Renal Nutrition—Where It Has Been and Where It Is Going †
Abstract
1. Introduction
2. The History of Renal Nutrition Since the 19th Century Encompasses Several Periods
- i.
- ii.
- iii.
- i.
- Patients with acute kidney injury (AKI)
- ii.
- Maintenance hemodialysis (MHD) patients
- iii.
- Chronic peritoneal dialysis (CPD) patients
- iv.
- Kidney transplant recipients
- v.
- Children with CKD
- vi.
- Macrominerals, especially sodium, potassium and phosphorus, in kidney disease
- vii.
- Vitamins and trace elements, especially iron, in kidney disease
- viii.
- Furthermore, developed during this time was the description of the syndrome of protein-energy wasting (PEW) in CKD [20]
- ix.
- Identification of the relationships between PEW, inflammation and adverse clinical outcomes.
Precision Nutrition
3. The Future of Renal Nutrition—The Immediate Future
3.1. Role of New Medicines That Modify Nutrient Biochemistry or Physiology
3.2. Reexamination of the Classification and Diagnostic Criteria for Protein-Energy Wasting (PEW) in Kidney Disease
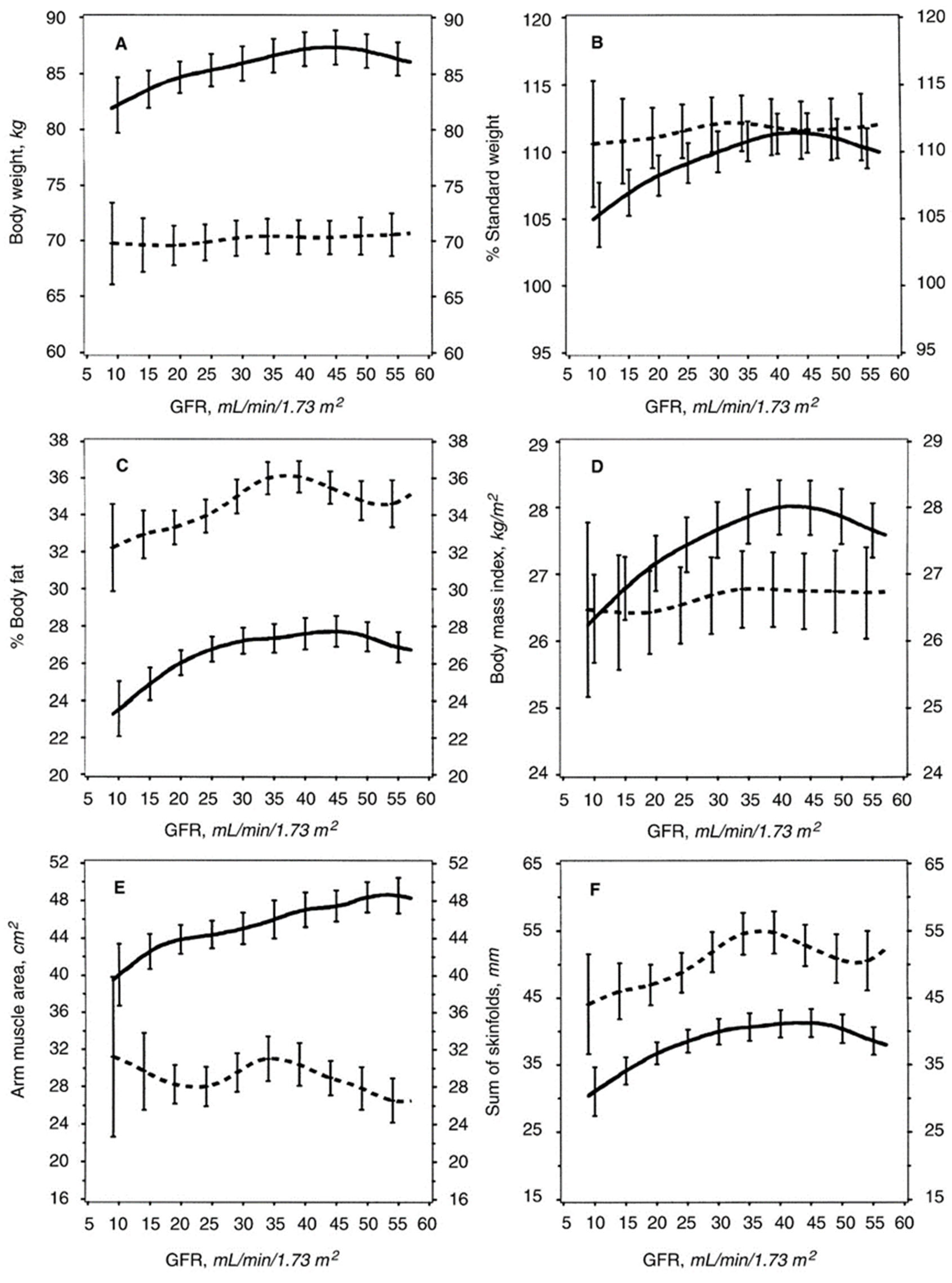
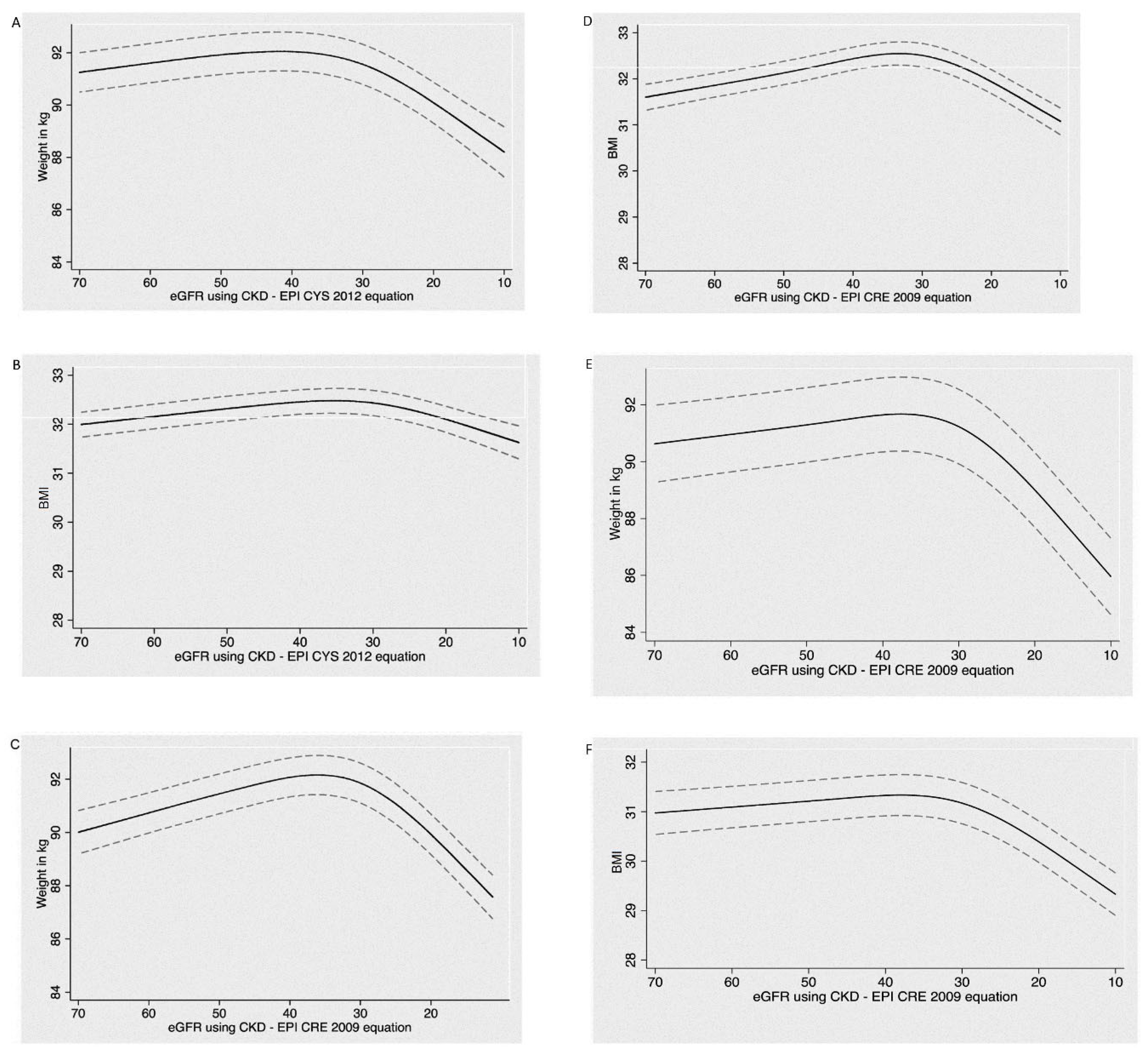
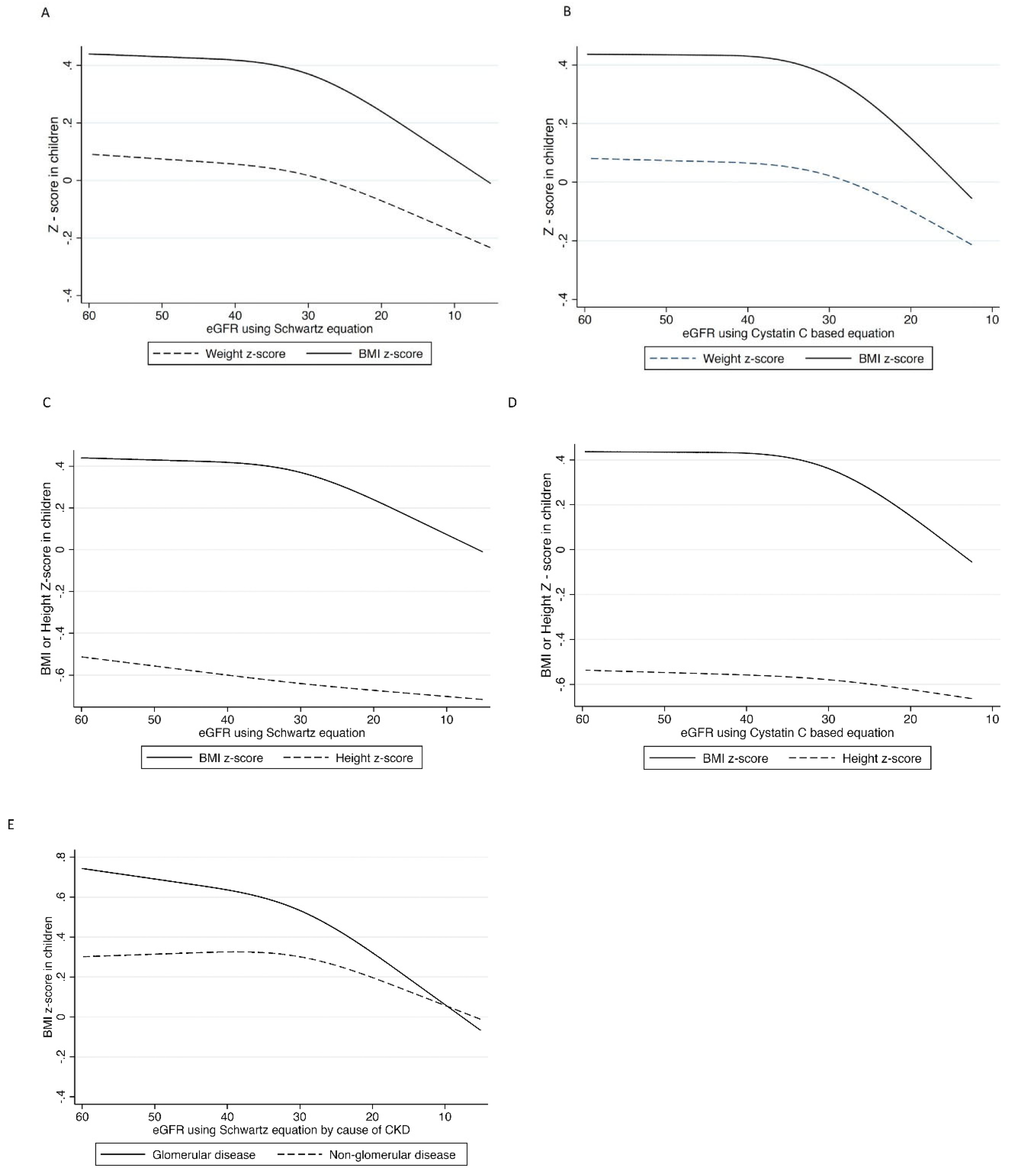
3.3. Examination of Why CKD Patients Often Lose Weight When the GFR Decreases to about 25–32 mL/min/1.73 m2, and Why a Large Weight Loss Is Associated with Increased Mortality?
3.4. Why Do CKD Patients Often Lose Weight When GFR Falls to about 30–35 mL/min/1.73 m2?
3.5. Why Is Large Weight Loss Associated with Increased Mortality in These CKD Patients?
3.6. The Human Microbiome
3.7. The Gut Microbiome in CKD
- i.
- Microbiota in the gut become altered (dysbiosis). The gut microbes may synthesize increased amounts or different types of compounds [42]
- ii.
- iii.
3.8. Plant-Based Diets for CKD Patients
3.9. The Problem of Adherence to High Fruit and Vegetable Diets (HFVDs)
