SARS-CoV-2 Infection Epidemiology Changes During Three Years of Pandemic in a Region in Central India
Abstract
1. Introduction
2. Method
2.1. Study Design, Period, and Settings
2.2. Sample Collection and Processing
2.3. Viral Nucleic Acid Extraction
2.4. Real-Time PCR
2.5. Statistical Analysis
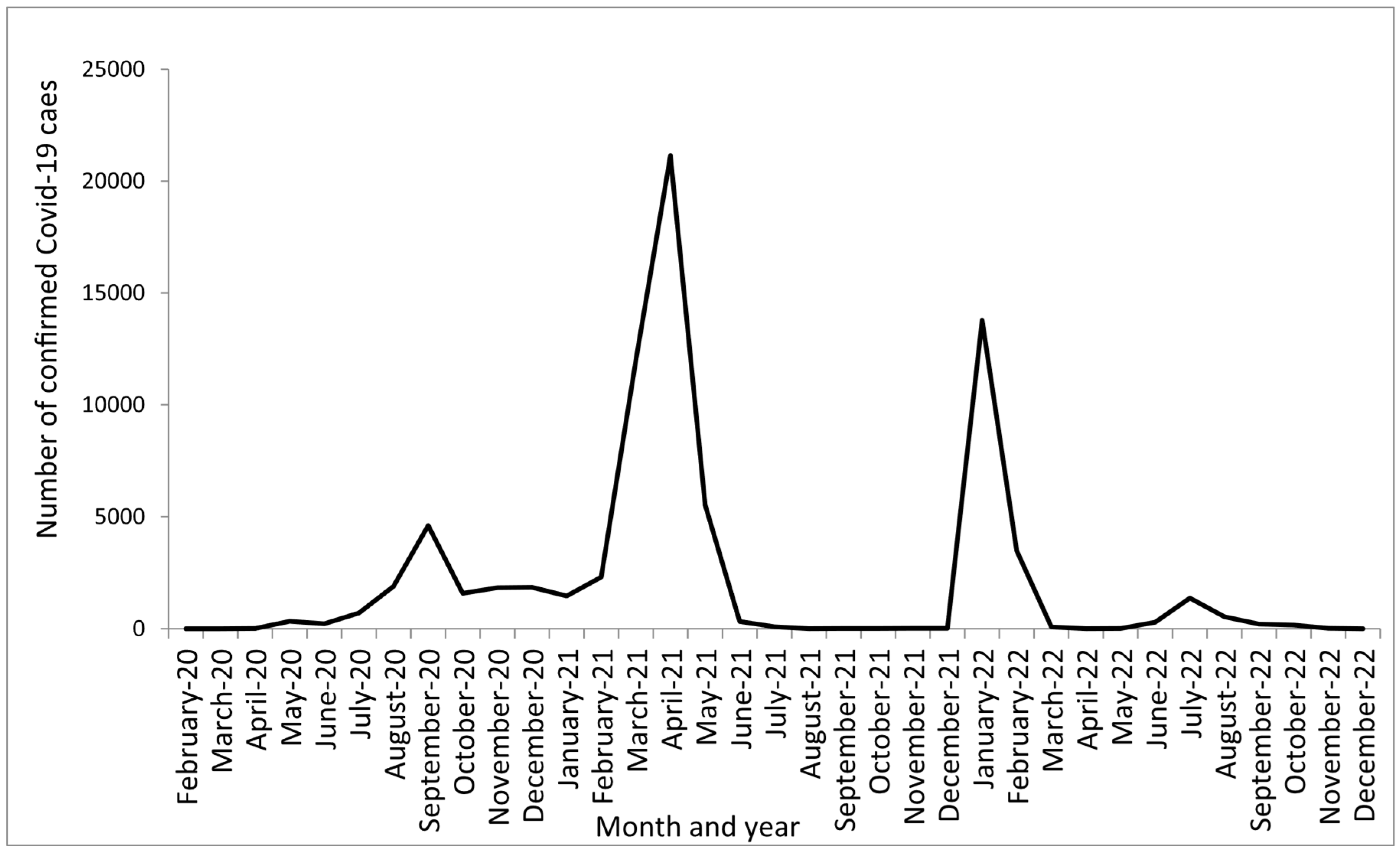
