Abstract
Background/Objectives: The impact of COVID-19 on male reproductive health remains unclear, with existing studies reporting conflicting results. This study aimed to evaluate longitudinal changes in sperm parameters, functional tests, and key markers related to the inflammasome complex in men diagnosed with COVID-19. Methods: In this experimental study, semen and blood samples were collected from 34 men at one- and three-months following COVID-19 diagnosis. Assessments included sperm parameters, chromatin damage, protamine deficiency, residual histones, and key inflammasome pathway markers (NLRP3, ASC, and caspase-1) in sperm samples. Additionally, blood levels of LH, testosterone, interleukin-6, and TNF-ɑ were measured. Results: While semen volume, sperm count, concentration, total motility, and DNA damage showed no significant changes, notable improvements were observed in sperm vitality, rapid motility, chromatin integrity, protamine content, and residual histones at three months compared to one-month post-diagnosis. Serum levels of LH, testosterone, and interleukin-6 remained stable, whereas TNF-ɑ levels significantly decreased after three months. Western blot analysis revealed a significant increase in NLRP3 protein expression after three months, while ASC and caspase-1 levels showed no significant changes. Conclusions: These results suggest a gradual recovery in sperm function over time after COVID-19 infection, highlighting possible implications for male reproductive health. Further studies with larger populations are necessary to better understand the underlying mechanisms and long-term effects of COVID-19 on male fertility.
1. Introduction
On 11 March 2020, COVID-19 was declared a global pandemic, and most research since has focused on its respiratory effects. However, its impact on other organs, including the testes and spermatogenesis, should not be overlooked. Although direct evidence of coronavirus-induced testicular damage is lacking, the limited protection of the blood–testis barrier against viruses such as HIV, hepatitis, mumps, and papilloma suggests a possible negative effect on sperm production and differentiation [1,2]. SARS-CoV-2 infects host cells through its spike (S) protein, which binds to angiotensin-converting enzyme 2 (ACE2), while host proteases such as TMPRSS2, cathepsins, basigin, elastase, and trypsin facilitate spike priming and membrane fusion. ACE2 is abundantly expressed in spermatogonia, Sertoli cells, Leydig cells, and myoid cells of the testes, indicating that these cells may be highly susceptible to viral infection. Although TMPRSS2 is less frequently co-expressed in testicular cells and sperm, it remains a key protease for viral entry, and its expression is enhanced by androgen-responsive elements, suggesting that higher androgen levels could increase susceptibility. Consequently, SARS-CoV-2 may gain access to germ cells within the testes, the epididymis, or even after ejaculation, thereby potentially impairing testicular function, sperm production, and male fertility [3]. In this regard, previous studies have stated that it is possible that the coronavirus, after entering the testes, in the epididymis, or even after ejaculation, can gain access to male germ cells and affect cellular function [2].
However, published data are controversial regarding the presence of coronavirus in the semen or the testis. In this regard, recently, a systematic review and meta-analysis were performed on the effect of SARS-CoV-2 on male reproductive function. The final conclusion of this study was: “COVID-19 and SARS-CoV-2 have been reported to have direct and indirect negative effects on male reproductive function, but there is an urgent need for further secondary analysis to confirm the impact of COVID-19 and SARS-CoV-2 on male reproductive function, and develop standard treatment strategies to help male patients recover from the attack of COVID-19 pandemic” [4].
Studies show that SARS-CoV-2 interaction with the ACE2 receptor activates the angiotensin II/AT1R pathway, inducing fibrosis, inflammation, and oxidative stress. This inflammatory mechanism involves NF-κB activation and increased IL-6 transcription. Additionally, NLRP3 inflammasome activation can cause seminal inflammation and elevate cytokines such as IL-1β and IL-18 [5]. Consequently, oxidative stress is a major contributor to male infertility, impairing testicular function, reducing sperm quality, and increasing sperm DNA damage [5]. In COVID-19, these inflammatory and oxidative processes may disrupt spermatogenesis and sperm function.
Reviewing the literature reveals contradictory results regarding the impact of COVID-19 on sperm function and fertility potential. Stigliani et al. (2023) found that semen quality assessed at least three months post-infection was not impaired [6], whereas Falahieh et al. (2021) reported significant improvements in sperm motility, DNA integrity, total antioxidant capacity (TAC), ROS, and malondialdehyde (MDA) levels at 120 days compared to 14 days post-diagnosis, attributing early impairments to oxidative stress [7]. A recent review also reported that sperm concentration, volume, progressive motility, and total motility decreased after SARS-CoV-2 infection, likely due to elevated ROS, sperm lipid peroxidation, and DNA damage, suggesting that men recovering from COVID-19 should evaluate their fertility potential through sperm parameters and functional tests [8].
In addition to elevated ROS levels, excessive activation of inflammatory factors is a key mediator of infertility. Inflammatory processes that contribute to COVID-19-related organ failure may also affect male fertility. COVID-19 patients show increased inflammatory cytokines such as IL-6 and TNF-α compared to healthy individuals, and hyperinflammation facilitates viral pathogenesis [9,10]. The NLRP3 protein, a central component of the most-studied NLRP3 inflammasome, contains a pyrin domain, a nucleotide oligomerization domain, and a leucine-rich repeat motif. Upon PAMP activation, NLRP3’s pyrin domain interacts with ASC, causing ASC polymerization and recruitment of pro-inflammatory caspases such as caspase-1 via its CARD domain [11]. Activation of the NLRP3 inflammasome is implicated in COVID-19 pathogenesis and certain reproductive disorders, including varicocele. SARS-CoV-2 may trigger NLRP3 activation in male reproductive organs via two mechanisms: (1) viral entry into Leydig and Sertoli cells, leading to organ dysfunction, and (2) ACE2 downregulation, increasing angiotensin II and promoting a pro-inflammatory testicular response [12]. Although several studies report the absence of the virus in semen, COVID-19 can damage testes through elevated pro-inflammatory cytokines. Extensive Sertoli and Leydig cell damage, observed in deceased patients, disrupts spermatogenesis and testosterone production [12,13,14,15].
