Impact of the COVID-19 Pandemic on Cervical Cancer Screening in Brazil: A Nationwide Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Variables
2.3. Statistical Analyses
2.4. Ethical Considerations
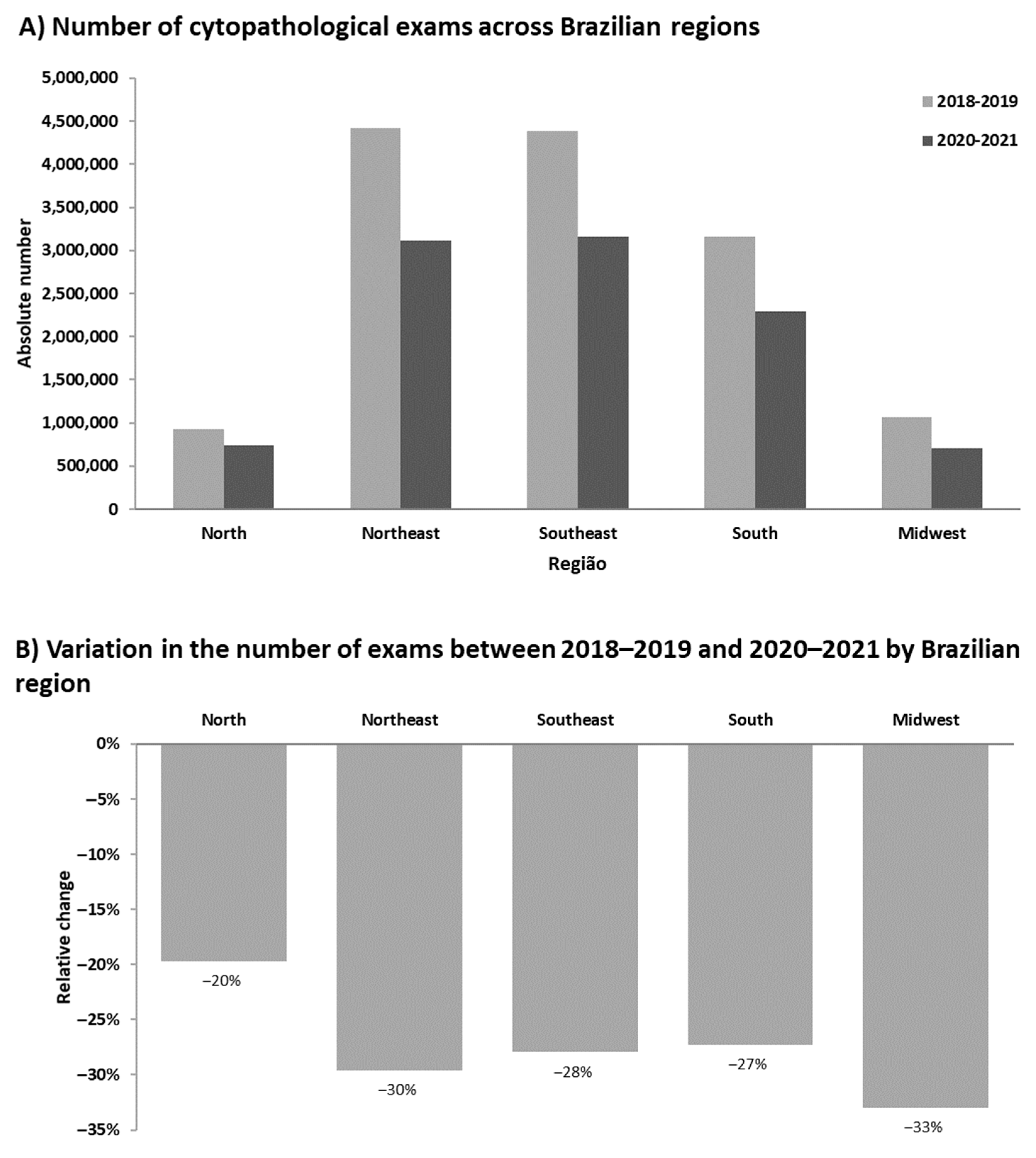
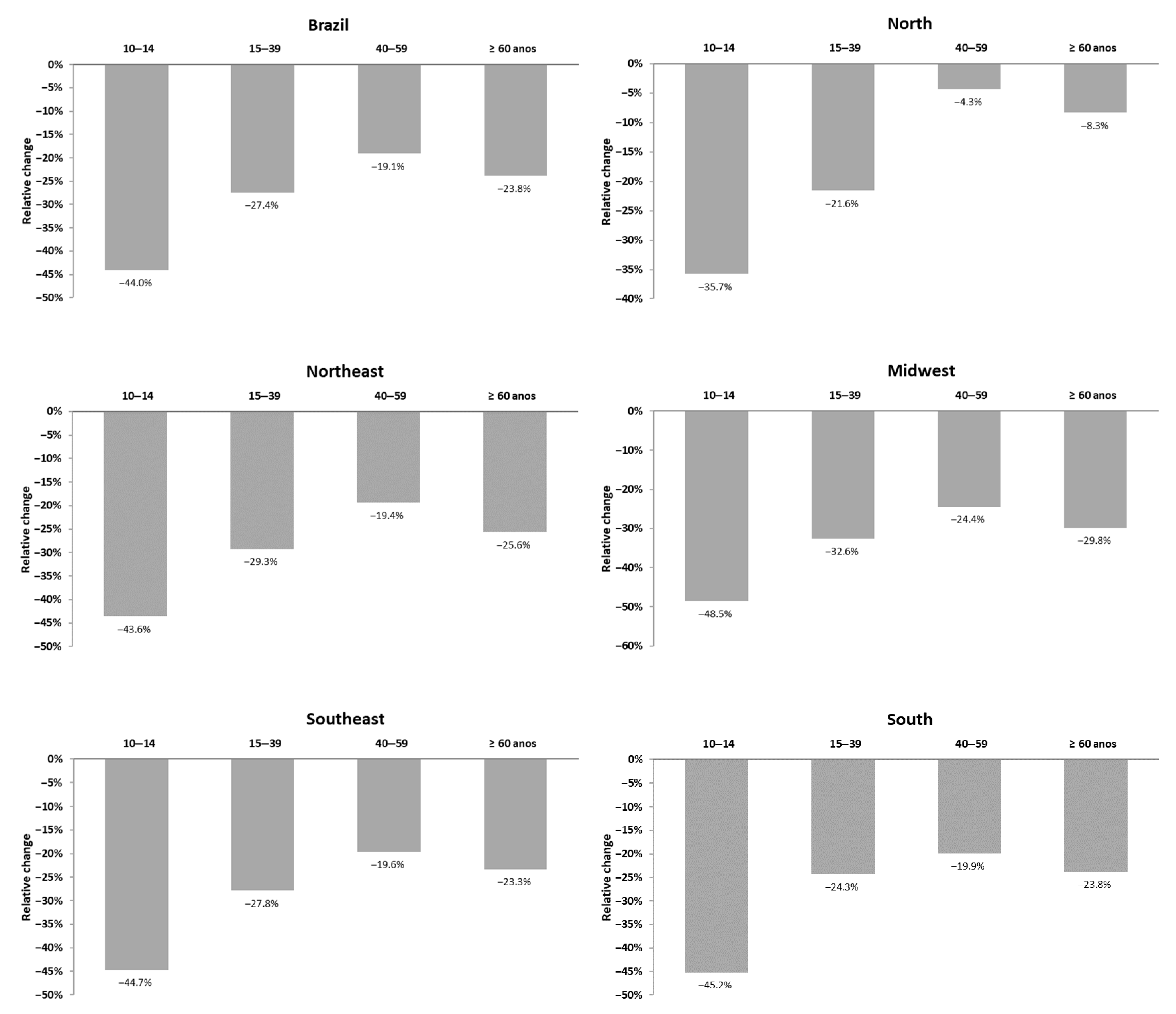
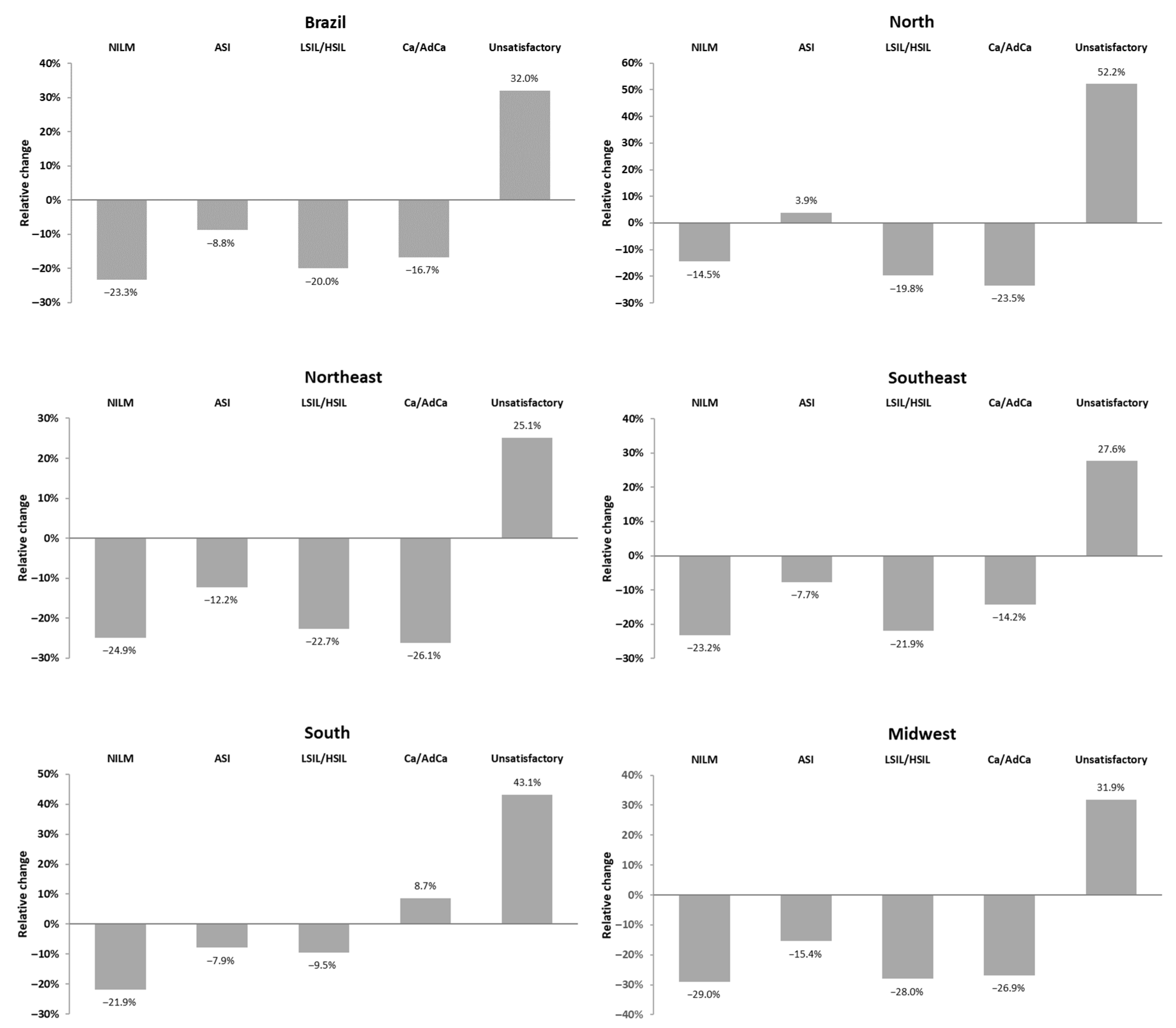
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SISCAN | Cancer Information System |
| NILM | Negative for Intraepithelial Lesion or Malignancy |
| ASC | Atypical Squamous Cells of Undetermined Significance |
| LSIL | Low-grade Squamous Intraepithelial Lesions |
| HSIL | High-grade Squamous Intraepithelial Lesions |
| Ca | Carcinoma |
| AdCa | Adenocarcinoma |
| OR | Odds ratios |
| 95% CI | 95% Confidence Intervals |
| ROC | Receiver Operating Characteristic |
References
- Löwy, I. Cancer, women, and public health: The history of screening for cervical cancer. História Ciências Saúde-Manguinhos 2010, 17 (Suppl. S1), 53–67. [Google Scholar] [CrossRef]
- de Oliveira Silva, B.L.A.; de Andrade Barros, R.A.; Lopes, I.M.R.S. O impacto da pandemia da COVID-19 no rastreamento do câncer de colo uterino em Teresina—PI. Res. Soc. Dev. 2021, 10, e2091010118768. [Google Scholar] [CrossRef]
- Militão, B.V.P.; Andrade, V.F.; Sousa, F.A.; Carneiro, I.D.; Cardoso, G.S.; de Freitas Mourão, T.; Coelho, J.P.; Guimarães, A.C.P. Repercussões da pandemia de Sars-Cov-2 na realização do exame de Papanicolaou: Um estudo epidemiológico. Rev. Eletrônica Acervo Saúde 2021, 13, e8869. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; De Miranda Correa, F.; Migowski, A. Short-term effects of the COVID-19 pandemic on cancer screening, diagnosis and treatment procedures in Brazil: A descriptive study, 2019–2020. Epidemiol. Serv. Saude 2022, 31, e2021405. [Google Scholar] [CrossRef] [PubMed]
- Vannabouathong, C.; Devji, T.; Ekhtiari, S.; Chang, Y.; Phillips, S.A.; Zhu, M.; Chagla, Z.; Main, C.; Bhandari, M. Novel coronavirus COVID-19 current evidence and evolving strategies. J. Bone Jt. Surg.-Am. Vol. 2020, 102, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.C.; Teixeira, L.A. Cancer education campaigns. Hist. Cienc. Saude-Manguinhos 2010, 17 (Suppl. S1), 223–241. [Google Scholar] [CrossRef]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.U.