Abstract
Objectives: This study aims to examine the serological and infectious characteristics of the Xokós indigenous community in Brazil, both prior to and following COVID-19 immunization; Methods: Immunofluorescence assays were employed to identify the SARS-CoV-2 viral antigen, while IgM and IgG antibody tests for COVID-19 were utilized to assess the participants’ infectious and serological profiles in July 2020, before the commencement of the COVID-19 vaccination campaign, and in March 2022, during the booster dose vaccination campaign; Results: The majority of participants (n = 22) were female, with an average age of 42.20 years. The most prevalent comorbidity was hypertension (60%; n = 9), followed by hypertension associated with diabetes (20%; n = 3). No statistically significant correlation was found between the timing of vaccination and the levels of antigens or IgM. However, the prevalence of reactive antigens and IgM was 13.3% (n = 4) in the pre-vaccination group and 3.3% (n = 1) in the post-vaccination group. A statistically significant difference in IgG production was observed before and after vaccination (χ2(1) = 39.095, p < 0.01), as well as differences in IgG antibody detection before and after vaccination and in the vaccines used. Participants showed a higher probability of reactive IgG antibodies following vaccination; Conclusions: Our data demonstrate the beneficial effects of vaccination on the indigenous community, highlighting that continued immunization is a crucial step in protecting indigenous health and preventing severe outbreaks and deaths associated with the disease.
1. Introduction
Indigenous peoples’ health is critically endangered by the spread of infectious diseases and their etiological agents, such as SARS-CoV-2 [1]. When public health policies fail to support these populations, they become more vulnerable to emerging illnesses compared to the non-Indigenous population [2]. As of 2023, Brazil is home to 1.7 million Indigenous people. In Sergipe, the smallest state in Brazil, only about 5% of the 4708 Indigenous people reside in Indigenous territories [3]. During the COVID-19 pandemic, complications from the virus led to the deaths of 963 Indigenous people in Brazil between 2020 and 2023 [4]. Over this period, nearly 70,000 cases were reported within the defined Indigenous territories [5].
The Kariri-XoKó ethnic group is one of the groups inhabiting these regions [6]. This ethnic group is found around Sergipe and Alagoas Brazilian states, with a significant presence on the island of São Pedro, near Porto da Folha in Sergipe [6]. The Xokós community in Sergipe, which includes 375 inhabitants, is part of this ethnic group [6]. Between 2020 and 2022, only 36 COVID-19 cases were reported to the Ministry of Health from the Xokó community, according to data from Brazil’s Indigenous Health Secretaria [5]. No COVID-19-related deaths were recorded in the community during the pandemic. It has been suggested that there is around 14% under-reporting of cases and 103% under-reporting of deaths in the Indigenous community in Brazil, attributed to the low numbers reported [7]. Additionally, Indigenous people experienced 110% higher incidence rates and 136% higher mortality rates compared to the non-Indigenous population.
Social distancing measures were implemented in these communities to prevent the spread of SARS-CoV-2 and mitigate its impact [8]. However, adhering to social separation and isolation is challenging because communal living is a fundamental cultural aspect of Indigenous life [9]. Moreover, the Brazilian health system’s overcrowding and saturation make it difficult to travel for testing and healthcare provision, hindering adequate support for Indigenous communities [10]. Despite this, the population often needs to relocate to access essential services [11]. According to Broering et al. (2023) [12], the introduction of SARS-CoV-2 into the Indigenous community may have been facilitated by visits to metropolitan areas and/or visits from medical personnel. The provision of pandemic materials such as masks, alcohol 70, and gel was not adequately managed for this community [13]. Additionally, there was a lack of proper educational and hygienic resources provided to Indigenous tribes [14].
