Renal Rehabilitation—Its Theory and Clinical Application to Patients Undergoing Daily Dialysis Therapy
Abstract
1. Introduction
2. Functional Status and Physical Frailty
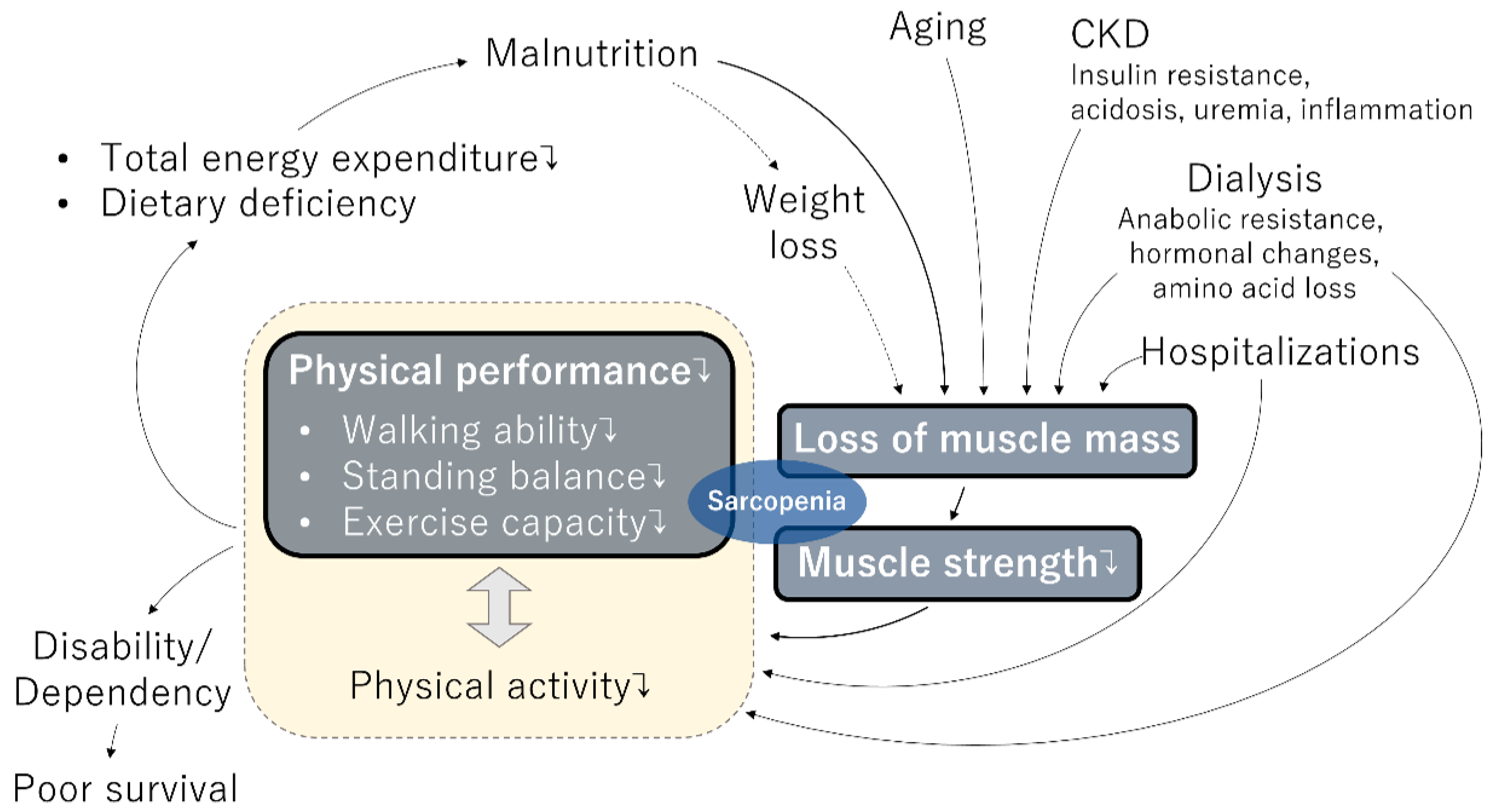
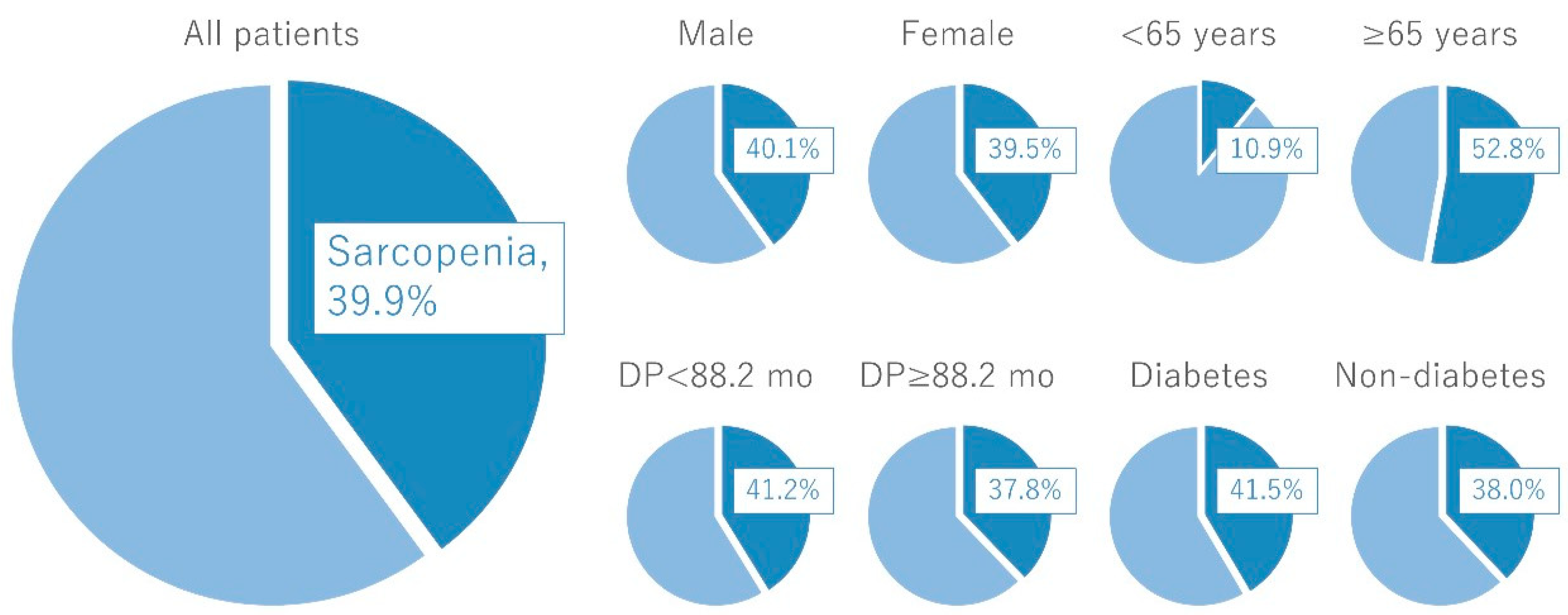
3. Sarcopenia in the Cycle of Frailty
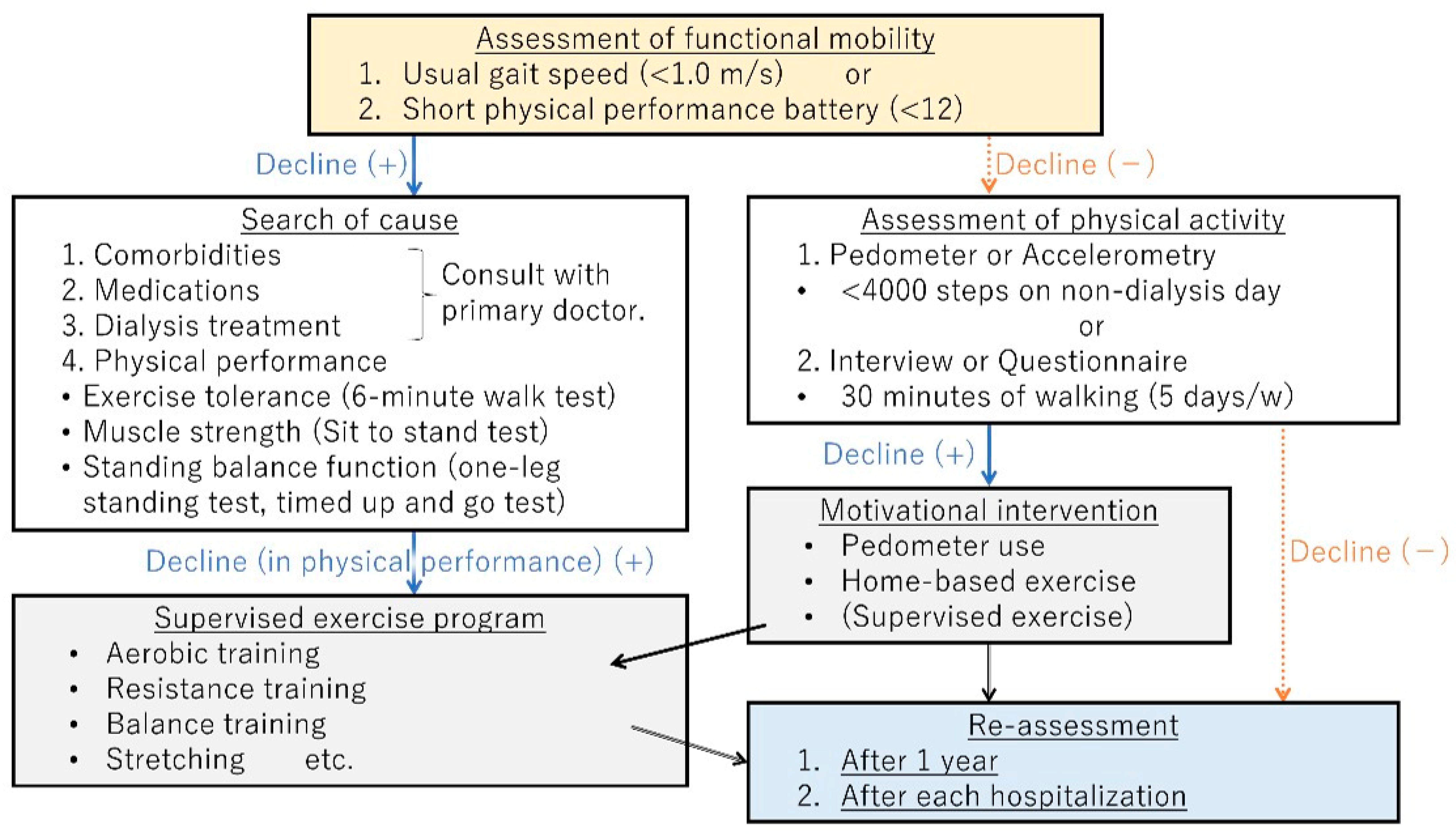
4. Management of Physical Frailty
5. Frailty and Renal Transplantation
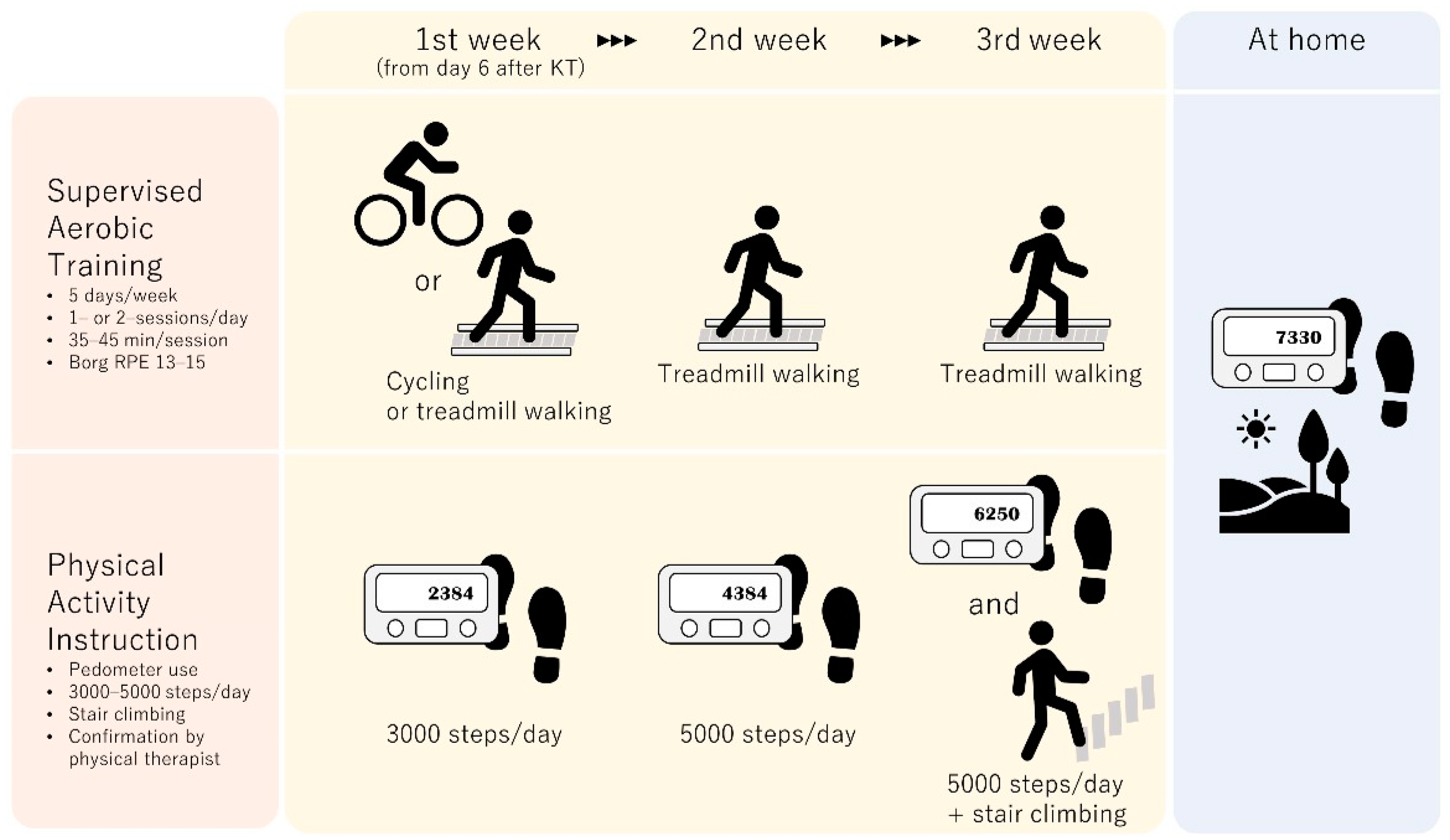
6. Exercise Intervention after Kidney Transplantation
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamer, R.A.; El Nahas, A.M. The burden of chronic kidney disease. BMJ 2006, 332, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Tong, L.; Tentori, F.; Akiba, T.; Karaboyas, A.; Gillespie, B.; Akizawa, T.; Pisoni, R.L.; Bommer, J.; Port, F.K. Clinical Practices and Outcomes in Elderly Hemodialysis Patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin. J. Am. Soc. Nephrol. 2011, 6, 1651–1662. [Google Scholar] [CrossRef]
- Kimata, N.; Tsuchiya, K.; Akiba, T.; Nitta, K. Differences in the Characteristics of Dialysis Patients in Japan Compared with Those in Other Countries. Blood Purif. 2015, 40, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41. [Google Scholar] [CrossRef]
- Farrington, K.; Covic, A.; Aucella, F.; Clyne, N.; De Vos, L.; Findlay, A.; Fouque, D.; Grodzicki, T.; Iyasere, O.; Jager, K.J.; et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR < 45 mL/min/1.73 m2). Nephrol. Dial. Transpl. 2016, 31, 1–66. [Google Scholar] [CrossRef]
- Yamagata, K.; Hoshino, J.; Sugiyama, H.; Hanafusa, N.; Shibagaki, Y.; Komatsu, Y.; Konta, T.; Fujii, N.; Kanda, E.; Sofue, T.; et al. Clinical practice guideline for renal rehabilitation: Systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren. Replace. Ther. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Tamura, M.K.; Covinsky, K.E.; Chertow, G.M.; Yaffe, K.; Landefeld, C.S.; McCulloch, C.E. Faculty Opinions recommendation of Functional status of elderly adults before and after initiation of dialysis. N. Engl. J. Med. 2009, 361, 1539–1547. [Google Scholar] [CrossRef]