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112, S92–S107. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, R.C.; Messias, A.P.; Moreira, O.A.; Leis, L.V.; Orsi, B.Z.; Testa, L.; Estevez-Diz, M.D.P. Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil. Ecancermedicalscience 2021, 15, 1299. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Chauhan, A.S.; Prinja, S.; Pandey, A.K. Impact of COVID-19 on Outcomes for Patients with Cervical Cancer in India. JCO Glob. Oncol. 2021, 7, 716–725. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, W.C.; Gonçalves, D.A.; Cruz, D.B. COVID-19: Local/regional inequalities and impacts over critical healthcare infrastructure in Brazil. Ambiente Soc. 2020, 23, e0114. [Google Scholar] [CrossRef]
- Ferezin, L.P.; Rosa, R.J.; Moura, H.S.D.; de Campos, M.C.T.; Delpino, F.M.; Nascimento, M.C.D.; Araújo, J.S.T.D.; Pinto, I.C.; Arcêncio, R.A. Disparities in Healthcare Utilization Among Vulnerable Populations During the COVID-19 Pandemic in Brazil: An Intersectional Analysis. Int. J. Environ. Res. Public Health 2025, 22, 831. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Santos Camargo, J.D.; Camargo, S.F.; Serquiz, N.; Alves Sarmento, A.C.; Gonçalves, A.K. Increased socioeconomic vulnerability in breast cancer diagnosis during the COVID-19 pandemic in Brazil. Clin. Epidemiol. Glob. Health 2024, 29, 101735. [Google Scholar] [CrossRef]
- Martins, T.R.; Witkin, S.S.; Mendes-Correa, M.C.; Godoy, A.S.D.; Cury, L.; Balancin, M.L.; Ab’Saber, A.M.; Peres, S.V.; Messias, S.; Tozetto Mendoza, T.R.; et al. Impact of the COVID-19 Pandemic on Cervical Cancer Screening in São Paulo State, Brazil. Acta Cytol. 2023, 67, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Antonini, M.; Miranda, M.L.; Vianna Junior, I.; Gonçalves, R.T.R.; Lopes, R.G.C. The Impact of The COVID-19 Pandemic on Cervical Cancer Screening: An Ecological Study on Pap Smears Test Conducted in Brazil. J. Community Med. Public Health Rep. 2023, 4. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Atty, A.T.d.M. Efeitos da Covid-19 na Atenção ao Câncer no Brasil: Impactos do Rastreamento ao Tratamento. Rev. Bras. Cancerol. 2025, 71, e-074848. [Google Scholar] [CrossRef]