Based on the evidence above, the present study aimed to comprehensively evaluate sperm function and oxidative stress, alongside key inflammasome-related markers, including ASC, caspase-1, and NLRP3. Additionally, we measured systemic inflammatory factors, specifically interleukin-6 (IL-6) and TNF-α, at one- and three-months following COVID-19 infection, to investigate the temporal effects of the disease on male reproductive function.
2. Materials and Methods
The study was approved by the ethics committee of the Royan Institute (IR.ACECR.ROYAN.REC.1400.140). All participants gave written informed consent and completed a questionnaire that included information regarding age, body weight, height, general health, lifestyle, consumption of alcohol, drugs, smoking, etc.
2.1. Experimental Design
Semen samples were obtained from 34 COVID-19 patients at 1 and 3 months after the COVID-19 diagnosis at the Nobel laboratory. We chose to examine the sperm samples one month after the disease peak because during the height of the illness, when symptoms such as high fever were prevalent, it was extremely challenging for the majority of participants to provide a sperm sample. To synchronize the sample collection, a period was selected when the subjects had nearly recovered, allowing us to examine the effect of this disease on sperm function.
Three semen samples for the Western blot technique were obtained from fertile men attending Esfahan Fertility and Infertility Center, who have at least one healthy child and were referred to this medical center for family balancing. Furthermore, they had not suffered from COVID-19 and had not exhibited any symptoms indicative of a COVID-19 infection during the past year since the delivery of the semen samples.
Eligible participants were men with SARS-CoV-2 infection confirmed by real-time PCR of pharyngeal or nasal swabs, who had previously received vaccination with AstraZeneca, Sinopharm, or Coviran Barkat, and who reported fever and chills during the acute phase of infection. Participants also provided semen samples at one- and three-months post-infection (calculated from the date of positive PCR), following 3–5 days of sexual abstinence before each collection.
Men were excluded if they were taking medications known to affect reproductive function; had a history of testicular torsion, cryptorchidism, testicular inflammation, atrophy, or surgery (including vasectomy); were older than 50 years; or reported erectile dysfunction or a prior history of mumps. Participants with semen volume < 0.5 mL or without proper liquefaction were also excluded.
Samples
Semen collection and analysis. Approximately 7–10 days before the one- or three-month post-COVID sampling time points, participants were contacted to remind them of semen collection procedures. Samples were collected via masturbation, either at home using sterile containers provided by the study center or at the clinic. Participants were instructed to abstain from sexual activity for 3–5 days prior to collection to maintain proper hygiene and to ensure that the initial portion of the ejaculate, which is sperm-rich, was not lost. Home-collected samples were transported to the laboratory within 30–60 min, kept at room or body temperature (20–37 °C), and protected from extreme heat or cold. Any issues during collection, such as loss of ejaculate or contamination, were reported to the study staff, and the time of collection, abstinence duration, and any collection-related problems were documented.
Semen samples from fertile men and individuals with COVID-19 infection were assessed in accordance with the World Health Organization (WHO, 2010) [16] guidelines. Fertile individuals were normozoospermic men, and the main sperm parameters of these individuals met the following criteria: sperm concentration exceeding 15 million/mL, a total sperm count of over 39 million per ejaculation, sperm motility exceeding 40%, progressive motility surpassing 32%, and normal morphology exceeding 4%. The sperm concentration was measured using a sperm-counting chamber (sperm meter, sperm processor; Garkheda, India) and a Labomed CxL optical microscope (magnification 20×, Labo America, Inc., Los Angeles, CA, USA), while sperm morphology and motility were assessed using a computer-assisted sperm analysis (CASA) system (VideoTesTSperm 2.1; Saint Petersburg, Russia). In addition, semen leukocyte and sperm viability were assessed using Endtz and eosin–nigrosin staining according to WHO-2010, respectively [16]. Supplementary Figure S1 illustrates each staining technique used in this study together with the principle of how the dye binds to the cell and what biological feature it reveals. Additionally, the expression of the main components of the NLRP3 inflammasome such as NLRP3, ASC, and caspase-1 was assessed by Western blotting. All procedures were carried out at SARA Laboratory (Tabriz, Iran).
2.2. Sperm Lipid Peroxidation and Intracytoplasmic ROS
Briefly, sperm lipid peroxidation was assessed using the BODIPY probe (BODIPY 581/591 C11 D3861, Molecular Probes) at a final concentration of 5 mmoL/L in approximately 2 million spermatozoa. The percentage of BODIPY-positive sperm was analyzed using a FACSCalibur fluorescence-activated cell sorter (Becton Dickinson, San Jose, CA, USA) [17]. Intracytoplasmic ROS was also assessed according to Kiani-Esfahani’s (2013) protocol [18] with minor alterations.