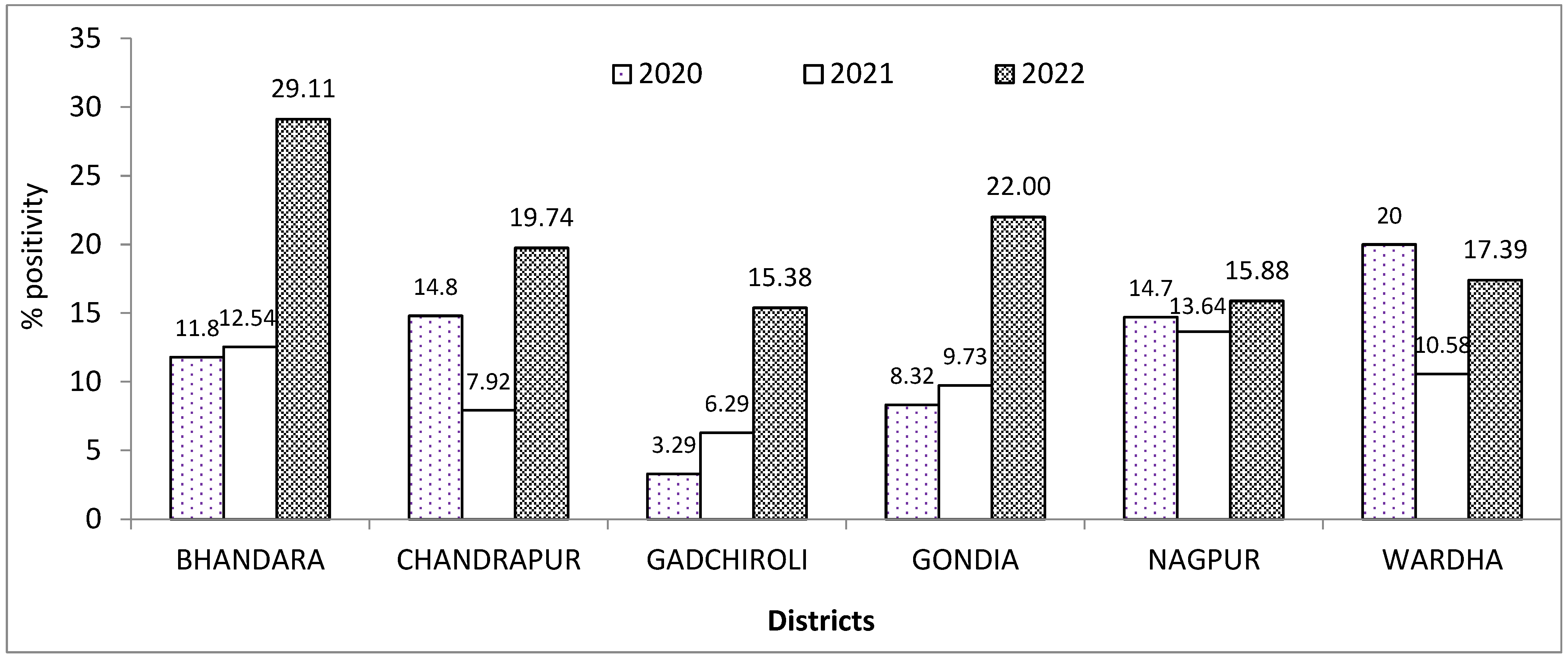
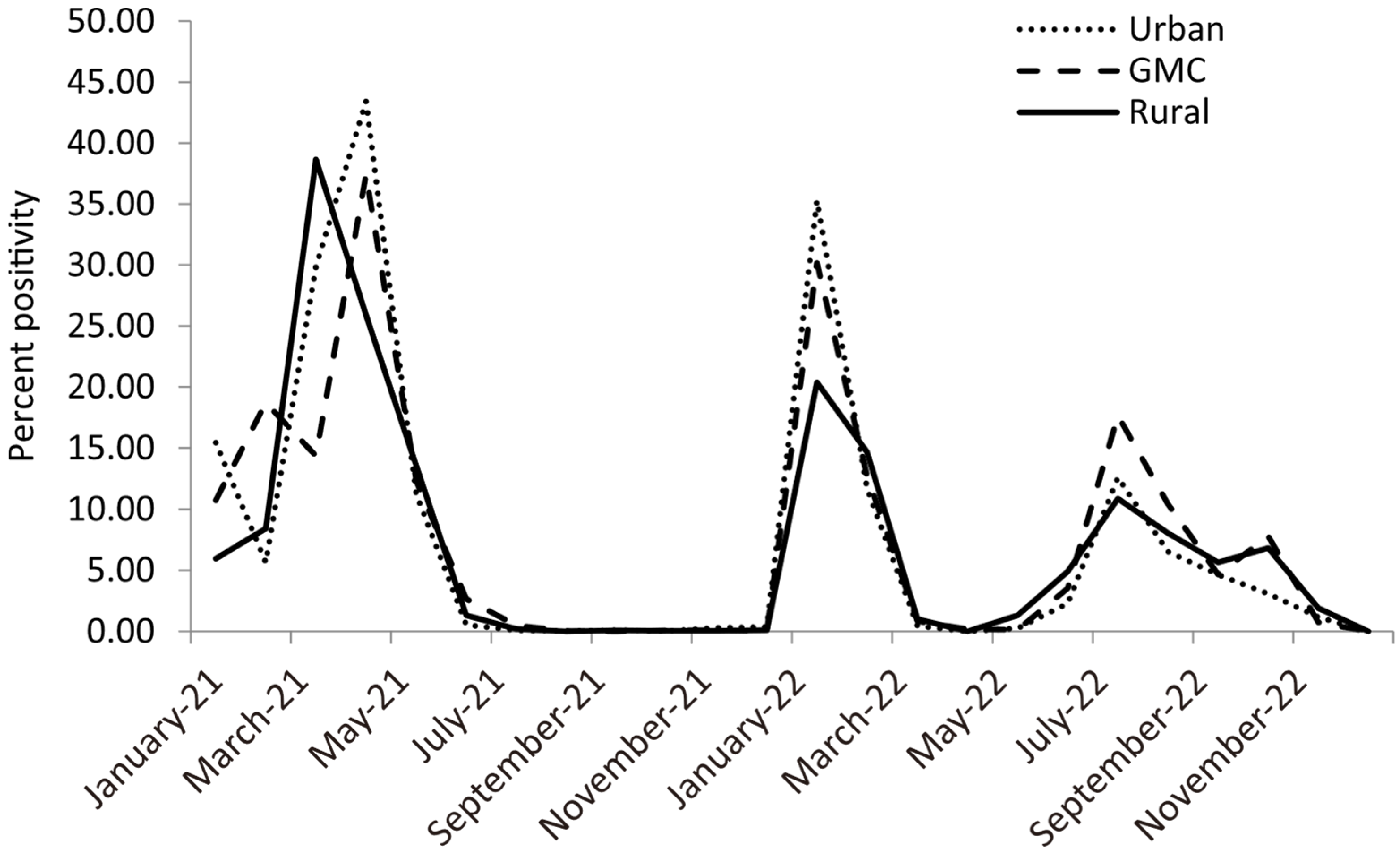
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du Toit, A. Outbreak of a novel coronavirus. Nat. Rev. Microbiol. 2020, 18, 123. [Google Scholar] [CrossRef]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Feehan, J.; Apostolopoulos, V. Is COVID-19 the worst pandemic? Maturitas 2021, 149, 56–58. [Google Scholar] [CrossRef]
- Blake, P.; Wadhwa, D. 2020 Year in Review: The Impact of COVID-19 in 12 Charts. 2020. Available online: https://blogs.worldbank.org/voices/2020-year-review-impact-covid-19-12-charts (accessed on 2 March 2023).