- Jassal, S.V.; Karaboyas, A.; Comment, L.A.; Bieber, B.; Morgenstern, H.; Sen, A.; Gillespie, B.W.; De Sequera, P.; Marshall, M.R.; Fukuhara, S.; et al. Functional Dependence and Mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2016, 67, 283–292. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Kamitani, T.; Roshanravan, B.; Fukuma, S.; Joki, N.; Fukagawa, M. Decline in the Functional Status and Mortality in Patients on Hemodialysis: Results from the Japan Dialysis Outcome and Practice Patterns Study. J. Ren. Nutr. 2019, 29, 504–510. [Google Scholar] [CrossRef]
- Heiland, E.G.; Welmer, A.-K.; Wang, R.; Santoni, G.; Angleman, S.; Fratiglioni, L.; Qiu, C. Association of mobility limitations with incident disability among older adults: A population-based study. Age Ageing 2016, 45, 812–819. [Google Scholar] [CrossRef]
- Goto, R.; Watanabe, H.; Haruta, J.; Tsutsumi, M.; Yokoya, S.; Maeno, T. Identification of prognostic factors for activities of daily living in elderly patients after hospitalization for acute infectious disease in Japan: A 6-month follow-up study. Geriatr. Gerontol. Int. 2017, 18, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Godfrey, S.; Ng, T.T.; Moorthi, R.; Liangos, O.; Ruthazer, R.; Jaber, B.L.; Levey, A.S.; Castaneda-Sceppa, C. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: A randomized pilot trial. Nephrol. Dial. Transpl. 2010, 25, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research G. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research Agenda for Frailty in Older Adults: Toward a Better Understanding of Physiology and Etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Prevalence of frailty in end-stage renal disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2017, 49, 1989–1997. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Taniguchi, Y.; Shimada, H.; Rakugi, H.; Walters, K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 347–353. [Google Scholar] [CrossRef]