- Duarte, M.B.O.; Argenton, J.L.P.; Carvalheira, J.B.C. Impact of COVID-19 in Cervical and Breast Cancer Screening and Systemic Treatment in São Paulo, Brazil: An Interrupted Time Series Analysis. JCO Glob. Oncol. 2022, 8, e2100371. [Google Scholar] [CrossRef] [PubMed]
- Loeb, A.M.; Gravitt, P.; Frank, A.; Puricelli Perin, D.M.; Duncan, K.; Eckert, L.; Almonte, M.; Broutet, N.; Rodman, J.; Oyebamiji, O.A.; et al. Assessing the global implications of the COVID-19 pandemic on the cervical cancer elimination initiative. PLoS Glob. Public Health 2025, 5, e0004419. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, M.; Shahil-Feroz, A.; Lofters, A.; Wong, J.P.H.; Prakash, V.; Pimple, S.; Anand, K.; Mishra, G. Surviving the Storm: The Impact of COVID-19 on Cervical Cancer Screening in Low- and Middle-Income Countries. Healthcare 2023, 11, 3079. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.K.; McElfish, P.A. Cancer Screening Recommendations During the COVID-19 Pandemic: Scoping Review. JMIR Cancer 2022, 8, e34392. [Google Scholar] [CrossRef]
- Tsui, J.; Llanos, A.A.M.; Doose, M.; Rotter, D.; Stroup, A. Determinants of abnormal cervical cancer screening follow-up and invasive cervical cancer among uninsured and underinsured women in New Jersey. J. Health Care Poor Underserved 2019, 30, 680–701. [Google Scholar] [CrossRef] [PubMed]
- Saraiya, M.; Cheung, L.C.; Soman, A.; Mix, J.; Kenney, K.; Chen, X.; Perkins, R.B.; Schiffman, M.; Wentzensen, N.; Miller, J. Risk of cervical precancer and cancer among uninsured and underserved women from 2009 to 2017. Am. J. Obstet. Gynecol. 2021, 224, 366.e1–366.e32. [Google Scholar] [CrossRef]
- Bashar, M.A.; Begam, N. Impact of COVID-19 Pandemic on Cancer Screening in India: Current Situation, Challenges and Way Forwards. Asian Pac. J. Cancer Care 2021, 6, 145–150. [Google Scholar] [CrossRef]
- Mantula, F.; Toefy, Y. Exploring the Impact of COVID-19 on Cervical Cancer Screening Services: A Qualitative Study of Healthcare Providers’ and Women’s Perspectives and Experiences. Health Serv. Insights 2024, 17, 11786329241275883. [Google Scholar] [CrossRef]