2.3. Sperm DNA Fragmentation by SCSA
Sperm DNA fragmentation was assessed by the sperm chromatin structure assay (SCSA) following the method described by Evenson et al. [19]. In brief, 1–2 million sperm were separated from seminal fluid, and the volume was adjusted to 1 mL TNE buffer (Tris-HCl/ NaCl/ EDTA). Subsequently, 1200 µL of acridine orange (Sigma, Saint Louis, MO, USA) staining solution was added to 200 µL of a diluted sperm sample with TNE in the control tube. For the test tube, 200 µL of diluted sperm sample with TNE was mixed with 400 µL acid–detergent solution for 30 s before adding 1200 µL of acridine orange staining. Finally, a FACSCalibur fluorescence-activated cell sorter (Becton Dickinson, San Jose, CA, USA) was employed to evaluate DNA fragmentation index (%DFI) and high DNA stainability (%HDS). Approximately 10,000 sperm were analyzed for each sample.
2.4. Sperm Protamine Deficiency Assessment by Chromomycin A3 (CMA3) Staining
In short, for each sample, two smears of washed spermatozoa fixed with Carnoy were taken. For staining, 200 µL CMA3 solution (0.25 mg/mL; Sigma, Saint Louis, MI, USA) was added to the smears. The slides were then rinsed 3 times with PBS 1×. At least 200 spermatozoa were evaluated using an epifluorescence microscope (Olympus, Hachioji, Tokyo, Japan) equipped with appropriate filters (460–470 nm) at ×100 magnification. Spermatozoa with low or insufficient protamine content appear light yellow, while spermatozoa with normal protamine content appear dark yellow [20].
2.5. Sperm Residual Histone Assessment by Aniline Blue Staining
In short, for each sample, two washed spermatozoa smears were taken. The slides were fixed with 3% glutaraldehyde (Merck, Darmstadt, Germany) and stained with 5% aqueous aniline blue (AB; Flinn Scientific, Inc., Batavia, IL, USA) in 4% acetic acid. The slides were then dehydrated in successive ethanol (Merck, Darmstadt, Germany) baths (70, 96 and 100%) and exposed to xylol (Merck, Darmstadt, Germany) for 5 min. The slides were then covered with Entellan (Merck, Darmstadt, Germany). For each sample, at least 200 spermatozoa were randomly counted using an optical microscope. Spermatozoa stained blue were considered to have an immature nucleus [21].
2.6. Sperm Chromatin Structure Assessment by Toluidine Blue Staining
Briefly, for each sample, two washed spermatozoa smears, freshly fixed with 96% ethanol–acetone, were taken. After 12 h, the slides were treated with 0.1 M HCl (Merck, Darmstadt, Germany) at 4 °C for 5 min and washed with distilled water (3 times for 2 min each). The slides were then covered with Toluidine Blue (TB; Merck, Darmstadt, Germany) solution (0.05% TB in 50% McIlvain citrate phosphate buffer, pH 3.5–4) for 5–10 min and washed with distilled water. Dehydration of the slides was carried out in successive ethanol baths (70, 96 and 100%). Finally, the slides were covered and mounted with xylene (Merck, Darmstadt, Germany) at room temperature (2–3 min), and the spermatozoa were counted under an optical microscope. For each sample, 200–500 spermatozoa were evaluated. Dark blue-stained spermatozoa were considered to have abnormal chromatin packaging [22].
2.7. Assessment of Serum Factors
Serum factors, including LH (Luteinizing Hormone; chemiluminescence immunoassay kit, Catalog No. CL1101-2; Autobio, Zhengzhou, China), testosterone (Siemens Healthineers Immulite 2000, Catalog No. 00630414962160; Erlangen, Germany), interleukin-6 (Human IL-6 ELISA Kit, Catalog No. 950.030.096; Diaclone, Besançon, France), and TNF-α (Human TNF-α ELISA Kit, LOT/BATCH AB181421; Abcam, Cambridge, UK) were measured according to the manufacturers’ protocols.
2.8. Statistical Analysis
The data from the present study were analyzed using IBM SPSS Statistics for Windows, version 26 (IBM, Armonk, New York, NY, USA). A paired-samples t-test was employed to compare intra-group differences at 3 months versus 1 month after the COVID-19 diagnosis in the participants. Additionally, we conducted an ANOVA analysis with the Tukey test to compare the protein levels of NLRP3, ASC, and caspase-1 between fertile men and those measured at 1 and 3 months after the COVID-19 diagnosis. For descriptive statistics, the results were expressed as mean ± standard error of the mean, except for participant age, which was presented as mean ± standard deviation. A p-value less than 0.05 was considered statistically significant.
3. Results
The mean age of males with COVID-19 was 34.68 ± 6.45 (21–47 years), and in fertile men, it was 36.63 ± 3.85 (31–44 years). In this study, sperm parameters, sperm functional tests, and main factors involved in the inflammasome were assessed and compared at 1 and 3 months after COVID-19 diagnosis.
As shown in Figure 1, the sperm parameters [concentration (146.93 ± 22.74 vs. 119.72 ± 18.19; p = 0.23), (count (410.72 ± 62.49 vs. 370.32 ± 73.98; p = 0.32), motility (53.58 ± 4.99 vs. 54.96 ± 5.85; p = 0.82), semen volume (3.30 ± 0.34 vs. 3.00 ± 0.27; p = 0.23), and semen leucocyte (0.44 ± 0.12 vs. 0.92 ± 0.24; p = 0.05)] were compared at 1 and 3 months after the COVID-19 diagnosis. The analysis clearly shows that the mean of sperm vitality (75.02 ± 1.89 vs. 55.00 ± 2.44; p < 0.001) and fast motility (14.56 ± 2.15 vs. 6.52 ± 1.57; p = 0.01) significantly improved after 3 months compared to 1 month from the diagnosis of COVID-19. The percentage of abnormal sperm morphology (97.08 ± 0.21 vs. 97.72 ± 0.29; p = 0.04), sperm lipid peroxidation (11.08 ± 0.75 vs. 17.54 ± 1.42; p = 0.001), and intracytoplasmic ROS (16.83 ± 2.6 vs. 29.7 ± 2.69; p < 0.002) significantly decreased after 3 months compared to 1 month from the diagnosis of COVID-19 in men.
