1. Introduction
Lactoferrin (Lf) is a component of whey protein found in most mammals. Human cells can produce lactoferrin, which is present wherever the body requires rapid and effective protection against external threats. Chemically, it is a glycoprotein that, due to its sequence homology with serum transferrin, is a member of the transferrin family, a protein group capable of binding to iron ions. As the name suggests (lacto + ferrin = milk + iron), it is a milk protein that binds to iron and helps balance its levels in the body [
1]. Lactoferrin (Lf) combines its iron-binding capacity with direct action on microbial membranes, conferring simultaneous bacteriostatic, bactericidal, and immunomodulatory functions. Moreover, it exhibits broad antiviral activity by preventing viral entry into host cells through binding to viral proteins or cellular receptors, while also enhancing the efficacy of antiviral drugs and modulating intracellular responses. Lf further demonstrates dose-dependent antifungal and antiparasitic effects, acting synergistically with conventional treatments to reduce infections caused by Candida and other pathogens, positioning it as a promising alternative against antifungal resistance [
2].
Beyond its antimicrobial activity, Lf displays multifunctional properties. It disrupts bacterial membranes, inhibits biofilm formation, and releases potent antimicrobial peptides. Additionally, Lf modulates immune responses by regulating cytokines, activating natural killer cells and macrophages, and supporting both innate and adaptive immunity. Notably, it also exhibits anticancer properties [
3].
Lf has shown potential benefits in metabolic and bone health. In osteoporosis models, it stimulates bone formation through synergistic interactions with hydroxyapatite and promotion of osteoblast activity, while enhancing vascularization in bone tissue, particularly among postmenopausal women. Concurrently, Lf regulates iron metabolism by increasing hemoglobin and erythrocyte levels, preventing nutritional deficiencies, and reducing lipid accumulation in the liver and serum [
4].
Its anticancer effects are mediated via multiple mechanisms, including cell cycle arrest at the G1/S checkpoint, induction of mitochondrial-dependent apoptosis, and suppression of metastatic progression. Furthermore, Lf can cross the blood–brain barrier, suggesting potential applications in neuromodulation and central nervous system-targeted therapies [
3].
Lf also binds and interacts with various metal ions, such as zinc, copper, manganese, and silver, enhancing its antioxidant and antimicrobial properties. Its ability to bind and transport iron supports host defense and regulates oxidative stress. Additionally, Lf contributes to gut health, bone growth, and wound healing, underscoring its versatility and therapeutic potential across multiple physiological processes [
4].
Human lactoferrin (hLf) shares 77% amino acid sequence similarity with its bovine counterpart (bLf). However, bovine lactoferrin is more widely studied due to its easier extraction. Between 25 and 75 mg of this protein is found in a glass of cow’s milk. However, bLf is not an ideal choice due to differences that may alter its antiviral and antimicrobial potential when used in human therapy, although some authors highlight its higher antimicrobial activity [
1].
Lactoferrin has been used since wartime in Europe, where knights used colostrum serum to clean wounds and prevent infections from worsening. It was first discovered in breast milk by scientist Johanson Bengt in 1960. Subsequently, the protein was found in the bodily fluids and various cells of some organisms [
5].
Rümke et al. [
6] discovered in 1971 that cancer patients exhibited elevated lactoferrin in serum, which was later confirmed in several types of cancer, making it a sign of this condition. In 1994, Mitra reported the first expression of hLf in plant cells, achieving its production in various plants such as tobacco, potato, rice, tomato, and carrot [
7]. In 2002, Van Berkel et al. developed transgenic cows that produced recombinant human lactoferrin (0.4–3.0 g/L), demonstrating that their biological activity was similar to that of natural protein [
8].
The appearance of the first cases of COVID-19 and the subsequent global pandemic have posed a great challenge for health systems around the world, as they were not prepared to provide this level of care. According to data from the Johns Hopkins University Coronavirus Research Center, as of 3 November 2023, 676,609,955 cases of COVID-19 and 6,881,955 deaths, respectively, had been reported worldwide [
9]. For this reason, the development of treatments and vaccines has been the result of the solid effort of nations and governments concerned with putting an end to the event that claimed the lives of millions of people around the world.
Although the fatality of the disease has decreased thanks to the application of vaccines, it is important to continue with research that will guide the development of new treatments in the future to combat similar situations or mutations of the virus. Lactoferrin (Lf), a protein found in various mucosal secretions and in neutrophil granules, has properties that classify it as a potential candidate for the treatment of COVID-19. In addition to its anti-inflammatory, antioxidant, and immunomodulatory activities, this protein has outstanding broad-spectrum antiviral capacity effective against several types of viruses, including SARS-CoV-2. Particularly, bovine lactoferrin (bLf) in liposomalized format improves its bioavailability, inhibiting the replication of SARS-CoV-2 and other coronaviruses. Lactoferrin is recognized as a nutritional additive by the United States Food and Drug Administration (FDA), which further affirms its importance for these types of studies [
10].
Previous studies have been published showing the relationship between the use of lactoferrin and its potential benefits in the treatment of COVID-19 patients. In a study published in 2021 [
11], a meta-analysis using PRISMA was conducted to evaluate the relationship between lactoferrin administration and the reduction in respiratory infection risk, reviewing data from nine randomized controlled trials, of which six were assessed. It was shown that using lactoferrin significantly reduced the likelihood of developing respiratory infections compared to the control, suggesting its fortification in infant formulas, as well as its potential benefits in managing chronic respiratory infections and in the complementary treatment of COVID-19.
Another meta-analysis conducted by the University of Newcastle, Newcastle, Australia, also using PRISMA, reviewed evidence on the effects of lactoferrin supplementation on inflammation, immune function, and respiratory infections in humans. A total of 25 studies (20 in adults) were included, and it was concluded that a daily dose of 200 mg of lactoferrin was effective in reducing systemic inflammation, while formulas with 35–833 mg/day showed potential to reduce the incidence of respiratory tract infections in children and infants [
12]. Other studies have demonstrated the safety and tolerability of bovine lactoferrin in hospitalized patients with moderate to severe COVID-19 [
13], which is a positive indicator for future studies on this topic.
The research aim of the present article is to carry out an exhaustive historical analysis of the literature available in three academic search engines: (i) Google Scholar, (ii) Elsevier, and (iii) PubMed, on the potential use of lactoferrin as a treatment for COVID-19. By rigorously searching and filtering through the articles obtained, information has been obtained on the different properties of lactoferrin in vivo models. With this, the effectiveness of lactoferrin has been evaluated as a viable treatment option applied to humans to contribute to the current effort to develop new drugs and address possible health crises in the future.
To further contribute to the existing body of research, the present study conducted a meta-analysis using the PRISMA methodology. From the database generated, specific articles were selected where the response variables would determine the inclusion criteria. In this case, the selected response variables focused on the effect of lactoferrin on a range of symptoms. This approach aims to systematically evaluate and synthesize evidence regarding the potential benefits of lactoferrin in the treatment of COVID-19. By leveraging rigorous data collection and analysis, this work seeks to provide a comprehensive perspective on the therapeutic possibilities of lactoferrin, contributing to the future understanding of its role in managing respiratory infections and its potential application in health emergencies.
2. Methodology
Initially, a separate database was created for each academic search engine: PubMed, Elsevier, and Google Scholar. A comprehensive multi-database strategy was then employed to minimize selection bias and ensure the robustness of our review. PubMed, as a specialized database in health sciences and biomedicine, primarily indexes articles from MEDLINE and selected journals. Its use of a controlled MeSH system with standardized terms provides highly precise but relatively limited results. Despite its restricted biomedical scope, PubMed offers robust filters by language, article type, and availability, making it particularly useful for focused medical research.
Elsevier, by contrast, is a portal that retrieves articles from its own journal collection across medicine, science, engineering, and social sciences. Searches are based on keywords in titles, abstracts, and author-defined terms, yielding an intermediate number of results. Although full-text access often requires a subscription, the platform provides advanced filtering options. Google Scholar, on the other hand, is a general academic search engine with the broadest coverage, indexing preprints, theses, books, conference proceedings, articles, and PDFs. Its indexing is less controlled, and results may include duplicates or non-peer-reviewed documents; however, its extensive scope captures literature not available in PubMed or Elsevier.
The search was conducted using the following keywords: COVID-19 and lactoferrin, COVID-19 and lactoferrin, SARS-COV-2 and lactoferrin, and SARS-COV-2 and lactoferrin, restricted to articles published between 2020 and the first quarter of 2025. Retrieved studies were classified according to their purpose (research or review) and study type (in vivo, in vitro, both, or none). As expected, some articles appeared as duplicates across searches; these were identified and eliminated to generate clean datasets. Finally, the three databases were merged, and an additional filtering process was applied to remove duplicate articles across platforms, yielding a final unified database with an accurate list of included studies.