3.10. What Type of Plant-Based Diets May Lower Dietary Phosphorus Uptake?
Grain-Based Foods
3.11. Effect of the Source of Dietary Phosphorus on Urinary Phosphorus Excretion
3.12. Can Medicines Substitute for Foods to Reduce Acidosis-Induced Progression of CKD or to Prevent or Treat Hyperphosphatemia?
3.13. Summary of Discussion on the Use of High Plant Diets to Control Acidosis and Hyperphosphatemia in Advanced CKD and Chronic Dialysis Patients
- A HFVD, as defined by Wesson and colleagues, is documented to reduce or prevent acidosis in CKD patients [71,72]. Since the PLAYDO diet may contain lower amounts of fruits and vegetables than the HFVD described by Wesson et al. [72] and may contain substantial animal-based protein, it is not clear how effective the PLAYDO diets are collectively at reducing the acid load and therefore, decreasing net body protein catabolism and slowing progression of CKD. It should be emphasized that grain-based foods are also plant foods, but still provide some acid (Table 1) [45].
- Any diet, vegan or omnivorous, that provides 0.60 g protein/kg/day is likely to be deficient in calcium and certain essential micronutrients and may require supplements to prevent calcium and micronutrient deficiencies. Such LPDs that are composed entirely or almost entirely of plant foods may also be deficient in some essential amino acids, especially methionine and lysine [73]. It is therefore important that the primarily plant-based LPDs prescribed for CKD patients should be designed with the assistance of an experienced renal dietitian.
- According to the tastes and preferences of the CKD patient, the potential benefits of plant dominant diets on alkalinization, decreased constipation, reduced intestinal phosphate absorption can be replicated with omnivorous LPDs that are augmented with alkali supplements, supplemental fiber, and intestinal phosphate binders.