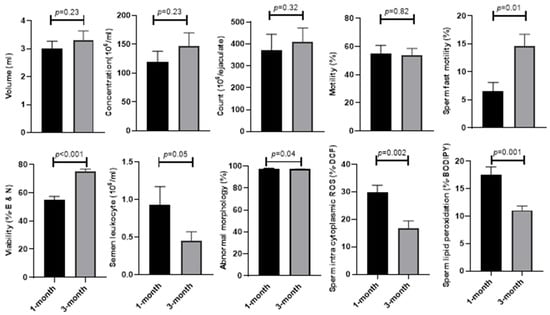
Figure 1.
Comparison of main semen parameters, sperm intracytoplasmic ROS, and sperm lipid peroxidation at 1 and 3 months after the COVID-19 diagnosis.
In addition, sperm functional parameters such as sperm abnormal chromatin, protamine deficiency, residual histones, and sperm DNA fragmentation were assessed. The results in Figure 2 demonstrate that unlike sperm DNA fragmentation (18.87 ± 1.71 vs. 22.16 ± 2.37; p = 0.17) and high DNA stainability (5.45 ± 0.52 vs. 5.25 ± 0.34; p = 0.93), where the means were similar after 3 months compared to 1 month from the diagnosis of COVID-19, the mean of sperm abnormal chromatin (12.28 ± 1.37 vs. 15.20 ± 1.38; p = 0.04), protamine deficiency (9.28 ± 2.5 vs. 10.74 ± 1.82; p = 0.02), and residual histones (26.48 ± 2.46 vs. 41.24 ± 3.47.; p < 0.001) significantly reduced after 3 months compared to 1 month from the diagnosis of COVID-19.
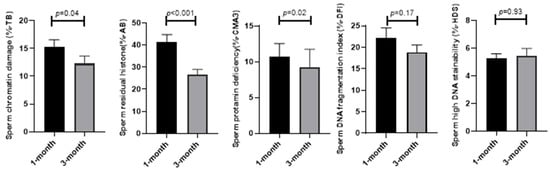
Figure 2.
Comparison of sperm functional parameters at 1 and 3 months after the COVID-19 diagnosis.
As demonstrated in Figure 3, serum factors in the blood such as LH (5.36 ± 0.64 vs. 6.08 ± 0.52; p = 0.17), testosterone (435.97 ± 29.19 vs. 411.56 ± 29.97; p = 0.3), and interleukin-6 (1.18 ± 0.17 vs. 2.35 ± 0.52; p = 0.39) did not change, while TNF-ɑ (112.3 ± 26.09 vs. 309.87 ± 29.63; p = 0.001) showed a significant improvement at 3 months compared to 1 month from the diagnosis of COVID-19.
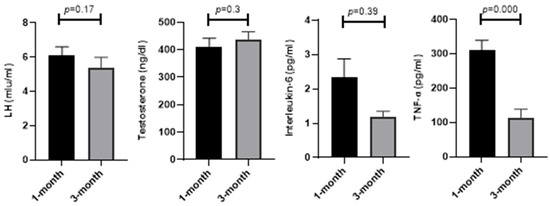
Figure 3.
Comparison of LH, testosterone, interleukin-6, and TNF-α at 1 and 3 months after COVID-19 diagnosis.
In addition, we assessed the expression of the main components of the NLRP3 inflammasome complex (NLRP3, ASC, and caspase-1) at the protein level by the Western blot technique in three individuals. The results in Figure 4 show that unlike the mean expression of ASC (1.93 ± 0.41 vs. 0.94 ± 0.07), and caspase-1 (1.18 ± 0.03 vs. 1.11 ± 0.05), which were not different after 3 months compared to 1 month from the diagnosis of COVID-19, the mean of NLRP3 protein (2.05 ± 0.35 vs. 0.86 ± 0.11; p = 0.03) significantly increased after 3 months compared to 1 month from the diagnosis of COVID-19.
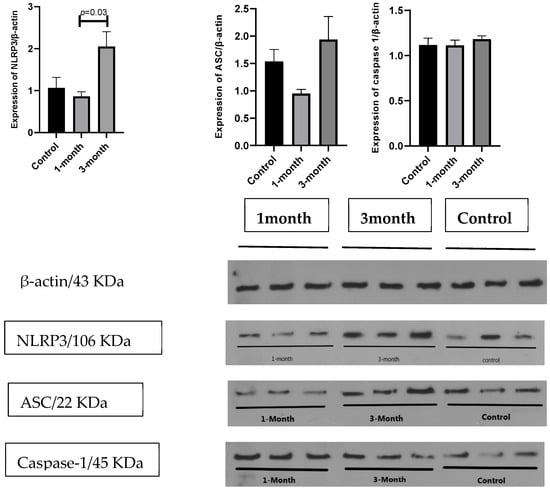
Figure 4.
Comparison of main markers involved in inflammasome pathway at 1 and 3 months after COVID-19 diagnosis.