These issues led to the prioritization of Indigenous populations in the COVID-19 vaccination campaign of 2021 [15]. Most Indigenous people in Brazil received the CoronaVac vaccine, the first available in the country, due to their high-risk status and priority for vaccination. Other vaccines, such as ChAdOx1 (Oxford-AstraZeneca®), BNT162b2 (Pfizer-BioNTech®), and Ad26.COV2.S (Janssen-cilag®), were also made available, and heterologous combinations were introduced to review the immunization schedule every four months [16]. To date, the Ministry of Health’s efforts have resulted in approximately 1,478,006 COVID-19 vaccine doses administered to the Indigenous community [17]. This early immunization facilitated the development of immunity against COVID-19 and its variants compared to non-Indigenous individuals [18], and mitigated the severity and mortality of the disease in these areas [19].
The Indigenous population has been significantly impacted by COVID-19 due to underlying socioeconomic and political challenges. Prioritizing its vaccination was a crucial strategy for health promotion and prevention, aiming to reduce case numbers and fatalities. This study investigates the infectious and serological profile of the Xokós community in Brazil before and after COVID-19 immunization to assess the pandemic’s effects on the local Indigenous population.
2. Materials and Methods
2.1. Study Population and Data Collection
This study was approved by the Brazilian Ethics Committee (CAAE 31018520.0.0000.5546) and involved 30 participants from the Xokós ethnic group within the indigenous community of Porto da Folha, a municipality located 110 miles from Sergipe’s capital, Aracaju. The total population of the Xokós community is 375 individuals. All participants or their guardians provided consent for participation. Inclusion criteria required participants to be 18 years of age or older, free from immunosuppressive diseases, residents of the Xokós community, and members of the Xokós ethnic group. Initially, we gathered data on participants’ age, symptom presence, pre-existing comorbidities, and details regarding their vaccination history and schedule. Following the questionnaire, participants underwent serological testing and antigen screening. Data collection occurred at two distinct times: in July 2020, prior to the initiation of the COVID-19 vaccination campaign, and in March 2022, two months after the commencement of the booster dose campaign in the country. Figure 1 illustrates the methodology of our study.
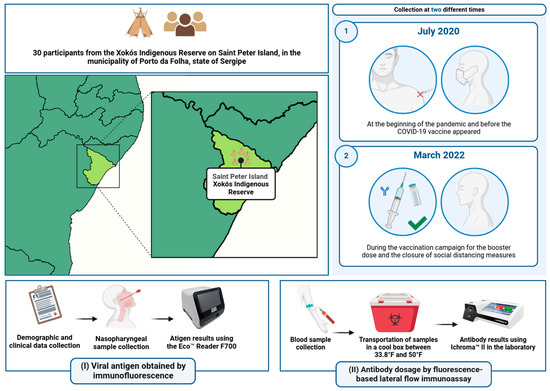
Figure 1.
Stages and processes of the study: steps (I) and (II) refer to the stages of the study methodology.
2.2. Collection and Laboratory Analysis
To assess the infectious profile of the participants, we conducted a Point of Care COVID-19 ECO F COVID-19 Ag® antigen test, developed by ECO Diagnóstica (Corinto, Minas Gerais, Brazil) (https://ecodiagnostica.com.br/diagnostico-rapido/eco-f-covid-19-ag/) (accessed on 2 July 2024) [20]. This test detects viral antigens in nasopharyngeal samples using an immunofluorescence technique. According to the manufacturer, the test has a sensitivity of 86% and a specificity of 95%. The immunoassay has a cut-off index (COI) of ≥1.0 for detectable results and <1.0 for non-detectable results.
Additionally, venous blood samples were collected from each participant in specific tubes for serum extraction. The samples were transported in a temperature-controlled thermal box, maintained between 33.8°F and 50°F, for subsequent laboratory analysis [21]. In the laboratory, we assessed the serological profile of participants by performing IgM and IgG antibody tests using the Ichroma™ COVID-19 Ab (Boditech, Chuncheon, Gangwon, Republic of Korea) (https://grupokovalent.com.br/reagente/ichroma-covid-19-ab/) (accessed on 2 July 2024) [22]. This device detects IgM and IgG anti-SARS-CoV-2 antibodies through immunofluorescence, with a sensitivity of 95.8% and a specificity of 97%. The device provides results by calculating antibody levels, which are expressed as a cut-off index (COI) for both IgM and IgG.
