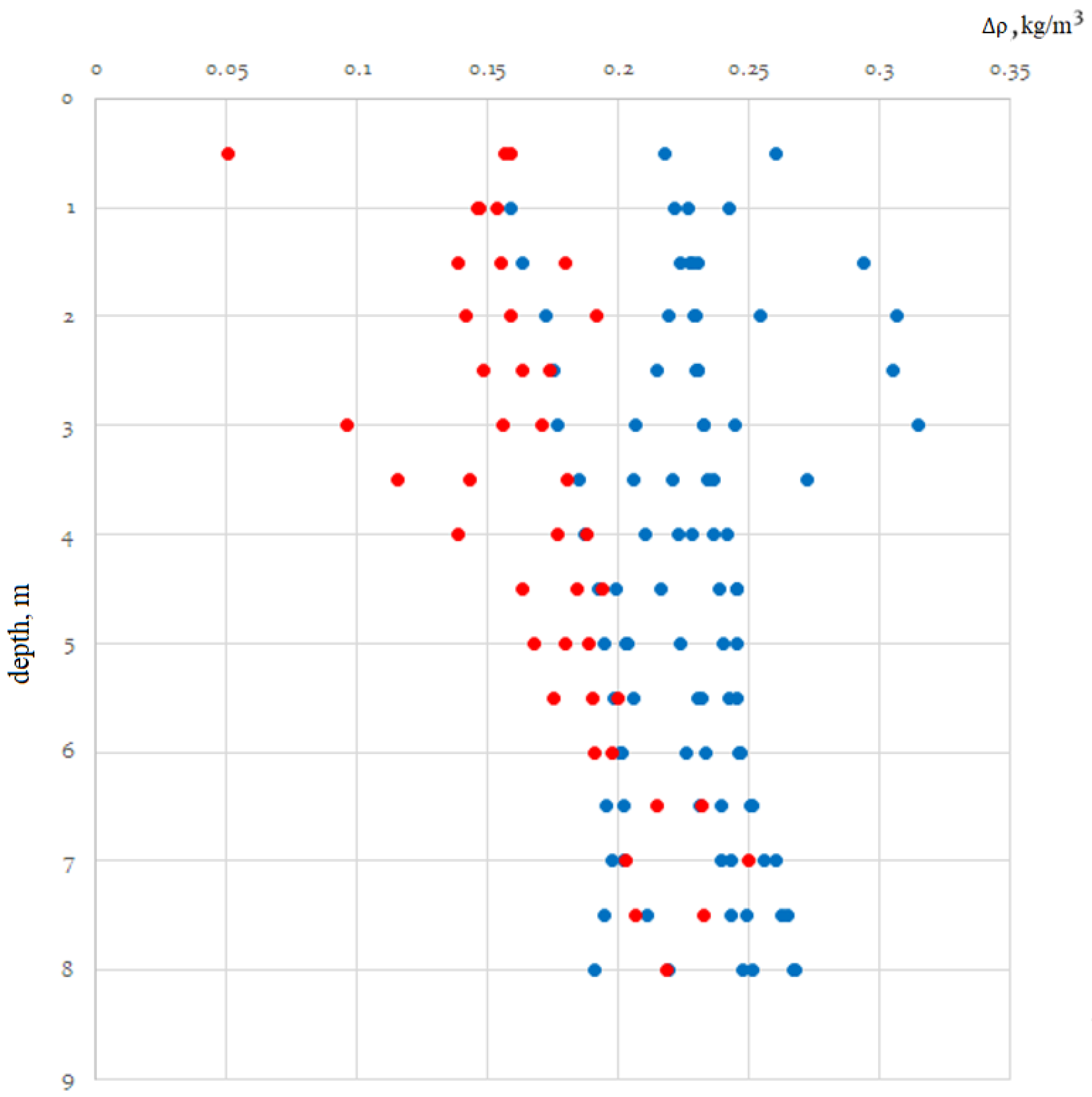
Therefore, the difference (delta) of densities can be used to estimate the amount of suspended matter in seawater:
where
Here,
SP =
SP(
T, P, χ); practical salinity is presented in the form of a special algorithm within the framework of the International Scale of Practical Salinity from 1978 [
19,
20].
The international Thermodynamic Equation Of state of Seawater TEOS-10 [
11] and the authors’ equation [
18] for calculating the density of seawater from measurements of the sound speed can be used, respectively, as equations for
ρCTD (11) and
ρSVP (12).