- To our knowledge, with the exception of the HFVD described by Wesson et al., there are no RCTs that demonstrate beneficial clinical outcomes (e.g., reduced rate of loss of GFR, less adverse cardiovascular events, decreased mortality) with PLADO diets as compared to similar LPDs that contain less plant protein but that have the same protein content and contain the medicines or supplements necessary to control blood pH, serum phosphate, bone-mineral disease, and fecal flow.
- The difficulty with the term, PLAYDO Diet, is the amount of plant foods present in the diet is not well-specified. There is a similar concern with the HFVD. Perhaps it would be helpful if the amount of plant food or plant food protein in these diets was defined more precisely.
3.14. Renal Nutrition in the More Distant Future?
- 1.
- Surveys of chronic dialysis patients indicate that food and fluid restriction are not uncommonly onerous [74,75,76]. In this regard, there are intestinal binders for potassium [77,78,79] and phosphorus [67,68,69,70]. An inhibitor of the Na/H exchanger iso-form 3 (NHE3) in the small intestine may suppress intestinal phosphate absorption [80]. Inhibitors of sodium absorption are under development [81,82,83,84]. Veverimer may bind hydrogen ion in the gut [85]. Diuretics, especially loop diuretics, may enhance the renal excretion of sodium, chloride and potassium, even in chronic dialysis patients who are not anuric.
- 2.
- Sodium-glucose co-transporter-2 (SGLT2) inhibitors stimulate tubular glomerular feedback to reduce intraglomerular hypertension and protect the kidney [86,87,88]. Glucagon-like peptide-1 (GLP-1) receptor agonists may improve serum glucose control [89,90,91]. Selective mineral corticoid antagonists may decrease blood pressure, improve glomerular hemodynamics and reduce renal fibrosis [92,93,94]. Hypoxia-inducible factor-proline hydroxylase inhibitors (HIF PHIs) enhance intestinal iron absorption and may improve anemia of CKD [95,96]. Calcimimetic medicines (cinecalcet, etalcalcetide) are used to treat hyperparathyroidism [97,98]. Oral, enteral or parenteral nutritional supplements are given to improve nutritional intake and prevent malnutrition in CKD patients. Dialysis treatments may also become more nutritionally relevant. For example, dialysate solutions may provide additional nutrients [99,100].
- 3.
- There will be more sophisticated methods for assessment of the patient’s nutritional status of micronutrients. For example, more effective methods may be developed for:
- -
- Simple measurements of serum or blood cell concentrations that can accurately indicate the body burden of an individual micronutrient.
- -
- Measurements may be developed to indicate the functionality, as well as the blood and individual tissue concentrations, of a micronutrient and also the presence of inhibitors or other modifiers of the physiology, metabolism or actions of micronutrients. As examples, in the uremic state, retained endogenous metabolites, increased hormone levels, medicines or metabolites of medicines might inhibit or enhance actions of micronutrients [101,102,103].
- -
- Altered Vitamin Function or Metabolism in CKD/ESKD.
- Erythrocyte transketolase (ETK) activity which requires the presence of the vitamin, thiamine pyrophosphate. However, low ETK activity has been found in patients who have normal blood thiamine levels [104].
- There is an increased daily need for pyridoxine hydrochloride (vitamin B6) in advanced CKD and chronic dialysis patients that exceeds the Recommended Dietary Allowance of vitamin B6 for normal adults [105].
- Membrane tetrahydrofolate (THF) transport is reported to be inhibited in advanced CKD [106].
- There is decreased vitamin B12 uptake by blood monocytes in advanced CKD patients [107].
- How do these alterations affect dietary needs for the respective vitamins or, for that matter, trace elements?
- How can we assess the daily vitamin and trace element needs and the state of vitamin and trace element nutriture of CKD and chronic dialysis patients? By function tests? By blood levels?
- How can we know whether CKD or chronic dialysis patients are receiving adequate amounts of every essential micronutrient?
- 4.
- There will be more definitive investigations of the role of the microbiota, and particularly the gut microbiome, as contributing causes of AKI or CKD, of the clinical manifestations of these disease states, and of the general health of AKI and CKD patients. The potential role of nutrition and nutrients for modifying the microbiome and its pathogenetic or health-enhancing effects in AKI and CKD patients will continue to be investigated.
3.15. Is Nutritional Care Clinically Valuable?
3.16. The SONG (Standard Outcomes in Nephrology) Initiative May Become Operative in Renal Nutrition
3.17. What May Be the Effects of SONG on Renal Nutrition?
3.18. Major Challenges to the Nutritional Treatment in CKD
3.19. A Different Institutional System for Nutritionally Managing People with Kidney Disease
3.20. Summary: Future of Renal Nutrition May Include
- Development of new medicines to help control absorption in the intestinal tract and facilitate removal of unwanted nutrients and their metabolites.
- The refinement of the classification and the criteria for diagnosis of PEW.
- Examine why people commonly lose weight when GFR decreases to 30–35 mL/min/1.73 m2 and why this weight loss is associated with increased mortality, or in children with CKD, an increased risk of developing ESRD.
- Continue the investigation of the gut microbiome, particularly with regard to how it influences human physiology and metabolism in CKD and ESRD and how it can be modified to make it more health enhancing.
- Continue to investigate the most effective uses of high fruit and vegetable diets and other plant-based diets vs. medicinal intake.
- Continued to explore the interactions and potential treatments of nutrient-medicine-hormonal interactions; for example, with regard to bone-mineral disorders.
- Develop and refine better methods for identifying altered vitamin and trace element metabolism, nutritional needs and deficiencies in kidney disease and kidney failure.
- Continue to develop more patient and family friendly nutritional therapy. The needs and feelings of the patients will become more central with regard to planning and implementing nutritional therapy.
- Explore the possible development of widely disseminated renal nutritional care centers, particularly in urban areas.