- Villain, P.; Carvalho, A.L.; Lucas, E.; Mosquera, I.; Zhang, L.; Muwonge, R.; Selmouni, F.; Sauvaget, C.; Basu, P.; IARC COVID-19 Impact Study Group. Cross-sectional survey of the impact of the COVID-19 pandemic on cancer screening programs in selected low- and middle-income countries: Study from the IARC COVID-19 impact study group. Int. J. Cancer 2021, 149, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Burger, E.A.; Castanon, A.; de Kok, I.M.; Hanley, S.J.; Rebolj, M.; Hall, M.T.; Jansen, E.E.; Killen, J.; O’Farrell, X.; et al. Impact of disruptions and recovery for established cervical screening programs across a range of high-income country program designs, using COVID-19 as an example: A modelled analysis. Prev. Med. 2021, 151, 106623. [Google Scholar] [CrossRef] [PubMed]
- Sabeena, S.; Ravishankar, N. The Short-Term Impact Of COVID-19 Pandemic on Cervical Cancer Screening: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2022, 23, 1497. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Rosas, T.; Cazap, E.; Delgado, L.; Ismael, J.; Bejarano, S.; Castro, C.; Castro, H.; Müller, B.; Gutiérrez-Delgado, F.; Santini, L.A.; et al. Social Distancing and Economic Crisis During COVID-19 Pandemic Reduced Cancer Control in Latin America and Will Result in Increased Late-Stage Diagnoses and Expense. JCO Glob. Oncol. 2021, 7, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Entiauspe, L.G.; Teixeira, L.O.; Mendoza-Sassi, R.A.; Gonçalves, C.V.; Gonçalves, P.; Martinez, A.M. Papilomavírus humano: Prevalência e genótipos encontrados em mulheres HIV positivas e negativas, em um centro de referência no extremo Sul do Brasil [Human papillomavirus: Prevalence and genotypes found among HIV-positive and negative women at a reference center in the far south of Brazil]. Rev. Soc. Bras. Med. Trop. 2010, 43, 260–263. [Google Scholar] [CrossRef][Green Version]