- Matsuzawa, R. Renal rehabilitation as a management strategy for physical frailty in CKD. Ren. Replace. Ther. 2022, 8, 1–9. [Google Scholar] [CrossRef]
- Hendriks, F.K.; Smeets, J.S.; van der Sande, F.M.; Kooman, J.P.; van Loon, L.J. Dietary Protein and Physical Activity Interventions to Support Muscle Maintenance in End-Stage Renal Disease Patients on Hemodialysis. Nutrients 2019, 11, 2972. [Google Scholar] [CrossRef]
- Vettoretti, S.; Caldiroli, L.; Armelloni, S.; Ferrari, C.; Cesari, M.; Messa, P. Sarcopenia is Associated with Malnutrition but Not with Systemic Inflammation in Older Persons with Advanced CKD. Nutrients 2019, 11, 1378. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.R.; Choi, M.J.; Kim, S.G.; Lee, Y.K.; Noh, J.W.; Kim, H.J.; Song, Y.R. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin. Nutr. 2014, 33, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Gungor, O.; Ulu, S.; Hasbal, N.B.; Anker, S.D.; Kalantar-Zadeh, K. Effects of hormonal changes on sarcopenia in chronic kidney disease: Where are we now and what can we do? J. Cachexia Sarcopenia Muscle 2021, 12, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Haugen, C.E.; Chu, N.; Ying, H.; Warsame, F.; Holscher, C.M.; Desai, N.M.; Jones, M.R.; Norman, S.P.; Brennan, D.C.; Garonzik-Wang, J.; et al. Frailty and Access to Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2019, 14, 576–582. [Google Scholar] [CrossRef] [PubMed]
- McAdams-DeMarco, M.A.; Law, A.; Salter, M.L.; Boyarsky, B.; Gimenez, L.F.; Jaar, B.; Walston, J.D.; Segev, D.L. Frailty as a Novel Predictor of Mortality and Hospitalization in Individuals of All Ages Undergoing Hemodialysis. J. Am. Geriatr. Soc. 2013, 61, 896–901. [Google Scholar] [CrossRef]
- Alfaadhel, T.A.; Soroka, S.D.; Kiberd, B.A.; Landry, D.; Moorhouse, P.; Tennankore, K.K. Frailty and Mortality in Dialysis: Evaluation of a Clinical Frailty Scale. Clin. J. Am. Soc. Nephrol. 2015, 10, 832–840. [Google Scholar] [CrossRef]