- Bhosale, S.; Kulkarni, A.P. Is a problem shared, a problem halved? Not always! The novel coronavirus COVID-19 outbreak. Indian J. Crit. Care Med. 2020, 24, 88–89. [Google Scholar] [CrossRef]
- Soni, A.R.; Amrit, K.; Shinde, A.M. COVID-19 and transportation of India: Influence on infection risk and greenhouse gas emissions. Environ. Dev. Sustain. 2022, 9, 7377–7392. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/table (accessed on 3 March 2023).
- Carta, M.G.; Orrù, G.; Littera, R.; Firinu, D.; Chessa, L.; Cossu, G.; Primavera, D.; Del Giacco, S.; Tramontano, E.; Manocchio, N.; et al. Comparing the responses of countries and National Health Systems to the COVID-19 pandemic: A critical analysis with a case-report series. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7868–7880. [Google Scholar] [CrossRef]
- India Fights Corona COVID-19 Dashboard. Available online: https://www.mygov.in/covid-19 (accessed on 3 March 2023).
- Tanna, J.; Singha, B.; Nayak, A.R.; Husain, A.A.; Raje, D.V.; Desai, S.A.; Deshmukh, M.; Mundhada, S.; Kashyap, R.S. Incidence and Epidemiological study of COVID-19 in Nagpur urban region (India) using Molecular testing. MedRxiv 2021. [Google Scholar] [CrossRef]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laxminarayan, R.; Jameel, S.; Sarkar, S. India’s Battle against COVID-19: Progress and Challenges. Am. J. Trop. Med. Hyg. 2020, 103, 1343–1347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bureau, W.O. India’s Double Mutant COVID Strain More Transmissible Than Other Variants: WHO. Outlook India. 2021. Available online: https://www.outlookindia.com/website/story/india-news-indias-double-mutant-covid-strain-more-transmissible-than-other-variants-who/382472 (accessed on 3 March 2023).
- Vaidyanathan, G. Coronavirus variants are spreading in India—what scientists know so far. Nature 2021, 593, 321–322. [Google Scholar] [CrossRef]
- Asrani, P.; Eapen, M.S.; Hassan, M.I.; Sohal, S.S. Implications of the second wave of COVID-19 in India. Lancet Respir. Med. 2021, 9, e93–e94. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; et al. SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Fast-spreading COVID variant can elude immune responses. Nature 2021, 589, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Times of India 2021. 2nd Wave Claimed 89,000 Lives in Maharashtra, Pune Worst Affected. Available online: http://timesofindia.indiatimes.com/articleshow/86542100.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst. (accessed on 3 March 2023).
- Ansari, A.A.Z.; Desai, H.D.; Sharma, K.; Jadeja, D.M.; Patel, R.; Patel, Y.; Desai, H.M. Prevalence and cross states comparison of case fatality rate and recovery rate of COVID 19/SARS-CoV-2 in India. J. Fam. Med. Prim. Care 2021, 10, 475–480. [Google Scholar] [CrossRef]
- Tarighi, P.; Eftekhari, S.; Chizari, M.; Sabernavaei, M.; Jafari, D.; Mirzabeigi, P. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur. J. Pharmacol. 2021, 895, 173890. [Google Scholar] [CrossRef]
- Leentjens, J.; van Haaps, T.F.; Wessels, P.F.; Schutgens, R.E.G.; Middeldorp, S. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021, 8, e524–e533. [Google Scholar] [CrossRef]