Results are interpreted as reactive for values equal to or greater than 1.1 and non-reactive for values less than 1.1. Values between 0.9 and 1.1 are considered indeterminate, necessitating a repeat test, according to the manufacturer. Results for the antigen test were provided to participants in a report within 30 min of collection, while serology results were made available within 24 h via QRcode®.
2.3. Data Analysis and Visualization
Data analysis and result visualization were conducted using IBM® SPSS® Statistics software (version 26.0 for Windows) [23]. We performed a descriptive analysis of the data and conducted various statistical tests, including the Cochran test, Fisher’s exact test, the odds ratio, and the chi-squared test. The Cochran test was utilized to determine the necessary sample size for the study. Fisher’s exact test analyzed the association between the detection of antigens and IgM antibodies before and after COVID-19 vaccination, as well as variations in IgG antibody production among different vaccine combinations. The odds ratio was employed to evaluate the relationship between the detection of SARS-CoV-2 antigens, IgM, and IgG antibodies, and to assess effect sizes by comparing the likelihood of symptoms before and after vaccination. Additionally, we used the chi-squared test to explore the association between symptoms before and after vaccination and to compare IgG antibody levels before and after vaccination. A p-value of <0.05 was considered statistically significant, indicating that the probability of the observed results occurring by chance is less than 5%.
3. Results
3.1. Descriptive Analysis of the Indigenous Peoples Tested in the Xokó Village
The mean age of the Indigenous participants was 42.20 years (SD = 18.80; 95% CI = 35.18–49.22). The majority of participants were female (n = 22). The mean age for women was 39.77 years (SD = 17.12) while the mean age for men was 48.87 years (SD = 22.71). Analysis of the questionnaire revealed that 15 participants had at least one type of comorbidity. Among those with comorbidities (n = 15; 50%), hypertension was the most common condition (60%; n = 9), followed by hypertension combined with diabetes (20%; n = 3). This information can be better visualized in Table 1.

Table 1.
Sociodemographic and clinical profile of the participants.
3.2. Variations in the Detection of SARS-CoV-2 Antigen, IgM, and Symptoms before and after Vaccination of the Indigenous People Tested
A sample of 30 participants was collected from the Xokó Indigenous population (n = 375). To determine the required sample size for the study, we used the Cochran model with a 5% margin of error and a 95% confidence interval. The standard error for the tested population was 16.04%, which may affect the significance of some statistical tests. In the pre-vaccination group (Group A), four (13.33%) participants showed signs of active infection, such as reactive viral antigens and IgM antibodies, with two of them presenting symptoms. The four participants were between 50 and 70 years old. However, in the post-vaccination group (Group B) only one participant had reactive IgM antibodies, but without detectable viral antigens or the appearance of symptoms. Fisher’s exact test was used to analyze differences in antigen and IgM antibody detection before and after COVID-19 vaccination. The results indicated no statistically significant association for either antigens or IgM antibodies (p > 0.05). To evaluate the effect of these variables, we performed an odds ratio analysis. This showed that pre-vaccination group A was 10.36 times (CI95% = 0.53 to 201.46; z = 1.54) more likely to have detectable SARS-CoV-2 antigens compared to post-vaccination group B. Additionally, the odds ratio indicated that group A was 4.46 times (CI95% = 0.47 to 42.52; z = 1.30) more likely to have reactive IgM antibodies than group B. The prevalence ratio showed that group A had 13.3% (n = 4) with detectable SARS-CoV-2 antigens and reactive IgM, whereas group B had no detectable antigens and only 3.3% (n = 1) with reactive IgM.