- Kim, J.C.; Shapiro, B.B.; Zhang, M.; Li, Y.; Porszasz, J.; Bross, R.; Feroze, U.; Upreti, R.; Kalantar-Zadeh, K.; Kopple, J.D. Daily physical activity and physical function in adult maintenance hemodialysis patients. J. Cachex Sarcopenia Muscle 2014, 5, 209–220. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kara, M.; Kaymak, B.; Frontera, W.; Ata, A.M.; Ricci, V.; Ekiz, T.; Chang, K.V.; Han, D.S.; Michail, X.; Quittan, M.; et al. Diagnosing sarcopenia: Functional perspectives and a new algorithm from the ISarcoPRM. J. Rehabil. Med. 2021, 53, jrm00209. [Google Scholar] [CrossRef]
- Shu, X.; Lin, T.; Wang, H.; Zhao, Y.; Jiang, T.; Peng, X.; Yue, J. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Kitamura, A.; Seino, S.; Abe, T.; Nofuji, Y.; Yokoyama, Y.; Amano, H.; Nishi, M.; Taniguchi, Y.; Narita, M.; Fujiwara, Y.; et al. Sarcopenia: Prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J. Cachex Sarcopenia Muscle 2020, 12, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kakita, D.; Matsuzawa, R.; Yamamoto, S.; Suzuki, Y.; Harada, M.; Imamura, K.; Yoshikoshi, S.; Imai, H.; Osada, S.; Shimokado, K.; et al. Simplified discriminant parameters for sarcopenia among patients undergoing hemodialysis. J. Cachexia Sarcopenia Muscle 2022, in press.
- Imamura, K.; Yamamoto, S.; Suzuki, Y.; Matsuzawa, R.; Harada, M.; Yoshikoshi, S.; Yoshida, A.; Matsunaga, A. Limitations of SARC-F as a Screening Tool for Sarcopenia in Patients on Hemodialysis. Nephron Exp. Nephrol. 2021, 146, 32–39. [Google Scholar] [CrossRef]
- Canaud, B.; Ye, X.; Usvyat, L.; Kooman, J.; van der Sande, F.; Raimann, J.; Wang, Y.; Kotanko, P. Clinical and predictive value of simplified creatinine index used as muscle mass surrogate in end-stage kidney disease haemodialysis patients—Results from the international MONitoring Dialysis Outcome initiative. Nephrol. Dial. Transplant. 2020, 35, 2161–2171. [Google Scholar] [CrossRef]