4. Discussions
The results of the present study show that there is no significant difference in parameters such as semen volume, sperm concentration, total sperm count, and motility three months after contracting COVID-19, compared to one month after the infection. However, significant improvements were observed in certain parameters three months after the disease, including rapid sperm motility and leukocyte count, as well as the integrity of sperm chromatin assessed by CMA3, AB, TB staining, and sperm oxidative stress level evaluated by DCF and Bodipy staining three months after the disease. In addition, the mean of sperm DNA damage decreased three months after contracting COVID-19, compared to one month after the infection, but the difference was not significant.
According to previous studies, it has been reported that the male reproductive system is susceptible to viral infections, indicating that the blood–testis barrier is not completely impermeable to the virus. In the case of COVID-19, the high expression of ACE2 in seminiferous tubule cells, including Leydig, Sertoli, and renal tubular cells, makes the testis a potential target for SARS-CoV-2 [8,23,24]. The recovery from such potential damage after one or two cycles of spermatogenesis is highly dependent on the severity of the disease.
One reason for the increase in fast sperm motility and viability three months after COVID-19, compared to one month after infection, may be the normalization of body temperature following the initial fever. Several studies have shown that acute fever (39–40 °C for 1–4 days) can negatively affect sperm parameters such as viability, count, and DNA integrity [25,26]. Fever-induced temperature stress is also associated with elevated oxidative stress. According to the literature, the main sources of ROS in semen are increased leukocytes and sperm with abnormal morphology. In our study, one month after infection, leukocyte count, abnormal sperm morphology, ROS, and lipid peroxidation were significantly higher than at three months.
In addition, the increased oxidative stress may induce abnormal chromatin packaging; however, we did not observe a rise in baseline sperm DNA damage (≈23% by SCSA), and the reduction after three months compared to one month was not statistically significant. These findings suggest that sperm quality is impaired at disease onset due to temperature and oxidative stress, but improves over time. Consistently, a previous study also reported no significant difference in sperm DNA fragmentation between recovery periods shorter or longer than 90 days [27]. Conversely, Shcherbitskaia et al. (2022) reported increased caspase activation, sperm DNA fragmentation, ROS, and nitric oxide in infected individuals [28]. Similarly, Veenith et al. (2022) found ROS production to be ~9 times higher in COVID-19 patients, especially in those requiring mechanical ventilation [29]. In agreement with our results, Fallahie et al. (2021) reported a 55.52% decrease in ROS, a 21.76% reduction in sperm DNA damage, and a 34.83% decline in malondialdehyde, along with a 31.52% increase in TAC 120 days post-diagnosis [7]. These findings suggest that although SARS-CoV-2 increases ROS and impairs sperm parameters, the damage diminishes over time.
A recent meta-analysis confirmed the negative effects of COVID-19 on semen quality, including reduced semen volume, sperm concentration, count, and motility [30]. Nevertheless, several studies support that while the adverse effects on sperm quality can be severe, they are largely reversible [31,32,33]. The discrepancy between studies reporting persistent severe sperm DNA damage and those (like ours) showing recovery may be due to differences in patient characteristics, severity of infection (e.g., high fever, systemic inflammation, or mechanical ventilation), timing of sample collection, and laboratory methods used to assess DNA fragmentation.
In this regard, different durations have been reported for recovery from the harmful effects of COVID-19, ranging from 74 days [32] to more than 90 days [27] or even 6 months [33]. Accordingly, there is still no exact knowledge about how long it takes for semen quality to fully recover after COVID-19.
An increase in seminal TAC level can be one of the factors contributing to the improvement in sperm parameters and reduction in sperm DNA damage after one or more cycles of spermatogenesis. This rise in TAC leads to a subsequent decrease in ROS levels and, consequently, an enhancement in sperm parameters and a reduction in sperm DNA damage. Notably, a previous study has shown that unlike the antioxidants SOD and catalase, which remained unchanged in the semen samples of men infected with COVID-19, the level of TAC in semen and sperm DNA integrity significantly increased 120 days after contracting COVID-19 compared to the 14-day mark. This increase in sperm DNA integrity may potentially be associated with alterations in the ROS concentration of seminal fluid during this period [7].
Regarding the inflammatory cytokines, our results showed that TNF-α was substantially higher than the normal range reported for normal human serum by the kit used (42 to 203 pg/mL). This value was significantly reduced by 3 months after COVID-19 contraction. Unlike TNF-α, the mean value for IL-6 was around the normal range (<2 pg/mL); this value was insignificantly reduced by 3 months after COVID-19. These observations are in line with the mitigation of inflammation and numerous studies assessing inflammatory cytokines after COVID-19 contraction and also after the recovery period, although the duration between the first and second tests may vary between studies [10,34,35,36]. Both TNF-α and IL-6 are produced by the immune system’s response to infections, a phenomenon commonly referred to as a cytokine storm. The cytokine storm contributes to the hyperinflammatory state and is potentially damaging to tissues [37]. With regard to the role of these two cytokines in the testes, it is initially important to note that the presence of coronavirus in the testis and semen is still a controversial issue, as very few studies have reported the presence of coronavirus in the testis or semen [38,39,40]. In this regard, Li et al. (2020) [10] demonstrated various abnormalities such as interstitial edema, congestion, and red blood cell exudation in the testes and epididymides, as well as thinning of seminiferous tubules from autopsied specimens from COVID-19 cases. Furthermore, they reported a significantly higher number of apoptotic cells within the seminiferous tubules, excessive presence of testicular CD3+ and CD68+ cells in the interstitial cells from autopsied specimens, and elevated seminal levels of IL-6, TNF-α, and MCP-1 (monocyte chemoattractant protein-1) in semen of COVID-19 patients compared to control men [10]. In contrast to Li et al. (2020), Shcherbitskaia et al. (2023) reported no significant alterations in the seminal concentration of IL-6 and TNF-α among COVID-19 patients when compared to the control group [10,36]. Interestingly, using a digital epidemiology approach and blood sampling, Shcherbitskaia et al. (2022) [28] discovered that post-acute sequelae persist for up to 24 months in 60% of patients following mild COVID-19. Analysis of plasma cytokines indicated a correlation with elevated levels of IL-1β, IL-6, and TNF, which may be secreted by overactivated monocytes/macrophages [28].