Finally, a filter was applied to the general database to discard all review-type articles and obtain only research articles. Of the latter, a new database was created containing the in vivo study articles, in which they were classified according to certain specific criteria to analyze the studies: purpose, study unit, number of patients, age/sex, comorbid conditions, study factor assignment, control group, study type assignment factor, applied methods, and measured variables.
Once this filter had been applied, the characteristics defined in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) methodology were followed to provide a minimum group of evidence-based elements for reporting in systematic and meta-analytic reviews to focus methodological clarity, precision in the choice and evaluation of research, and ensure the reproducibility of the findings with the results of the report.
From the latest database, the PRISMA checklist is examined, which consists of 27 essential elements to ensure the transparency and quality of the report. This checklist covers key aspects such as search design, study selection, risk of bias assessment, and synthesis of results. In addition, PRISMA recommends the inclusion of a flowchart to describe the identification, selection, and exclusion process concerning the total number of research articles retrieved, duplicates discarded, and studies excluded.
2.1. Methodology Meta-Analysis
To evaluate the effects of lactoferrin treatment on fatigue, diarrhea, headache, and anosmia/ageusia symptoms in SARS-CoV-2 patients, a meta-analysis was conducted using a mixed-effects model with random effects. This approach accounted for correlated data due to repeated measurements within the same articles, capturing variations in treatment response over time. The analysis included studies that met the following criteria:
The presence of a control group, defined as patients who did not receive lactoferrin, received only standard treatment, were assessed before receiving the treatment, or were compared with a treatment with a longer exposure period to the treatment.
The presence of a lactoferrin-treated group as an experimental group.
The included studies compared concurrent control and experimental groups, as well as changes over time within the same study. For instance, if a study measured the same group before and after lactoferrin treatment, or if a treated group was assessed at different time points, these comparisons were included under the assumption that a longer exposure to lactoferrin could yield observable effects. Additionally, in cases where a control group was compared to two different lactoferrin doses, the same control group was used for both comparisons.
The selected studies exhibited heterogeneity in several aspects, including (
Table 1): (i) Administered dose and type of lactoferrin, (ii) Presence of other active compounds combined with lactoferrin, and (iii) Follow-up time to measure the treatment response.
Log (Odds Ratios) and their standard errors were calculated as measures of effect to quantify the impact of lactoferrin on reducing the symptoms. Only studies reporting symptoms as present or absent were included, and this criterion was applied consistently to all symptoms evaluated in the meta-analysis. For fatigue, the meta-analysis included 15 observations from four articles, with effect sizes derived from different time points within the same studies. Similarly, for diarrhea symptoms, 20 observations from five articles were analyzed. For headache and anosmia/ageusia symptoms, the analysis included 24 and 29 observations, respectively, from five articles. Since some studies did not distinguish between anosmia and ageusia symptoms, these symptoms were pooled together. For muscular pain, 12 observations from four articles were included; studies that reported either muscular pain, weakness, or rhabdomyolysis were considered.
In time points where the proportions remained unchanged for both the lactoferrin treatment and the control groups, subsequent observations were excluded from the meta-analysis to avoid overcounting the effect when a stable response appeared to have been reached. Similarly, time points with only one reported case of a symptom, either in the treatment or control group, were not included, as such sparse data can lead to unreliable estimates and compromise the validity of the meta-analytic results.
To account for the potential correlation among observations within the same article, a multivariate meta-analysis model with random effects by article was employed. The model was fitted using restricted maximum likelihood (REML), as this method provides unbiased estimates of variance components in random-effects models, particularly when the number of studies is limited. Additionally, REML adjusts for the loss of degrees of freedom associated with fixed-effects estimation, making it more precise in heterogeneity estimation. A heterogeneity test was subsequently conducted to assess whether the observed variation in effect sizes could be attributed solely to chance.
2.2. Meta-Regression
Subgroup analyses or meta-regression were conducted only for symptoms showing a significant overall effect in the primary meta-analysis. Since the absence of a detectable effect suggests that variability is likely due to random error rather than systematic differences, exploring moderators for non-significant outcomes would not be informative.
Fatigue was the only symptom with a significant overall effect. Subgroup analyses based on lactoferrin type or dosage were considered; however, these variables were strongly confounded with individual studies. Each trial used a different formulation (liposomal bovine lactoferrin, non-liposomal bovine lactoferrin, or a bovine colostrum/egg combination), and dosages were inherently linked to the product type. Consequently, subgroup or meta-regression analyses for these factors would primarily reflect between-study differences rather than true moderator effects. In contrast, intervention duration varied both within and across studies, making it a suitable moderator. Accordingly, a meta-regression was performed to assess the effect of treatment length on fatigue.
3. Results
The results obtained from the individual database by the search engine are shown in
Figure 1,
Figure 2,
Figure 3 and
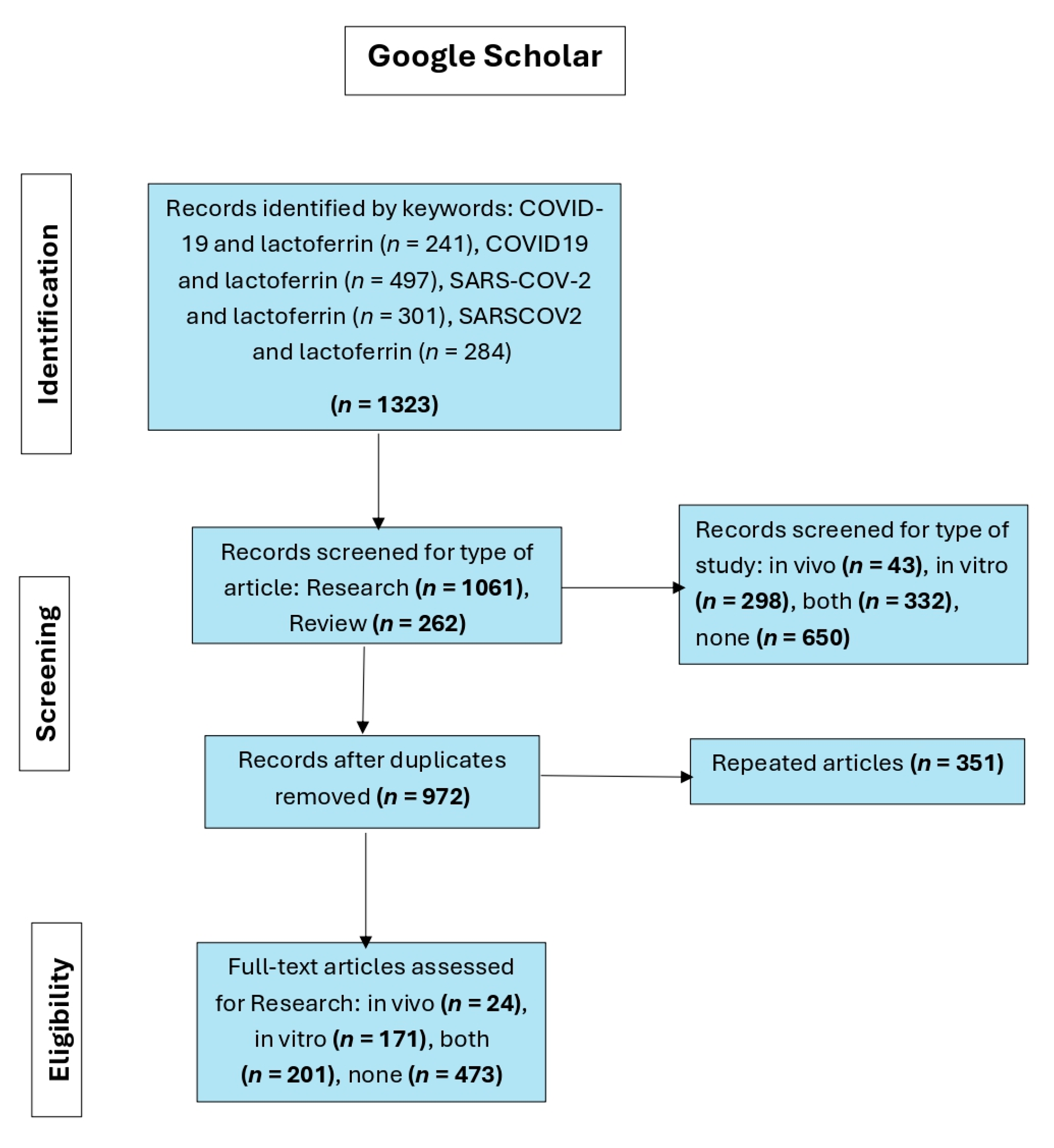
Figure 4. The first flowchart (
Figure 1) represents the process of selecting articles in a systematic review of the relationship between lactoferrin and COVID-19 based on searches in Google Scholar.