- Yamamoto, S.; Matsuzawa, R.; Hoshi, K.; Suzuki, Y.; Harada, M.; Watanabe, T.; Isobe, Y.; Imamura, K.; Osada, S.; Yoshida, A.; et al. Modified Creatinine Index and Clinical Outcomes of Hemodialysis Patients: An Indicator of Sarcopenia? J. Ren. Nutr. 2021, 31, 370–379. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Roshanravan, B. Management of Physical Frailty in Patients Requiring Hemodialysis Therapy. Contrib. Nephrol. 2018, 196, 101–109. [Google Scholar] [CrossRef]
- Roshanravan, B.; Gamboa, J.; Wilund, K. Exercise and CKD: Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. Am. J. Kidney Dis. 2017, 69, 837–852. [Google Scholar] [CrossRef]
- Yamamoto, S.; Matsuzawa, R.; Abe, Y.; Hoshi, K.; Yoneki, K.; Harada, M.; Watanabe, T.; Shimoda, T.; Suzuki, Y.; Matsunaga, Y.; et al. Utility of Regular Management of Physical Activity and Physical Function in Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 1505–1515. [Google Scholar] [CrossRef]
- Kojima, G. Quick and Simple FRAIL Scale Predicts Incident Activities of Daily Living (ADL) and Instrumental ADL (IADL) Disabilities: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2018, 19, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, R.; Matsunaga, A.; Wang, G.; Yamamoto, S.; Kutsuna, T.; Ishii, A.; Abe, Y.; Yoneki, K.; Yoshida, A.; Takahira, N. Relationship Between Lower Extremity Muscle Strength and All-Cause Mortality in Japanese Patients Undergoing Dialysis. Phys. Ther. 2014, 94, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ren, Y.; Wang, H.; Bai, Y.; Huang, L. Daily Step Counts in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Observational Studies. Front. Med. 2022, 17, 842423. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Kaysen, G.A.; Dalrymple, L.S.; Grimes, B.A.; Glidden, D.; Anand, S.; Chertow, G.M. Association of Physical Activity with Survival among Ambulatory Patients on Dialysis: The Comprehensive Dialysis Study. Clin. J. Am. Soc. Nephrol. 2012, 8, 248–253. [Google Scholar] [CrossRef]