The chi-squared test was conducted, revealing a statistically significant association (χ2(1) = 10.48, p < 0.01; Φ = 0.42) between the symptoms experienced by the indigenous population before and after vaccination. To evaluate the effect size, the odds ratio was employed, which indicated that indigenous people were 16.79 times more likely to exhibit symptoms before vaccination compared to after. The prevalence ratio showed 36.7% (n = 11) for group A (pre-vaccination) and 3.3% (n = 1) for group B (post-vaccination).
3.3. Variations in the Detection of IgG Antibodies before and after Vaccination, and the Relationship between the Vaccines Used by the Indigenous Peoples Tested
In the pre-vaccination group, only five participants were reactive for IgG antibody production. In contrast, 29 participants in the post-vaccination group were reactive for IgG antibodies. A chi-square test was employed to determine whether there was a difference in IgG antibody production before and after vaccination among the indigenous individuals tested. The results indicated a statistically significant difference (χ2(1) = 39.095, p < 0.01; Φ = 0.807). Figure 2 illustrates IgG antibody reactivity in the pre-vaccination and post-vaccination groups. The odds ratio analysis revealed that, after vaccination, the indigenous people were 145.00 times more likely to have reactive IgG antibodies compared to before vaccination (CI95% = 15.86 to 1325.36; z = 4.41). Two heterologous vaccine combinations were used: CoronaVac/BNT162b2 (n = 14) and CoronaVac/ChadOx1 (n = 16). Fisher’s exact test was conducted to assess differences in IgG production between these combined vaccines. No statistical difference was found (p > 0.05).
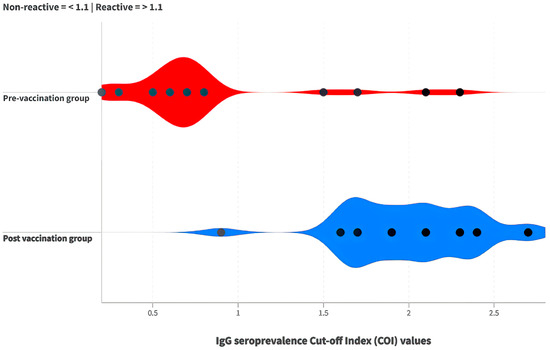
Figure 2.
Comparison of cut-off index (COI) values for IgG antibodies between the pre-vaccination (red) and post-vaccination groups (blue).
4. Discussion
In this study, we assessed the incidence and serological profile of COVID-19 cases within a community of indigenous people in the Brazilian state of Sergipe. It was observed that, prior to vaccination, there was an elevated risk of SARS-CoV-2 infection, significant symptom manifestation, and low reactivity of anti-SARS-CoV-2 IgG memory antibodies. This susceptibility reflected the limited availability of vaccines among indigenous populations, compounded by the absence of health policies that included them in the vaccination program [24]. Additionally, cultural and structural barriers exacerbated the virus’s spread, as many preventive measures, such as social distancing, mask wearing, and hand washing, were not adhered to. The community’s isolation on an island, coupled with barriers to river access, also posed challenges for timely medical support [24]. These issues are closely related to the social injustice, poverty, and lack of health education faced by indigenous peoples [25,26].
We observed a large standard deviation in the mean age of the participants, indicating a diverse age range, as detailed in Supplementary Table S1. Additionally, the predominance of females in the study group reflects the demographic pattern of Sergipe, which has a higher proportion of women [27]. The community investigated exhibited high rates of diabetes and hypertension, which is concerning as these conditions further increase the risk of COVID-19 complications. These health challenges are compounded by the broader impact of COVID-19 on indigenous populations. According to Zhang et al. (2022) [28] and Ejaz et al. (2020) [29], comorbidities such as hypertension enhance the expression of the angiotensin-converting enzyme (ACE)-2 receptor, facilitating the entry of the SARS-CoV-2 virus into human cells. We propose that the high prevalence of comorbidities, particularly hypertension, among indigenous people may have contributed to the increased detection of antigens and reactive IgM antibodies prior to vaccination.