Author Contributions
Funding
Conflicts of Interest
References
- Kopple, J.D.B.; Jerrilynn, D. History of Dietary Protein Treatment for Non-dialyzed Chronic Kidney Disease Patients. In Nutrition in Kidney Disease, 3rd ed.; Burrowes, J., Kovesdy, C.P., Byham-Gray, L.D., Eds.; Humana Press: Totowa, NJ, USA, 2022. [Google Scholar]
- Giovannetti, S.; Maggiore, Q. A low-nitrogen diet with proteins of high biological value for severe chronic uræmia. Lancet 1964, 283, 1000–1003. [Google Scholar] [CrossRef]
- Monasterio, G.; Giovannetti, S.; Maggiore, Q. The place of the low protein diet in the treatment of chronic uraemia. Panminerva Medica 1965, 7, 479–484. [Google Scholar] [PubMed]
- Giovannetti, S. Dietary Treatment of Chronic Renal Failure: Why Is It Not Used More Frequently? Nephron 1985, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Pluvio, M.; Di Guida, G.; Savoia, S.; Di Serfino, A. Modulated nitrogen intake for patients on low-protein diets. Am. J. Clin. Nutr. 1980, 33, 1638–1641. [Google Scholar] [CrossRef]
- Giordano, C. The Role of Diet in Renal Disease. Hosp. Pract. 1977, 12, 113–119. [Google Scholar] [CrossRef]
- Kopple, J.D.; Shinaberger, J.H.; Coburn, J.W.; Sorensen, M.K.; Rubini, M.E. Evaluating modified protein diets for uremia. J. Am. Diet. Assoc. 1969, 54, 481–485. [Google Scholar] [CrossRef]
- Kopple, J.D.; Coburn, J.W. Metabolic studies of low protein diets in uremia. Medicine 1973, 52, 583–595. [Google Scholar] [CrossRef]
- Alvestrand, A.; Furst, P.; Bergstrom, J. Plasma and muscle free amino acids in uremia: Influence of nutrition with amino acids. Clin. Nephrol. 1982, 18, 297–305. [Google Scholar]
- Bergström, J.; Ahlberg, M.; Alvestrand, A.; Fürst, P. Amino Acid Therapy for Patients with Chronic Renal Failure. Transfus. Med. Hemotherapy 1987, 14, 8–11. [Google Scholar] [CrossRef]
- Fürst, P. Amino acid metabolism in uremia. J. Am. Coll. Nutr. 1989, 8, 310–323. [Google Scholar] [CrossRef]
- Walser, M. Keto Acid Therapy in Chronic Renal Failure. Nephron Exp. Nephrol. 1978, 21, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Walser, M.; Steinman, T.I.; Hill, S.; Zeger, S.; Tungsanga, K. The Effect of a Keto Acid–Amino Acid Supplement to a Restricted Diet on the Progression of Chronic Renal Failure. N. Engl. J. Med. 1984, 311, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The Effects of Dietary Protein Restriction and Blood-Pressure Control on the Progression of Chronic Renal Disease. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a Very Low-Protein Diet on Outcomes: Long-term Follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Mircescu, G.; Gârneaţă, L.; Stancu, S.H.; Căpuşă, C. Effects of a Supplemented Hypoproteic Diet in Chronic Kidney Disease. J. Ren. Nutr. 2007, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, M.; Bellizzi, V.; Chauveau, P.; Cupisti, A.; Ecder, T.; Fouque, D.; Garneata, L.; Lin, S.; Mitch, W.E.; Teplan, V.; et al. Protein-Restricted Diets Plus Keto/Amino Acids—A Valid Therapeutic Approach for Chronic Kidney Disease Patients. J. Ren. Nutr. 2012, 22, S1–S21. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low–Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Fouque, D.; Chen, J.; Chen, W.; Garneata, L.; Hwang, S.J.; Kalantar-Zadeh, K.; Kopple, J.D.; Mitch, W.E.; Piccoli, G.; Teplan, V.; et al. Adherence to ketoacids/essential amino acids-supplemented low protein diets and new indications for patients with chronic kidney disease. BMC Nephrol. 2016, 17, 63. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Stover, P.J.; King, J.C. More Nutrition Precision, Better Decisions for the Health of Our Nation. J. Nutr. 2020, 150, 3058–3060. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Moore, L.W. Precision Nutrition and Personalized Diet Plan for Kidney Health and Kidney Disease Management. J. Ren. Nutr. 2020, 30, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Chen, X.; Wei, G.; Raj, D.; Raphael, K.L.; Boucher, R.; Chonchol, M.B.; Murtaugh, M.A.; Greene, T. Associations of Protein−Energy Wasting Syndrome Criteria with Body Composition and Mortality in the General and Moderate Chronic Kidney Disease Populations in the United States. Kidney Int. Rep. 2017, 2, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Moreau-Gaudry, X.; Jean, G.; Genet, L.; Lataillade, D.; Legrand, E.; Kuentz, F.; Fouque, D. A Simple Protein–Energy Wasting Score Predicts Survival in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2014, 24, 395–400. [Google Scholar] [CrossRef]
- Kopple, J.D.; Greene, T.; Chumlea, W.C.; Hollinger, D.; Maroni, B.J.; Merrill, D.; Scherch, L.K.; Schulman, G.; Wang, S.-R.; Zimmer, G.S. Relationship between nutritional status and the glomerular filtration rate: Results from the MDRD Study. Kidney Int. 2000, 57, 1688–1703. [Google Scholar] [CrossRef]
- Ku, E.; Kopple, J.D.; Johansen, K.L.; McCulloch, C.E.; Go, A.S.; Xie, D.; Lin, F.; Hamm, L.L.; He, J.; Kusek, J.W.; et al. Longitudinal Weight Change During CKD Progression and Its Association with Subsequent Mortality. Am. J. Kidney Dis. 2018, 71, 657–665. [Google Scholar] [CrossRef]
- Ku, E.; Kopple, J.D.; McCulloch, C.E.; Warady, B.A.; Furth, S.L.; Mak, R.H.; Grimes, B.A.; Mitsnefes, M. Associations Between Weight Loss, Kidney Function Decline, and Risk of ESRD in the Chronic Kidney Disease in Children (CKiD) Cohort Study. Am. J. Kidney Dis. 2018, 71, 648–656. [Google Scholar] [CrossRef]