- The WHORapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group: Association between Administration of Systemic Corticosteroids Mortality among Critically Ill Patients with COVID-19: AMeta-analysis. JAMA 2020, 324, 1330–1341. [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B.; Momen-Heravi, M. An Insight into the Sex Differences in COVID-19 Patients: What are the Possible Causes? Prehosp. Disaster Med. 2020, 35, 438–441. [Google Scholar] [CrossRef]
- Penna, C.; Mercurio, V.; Tocchetti, C.G.; Pagliaro, P. Sex-related differences in COVID-19 lethality. Br. J. Pharmacol. 2020, 177, 4375–4385. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Cossio, Y.; Aller, M.B.; Abadias, M.J.; Domínguez, J.M.; Romea, M.S.; Barba, M.À.; Rodríguez, M.I.; Roman, A.; Salazar, A. Comparing the first and second waves of COVID-19 in a tertiary university hospital in Barcelona. F1000Res 2021, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.N.; Tuite, A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: A retrospective cohort study in Ontario, Canada. CMAJ 2021, 193, E1619–E1625. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Vinay, T.G.; Kumar, K.A.; Wahl, B.; Lewnard, J.A. SARS-CoV-2 infection and mortality during the first epidemic wave in Madurai, south India: A prospective, active surveillance study. Lancet Infect. Dis. 2021, 21, 1665–1676. [Google Scholar] [CrossRef]
- Shil, P.; Atre, N.M.; Tandale, B.V. Epidemiological findings for the first and second waves of COVID-19 pandemic in Maharashtra, India. Spat. Spatiotemporal Epidemiol. 2022, 41, 100507. [Google Scholar] [CrossRef]
- Agarwala, P.; Bhargava, A.; Gahwai, D.K.; Negi, S.S.; Shukla, P.; Dayama, S. Epidemiological Characteristics of the COVID-19 Pandemic During the First and Second Waves in Chhattisgarh, Central India: A Comparative Analysis. Cureus 2022, 14, e24131. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Wahl, B.; Dudala, S.R.; Gopal, K.; Mohan, B.C.; Neelima, S.; Jawahar Reddy, K.S.; Radhakrishnan, J.; Lewnard, J.A. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science 2020, 370, 691–697. [Google Scholar] [CrossRef]
- Porcheddu, R.; Serra, C.; Kelvin, D.; Kelvin, N.; Rubino, S. Similarity in Case Fatality Rates (CFR) of COVID-19/SARS-CoV-2 in Italy and China. J. Infect. Dev. Ctries. 2020, 14, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Akande, O.W.; Elimian, K.O.; Igumbor, E.; Dunkwu, L.; Kaduru, C.; Olopha, O.O.; Ohanu, D.O.; Nwozor, L.; Agogo, E.; Aruna, O.; et al. Epidemiological comparison of the first and second waves of the COVID-19 pandemic in Nigeria, February 2020-April 2021. BMJ Glob. Health 2021, 6, e007076. [Google Scholar] [CrossRef]
- Otshudiema, J.O.; Folefack, G.L.T.; Nsio, J.M.; Mbala-Kingebeni, P.; Kakema, C.H.; Kosianza, J.B.; Mfumu, A.K.; Saidi, G.N.; Kabongo, P.M.; Okum, R.; et al. Epidemiological Comparison of Four COVID-19 Waves in the Democratic Republic of the Congo, March 2020-January 2022. J. Epidemiol. Glob. Health 2022, 12, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Salyer, S.J.; Maeda, J.; Sembuche, S.; Kebede, Y.; Tshangela, A.; Moussif, M.; Ihekweazu, C.; Mayet, N.; Abate, E.; Ouma, A.O.; et al. The first and second waves of the COVID-19 pandemic in Africa: A cross-sectional study. Lancet 2021, 397, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zheng, C.; Shi, Q.; Xiao, K.; Wang, L.; Liu, Z.; Li, Z.; Dong, X. Evolving trend change during the COVID-19 pandemic. Front. Public Health 2022, 10, 957265. [Google Scholar] [CrossRef] [PubMed]