- Lopes, A.A.; Lantz, B.; Morgenstern, H.; Wang, M.; Bieber, B.; Gillespie, B.W.; Li, Y.; Painter, P.; Jacobson, S.H.; Rayner, H.C.; et al. Associations of Self-Reported Physical Activity Types and Levels with Quality of Life, Depression Symptoms, and Mortality in Hemodialysis Patients: The DOPPS. Clin. J. Am. Soc. Nephrol. 2014, 9, 1702–1712. [Google Scholar] [CrossRef]
- O’Hare, A.M.; Tawney, K.; Bacchetti, P.; Johansen, K.L. Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am. J. Kidney Dis. 2003, 41, 447–454. [Google Scholar] [CrossRef]
- Stack, A.G.; Molony, D.A.; Rives, T.; Tyson, J.; Murthy, B.V. Association of physical activity with mortality in the US dialysis population. Am. J. Kidney Dis. 2005, 45, 690–701. [Google Scholar] [CrossRef]
- Tentori, F.; Elder, S.J.; Thumma, J.; Pisoni, R.L.; Bommer, J.; Fissell, R.B.; Fukuhara, S.; Jadoul, M.; Keen, M.L.; Saran, R.; et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Correlates and associated outcomes. Nephrol. Dial. Transpl. 2010, 25, 3050–3062. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Matsunaga, A.; Wang, G.; Kutsuna, T.; Ishii, A.; Abe, Y.; Takagi, Y.; Yoshida, A.; Takahira, N. Habitual Physical Activity Measured by Accelerometer and Survival in Maintenance Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 2010–2016. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Roshanravan, B.; Shimoda, T.; Mamorita, N.; Yoneki, K.; Harada, M.; Watanabe, T.; Yoshida, A.; Takeuchi, Y.; Matsunaga, A. Physical Activity Dose for Hemodialysis Patients: Where to Begin? Results from a Prospective Cohort Study. J. Ren. Nutr. 2018, 28, 45–53. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W. ; American College of Sports Medicine. ACSM’s Exercise for Older Adults, 1st ed.; Lippincott Williams & Wilkins: New York, NY, USA, 2014. [Google Scholar]
- Shimoda, T.; Matsuzawa, R.; Yoneki, K.; Harada, M.; Watanabe, T.; Matsumoto, M.; Yoshida, A.; Takeuchi, Y.; Matsunaga, A. Changes in physical activity and risk of all-cause mortality in patients on maintenance hemodialysis: A retrospective cohort study. BMC Nephrol. 2017, 18, 154. [Google Scholar] [CrossRef]