Initially, 1323 records were identified using the keywords: COVID-19 and lactoferrin, COVID19 and lactoferrin, SARS-COV-2 and lactoferrin, and SARSCOV2 and lactoferrin. From these records, 1061 were research studies and 262 were reviews. Then, they were filtered according to the type of study, resulting in 650 unrelated articles and 673 distributed among in vivo, in vitro, and combined studies. Articles were considered irrelevant if, despite being retrieved by the search engine, they did not provide information on or demonstrate a relationship between COVID-19 and lactoferrin. Studies lacking in vivo or in vitro data were also deemed irrelevant. Subsequently, 351 duplicate articles were removed, leaving 972 unique records. After a full-text review, 24 in vivo studies, 171 in vitro studies, and 201 mixed studies were selected, while 473 articles were not considered for not meeting the eligibility criteria.
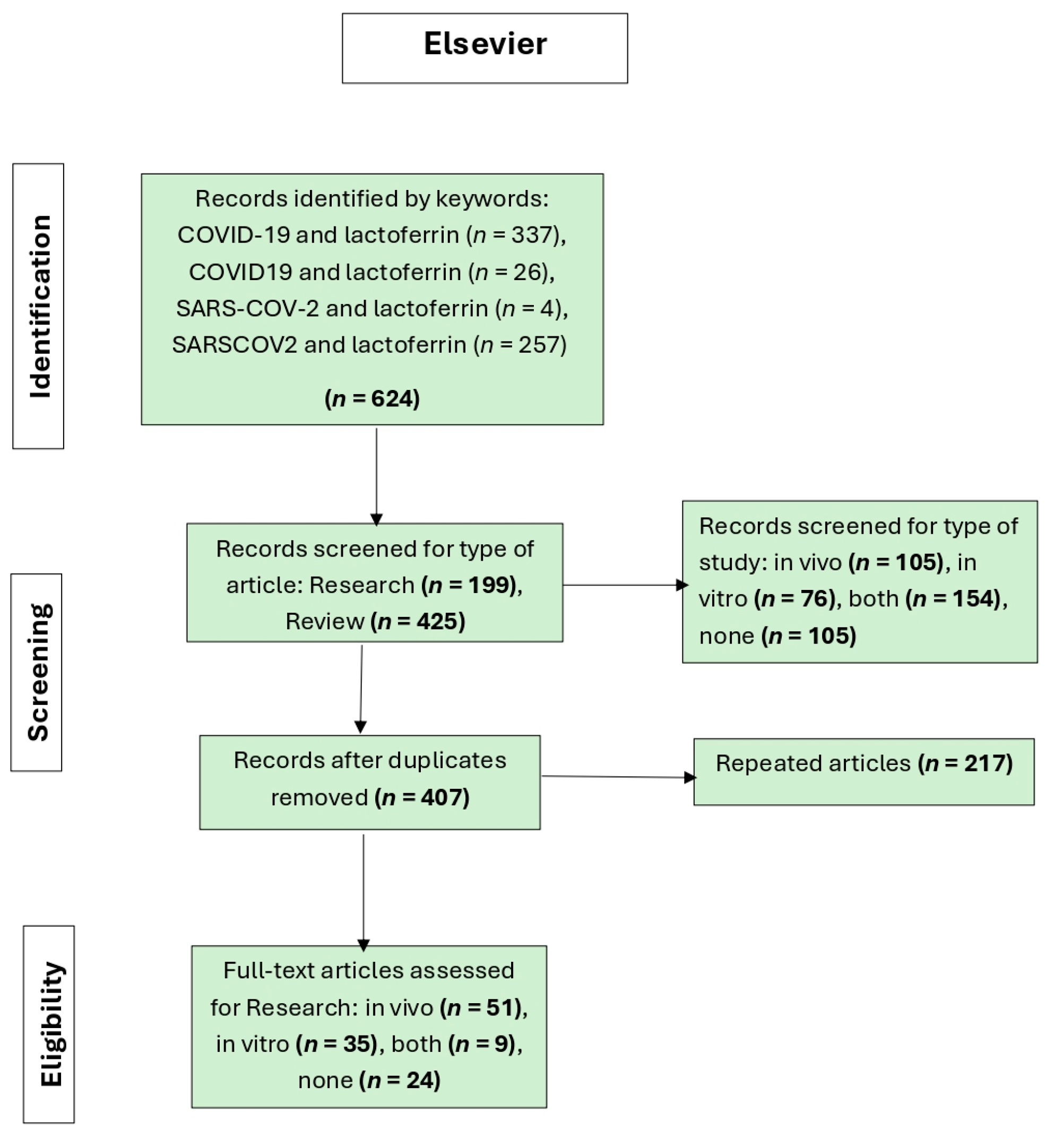
The second flowchart (
Figure 2) shows the article selection process in a systematic review on lactoferrin and COVID-19 using the Elsevier database. A total of 624 records were identified through the keywords: COVID-19 and lactoferrin, COVID19 and lactoferrin, SARS-COV-2 and lactoferrin, and SARSCOV2 and lactoferrin. From these records, 199 were research studies and 425 were reviews. The articles were then classified according to the type of study, resulting in 105 in vivo, 76 in vitro, 154 combined, and 105 irrelevant studies. Articles were considered irrelevant if, despite being retrieved by the search engine, they did not provide information on or demonstrate a relationship between COVID-19 and lactoferrin. Studies lacking in vivo or in vitro data were also deemed irrelevant. After removing 217 duplicate articles, 407 unique records remained. In the full-text evaluation phase, 51 in vivo studies, 35 in vitro studies, and 9 mixed studies were included, while 24 articles were not considered for not meeting the eligibility criteria.
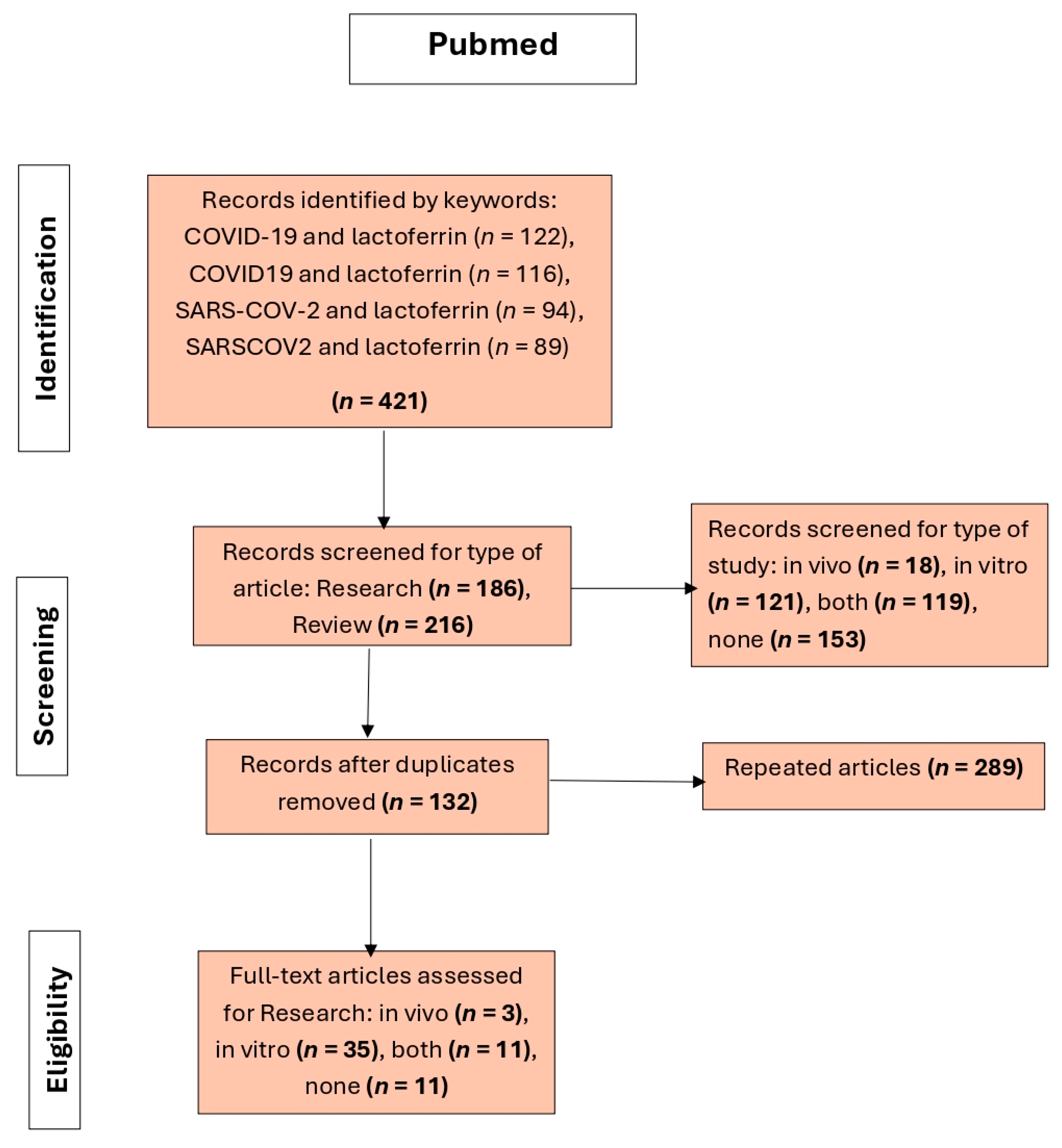
The third flowchart (
Figure 3) shows the article selection process in a systematic review on lactoferrin and COVID-19 using the PubMed database. A total of 421 records were identified through the keywords: COVID-19 and lactoferrin, COVID19 and lactoferrin, SARS-COV-2 and lactoferrin, and SARSCOV2 and lactoferrin. From these records, 186 were research studies and 216 were reviews. The articles were then filtered according to the type of study, resulting in 18 in vivo studies, 121 in vitro studies, 119 combined studies, and 153 irrelevant articles. Articles were considered irrelevant if, despite being retrieved by the search engine, they did not provide information on or demonstrate a relationship between COVID-19 and lactoferrin. Studies lacking in vivo or in vitro data were also deemed irrelevant. After removing 289 duplicate articles, 132 unique records remained. In the full-text evaluation phase, 3 in vivo studies, 35 in vitro studies, and 11 mixed studies were included, while 11 articles were not considered for not meeting the eligibility criteria.