- Hyun, Y.Y.; Lee, K.-B.; Han, S.H.; Kim, Y.H.; Kim, Y.-S.; Lee, S.W.; Oh, Y.K.; Chae, D.W.; Ahn, C. Nutritional Status in Adults with Predialysis Chronic Kidney Disease: KNOW-CKD Study. J. Korean Med Sci. 2017, 32, 257–263. [Google Scholar] [CrossRef]
- Chang, T.I.; Ngo, V.; Streja, E.; Chou, J.A.; Tortorici, A.R.; Kim, T.H.; Soohoo, M.; Gillen, D.; Rhee, C.M.; Kovesdy, C.P.; et al. Association of body weight changes with mortality in incident hemodialysis patients. Nephrol. Dial. Transplant. 2017, 32, 1549–1558. [Google Scholar] [CrossRef][Green Version]
- Mehta, R.; Cai, X.; Lee, J.; Xie, D.; Wang, X.; Scialla, J.; Anderson, A.H.; Taliercio, J.; Dobre, M.; Chen, J.; et al. Serial Fibroblast Growth Factor 23 Measurements and Risk of Requirement for Kidney Replacement Therapy: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2020, 75, 908–918. [Google Scholar] [CrossRef]
- Isakova, T.; Cai, X.; Lee, J.; Mehta, R.; Zhang, X.; Yang, W.; Nessel, L.; Anderson, A.H.; Lo, J.; Porter, A.; et al. Longitudinal Evolution of Markers of Mineral Metabolism in Patients With CKD: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2020, 75, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shiizaki, K.; Kuro-O, M.; Moe, O.W. Fibroblast Growth Factor 23 and Klotho: Physiology and Pathophysiology of an Endocrine Network of Mineral Metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Kuro-O, M.; Moe, O.W. FGF23-αKlotho as a paradigm for a kidney-bone network. Bone 2017, 100, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Jang, H.R.; Gandolfo, M.T.; Ko, G.J.; Satpute, S.; Racusen, L.; Rabb, H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am. J. Physiol.-Ren. Physiol. 2009, 297, F1457–F1465. [Google Scholar] [CrossRef]
- Noel, S.; Martina-Lingua, M.N.; Bandapalle, S.; Pluznick, J.; Hamad, A.R.A.; Peterson, D.A.; Rabb, H. Intestinal Microbiota-Kidney Cross Talk in Acute Kidney Injury and Chronic Kidney Disease. Nephron Exp. Nephrol. 2014, 127, 139–143. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.; Smith, G.; Ross, R. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Noce, A.; Marchetti, M.; Marrone, G.; Di Renzo, L.; Di Lauro, M.; Di Daniele, F.; Albanese, M.; Di Daniele, N.; De Lorenzo, A. Link between gut microbiota dysbiosis and chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2057–2074. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. CKD impairs barrier function and alters microbial flora of the intestine. Curr. Opin. Nephrol. Hypertens. 2012, 21, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E. The Continuum of Acid Stress. Clin. J. Am. Soc. Nephrol. 2021, 16, 1292–1299. [Google Scholar] [CrossRef]
- Macfarlane, B.J.; Bezwoda, W.R.; Bothwell, T.H.; Baynes, R.D.; Bothwell, E.J.; MacPhail, A.P.; Lamparelli, R.D.; Mayet, F. Inhibitory effect of nuts on iron absorption. Am. J. Clin. Nutr. 1988, 47, 270–274. [Google Scholar] [CrossRef]
- Helfrich, A. Determination of phytic acid and its degradation products by ion-pair chromatography (IPC) coupled to inductively coupled plasma-sector field-mass spectrometry (ICP-SF-MS). J. Anal. At. Spectrom. 2004, 19, 1330–1334. [Google Scholar] [CrossRef]
- Wyatt, C.J.; Triana-Tejas, A. Soluble and Insoluble Fe, Zn, Ca, and Phytates in Foods Commonly Consumed in Northern Mexico. J. Agric. Food Chem. 1994, 42, 2204–2209. [Google Scholar] [CrossRef]
- Liang, J.; Han, B.-Z.; Nout, M.R.; Hamer, R.J. Effects of soaking, germination and fermentation on phytic acid, total and in vitro soluble zinc in brown rice. Food Chem. 2008, 110, 821–828. [Google Scholar] [CrossRef]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian Compared with Meat Dietary Protein Source and Phosphorus Homeostasis in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 257–264. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Armstrong, C.L.; Janda, K.; Ponsler-Sipes, K.; Asplin, J.R.; Moe, S.M. The Effect of a Diet Containing 70% Protein from Plants on Mineral Metabolism and Musculoskeletal Health in Chronic Kidney Disease. Am. J. Nephrol. 2014, 40, 582–591. [Google Scholar] [CrossRef]
- Vorland, C.J.; Lachcik, P.J.; Aromeh, L.O.; Moe, S.M.; Chen, N.X.; Gallant, K.M.H. Effect of dietary phosphorus intake and age on intestinal phosphorus absorption efficiency and phosphorus balance in male rats. PLoS ONE 2018, 13, e0207601. [Google Scholar] [CrossRef] [PubMed]
- Mannon, E.C.; O’Connor, P.M. Alkali supplementation as a therapeutic in chronic kidney disease: What mediates protection? Am. J. Physiol.-Ren. Physiol. 2020, 319, F1090–F1104. [Google Scholar] [CrossRef] [PubMed]
- Melamed, M.L.; Horwitz, E.J.; Dobre, M.A.; Abramowitz, M.K.; Zhang, L.; Lo, Y.; Mitch, W.E.; Hostetter, T.H. Effects of Sodium Bicarbonate in CKD Stages 3 and 4: A Randomized, Placebo-Controlled, Multicenter Clinical Trial. Am. J. Kidney Dis. 2020, 75, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Greene, T.; Wei, G.; Bullshoe, T.; Tuttle, K.; Cheung, A.K.; Beddhu, S. Sodium Bicarbonate Supplementation and Urinary TGF-β1 in Nonacidotic Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Sahoo, J.; Vairappan, B.; Haridasan, S.; Parameswaran, S.; Priyamvada, P.S. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial. Nephrol. Dial. Transplant. 2020, 35, 121–129. [Google Scholar] [CrossRef]
- Mahajan, A.; Simoni, J.; Sheather, S.J.; Broglio, K.R.; Rajab, M.; Wesson, D.E. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010, 78, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Phisitkul, S.; Khanna, A.; Simoni, J.; Broglio, K.; Sheather, S.; Rajab, M.H.; Wesson, D.E. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010, 77, 617–623. [Google Scholar] [CrossRef]