- Yamamoto, S.; Matsuzawa, R.; Hoshi, K.; Harada, M.; Watanabe, T.; Suzuki, Y.; Isobe, Y.; Imamura, K.; Osada, S.; Yoshida, A.; et al. Impact of Physical Activity on Dialysis and Nondialysis Days and Clinical Outcomes Among Patients on Hemodialysis. J. Ren. Nutr. 2021, 31, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Rueda, R.; Bansal, S.; Kasinath, B.S.; Sharma, K.; Lorenzo, C. Fatigue characteristics on dialysis and non-dialysis days in patients with chronic kidney failure on maintenance hemodialysis. BMC Nephrol. 2021, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, H.; Tsujita, M.; Yazawa, M.; Kawaguchi, T.; Hoshino, J.; Kohzuki, M.; Ito, O.; Yamagata, K.; Shibagaki, Y.; Sofue, T. The efficacy of exercise training in kidney transplant recipients: A meta-analysis and systematic review. Clin. Exp. Nephrol. 2018, 23, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Matsuzawa, R.; Kamitani, T.; Hoshi, K.; Ishii, D.; Noguchi, F.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Maekawa, E.; et al. Efficacy of Exercise Therapy Initiated in the Early Phase After Kidney Transplantation: A Pilot Study. J. Ren. Nutr. 2020, 30, 518–525. [Google Scholar] [CrossRef]

| Component | Questions and Measurements | Answer |
|---|---|---|
| Shrinking | Have you unintentionally lost 2 or more kg in the past 6 months? | Yes = 1 |
| No = 0 | ||
| Weakness | Grip strength <28 kg in men or 18 kg in women | Yes = 1 |
| No = 0 | ||
| Exhaustion | In the past 2 weeks, have you felt tired without reason? | Yes = 1 |
| No = 0 | ||
| Slowness | Gait speed <1.0 m/s | Yes = 1 |
| No = 0 | ||
| Low activity | Do you engage in a moderate level of physical exercise or sports? | No to both questions = 1 |
| Do you engage in a low level of physical exercise aimed at health? | Other = 0 |
| Working Group | (A) Low Muscle Mass | (B) Low Muscle Strength | (C) Low Physical Performance | Diagnosis |