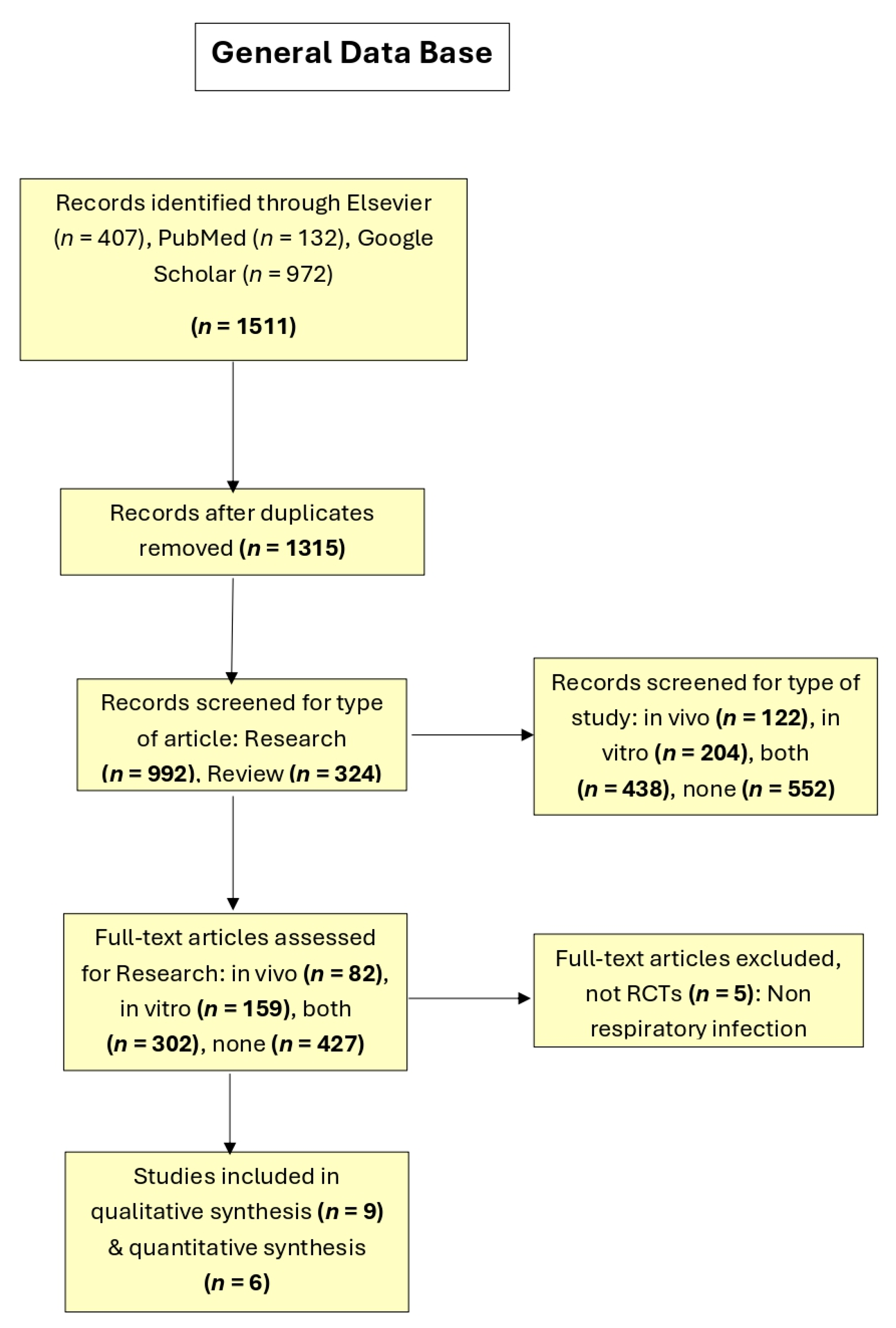
Additionally, the results of the general database are presented in
Figure 4, which includes the filtered bases of the three search engines (i.e., Google Scholar, Elsevier, and PubMed), including the results of the applied filters: total articles excluding duplicates and subsequently the classification of research-type articles according to their type of study (in vivo, in vitro, both, or neither). Finally, with 82 research and in vivo study articles, a database was created, presented below.
3.1. Description of Studies Included in the Meta-Analysis
Nine publications evaluated the effect of lactoferrin in SARS-CoV-2 patients and included a control group—either receiving a placebo or standard care—or assessed patients before and after treatment. For the meta-analysis, only seven of these studies were included (
Table 1). One study was excluded because symptoms were reported solely as scores, while another was excluded because it did not provide the number of patients with or without specific symptoms, nor the types of symptoms assessed, making it impossible to extract data on symptom presence or absence for calculating log (Odds Ratios).
Additionally, in some cases, symptoms were reported only at baseline (although data were provided by the authors and included in the meta-analysis) or were not the primary objective of the study.
These articles reported 31 different symptoms, with an average of 9.5 ± 1.96 symptoms per study (mean ± SE). Among them, five symptoms were the most frequently reported: fatigue (5 studies), diarrhea (6 studies), headache (5 studies), and anosmia/ageusia disturbances (5/4 studies). All these symptoms were considered for the meta-analysis, but not all studies were included due to the inability to obtain log (Odds Ratios) (see above): fatigue (4 studies), diarrhea (5 studies), headache (5 studies), anosmia/ageusia disturbances (5 studies), and muscle pain (4 studies). The type of lactoferrin, dose administered to patients, and study duration varied among the studies included in the meta-analysis (
Table 1).
Of the seven studies included, four were randomized controlled trials (RCTs) (57.1%), and three were non-randomized studies (42.9%). All studies were published in recent years and conducted primarily in adult populations (≥18 years old), with the female proportion ranging from 27.8% to 55.6% across groups or studies; one study included pediatric patients (<18 years). All trials focused on COVID-19 patients, with disease severity ranging from asymptomatic to moderate or severe symptoms. SARS-CoV-2 infection was confirmed using various diagnostic methods, including rapid antigen tests, IgM/IgG rapid tests, and reverse transcriptase polymerase chain reaction (RT-PCR) from nasopharyngeal swabs.
The sample sizes across the studies ranged from 54 to 218 participants, with a total of 793 subjects included in this meta-analysis. Most studies assessed objective symptoms (n = 6, 85.7%). The intervention duration ranged from 5 to 30 days, with follow-up periods extending up to 18 months in one pediatric cohort. Lactoferrin (bLf) was administered in varying dosages and formulations, including oral capsules, sachets, liposomal syrups, and intranasal sprays. Daily doses ranged from 100 mg to 1 g in adults and from 600 to 800 mg/day in children, depending on age. Some studies combined bLf with other ingredients such as vitamin C, zinc, or conventional modern treatments.