In the pre-vaccination group, four participants exhibited parameters indicative of active infection and two of them were symptomatic. Since antibody development can be host-dependent and may take time, most patients tend to seroconvert within seven to eleven days after antigen exposure, though some may produce antibodies earlier, as observed in previous studies [30]. Although we did not categorize the symptoms, it is plausible that these individuals showed signs consistent with respiratory infections, potentially including symptoms of COVID-19. Moreover, only 3.3% (1/30) of the antigens were detected after vaccination, with the majority of patients showing a reduction in symptoms and an increase in reactive IgG antibodies. A study conducted in the Brazilian Amazon during the early stages of COVID-19 vaccination also observed an increase in IgG antibodies among populations from three indigenous tribes, a finding that supports our results [31].
The development of hybrid immunity, which can enhance levels of neutralizing antibodies as demonstrated in a previous study by our group [32], along with the completion of the recommended vaccination schedule of at least two doses by all indigenous individuals, may be associated with the observed increase in IgG antibody production and reduction in symptoms. Additionally, research on the time-dependent nature of immune responses indicates that variations in antibody levels, including IgG, are influenced by changes in disease prevalence and individual immune responses [33]. This underscores the importance of adhering to the vaccination schedule to prevent and safeguard the health of these populations, despite the under-reporting of cases and deaths in these areas due to limited access to public policies and health services.
Two doses of the vaccine were administered to our participants, utilizing the heterologous combinations CoronaVac/BNT162b2 (14/30) and CoronaVac/ChAdOx1 (16/30). The absence of a difference in neutralization against the virus between these combinations suggests that both vaccine regimens effectively contributed to the production of neutralizing antibodies. Pescarini et al. (2023) [19] reported that a trial involving indigenous patients demonstrated the effectiveness of the second dose in reducing mortality and the progression of COVID-19. However, since vaccine effectiveness tends to decline after six months, it is crucial to update vaccinations [34].
The significance of this study lies in its potential to directly inform the development of strategies aimed at preserving and safeguarding the health of these indigenous populations. Indigenous communities have been characterized by social vulnerability since the colonization process, particularly concerning health systems. According to the United Nations [35], immunization is one way to fulfill the moral responsibility of protecting indigenous peoples and promoting their health.
This study does, however, have certain limitations. For instance, we did not validate the individuals’ viral strain using RT-PCR, the gold standard for virus detection. Additionally, we did not obtain information regarding potential side effects of the vaccination, which could have been useful for understanding the reactions within this population. Moreover, the sample size is relatively small, comprising only 30 individuals from the first and second tests out of the 375 residents in the remote village. Nevertheless, this study is the first to evaluate the seroprevalence and infection rate of COVID-19 in this indigenous community. Notably, there were no reported COVID-19-related deaths in the community under investigation during the study period.
In summary, our data demonstrate that vaccination has a positive impact on the indigenous community, highlighting the importance of ongoing immunization as a critical measure for protecting indigenous health and reducing serious disease outbreaks and fatalities. However, the prevalence of comorbidities underscores the challenges that indigenous peoples have historically faced, including cultural barriers. To achieve a comprehensive approach and provide more effective and enduring protection, health policies must address the specific needs and cultural aspects of indigenous communities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid4090104/s1; Table S1: Before vaccination campaign; Table S2: After vaccination campaign.
Author Contributions
Conceptualization, E.E.D.S., M.d.S.B. and R.S.S.; methodology, R.S.S., M.d.S.B. and E.E.D.S.; data curation, E.E.D.S., M.d.S.B., R.S.S., P.C.d.J., D.M.R.R.S., P.H.M.M., J.B.d.S. and L.P.B.; investigation, E.E.D.S., M.d.S.B., R.S.S., P.C.d.J., D.M.R.R.S., P.H.M.M., J.B.d.S. and L.P.B.; project administration, L.P.B.; software, R.S.S.; supervision, L.P.B. and A.G.G.; writing—original draft, E.E.D.S., M.d.S.B., R.S.S. and L.P.B.; writing—review and editing, P.C.d.J., A.G.G., L.A.d.M.S. and L.P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Research in Brazil (Comite de etica em pesquisa com seres humanos; CEP platform) under the protocol code 31018520.0.0000.5546 in September 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
If you are interested in further data on the results, please contact the corresponding authors.