- Ribeiro, A.; Corrêa, F.M.; Migowski, A.; Leal, A.; Martins, S.; Raiol, T.; Marques, C.P.; Torres, K.L.; Novetsky, A.P.; Marcus, J.Z.; et al. Rethinking cervical cancer screening in Brazil post COVID-19: A global opportunity to adopt higher impact strategies. Cancer Prev. Res. 2021, 14, 919–926. [Google Scholar] [CrossRef]
- Elfström, K.M.; Arnheim-Dahlström, L.; von Karsa, L.; Dillner, J. Cervical Cancer Screening in Europe: Quality Assurance and Organisation of Programmes. Eur. J. Cancer 2015, 51, 950–968. [Google Scholar] [CrossRef] [PubMed]
- Kalliala, I.; Athanasiou, A.; Veroniki, A.A.; Salanti, G.; Efthimiou, O.; Raftis, N.; Bowden, S.; Paraskevaidi, M.; Aro, K.; Ar-byn, M.; et al. Incidence and Mortality from Cervical Cancer and Other Malignancies after Treatment of Cervical Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis of the Literature. Ann. Oncol. 2020, 31, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.E.L.; Zielonke, N.; Gini, A.; Anttila, A.; Segnan, N.; Vokó, Z.; Ivanuš, U.; McKee, M.; de Koning, H.J.; de Kok, I.M.C.M.; et al. Effect of Organised Cervical Cancer Screening on Cervical Cancer Mortality in Europe: A Systematic Review. Eur. J. Cancer 2020, 127, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Fuzzell, L.; Lake, P.; Brownstein, N.C.; Fontenot, H.B.; Whitmer, A.; Michel, A.; McIntyre, M.; Rossi, S.L.; Kajtezovic, S.; Vadaparampil, S.T.; et al. Examining the perceived impact of the COVID-19 pandemic on cervical cancer screening practices among clinicians practicing in Federally Qualified Health Centers: A mixed methods study. eLife 2023, 12, e86358. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.C.B.; Garnelo, L.; Herkrath, F.J. Barriers to Access the Pap Smear Test for Cervical Cancer Screening in Rural Riverside Populations Covered by a Fluvial Primary Healthcare Team in the Amazon. Int. J. Environ. Res. Public Health 2022, 19, 4193. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, R.S.; dos Santos, H.L.P.C.; Prado, N.M.D.B.L.; Bittencourt, R.G.; Biscarde, D.G.D.S.; Santos, A.M.D. Controle do Câncer do Colo do Útero na Atenção Primária à Saúde em Países Sul-Americanos: Revisão Sistemática. Rev. Panam. Salud Pública 2022, 46, e107. [Google Scholar] [CrossRef] [PubMed]
| Variables | Period | p-Value * | ||
|---|---|---|---|---|