Among the included trials, three were double-blinded RCTs, while the others were either non-randomized or retrospective analyses. Two studies employed a placebo-controlled design, whereas others compared lactoferrin with standard of care, a combination of modern medications, or pre-post treatment evaluations. Most patients had mild to moderate COVID-19, and some studies excluded critically ill patients. Oxygen saturation levels were typically above 93%, or other clinical stability criteria were applied.
The effects of bLf supplementation on COVID-19 symptoms and recovery were evaluated at various time points, including days 5, 7, 14, 21, and 32. One study extended follow-up to 18 months to assess long-term gastrointestinal symptom evolution in children.
Regarding adverse reactions, three studies did not assess this aspect (Serrano et al., 2020; Agahtani et al., 2021; Mariani et al., 2024) [
15,
16,
19], although one of them noted that bLf has an excellent safety and tolerability profile. Bhandari et al. (2023) [
18] reported no adverse reactions, but the method of follow-up was not specified. In contrast, Campione et al. (2021) and Mann et al. (2023) documented adverse reactions to liposomal bLf and a mixture of hen egg white and bovine colostrum, respectively [
14,
17]. Campione et al. (2021) reported two cases of gastrointestinal reactions that resolved spontaneously, while Mann et al. (2023) observed nine mild-to-moderate gastrointestinal reactions (six in the placebo group and three in the experimental group) [
17].
It is important to note that in one study (Matino et al., 2023) [
13], the primary objective was not to assess lactoferrin’s effect on the reduction in COVID-19-associated symptoms. Nevertheless, adverse reactions were reported in both control and experimental groups, and this information was included in the meta-analysis. Overall, 106 adverse reactions were documented—57 in the lactoferrin group and 49 in the placebo group—and were monitored daily during hospitalization and at discharge.
One of the studies (Mann et al., 2023) [
17] reported a significant reduction in the presence of some symptoms (e.g., fever, joint pain/malaise, chest pain, and sore throat) in the lactoferrin-treated group. Three other studies (Campione et al., 2021, Serrano et al., 2020, and Bhandhari et al., 2023) [
14,
15,
18] also reported a reduction in symptom presence; however, no statistical test was performed. Two studies (Algahtani et al., 2021, Mariani et al., 2024) [
16,
19] reported no significant difference in symptom presence compared to the control. One study that measured symptoms as a variable to control for possible adverse reactions to lactoferrin did not report an overall effect; however, they conducted a χ
2 test, which showed a significantly lower number of diarrhea cases in the lactoferrin-treated group [
13]. Regarding the two studies excluded from the meta-analysis, Rosa et al. (2021) reported that the time to symptom resolution in COVID-19 patients did not differ between the bLf treatment group and the control group. Notably, they observed a significant correlation between age and bLf effectiveness in reducing symptom duration, particularly in patients aged 40 years and older [
20]. Meanwhile, Redel et al. (2021) conducted a randomized, double-blind, placebo-controlled trial to assess the effect of lactoferrin on fatigue, anxiety, depression, cognitive failure, and muscle strength. They found that lactoferrin did not significantly reduce these symptoms [
21].
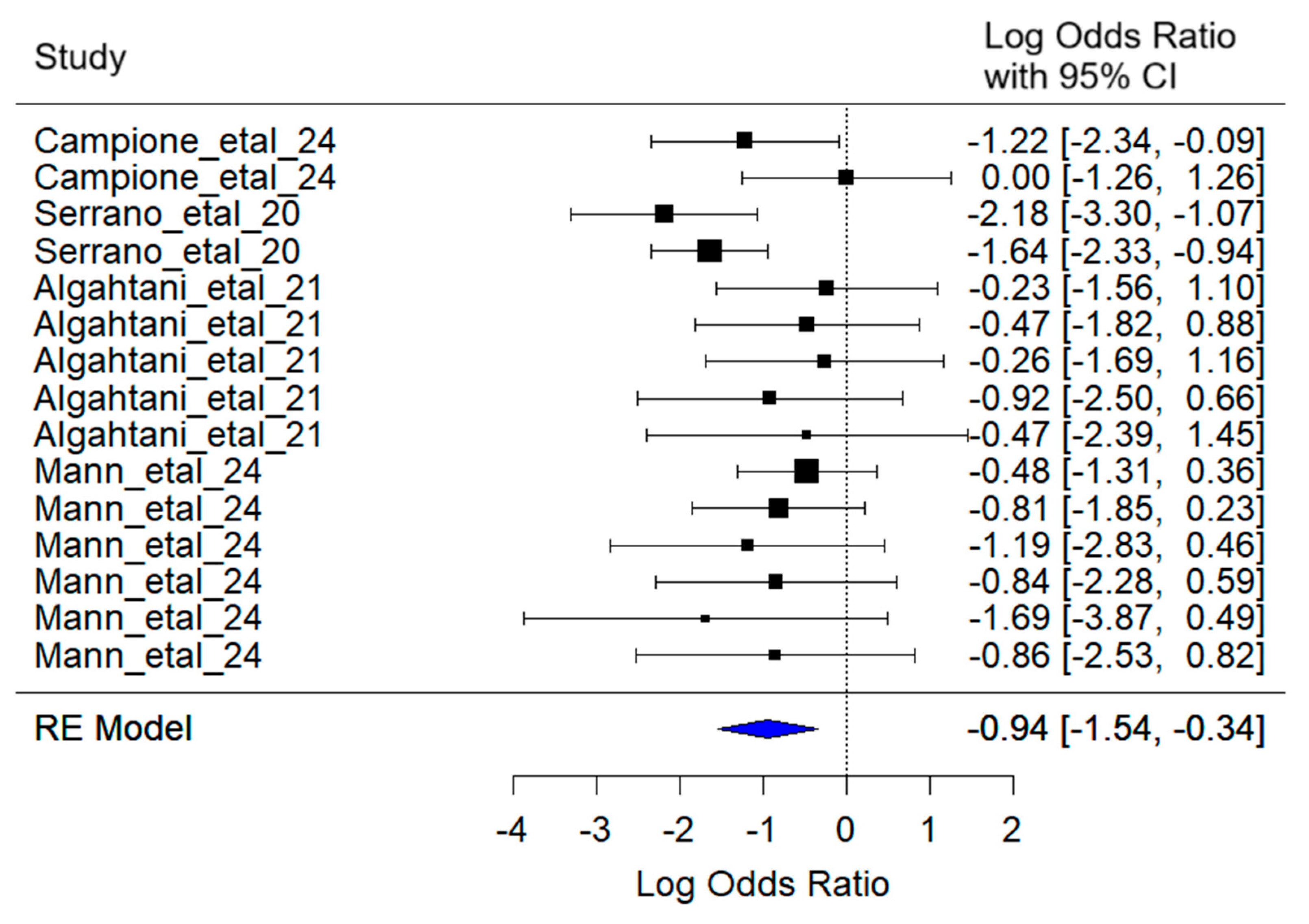
3.2. Fatigue
The mixed-effects meta-analysis yielded the following key results (
Figure 5): Considering random effects by article, a between-article variance of σ
2 = 0.27 was estimated, with an associated standard deviation of 0.52. This indicates moderate variability in effect sizes attributable to specific differences among the studies included.
Regarding heterogeneity, the heterogeneity test [Q(df = 16) = 15.46, p = 0.34] did not detect statistically significant evidence of excessive heterogeneity among studies. This suggests that the observed variation in effect sizes can be reasonably explained by the model.
The combined effect estimate for the log (Odds Ratio) was −0.94 (95% CI: −1.54 to −0.34), indicating that lactoferrin administration significantly reduces the probability of fatigue in SARS-CoV-2 patients compared to controls (z = −3.05, p = 0.002). Patients treated with lactoferrin had approximately 61% lower odds of experiencing fatigue compared to those in the control group.