In this study, we also evaluated three key components of the inflammatory pathway. The results showed that, among these components, NLRP3 levels were significantly higher at three months compared to one month, while they did not differ significantly from those of fertile individuals who had not contracted COVID-19. A similar trend was also observed for ASC, but the difference was insignificant. Although one expects to see an increase in NLPR3, ASC, and caspase-1 in these individuals, the delay is likely related to a phenomenon called “resolution of acute infection”. In this state, as the infection progresses, the immune system works to eliminate the virus, and the inflammatory response is expected to gradually subside but the resolution of the acute infection involves multiple processes, including the clearance of the virus, repair of damaged tissues, and restoration of normal physiological functions, a state indicative of ongoing immune activity related to the resolution phase. This might involve the modulation of inflammatory responses as part of the healing process and the restoration of normal tissue function [41,42]. It is important to note that the immune response is a dynamic and highly regulated process, and the exact mechanisms can vary among individuals. Factors such as the severity of the infection, the individual’s overall health, and the presence of other underlying conditions can influence the trajectory of the immune response [43] and the levels of specific immune markers like NLRP3 over time [44]. An alternative explanation is that the inflammasome pathway may become activated for clearance of damaged tissue after the contraction of viruses, but among the inflammasome components, NLRP3 is relatively more stable and may explain our observations [45]. In summary, an increase in NLRP3 levels three months after COVID-19 may reflect the ongoing efforts of the immune system to restore balance and homeostasis after the acute phase of infection.
This study has two major limitations. First, we did not have baseline normal values for all parameters prior to COVID-19 infection. Second, due to financial constraints, Western blot analysis could only be performed on a limited number of samples (n = 3 per group); therefore, this limitation should be considered when interpreting the findings, and future studies with larger sample sizes are warranted to validate these results.
In conclusion, semen volume, sperm count, concentration, total motility, and DNA damage did not change significantly one and three months after COVID-19 infection, while rapid motility, vitality, morphology, oxidative stress, chromatin integrity, plasma TNF-α, and leukocyte count improved after three months. Plasma LH, testosterone, and IL-6 remained unchanged, and only NLRP3 showed a significant increase among inflammasome factors. Since sperm chromatin maturation was impaired despite non-significant DNA damage, clinical decisions should be made cautiously. Before assisted reproduction or conception, assessment of sperm DNA integrity through fragmentation testing is advisable. Overall, these findings suggest a gradual recovery of sperm function following COVID-19, with important implications for male reproductive health, though larger studies are needed to clarify mechanisms and long-term effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid5090152/s1, Supplementary Figure S1. Overview of the principles and applications of the staining techniques employed in this study.
Author Contributions
B.G., B.N., M.S., E.K.H., M.J.H.: material preparation, data collection, experimental procedures. N.T., A.A.: Head of Nobel Laboratory, facilitating sample collection, confirming patients’ COVID-19 status through molecular testing, and granting final approval of the manuscript. M.H.N.-E. and M.T.: conceptualization, study design, coordination, data analysis, manuscript drafting, revision, and final approval. All authors have read and agreed to the published version of the manuscript.
Funding
National Institute for Medical Research Development (Grant No: 4002299).
Institutional Review Board Statement
This study was approved by the Ethics Committee of Royan Institute (IR.ACECR.ROYAN.REC.1400.140, 25 January 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
The authors would like to express their gratitude to the staff of the “Animal Biotechnology Department of the Royan Institute”, and “Isfahan Fertility and Infertility Center, Isfahan, Iran” for their full support throughout this work. In addition, we would like to acknowledge the contribution of a chat-based artificial intelligence tool in writing and editing this article. While the content and writing of this article were primarily performed by the authors themselves, chat-based AI was only used for editing purposes to enhance the clarity and coherence of the text.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- Bhat, E.A.; Sajjad, N.; Ali, A.; Aldakheel, F.M.; Mateen, A.; Alqahtani, M.S.; Syed, R. SARS-CoV-2: Insight in genome structure, pathogenesis and viral receptor binding analysis—An updated review. Int. Immunopharmacol. 2021, 95, 107493. [Google Scholar] [CrossRef]