- Gadola, L.; Noboa, O.; Márquez, M.N.; Rodriguez, M.J.; Nin, N.; Boggia, J.; Ferreiro, A.; García, S.; Ortega, V.; Musto, M.L.; et al. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int. 2004, 65, 1224–1230. [Google Scholar] [CrossRef]
- Tanner, G.A. Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J. Am. Soc. Nephrol. 1998, 9, 1242–1248. [Google Scholar] [CrossRef]
- Torres, V.E.; Mujwid, D.K.; Wilson, D.M.; Holley, K.H. Renal cystic disease and ammoniagenesis in Han:SPRD rats. J. Am. Soc. Nephrol. 1994, 5, 1193–1200. [Google Scholar] [CrossRef]
- Wesson, D.E.; Simoni, J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010, 78, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Hostetter, M.K.; Hostetter, T.H. Increased Ammoniagenesis as a Determinant of Progressive Renal Injury. Am. J. Kidney Dis. 1991, 17, 654–657. [Google Scholar] [CrossRef]
- Clark, E.C.; Nath, K.A.; Hostetter, M.K.; Hostetter, T.H. Role of ammonia in tubulointerstitial injury. Miner. Electrolyte Metab. 1990, 16, 315–321. [Google Scholar] [PubMed]
- Wesson, D.E.; Dolson, G.M. Endothelin-1 increases rat distal tubule acidification in vivo. Am. J. Physiol.-Ren. Physiol. 1997, 273, F586–F594. [Google Scholar] [CrossRef]
- Khanna, A.; Simoni, J.; Hacker, C.; Duran, M.J.; Wesson, D.E. Increased endothelin activity mediates augmented distal nephron acidification induced by dietary protein. Trans. Am. Clin. Climatol. Assoc. 2005, 116, 239–256. [Google Scholar] [CrossRef]
- Qunibi, W.Y.; Hootkins, R.E.; McDowell, L.L.; Meyer, M.S.; Simon, M.; Garza, R.O.; Pelham, R.W.; Cleveland, M.V.; Muenz, L.R.; He, D.Y.; et al. Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study). Kidney Int. 2004, 65, 1914–1926. [Google Scholar] [CrossRef]
- Hervás, J.G.; Prados, D.; Cerezo, S. Treatment of hyperphosphatemia with sevelamer hydrochloride in hemodialysis patients: A comparison with calcium acetate. Kidney Int. 2003, 63, S69–S72. [Google Scholar] [CrossRef]
- Chen, N.; Wu, X.; Ding, X.; Mei, C.; Fu, P.; Jiang, G.; Li, X.; Chen, J.; Liu, B.; La, Y.; et al. Sevelamer carbonate lowers serum phosphorus effectively in haemodialysis patients: A randomized, double-blind, placebo-controlled, dose-titration study. Nephrol. Dial. Transplant. 2014, 29, 152–160. [Google Scholar] [CrossRef]
- Chiang, S.-S.; Chen, J.-B.; Yang, W.-C. Lanthanum carbonate (Fosrenol®) efficacy and tolerability in the treatment of hyperphosphatemic patients with end-stage renal disease. Clin. Nephrol. 2005, 63, 461–470. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef]
- Wesson, D.E.; Kitzman, H.; Montgomery, A.; Mamun, A.; Parnell, W.; Vilayvanh, B.; Tecson, K.M.; Allison, P. A population health dietary intervention for African American adults with chronic kidney disease: The Fruit and Veggies for Kidney Health randomized study. Contemp. Clin. Trials Commun. 2020, 17, 100540. [Google Scholar] [CrossRef]
- Khor, B.-H.; Tallman, D.A.; Karupaiah, T.; Khosla, P.; Chan, M.; Kopple, J.D. Nutritional Adequacy of Animal-Based and Plant-Based Asian Diets for Chronic Kidney Disease Patients: A Modeling Study. Nutrients 2021, 13, 3341. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Shapiro, B.B.; Feroze, U.; Kim, J.C.; Zhang, M.; Li, Y.; Martin, D.J. Hemodialysis treatment engenders anxiety and emotional distress. Clin. Nephrol. 2017, 88, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.L.; Held, I.; Khawnekar, D.; Rutherford, P. Differences in Knowledge, Stress, Sensation Seeking, and Locus of Control Linked to Dietary Adherence in Hemodialysis Patients. Front. Psychol. 2016, 7, 1864. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Mullan, J.; Mansfield, K. An integrative review of the methodology and findings regarding dietary adherence in end stage kidney disease. BMC Nephrol. 2017, 18, 318. [Google Scholar] [CrossRef]
- Bakris, G.L.; Pitt, B.; Weir, M.R.; Freeman, M.W.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Zawadzki, R.; Berman, L.; Bushinsky, D.A. Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and Diabetic Kidney Disease. JAMA 2015, 314, 151–161. [Google Scholar] [CrossRef]
- Agarwal, R.; Rossignol, P.; Romero, A.; Garza, D.; Mayo, M.R.; Warren, S.; Ma, J.; White, W.B.; Williams, B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019, 394, 1540–1550. [Google Scholar] [CrossRef]
- Rastogi, A.; Hanna, R.M.; Mkrttchyan, A.; Khalid, M.; Yaqoob, S.; Shaffer, K.; Dhawan, P.; Nobakht, N.; Kamgar, M.; Goshtaseb, R.; et al. Sodium zirconium cyclosilicate for the management of chronic hyperkalemia in kidney disease, a novel agent. Expert Rev. Clin. Pharmacol. 2021, 14, 1055–1064. [Google Scholar] [CrossRef]
- Rieg, J.D.; Chavez, S.D.L.M.; Rieg, T. Novel developments in differentiating the role of renal and intestinal sodium hydrogen exchanger 3. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R1186–R1191. [Google Scholar] [CrossRef]
- Spencer, A.G.; Greasley, P.J. Pharmacologic inhibition of intestinal sodium uptake. Curr. Opin. Nephrol. Hypertens. 2015, 24, 410–416. [Google Scholar] [CrossRef]