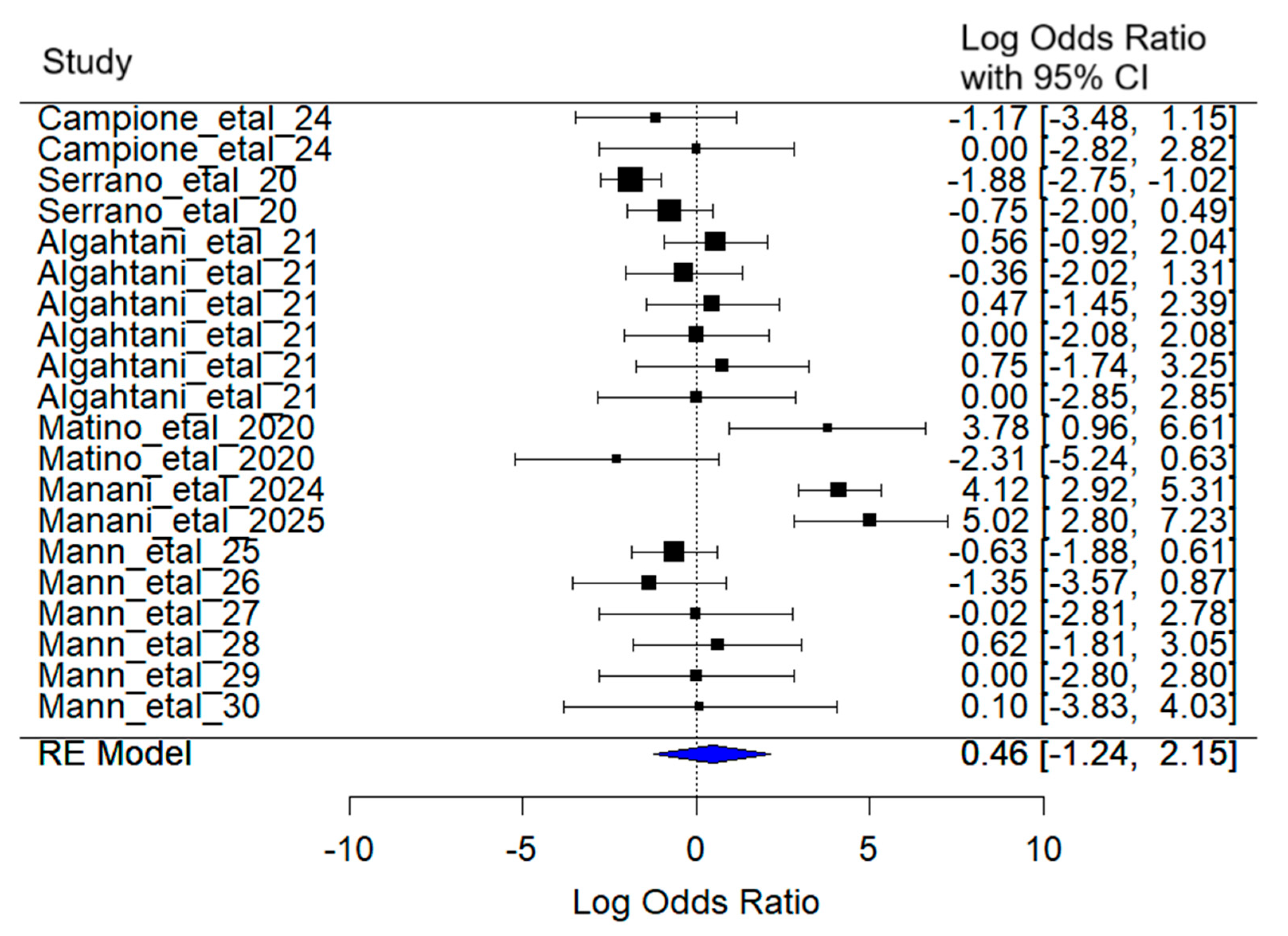
3.3. Diarrhea
The mixed-effects meta-analysis yielded the following results (
Figure 6): Considering random effects by article, the between-article variance was estimated at σ
2 = 4.07, with an associated standard deviation of 2.02. This reflects substantial variability in effect sizes attributable to differences among the included studies.
Regarding heterogeneity, the test for heterogeneity [Q(df = 19) = 98.92, p < 0.001] reveals statistically significant evidence of substantial heterogeneity among studies, supporting the use of a random-effects model.
The combined effect estimate for the log (Odds Ratio) was 0.45 (95% CI: −1.24 to 2.15). Although a increase in the probability of diarrhea symptoms was observed in SARS-CoV-2 patients treated with lactoferrin compared to the control group, this effect was not statistically significant (z = −0.53, p = 0.60), and the confidence interval includes zero, indicating uncertainty about the direction and magnitude of the effect.
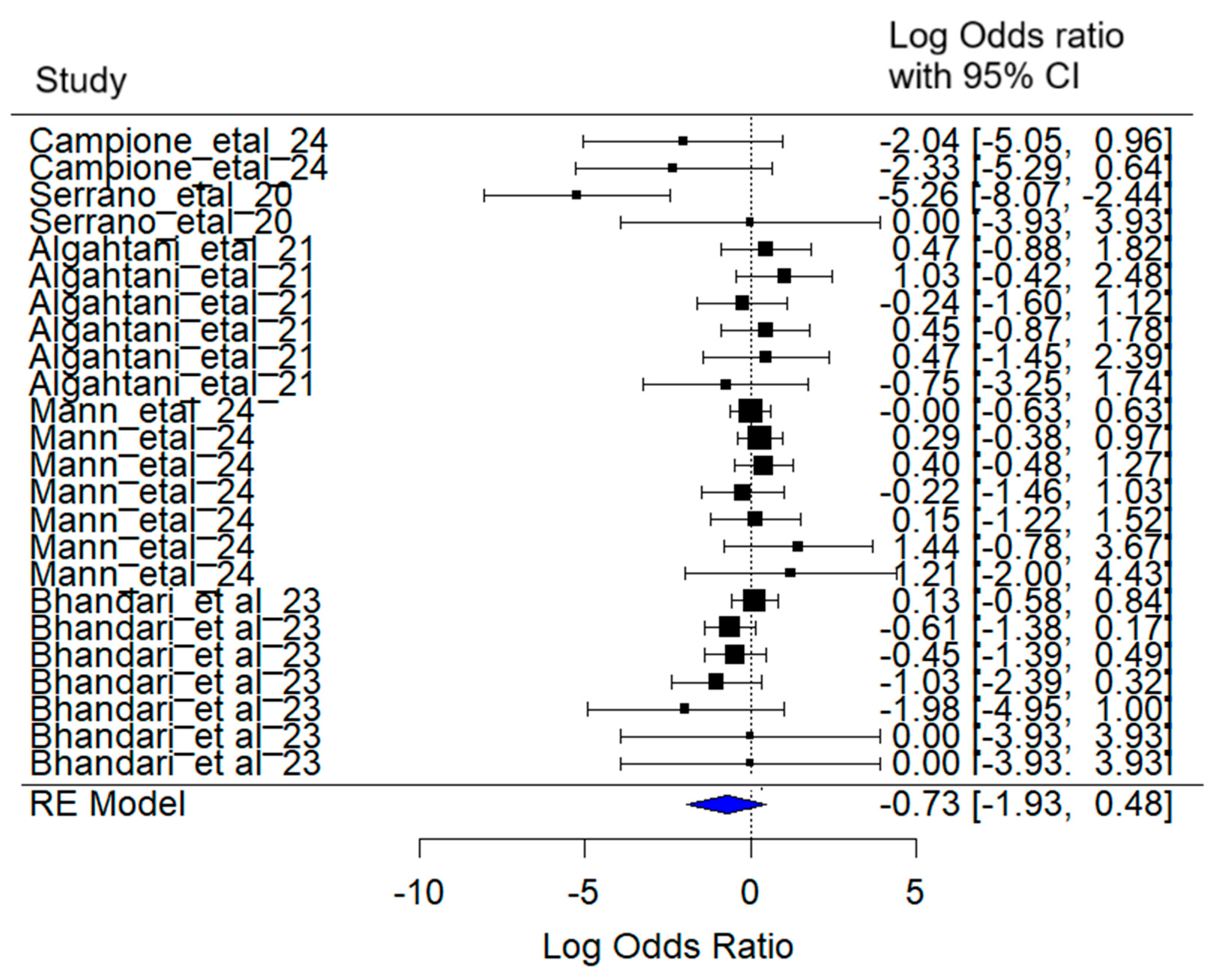
3.4. Headache
The mixed-effects meta-analysis yielded the following results (
Figure 7): Considering random effects by article, a between-article variance of σ
2 = 1.50 was estimated, with an associated standard deviation of 1.23. This indicates moderate variability in effect sizes attributable to specific differences among the included studies.
Regarding heterogeneity, the heterogeneity test [Q (df = 23) = 32.00, p = 0.10] did not detect statistically significant evidence of excessive heterogeneity among studies.
The combined effect estimate for the log (Odds Ratio) was −0.73 (95% CI: −1.93 to 0.47), suggesting a reduction in the probability of experiencing the headache symptom in SARS-CoV-2 patients treated with lactoferrin. However, this reduction was not statistically significant (z = −1.18, p = 0.24).