- Aitken, R.J. COVID-19 and human spermatozoa—Potential risks for infertility and sexual transmission. Andrology 2021, 9, 48–52. [Google Scholar] [CrossRef]
- Luddi, A.; Luongo, F.P.; Dragoni, F.; Fiaschi, L.; Vicenti, I.; Lupetti, P.; Gentile, M.; Paccagnini, E.; Haxhiu, A.; Ponchia, R.; et al. Cellular and Molecular Mechanisms of In Vivo and In Vitro SARS-CoV-2 Infection: A Lesson from Human Sperm. Cells 2022, 11, 2631. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Li, F.; Zhang, Q.; Wang, T.; Qiang, L.; Yang, Q. Impacts of COVID-19 and SARS-CoV-2 on male reproductive function: A systematic review and meta-analysis protocol. BMJ Open 2022, 12, e053051. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Fabi, C.; Sugoni, C.; Bellet, M.M.; Costantini, C.; Luca, G.; Brancorsini, S. The role of NLRP3 inflammasome activation and oxidative stress in varicocele-mediated male hypofertility. Int. J. Mol. Sci. 2022, 23, 5233. [Google Scholar] [CrossRef]
- Stigliani, S.; Massarotti, C.; Bovis, F.; Maccarini, E.; Anserini, P.; Scaruffi, P. Semen parameters and male reproductive potential are not adversely affected after three or more months of recovery from COVID-19 disease. Front. Reprod. Health 2023, 4, 1114308. [Google Scholar] [CrossRef] [PubMed]
- Falahieh, F.M.; Zarabadipour, M.; Mirani, M.; Abdiyan, M.; Dinparvar, M.; Alizadeh, H.; Paktinat, S.; Hosseinirad, H. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod. Fertil. Dev. 2021, 33, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Guo, Z.; Sang, Z.; Xin, Q.; Chen, F. Effect of COVID-19 on sperm parameters: Pathologic alterations and underlying mechanisms. J. Assist. Reprod. Genet. 2023, 40, 1623–1629. [Google Scholar] [CrossRef]
- Renu, K.; Subramaniam, M.D.; Chakraborty, R.; Myakala, H.; Iyer, M.; Bharathi, G.; Siva, K.; Vellingiri, B.; Gopalakrishnan, A.V. The role of Interleukin-4 in COVID-19 associated male infertility–A hypothesis. J. Reprod. Immunol. 2020, 142, 103213. [Google Scholar] [CrossRef]
- Li, H.; Xiao, X.; Zhang, J.; Zafar, M.I.; Wu, C.; Long, Y.; Lu, W.; Pan, F.; Meng, T.; Zhao, K.; et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020, 28, 100604. [Google Scholar] [CrossRef]
- Tavalaee, M.; Rahmani, M.; Drevet, J.R.; Nasr-Esfahani, M.H. The NLRP3 inflammasome: Molecular activation and regulation in spermatogenesis and male infertility; a systematic review. Basic Clin. Androl. 2022, 32, 8. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.; Kohan-Ghadr, H.R.; Bazrafkan, M.; Amorim, C.A.; Askari, M.; Zakeri, A.; Mousavi, S.N.; Kafaeinezhad, R.; Afradiasbagharani, P.; Esfandyari, S.; et al. Rescuing fertility during COVID-19 infection: Exploring potential pharmacological and natural therapeutic approaches for comorbidity, by focusing on NLRP3 inflammasome mechanism. J. Assist. Reprod. Genet. 2023, 40, 1173–1185. [Google Scholar] [CrossRef]
- Abdelhamid, M.H.; Fellah, A.A.; Elmarghani, A.; Al Msellati, I.A. An assessment of men semen alterations in SARS-CoV-2: Is fever the principal concern? Reprod. Sci. 2023, 30, 72–80. [Google Scholar] [CrossRef]
- Peirouvi, T.; Aliaghaei, A.; Eslami Farsani, B.; Ziaeipour, S.; Ebrahimi, V.; Forozesh, M.; Ghadipasha, M.; Mahmoudiasl, G.-R.; Aryan, A.; Moghimi, N.; et al. COVID-19 disrupts the blood–testis barrier through the induction of inflammatory cytokines and disruption of junctional proteins. Inflamm. Res. 2021, 70, 1165–1175. [Google Scholar] [CrossRef]
- Yang, M.; Chen, S.; Huang, B.O.; Zhong, J.M.; Su, H.; Chen, Y.J.; Cao, Q.; Ma, L.; He, J.; Li, X.-F.; et al. Pathological findings in the testes of COVID-19 patients: Clinical implications. Eur. Urol. Focus. 2020, 6, 1124–1129. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Aitken, R.J.; Wingate, J.K.; De Iuliis, G.N.; McLaughlin, E.A. Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. MHR. Mol. Hum. Reprod. 2007, 13, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kiani-Esfahani, A.; Bahrami, S.; Tavalaee, M.; Deemeh, M.R.; Mahjour, A.A.; Nasr-Esfahani, M.H. Cytosolic and mitochondrial ROS: Which one is associated with poor chromatin remodeling? Syst. Biol. Reprod. Med. 2013, 59, 352–359. [Google Scholar] [CrossRef]
- Evenson, D.P. Sperm chromatin structure assay (SCSA®) for fertility assessment. Curr. Protoc. 2022, 2, e508. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, F.G.; Nasr-Esfahani, M.H.; Valojerdi, M.R.; Taki Al-Taraihi, T.M. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J Assist. Reprod. Genet. 2000, 17, 60–66. [Google Scholar] [CrossRef]
- Franken, D.R.; Franken, C.J.; De La Guerre, H.; De Villiers, A. Normal sperm morphology and chromatin packaging: Comparison between aniline blue and chromomycin A3 staining. Andrologia 1999, 31, 361–366. [Google Scholar] [CrossRef]