- Linz, B.; Hohl, M.; Reil, J.C.; Böhm, M.; Linz, D. Inhibition of NHE3-mediated Sodium Absorption in the Gut Reduced Cardiac End-organ Damage Without Deteriorating Renal Function in Obese Spontaneously Hypertensive Rats. J. Cardiovasc. Pharmacol. 2016, 67, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Rosenbaum, D.P.; Yang, Y.; Chertow, G.M. A Randomized Trial of Tenapanor and Phosphate Binders as a Dual-Mechanism Treatment for Hyperphosphatemia in Patients on Maintenance Dialysis (AMPLIFY). J. Am. Soc. Nephrol. 2021, 32, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Tenapanor: First Approval. Drugs 2019, 79, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Mathur, V.; Tangri, N.; Stasiv, Y.; Parsell, D.; Li, E.; Klaerner, G.; Bushinsky, D.A. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: A multicentre, randomised, blinded, placebo-controlled, 40-week extension. Lancet 2019, 394, 396–406. [Google Scholar] [CrossRef]
- Sarafidis, P.; Ortiz, A.; Ferro, C.J.; Halimi, J.-M.; Kreutz, R.; Mallamaci, F.; Mancia, G.; Wanner, C. Sodium—Glucose co-transporter-2 inhibitors for patients with diabetic and nondiabetic chronic kidney disease: A new era has already begun. J. Hypertens. 2021, 39, 1090–1097. [Google Scholar] [CrossRef]
- Herrington, W.G.; Preiss, D.; Haynes, R.; Von Eynatten, M.; Staplin, N.; Hauske, S.J.; George, J.T.; Green, J.B.; Landray, M.J.; Baigent, C.; et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: A rationale for the EMPA-KIDNEY study. Clin. Kidney J. 2018, 11, 749–761. [Google Scholar] [CrossRef]
- Sen, T.; Heerspink, H.J. A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors. Cell Metab. 2021, 33, 732–739. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2020, 46, 101102. [Google Scholar] [CrossRef]
- Greco, E.V.; Russo, G.; Giandalia, A.; Viazzi, F.; Pontremoli, R.; De Cosmo, S. GLP-1 Receptor Agonists and Kidney Protection. Medicina 2019, 55, 233. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Girerd, S.; Jaisser, F. Mineralocorticoid receptor antagonists and kidney diseases: Pathophysiological basis. Kidney Int. 2019, 96, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Joharapurkar, A.; Jain, M. Role of mineralocorticoid receptor antagonists in kidney diseases. Drug Dev. Res. 2021, 82, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Carroll, K.; Perkovic, V.; Solomon, S.; Jha, V.; Johansen, K.L.; Lopes, R.D.; Macdougall, I.C.; Obrador, G.T.; Waikar, S.S.; et al. Daprodustat for the Treatment of Anemia in Patients Undergoing Dialysis. N. Engl. J. Med. 2021, 385, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Mima, A. Hypoxia-inducible factor-prolyl hydroxylase inhibitors for renal anemia in chronic kidney disease: Advantages and disadvantages. Eur. J. Pharmacol. 2021, 912, 174583. [Google Scholar] [CrossRef]
- Block, G.A.; Bushinsky, D.A.; Cheng, S.; Cunningham, J.; Dehmel, B.; Drueke, T.B.; Ketteler, M.; KewalRamani, R.; Martin, K.J.; Moe, S.M.; et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis with Secondary Hyperparathyroidism. JAMA 2017, 317, 156–164. [Google Scholar] [CrossRef]
- Friedl, C.; Zitt, E. Role of etelcalcetide in the management of secondary hyperparathyroidism in hemodialysis patients: A review on current data and place in therapy. Drug Des. Dev. Ther. 2018, 12, 1589–1598. [Google Scholar] [CrossRef]
- Kopple, J.D.; Bernard, D.; Messana, J.; Swartz, R.; Bergström, J.; Lindholm, B.; Lim, V.; Brunori, G.; Leiserowitz, M.; Bier, D.M.; et al. Treatment of malnourished CAPD patients with an amino acid based dialysate. Kidney Int. 1995, 47, 1148–1157. [Google Scholar] [CrossRef][Green Version]
- Feinstein, E.I.; Collins, J.F.; Blumenkrantz, M.J.; Roberts, M.; Kopple, J.D. Nutritional hemodialysis. In Progress in Artificial Organs, 1st ed.; ISAO Press: Cleveland, OH, USA, 1984; Volume 1, pp. 421–426. [Google Scholar]
- Raskin, N.H.; Fishman, R.A. Pyridoxine-Deficiency Neuropathy Due to Hydralazine. N. Engl. J. Med. 1965, 273, 1182–1185. [Google Scholar] [CrossRef]
- Levy, L.; Higgins, L.J.; Burbridge, T.N. Isoniazid-induced vitamin B6 deficiency. Metabolic studies and preliminary vitamin B6 excretion studies. Am. Rev. Respir. Dis. 1967, 96, 910–917. [Google Scholar] [CrossRef]
- Bastl, C.; Finkelstein, F.O.; Sherwin, R.; Hendler, R.; Felig, P.; Hayslett, J.P. Renal extraction of glucagon in rats with normal and reduced renal function. Am. J. Physiol.-Ren. Physiol. 1977, 233, F67–F71. [Google Scholar] [CrossRef] [PubMed]
- Descombes, E.; Hanck, A.B.; Fellay, G. Water soluble vitamins in chronic hemodialysis patients and need for supplementation. Kidney Int. 1993, 43, 1319–1328. [Google Scholar] [CrossRef]
- Kopple, J.D.; Mercurio, K.; Blumenkrantz, M.J.; Jones, M.R.; Tallos, J.; Roberts, C.; Card, B.; Saltzman, R.; Casciato, D.A.; Swendseid, M.E. Daily requirement for pyridoxine supplements in chronic renal failure. Kidney Int. 1981, 19, 694–704. [Google Scholar] [CrossRef]
- Jennette, J.C.; Goldman, I.D. Inhibition of the membrane transport of folates by anions retained in uremia. J. Lab. Clin. Med. 1975, 86, 834–843. [Google Scholar] [PubMed]
- Obeid, R.; Kuhlmann, M.; Kirsch, C.-M.; Herrmann, W. Cellular Uptake of Vitamin B12 in Patients with Chronic Renal Failure. Nephron Exp. Nephrol. 2005, 99, c42–c48. [Google Scholar] [CrossRef]
- DeLuca, H.F. The kidney as an endocrine organ involved in the function of vitamin D. Am. J. Med. 1975, 58, 39–47. [Google Scholar] [CrossRef]
- Vaziri, N.; Said, H.; Hollander, D.; Barbari, A.; Patel, N.; Dang, D.; Kariger, R. Impaired Intestinal Absorption of Riboflavin in Experimental Uremia. Nephron Exp. Nephrol. 1985, 41, 26–29. [Google Scholar] [CrossRef]
- Said, H.M.; Vaziri, N.D.; Kariger, R.K.; Hollander, D. Intestinal absorption of 5-methyltetrahydrofolate in experimental uremia. Acta Vitaminol. Et Enzymol. 1984, 6, 339–346. [Google Scholar]