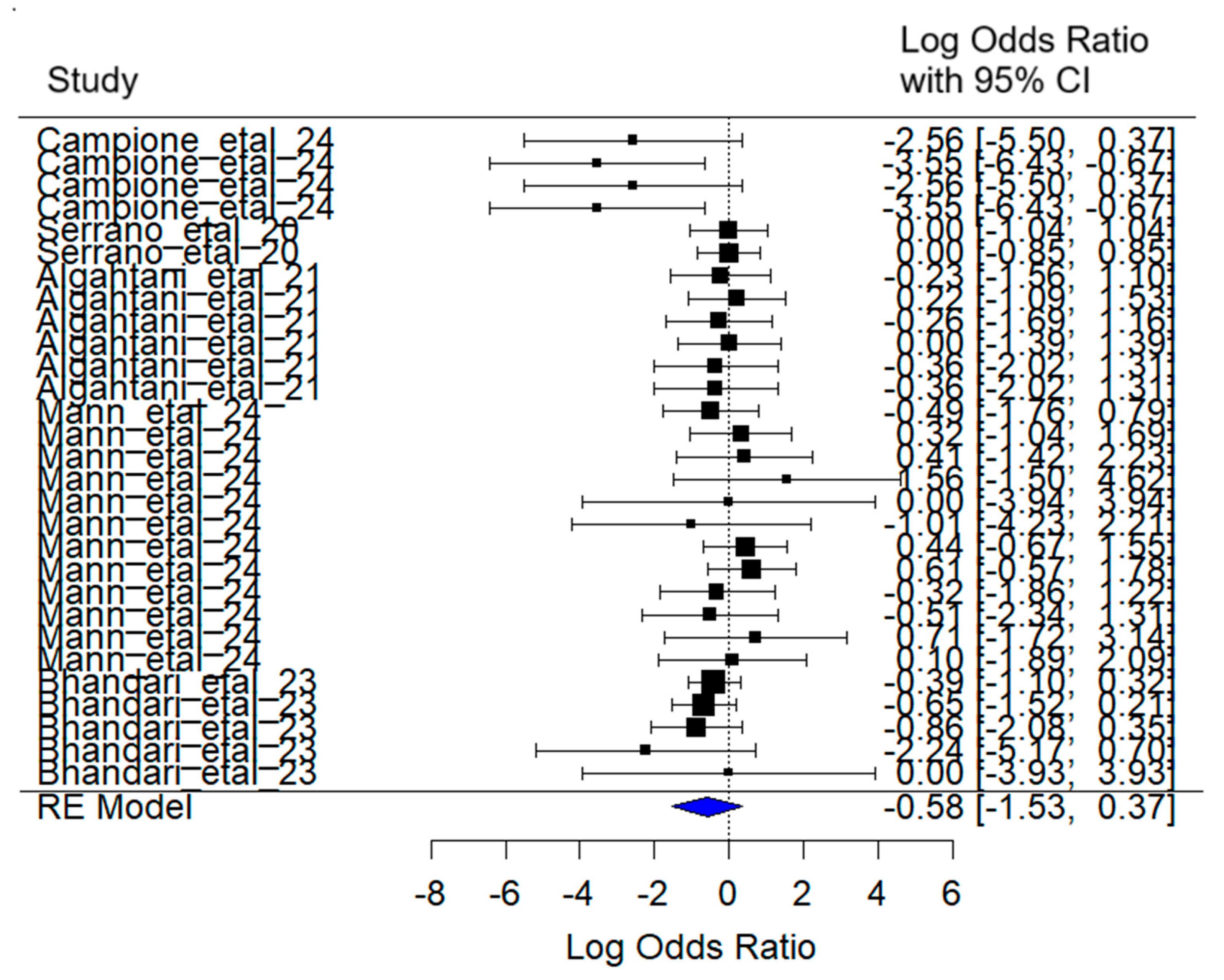
3.5. Anosmia/Ageusia
The mixed-effects meta-analysis yielded the following results (
Figure 8): Considering random effects by article, a between-article variance of σ
2 = 1.02 was estimated, with an associated standard deviation of 1.00. This indicates moderate variability in effect sizes attributable to specific differences among the studies included.
Regarding heterogeneity, the heterogeneity test [Q (df = 28) = 27.00, p = 0.52] did not detect statistically significant evidence of excessive heterogeneity among studies.
The combined effect estimate for the log (Odds Ratio) was –0.58 (95% CI: −1.52 to 0.37), suggesting a reduction in the probability of experiencing the anosmia/ageusia symptom in SARS-CoV-2 patients treated with lactoferrin. However, this reduction was not statistically significant (z = −1.20, p = 0.23).
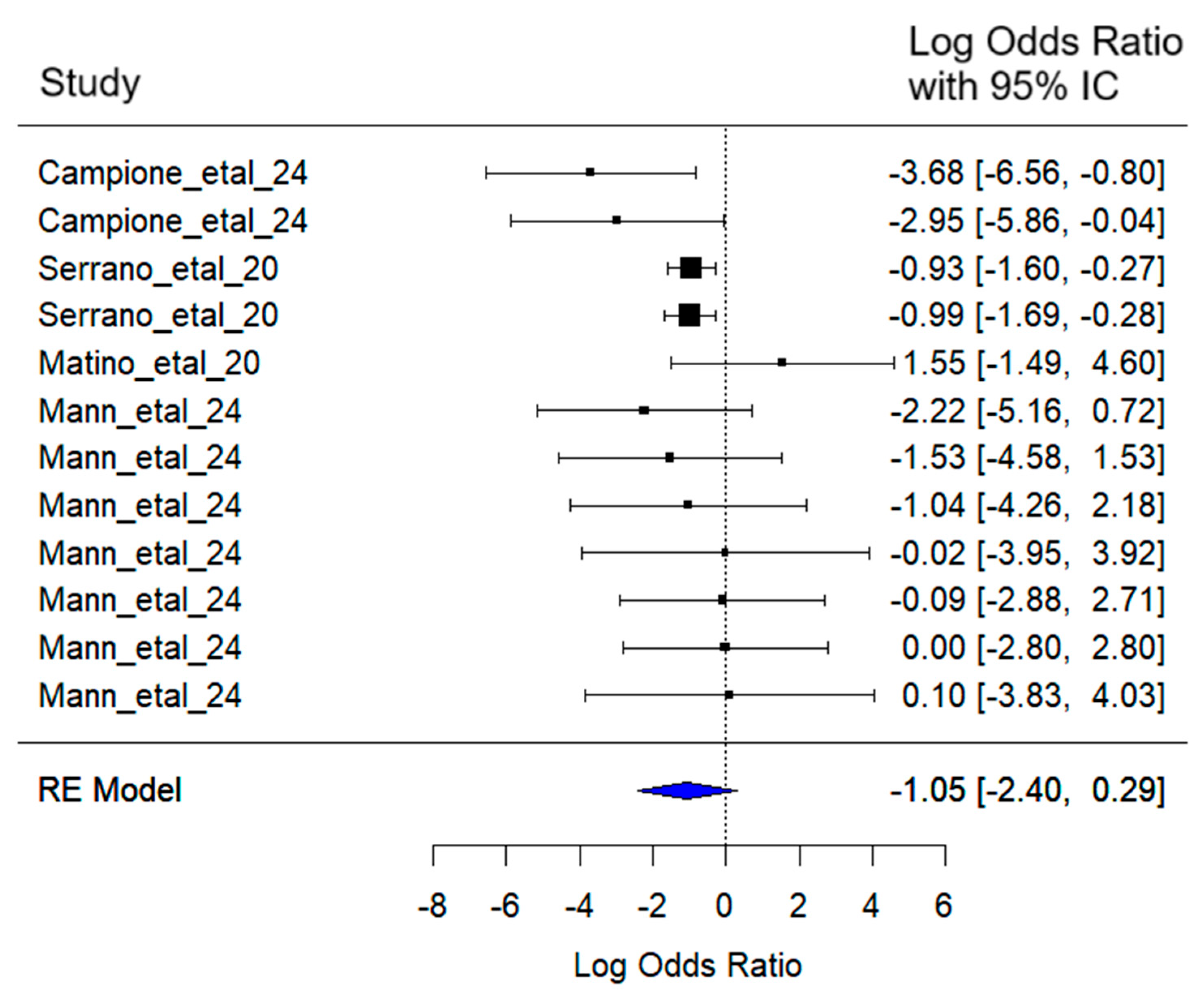
3.6. Muscular Pain
The mixed-effects meta-analysis yielded the following results (
Figure 9): Considering random effects by article, a between-article variance of σ
2 = 1.22 was estimated, with an associated standard deviation of 1.10. This indicates moderate variability in effect sizes attributable to specific differences among the included studies.
Regarding heterogeneity, the heterogeneity test [Q (df = 11) = 10.00, p = 0.53] did not detect statistically significant evidence of excessive heterogeneity among studies.