| Year | 2020 | 2021 | 2022 | p-Value |
|---|---|---|---|---|
| Overall Positivity | 13,035 (13.29) | 42,909 (13.66) | 19,936 (15.91) | <0.001 |
| District | <0.001 | |||
| Bhandara | 1807 (13.86) | 73 (0.17) | 23 (0.11) | |
| Chandrapur | 49 (0.38) | 27 (0.07) | 15 (0.07) | |
| Gadchiroli | 304 (2.33) | 11 (0.03) | 4 (0.02) | |
| Gondia | 55 (0.42) | 22 (0.05) | 11 (0.06) | |
| Nagpur | 10,402 (79.80) | 42,275 (98.52) | 19,791 (99.28) | |
| Wardha | 22 (0.16) | 22 (0.05) | 8 (0.04) | |
| Other | 396 (3.04) | 479 (1.11) | 84 (0.42) | |
| Gender | <0.001 | |||
| Male | 7935 (60.88) | 24,811 (57.82) | 11,640 (58.39) | |
| Female | 5099 (39.11) | 18,096 (42.17) | 8294 (41.60) | |
| Transgender | 1 (0.01) | 2 (0.01) | 2 (0.01) | |
| Age Group (years) | <0.001 | |||
| 1–15 | 918 (7.04) | 3235 (7.54) | 1707 (8.56) | |
| 16–30 | 3476 (26.66) | 12,121 (28.25) | 6735 (33.80) | |
| 31–45 | 3876 (29.74) | 14,275 (33.27) | 6192 (31.05) | |
| 45+ | 4765 (36.56) | 13,278 (30.94) | 5302 (26.59) | |
| Vaccination | <0.001 | |||
| Yes | 0 | 10,381 (3.30) | 10,105 (8.06) | |
| No | 0 | 303,537 (96.69) | 115,237 (91.93) | |
| Hospitalization | <0.001 | |||
| Yes | 5203 (5.30) | 2903 (0.92) | 266 (0.21) | |
| No | 92,857 (94.70) | 311,015 (99.08) | 125,076 (99.79) | |
| Clinical Status | <0.001 | |||
| Symptomatic | 9264 (9.44) | 7651 (2.43) | 6230 (4.98) | |
| Asymptomatic | 88,796 (90.56) | 306,267 (97.57) | 119,112 (95.02) |
| Parameter | 2020 | 2021 | 2022 |
|---|---|---|---|
| Cumulative confirmed cases | 8607 | 6882 | 672 |
| Deaths | 1562 | 2525 | 103 |
| Case fatality ratio (CFR) | 18.14% | 36.68% | 15.32% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshmukh, P.; Bhise, S.; Kokate, S.; Mategadikar, P.; Rahangdale, H.; Rahangdale, V.; Shrikhande, S.; Pathan, S.; Damodare, A.; Baghele, S.; et al. SARS-CoV-2 Infection Epidemiology Changes During Three Years of Pandemic in a Region in Central India. COVID 2025, 5, 125. https://doi.org/10.3390/covid5080125
Deshmukh P, Bhise S, Kokate S, Mategadikar P, Rahangdale H, Rahangdale V, Shrikhande S, Pathan S, Damodare A, Baghele S, et al. SARS-CoV-2 Infection Epidemiology Changes During Three Years of Pandemic in a Region in Central India. COVID. 2025; 5(8):125. https://doi.org/10.3390/covid5080125
Chicago/Turabian StyleDeshmukh, Pravin, Swati Bhise, Sandeep Kokate, Priyanka Mategadikar, Hina Rahangdale, Vaishali Rahangdale, Sunanda Shrikhande, Sana Pathan, Anuradha Damodare, Sachin Baghele, and et al. 2025. "SARS-CoV-2 Infection Epidemiology Changes During Three Years of Pandemic in a Region in Central India" COVID 5, no. 8: 125. https://doi.org/10.3390/covid5080125
APA StyleDeshmukh, P., Bhise, S., Kokate, S., Mategadikar, P., Rahangdale, H., Rahangdale, V., Shrikhande, S., Pathan, S., Damodare, A., Baghele, S., Gajbhiye, J., & Shahu, P. (2025). SARS-CoV-2 Infection Epidemiology Changes During Three Years of Pandemic in a Region in Central India. COVID, 5(8), 125. https://doi.org/10.3390/covid5080125