- Vaziri, N.D.; Hollander, D.; Hung, E.K.; Vo, M.; Dadufalza, L. Impaired intestinal absorption of vitamin D3 in azotemic rats. Am. J. Clin. Nutr. 1983, 37, 403–406. [Google Scholar] [CrossRef]
- Alqarni, A.M.; Zeidler, M.P. How does methotrexate work? Biochem. Soc. Trans. 2020, 48, 559–567. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.-F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachex Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hamidianshirazi, M.; Shafiee, M.; Ekramzadeh, M.; Jahromi, M.T.; Nikaein, F. Diet therapy along with Nutrition Education can Improve Renal Function in People with Stages 3-4 chronic kidney disease who do not have diabetes. (A randomized controlled trial). Br. J. Nutr. 2022, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Signoriello, S.; Minutolo, R.; Di Iorio, B.; Nazzaro, P.; Garofalo, C.; Calella, P.; Chiodini, P.; De Nicola, L.; Torraca, S.; et al. No additional benefit of prescribing a very low-protein diet in patients with advanced chronic kidney disease under regular nephrology care: A pragmatic, randomized, controlled trial. Am. J. Clin. Nutr. 2022, 115, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Lodebo, B.T.; Shah, A.; Kopple, J.D. Is it Important to Prevent and Treat Protein-Energy Wasting in Chronic Kidney Disease and Chronic Dialysis Patients? J. Ren. Nutr. 2018, 28, 369–379. [Google Scholar] [CrossRef]
- Evangelidis, N.; Sautenet, B.; Madero, M.; Tong, A.; Ashuntantang, G.; Sanabria, L.C.; de Boer, I.H.; Fung, S.; Gallego, D.; Levey, A.S.; et al. Standardised Outcomes in Nephrology—Chronic Kidney Disease (SONG-CKD): A protocol for establishing a core outcome set for adults with chronic kidney disease who do not require kidney replacement therapy. Trials 2021, 22, 1–8. [Google Scholar] [CrossRef]
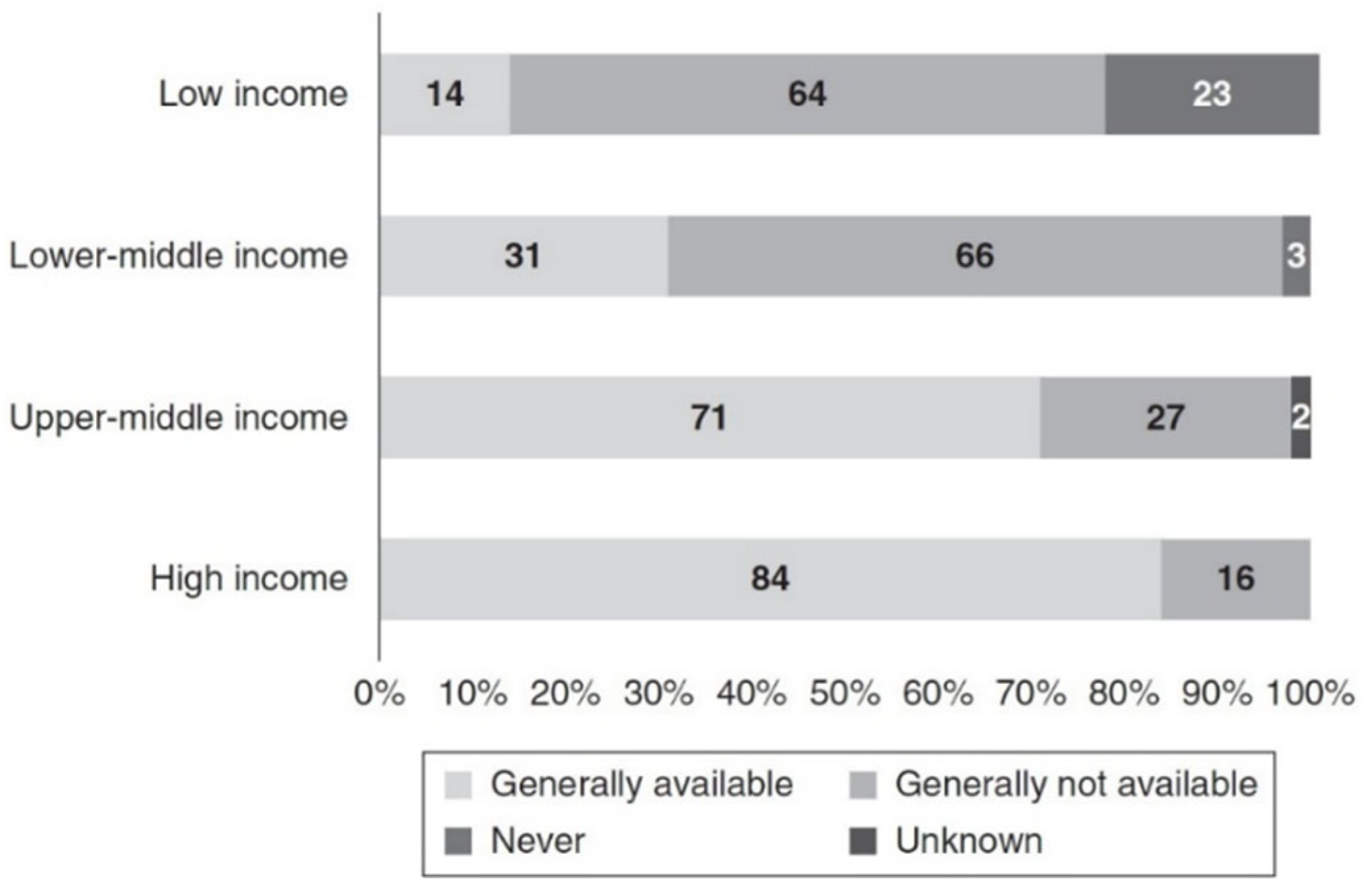
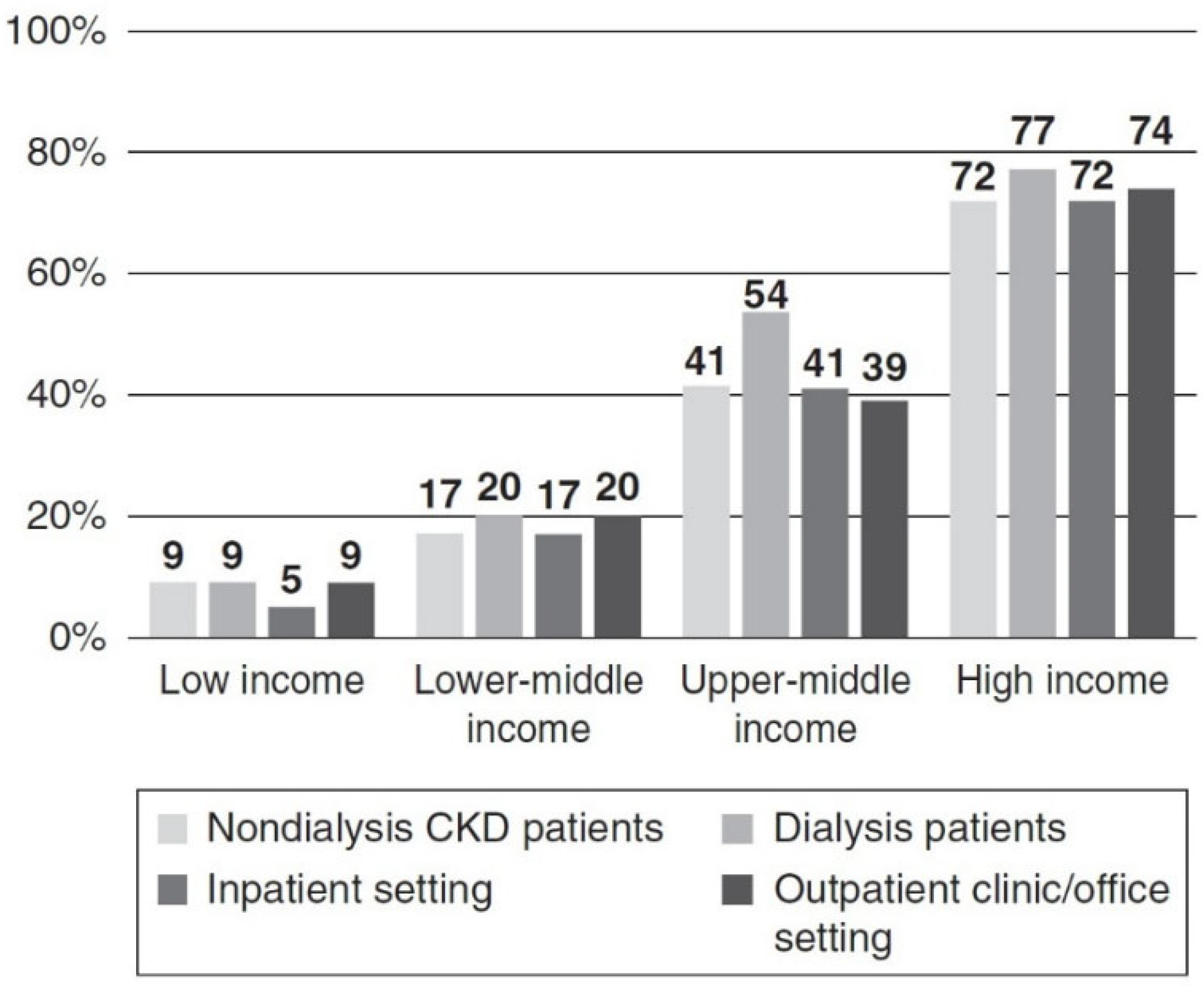
- Wang, A.Y.-M.; Okpechi, I.G.; Ye, F.; Kovesdy, C.P.; Brunori, G.; Burrowes, J.D.; Campbell, K.; Damster, S.; Fouque, D.; Friedman, A.N.; et al. Assessing Global Kidney Nutrition Care. Clin. J. Am. Soc. Nephrol. 2022, 17, 38–52. [Google Scholar] [CrossRef]
| Food | USDA Recommended | Average Intake in the United States | Study Participants | Study Participants Given F + V | Mediterranean Diet | DASH Diet | Vegan Diet |
|---|---|---|---|---|---|---|---|
| Meat/seafood | 13.61 | 22.55 | 27.45 | 25.21 | 6.03 | 22.83 | 0 |
| Vegetables | −24.91 | −12.46 | − 5.75 | −13.96 | −22.63 | −20.19 | −6.52 |
| Fruit | −1.81 | −0.91 | 6.23 | −18.68 | −10.04 | −5.91 | −13.72 |
| Grains | 6.34 | 6.43 | 10.64 | 9.57 | 18.26 | 8.15 | 27.6 |
| Dairy | 10.16 | 11.21 | 23.31 | 23.31 | 7.99 | 5.77 | 0 |
| Oils | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0 |
| Total | 3.4 | 26.83 | 61.89 | 25.46 | −0.39 | 10.63 | 7.36 |
| Food | Phytate (In Milligrams per 100 g of Dry Weight) |
|---|---|
| Brazil nuts | 1719 |
| Cocoa powder | 1684–1796 |
| Brown rice | 1250 |
| Oat flakes | 1174 |
| Almond | 1138–1400 |
| Walnut | 982 |
| Peanut roasted | 952 |
| Peanuts ungerminated | 821 |
| Lentils | 779 |
| Peanuts germinated | 610 |
| Hazel nuts | 648–1000 |
| Wild rice flour | 634–752.5 |
| Yam meal | 637 |
| Refried beans | 622 |
| Corn tortillas | 448 |
| Coconut | 357 |
| Corn | 367 |
| Entire coconut meat | 270 |
| White flour | 258 |
| White flour tortillas | 123 |
| Polished rice | 11.5–66 |
| Strawberries | 12 |
| Potential Benefit | Potential Limitations |
|---|---|
| 1. Reduces intestinal phosphorus absorption. | The reduction in intestinal phosphate absorption by plant foods is rather modest. Such medicines as phosphate binders or tenapanor also reduce phosphate absorption. LPDs are usually lower in phosphorus and therefore cause less intestinal phosphorus absorption. |
| 2. Diets high in vegetables and fruits can alkalize blood, urine and, potentially, the kidneys. | Sodium bicarbonate, solutions of sodium citrate and citric acid, and the resin veverimer also can alkalinize, neutralize acid or bind protons. |
| 3. High dietary fiber may enhance GI motility and reduce risk of hyperkalemia in advanced CKD. | Constipation usually is not a problem in CKD patients and often can be prevented with fiber supplements or other changes in the diet. |
| 4. Animal studies indicate high plant diets may improve the microbiome and reduce renal inflammation and oxidative stress. | There are no randomized prospective clinical trials (RCTS) in humans with CKD that demonstrate these beneficial outcomes. |
| 5. Animal studies show high plant diets produce less trimethylamine oxide, p-cresyl phosphate, p-cresyl sulfate and indoxyl acetic acid. | There are no RCTS that demonstrate beneficial clinical outcomes in humans with CKD from any such changes in production. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopple, J.D.; Ekramzadeh, M. Renal Nutrition—Where It Has Been and Where It Is Going. Kidney Dial. 2022, 2, 512-533. https://doi.org/10.3390/kidneydial2040046
Kopple JD, Ekramzadeh M. Renal Nutrition—Where It Has Been and Where It Is Going. Kidney and Dialysis. 2022; 2(4):512-533. https://doi.org/10.3390/kidneydial2040046
Chicago/Turabian StyleKopple, Joel D., and Maryam Ekramzadeh. 2022. "Renal Nutrition—Where It Has Been and Where It Is Going" Kidney and Dialysis 2, no. 4: 512-533. https://doi.org/10.3390/kidneydial2040046
APA StyleKopple, J. D., & Ekramzadeh, M. (2022). Renal Nutrition—Where It Has Been and Where It Is Going. Kidney and Dialysis, 2(4), 512-533. https://doi.org/10.3390/kidneydial2040046