| 2018–2019 | 2020–2021 | 2022–2023 | ||
| Monthly volume of cytopathological exams in Brazil | 1,161,819 ± 114,720 | 834,783 ± 228,871 | 1,252,218 ± 226,578 | <0.01 |
| Volume of cytopathological exams by Region | 2,788,366 ± 1,718,076 | 2,003,480 ± 1,216,074 | 3,005,323 ± 1,741,790 | 0.013 |
| Variables | Test Statistic | p-Value * |
|---|---|---|
| Monthly volume of cytopathological exams in Brazil | 5.121 | <0.01 |
| Cytopathological exams by region | 3.467 | 0.026 |
| Cytopathological exams by report type in the North Region | −0.405 | 0.686 |
| Cytopathological exams by report type in the Northeast Region | −1.214 | 0.225 |
| Cytopathological exams by report type in the Southeast Region | −1.483 | 0.138 |
| Cytopathological exams by report type in the South Region | −1.214 | 0.225 |
| Cytopathological exams by report type in the Central-West Region | −1.483 | 0.138 |
| Cytopathological exams by report type in Brazil | −1.214 | 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, K.K.A.d.C.; de Souza, A.T.B.; Camargo, S.F.; Camargo, J.D.d.A.S.; Crispim, J.C.d.O. Impact of the COVID-19 Pandemic on Cervical Cancer Screening in Brazil: A Nationwide Population-Based Study. COVID 2025, 5, 151. https://doi.org/10.3390/covid5090151
Gomes KKAdC, de Souza ATB, Camargo SF, Camargo JDdAS, Crispim JCdO. Impact of the COVID-19 Pandemic on Cervical Cancer Screening in Brazil: A Nationwide Population-Based Study. COVID. 2025; 5(9):151. https://doi.org/10.3390/covid5090151
Chicago/Turabian StyleGomes, Kayonaria Kardenia Alves da Costa, Amaxsell Thiago Barros de Souza, Sávio Ferreira Camargo, Juliana Dantas de Araújo Santos Camargo, and Janaina Cristiana de Oliveira Crispim. 2025. "Impact of the COVID-19 Pandemic on Cervical Cancer Screening in Brazil: A Nationwide Population-Based Study" COVID 5, no. 9: 151. https://doi.org/10.3390/covid5090151
APA StyleGomes, K. K. A. d. C., de Souza, A. T. B., Camargo, S. F., Camargo, J. D. d. A. S., & Crispim, J. C. d. O. (2025). Impact of the COVID-19 Pandemic on Cervical Cancer Screening in Brazil: A Nationwide Population-Based Study. COVID, 5(9), 151. https://doi.org/10.3390/covid5090151