|---|---|---|---|---|
| IWGS (2011) [28] | ASM/height2 (DXA): men ≤7.23 kg/m2, women ≤5.67 kg/m2 | - | Gait speed: <1.0 m/s |
|
| EWGSOP2 (2019) [29] | ASM (BIA or DXA): men <20 kg, women <15 kg or ASM/height2 (BIA or DXA): men <7.0 kg/m2, women <6.0 kg/m2 | Handgrip strength: men <27 kg, women <16 kg or five-time chair stand time: >15 s | Gait speed: ≤0.8 m/s or SPPB: ≤8 points or timed up and go test: ≥20 s or 400 m walk test: non-completion or ≥6 min for completion |
|
| AWGS (2020) [27] | ASM/height2 (BIA): men <7.0 kg/m2, women <5.7 kg/m2 or ASM/height2 (DXA): men <7.0 kg/m2, women <5.4 kg/m2 | Handgrip strength: men <28 kg, women <18 kg | Gait speed: <1.0 m/s or SPPB: ≤9 points or five-time chair stand time: ≥12 s |
|
| ISPRM (2021) [30] | STAR (ultrasound): men <1.4, women <1.0 | Handgrip strength: men <32 kg, women <19 kg or five-time chair stand time: ≥12 s | Rise from a chair: inability or gait speed: ≤0.8 m/s |
|
| In the following cases, exercise therapy should be stopped or exercise intensity should be changed: |
|---|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzawa, R.; Kakita, D. Renal Rehabilitation—Its Theory and Clinical Application to Patients Undergoing Daily Dialysis Therapy. Kidney Dial. 2022, 2, 565-575. https://doi.org/10.3390/kidneydial2040051
Matsuzawa R, Kakita D. Renal Rehabilitation—Its Theory and Clinical Application to Patients Undergoing Daily Dialysis Therapy. Kidney and Dialysis. 2022; 2(4):565-575. https://doi.org/10.3390/kidneydial2040051
Chicago/Turabian StyleMatsuzawa, Ryota, and Daisuke Kakita. 2022. "Renal Rehabilitation—Its Theory and Clinical Application to Patients Undergoing Daily Dialysis Therapy" Kidney and Dialysis 2, no. 4: 565-575. https://doi.org/10.3390/kidneydial2040051
APA StyleMatsuzawa, R., & Kakita, D. (2022). Renal Rehabilitation—Its Theory and Clinical Application to Patients Undergoing Daily Dialysis Therapy. Kidney and Dialysis, 2(4), 565-575. https://doi.org/10.3390/kidneydial2040051