- Tsarev, I.; Bungum, M.; Giwercman, A.; Erenpreisa, J.; Ebessen, T.; Ernst, E.; Erenpreiss, J. Evaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessment. Hum. Reprod. 2009, 24, 1569–1574. [Google Scholar] [CrossRef]
- Fan, C.; Lu, W.; Li, K.; Ding, Y.; Wang, J. ACE2 expression in kidney and testis may cause kidney and testis infection in COVID-19 patients. Front. Med. 2021, 7, 563893. [Google Scholar] [CrossRef]
- Liu, W.; Han, R.; Wu, H.; Han, D. Viral threat to male fertility. Andrologia 2018, 50, e13140. [Google Scholar] [CrossRef]
- Evenson, D.P.; Jost, L.K.; Corzett, M.; Balhorn, R. Characteristics of human sperm chromatin structure following an episode of influenza and high fever: A case study. Asian. J. Androl. 2000, 21, 739–746. [Google Scholar] [CrossRef]
- Sergerie, M.; Mieusset, R.; Croute, F.; Daudin, M.; Bujan, L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil. Steril. 2007, 88, 970-e1. [Google Scholar] [CrossRef]
- Ruan, Y.; Hu, B.; Liu, Z.; Liu, K.; Jiang, H.; Li, H.; Li, R.; Luan, Y.; Liu, X.; Yu, G.; et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation. Andrology 2021, 9, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Shcherbitskaia, A.D.; Komarova, E.M.; Milyutina, Y.P.; Ishchuk, M.A.; Sagurova, Y.M.; Safaryan, G.K.; Lesik, E.A.; Gzgzyan, A.M.; Bespalova, O.N.; Kogan, I.Y. Oxidative stress markers and sperm DNA fragmentation in men recovered from COVID-19. Int. J. Mol. Sci. 2022, 23, 10060. [Google Scholar] [CrossRef] [PubMed]
- Veenith, T.; Martin, H.; Le Breuilly, M.; Whitehouse, T.; Gao-Smith, F.; Duggal, N.; Lord, J.M.; Mian, R.; Sarphie, D.; Moss, P. High generation of reactive oxygen species from neutrophils in patients with severe COVID-19. Sci. Rep. 2022, 12, 10484. [Google Scholar] [CrossRef]
- Xie, Y.; Mirzaei, M.; Kahrizi, M.S.; Shabestari, A.M.; Riahi, S.M.; Farsimadan, M.; Roviello, G. SARS-CoV-2 effects on sperm parameters: A meta-analysis study. J. Assist. Reprod. Genet. 2022, 39, 1555–1563. [Google Scholar] [CrossRef]
- Donders, G.G.; Bosmans, E.; Reumers, J.; Donders, F.; Jonckheere, J.; Salembier, G.; Stern, N.; Ombelet, W.; Depuydt, C.E. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: A prospective, observational study and validation of the SpermCOVID test. Fertil. Steril. 2022, 117, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.H.; Sang, M.Y.; Bai, S.; Ma, H.; Wan, Y.Y.; Jiang, X.H.; Zhang, Y.-W.; Xu, B.; Chen, H.; Zheng, X.-Y.; et al. Semen parameters in men recovered from COVID-19. Asian J. Androl. 2021, 23, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, K.; Ruan, Y.; Wei, X.; Wu, Y.; Feng, H.; Deng, Z.; Liu, J.; Wang, T. Evaluation of mid-and long-term impact of COVID-19 on male fertility through evaluating semen parameters. Transl. Androl. Urol. 2022, 11, 159. [Google Scholar] [CrossRef]
- Mustafa, M.I.; Abdelmoneim, A.H.; Mahmoud, E.M.; Makhawi, A.M. Cytokine storm in COVID-19 patients, its impact on organs and potential treatment by QTY code-designed detergent-free chemokine receptors. Mediat. Inflamm. 2020, 2020, 8198963. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.-S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Shcherbitskaia, A.D.; Komarova, E.M.; Milyutina, Y.P.; Sagurova, Y.M.; Ishchuk, M.A.; Mikhel, A.V.; Ob’edkova, K.V.; Lesik, E.A.; Gzgzyan, A.M.; Tapilskaya, N.I.; et al. Age-related COVID-19 influence on male fertility. Int. J. Mol. Sci. 2023, 24, 15742. [Google Scholar] [CrossRef]
- Silva, M.J.; Ribeiro, L.R.; Gouveia, M.I.; Marcelino, B.D.; Santos, C.S.; Lima, K.V.; Lima, L.N.G.C. Hyperinflammatory response in COVID-19: A systematic review. Viruses 2023, 15, 553. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.M.; Bonuccelli, D.; Giannini, R.; Macerola, E.; Vignali, P.; Ugolini, C.; Torregrossa, L.; Proietti, A.; Pistello, M.; Basolo, A.; et al. COVID-19 autopsy cases: Detection of virus in endocrine tissues. J. Endocrinol. Investig. 2022, 45, 209–214. [Google Scholar] [CrossRef]
- Ata, B.; Vermeulen, N.; Mocanu, E.; Gianaroli, L.; Lundin, K.; Rautakallio-Hokkanen, S.; Tapanainen, J.S.; Veiga, A. SARS-CoV-2, fertility and assisted reproduction. Hum. Reprod. Update 2023, 29, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, C.; Garolla, A.; Maccarini, E.; Scaruffi, P.; Stigliani, S.; Anserini, P.; Foresta, C. SARS-CoV-2 in the semen: Where does it come from? Andrology 2021, 9, 39–41. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Spite, M. Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef]
- Shin, E.C.; Sung, P.S.; Park, S.H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 2016, 16, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the human innate immune system. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Marrone, L.; Peace, C.G.; O’Neill, L.A. NLRP3, the inflammasome and COVID-19 infection. QJM Int. J. Med. 2023, 116, 502–507. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Q.; Liang, W.; Yan, R.; Tong, L.; Jia, M.; Zhao, C.; Zhao, W. TRIM28 SUMOylates and stabilizes NLRP3 to facilitate inflammasome activation. Nat. Commun. 2021, 12, 4794. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).