Acknowledgments
We acknowledge the people of the Xokó indigenous community, as well as the municipality of Porto da Folha. We thank the Government of the State of Sergipe, as well as the state health department. Finally, we thank the MPT, MPE, and MPF.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pontes, G.S.; Silva, J.d.M.; Pinheiro-Silva, R.; Barbosa, A.N.; Santos, L.C.; Ramalho, A.d.P.Q.; Alves, C.E.d.C.; da Silva, D.F.; de Oliveira, L.C.; da Costa, A.G.; et al. Increased vulnerability to SARS-CoV-2 infection among indigenous people living in the urban area of Manaus. Sci. Rep. 2021, 11, 17534. [Google Scholar] [CrossRef] [PubMed]
- Sansone, N.M.S.; Boschiero, M.N.; Ortega, M.M.; Ribeiro, I.A.; Peixoto, A.O.; Mendes, R.T.; Marson, F.A.L. Severe Acute Respiratory Syndrome by SARS-CoV-2 Infection or Other Etiologic Agents Among Brazilian Indigenous Population: An Observational Study from the First Year of Coronavirus Disease (COVID)-19 Pandemic. Lancet Reg. Health—Am. 2022, 8, 100177. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Dados do Censo 2022 Revelam Que o Brasil Tem 1,7 Milhão de Indígenas. Available online: https://www.gov.br/funai/pt-br/assuntos/noticias/2023/dados-do-censo-2022-revelam-que-o-brasil-tem-1-7-milhao-de-indigenas#:~:text=Em%202022%2C%20o%20n%C3%BAmero%20de,da%20popula%C3%A7%C3%A3o%20total%20do%20pa%C3%ADs (accessed on 29 June 2024).
- Socioambiental. COVID-19 e os Povos Indígenas. Plataforma de Monitoramento da Situação Indígena na Pandemia do Novo Coronavírus (COVID-19) No Brasil. Available online: https://covid19.socioambiental.org/index.html (accessed on 29 June 2024).
- Brasil. Painel COVID-19 SESAI (SasiSUS). Available online: https://infoms.saude.gov.br/extensions/sesai_covid/sesai_covid.html (accessed on 29 June 2024).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Distribution of Indigenous People in Brazil. Available online: https://indigenas.ibge.gov.br/mapas-indigenas-2.html (accessed on 29 June 2024).
- Fellows, M.; Paye, V.; Alencar, A.; Nicácio, M.; Castro, I.; Coelho, M.E.A.; Silva, C.V.J.; Bandeira, M.; Lourival, R.; Basta, P.C. Under-Reporting of COVID-19 Cases Among Indigenous Peoples in Brazil: A New Expression of Old Inequalities. Front. Psychiatry 2021, 12, 638359. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Recommendations of the National Health Council. Available online: https://conselho.saude.gov.br/recomendacoes-cns/1163-recomendac-a-o-n-036-de-11-de-maio-de-2020 (accessed on 29 June 2024).