The combined effect estimate for the log (Odds Ratio) was −1.05 (95% CI: −2.40 to 0.29), suggesting a reduction in the probability of experiencing muscular pain symptoms in SARS-CoV-2 patients treated with lactoferrin. However, this reduction was not statistically significant (z = −1.53, p = 0.12).
Given the heterogeneity in study designs, treatment dosages, and follow-up times, the results should be interpreted with caution. While the meta-analysis provides valuable insights into the potential effects of lactoferrin, further well-designed, randomized controlled trials are necessary to confirm these findings and minimize sources of bias.
3.7. Meta-Regression Results
Meta-regression analysis was conducted only for fatigue, the sole symptom showing a significant overall effect. The results indicated that treatment duration did not significantly influence the effect size of lactoferrin on fatigue (z = −0.06, p = 0.95), suggesting that longer treatment periods were not associated with greater reductions in fatigue.
4. Discussion
Lactoferrin is a glycoprotein with immunomodulatory and anti-inflammatory properties that contribute to improving overall health and reducing fatigue. Specifically, as an iron-binding glycoprotein, it can capture and transport free iron, preventing its accumulation and improving its bioavailability for physiological processes such as hemoglobin synthesis. This leads to better tissue oxygenation and, consequently, a reduced sensation of fatigue, particularly in individuals with iron deficiency or mild anemia. In this regard, a clinical trial in pregnant women with iron-deficiency anemia demonstrated that the oral administration of 100 mg of lactoferrin significantly improved hemoglobin and ferritin levels while reducing self-reported fatigue [
22].
Additionally, lactoferrin may alleviate fatigue by downregulating pro-inflammatory cytokines such as TNF-α and IL-6. Lepanto et al. 2019 [
23] reported a reduction in inflammation-associated fatigue, highlighting lactoferrin’s anti-inflammatory and immune-modulating properties through its ability to decrease pro-inflammatory cytokine levels linked to fatigue.
On the other hand, fatigue associated with COVID-19 is primarily related to persistent systemic inflammation and sustained immunometabolic dysfunction following the acute phase of infection. During and after a SARS-CoV-2 infection, elevated levels of pro-inflammatory cytokines such as IL-6 and TNF-α are commonly observed, contributing to chronic fatigue. High production of these cytokines, oxidative stress, mitochondrial dysfunction, and neuroimmune alterations result in a state of physical and cognitive exhaustion [
24,
25]. This mechanism underlies post-COVID syndrome, characterized by prolonged fatigue and functional impairment even in the absence of an active infection [
26]. Furthermore, lactoferrin modulates the production of pro-inflammatory cytokines such as IL-6 and TNF-α [
27], promotes the proliferation of immunocompetent cells, and reduces oxidative stress [
28]. Collectively, these actions help optimize mitochondrial function and energy metabolism [
29], ultimately contributing to a reduction in fatigue symptoms
The present meta-analysis revealed a reduction in the presence of the symptoms explored, with a statistically significant effect observed only for fatigue. This result is promising, as only one study included in the meta-analysis and one study excluded from it reported significant improvements in certain symptoms [
17]. The remaining studies either reported non-significant effects or suggested potential benefits without conducting statistical tests. This meta-analysis contributes by applying a statistical approach to a body of research that, until now, had largely relied on descriptive outcomes. These findings highlight the potential of lactoferrin to alleviate fatigue, a common and persistent symptom in COVID-19 patients.
Among the studies included in the meta-analysis, there was considerable variation in the type and dosage of lactoferrin administered. However, most trials used similar formulations and dosages, which limited the ability to examine these factors as potential moderators for fatigue. In contrast, treatment duration varied across studies, allowing its impact to be assessed.
The meta-regression indicated that treatment duration did not significantly influence the effect size of lactoferrin on fatigue symptoms, suggesting that longer treatment periods did not lead to greater improvements. One possible explanation is that the beneficial effects of lactoferrin on fatigue emerge early after treatment initiation, with additional treatment time offering little added benefit. These results align with Serrano et al. (2020) [
15], who reported that patients experienced symptom improvement within the first three to four days of lactoferrin treatment. Early improvement may enhance energy levels and provide symptom relief, which could positively affect patients’ emotional well-being. As noted by Serrano et al. (2020) [
15], the positive effects of lactoferrin are further supported by its simplicity and favorable safety profile, with few adverse reactions reported across all age groups, from children to the elderly.
Regarding symptom assessment, considerable variability was observed across studies in both the types of symptoms recorded and the experimental designs employed. Although four studies were randomized controlled trials (RCTs), only two included a placebo and were double-blinded, features that enhance the reliability and internal validity of their findings. Notably, the study by Mann et al. (2023) [
17], which reported a significant effect on symptom relief, was among the better-designed trials, incorporating both double-blinding and a placebo control.
Although no significant effect of lactoferrin was observed for symptoms other than fatigue, a consistent trend toward symptom reduction was noted, particularly for anosmia/ageusia, headache, and muscle pain. The absence of standardized protocols across the studies included in the meta-analysis may have limited the ability to detect a potential effect of lactoferrin on these outcomes. More rigorously designed trials—such as randomized, double-blind, placebo-controlled studies—are needed to better assess whether lactoferrin can influence symptoms beyond fatigue. Additionally, future research should explore different dosages and formulations of lactoferrin to help establish optimized treatment protocols for COVID-19 patients.
Although fatigue was the only symptom for which a statistically significant effect of lactoferrin was found, its improvement may have meaningful implications for patients with SARS-CoV-2. An enhanced sense of energy could contribute to a better quality of life, potentially increasing patients’ motivation to adhere to treatment, engage in therapy, or resume their previous daily routines.
Ongoing fatigue is the most frequently reported symptom in individuals with Long COVID, independently from how severe their initial infection was or whether they experienced respiratory problems [
30]. In a study by Walker et al. (2023), [
31] which involved treatment-seeking individuals with post-COVID-19 syndrome (PCS), self-reported symptoms were related to health-related quality of life (HRQoL), the ability to work, and the capacity to perform everyday activities. The study found that fatigue was the primary symptom associated with severe functional impairment, as measured by the Work and Social Adjustment Scale (WSAS > 20; scores range from 0 to 40, with values above 20 indicating moderately severe or greater impairment in daily functioning), and with low HRQoL. Notably, patients in this study reported higher levels of fatigue than individuals diagnosed with stroke, inflammatory bowel disease, or anaemic cancer.
Similarly, Vélez Santamaría et al. (2023) [
32] reported that COVID-19 patients experiencing greater fatigue also showed a more pronounced decline in quality of life. Furthermore, they observed that, based on the severity of fatigue, a significant proportion of patients with Long COVID met the diagnostic criteria for Chronic Fatigue Syndrome (CFS), with 92.4% fulfilling this condition.
Thus, these findings highlight the clinical importance of fatigue and reinforce the potential value of interventions, such as lactoferrin, which may contribute to its relief. Even modest reductions in fatigue could result in meaningful improvements in quality of life and enhance patients’ ability to return to work, engage in therapy, and resume daily activities. For example, Uesaki et al. (2018) [
33] reported that lactoferrin administration in individuals with sleep difficulties improved sleep quality, enhanced daily mood, and positively influenced the gut environment, ultimately promoting overall well-being.
A key limitation of this review is the notable variability among the included studies. Differences in study design, sample size, treatment duration, lactoferrin dosage, and the type of formulation used (e.g., oral capsules, liposomal syrups, intranasal sprays) may have influenced the observed outcomes and contributed to heterogeneity in the results. Furthermore, variations in patient characteristics, such as disease severity, comorbidities, follow up of adverse reactions, and timing of intervention, complicate direct comparisons across studies. Although four randomized controlled trials were included, several studies employed non-randomized or open-label designs, which may introduce bias and limit the strength of the conclusions. Taken together, these factors highlight the need to interpret the findings with caution and underscore the importance of conducting larger, placebo-controlled, and double-blinded trials to establish more definitive evidence.
The review of lactoferrin as a supportive intervention for alleviating COVID-19-related fatigue suggests potential benefits; however, current evidence remains limited. Further research is needed to validate and expand these observations. Future studies should prioritize large-scale, placebo-controlled, double-blinded clinical trials with robust experimental designs and appropriate statistical analyses to strengthen the evidence base. Efforts to standardize lactoferrin dosing regimens, treatment durations, and formulations (e.g., oral vs. intranasal delivery) will be critical to improving comparability across studies. Additionally, exploring its effects in different patient populations, including those with varying comorbidities or severity of post-acute COVID-19 syndrome, could help identify subgroups most likely to benefit. Finally, mechanistic studies investigating the immunomodulatory and metabolic pathways underlying lactoferrin’s effects may provide further insights to guide its therapeutic application.