- Lana, R.M.; Codeço, C.T.; Santos, R.V.; Cunha, B.; Coelho, F.C.; Cruz, O.G.; Caldas, A.D.R.; Souza, M.C.d.; Bastos, L.S.; Pontes, A.L.d.M.; et al. Vulnerability of indigenous populations to the COVID-19 pandemic in Brazil and the challenges of monitoring it. In COVID-19 in Brazil: Epidemiological Scenarios and Health Surveillance, 1st ed.; SciELO Books; Editora Fiocruz: Rio de Janeiro, Brazil, 2021; Volume 1, pp. 127–142. [Google Scholar]
- Mendes, A.M.; Leite, M.S.; Langdon, E.J.; Grisotti, M. The challenge of providing primary health care to indigenous people in Brazil. Rev. Panam. Salud Pública 2018, 42, e184. [Google Scholar] [CrossRef] [PubMed]
- Gracey, M.; King, M. Indigenous health part 1: Determinants and disease patterns. Lancet 2009, 374, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Broering, L.; Padilha, M.I.; Costa, R.d.; Mazera, M.S. Implications of the pandemic for the construction of nurses’ identity based on the journalistic media. Rev. Bras. Enferm. 2023, 76, e20220245. [Google Scholar] [CrossRef] [PubMed]
- United Nations. COVID-19 and Indigenous Peoples. n.d. Available online: https://social.desa.un.org/issues/indigenous-peoples/covid-19-and-indigenous-peoples (accessed on 30 June 2024).
- UNFPA. Implications of COVID-19 for Indigenous People in Latin America and the Caribbean. Available online: https://lac.unfpa.org/sites/default/files/pub-pdf/1-Covid-Indigenas-ENG.pdf (accessed on 30 June 2024).
- Ministry of Health. Vaccination Priority: Technical Note N. 155/2021-CGPNI/DEIDT/SVS/MS. Available online: https://www.gov.br/saude/pt-br/assuntos/covid-19/notas-tecnicas/2021/nota-tecnica-no-155-2021-cgpni-deidt-svs-ms.pdf/view (accessed on 30 June 2024).
- Butantan. What Are the Differences between the COVID-19 Vaccines Being Administered in Brazil? Available online: https://butantan.gov.br/covid/butantan-tira-duvida/tira-duvida-noticias/quais-sao-as-diferencas-entre-as-vacinas-contra-covid-19-que-estao-sendo-aplicadas-no-brasil (accessed on 30 June 2024).
- Vaccinometer. Vaccine Monitoring Panel. Ministry of Health: COVID-19 Vaccinometer. Available online: https://infoms.saude.gov.br/extensions/SEIDIGI_DEMAS_Vacina_C19/SEIDIGI_DEMAS_Vacina_C19.html (accessed on 30 June 2024).
- World Health Organization (WHO). Coronavirus Disease (COVID-19): Vaccines and Vaccine Safety. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines (accessed on 30 June 2024).
- Pescarini, J.M.; Cardoso, A.M.; Santos, R.V.; Scaff, P.F.; Paixao, E.S.; Ranzani, O.T.; Cerqueira-Silva, T.; Boaventura, V.S.; Bertoldo-Junior, J.; de Oliveira, V.A.; et al. Vaccine coverage and effectiveness against laboratory-confirmed symptomatic and severe COVID-19 in indigenous people in Brazil: A cohort study. BMC Public Health 2023, 23, 1267. [Google Scholar] [CrossRef] [PubMed]
- ECO Diagnóstica. COVID-19 Ag ECO Teste. Available online: https://ecodiagnostica.com.br/diagnostico-rapido/covid-19-ag-eco-teste/ (accessed on 2 July 2024).
- Ministry of Health. Portaria Conjunta nº 370, 7 May 2014. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sas/2014/poc0370_07_05_2014.html (accessed on 6 September 2024).
- Boditech. ichromaTM COVID-19 Ab. Available online: https://grupokovalent.com.br/wp-content/uploads/2021/11/BL3686-REV02-08-2020-ICHROMA-COVID-19-AB-Boditech.pdf (accessed on 2 July 2024).
- IBM. Software IBM SPSS. Available online: https://www.ibm.com/br-pt/spss (accessed on 2 July 2024).
- Machado, F.C.G.; Ferron, M.M.; Barddal, M.T.d.M.; Nascimento, L.A.; Rosalen, J.; Avelino-Silva, V.I. COVID-19 vaccination, incidence, and mortality rates among indigenous populations compared to the general population in Brazil: Describing trends over time. Lancet Reg. Health—Am. 2022, 13, 100319. [Google Scholar] [CrossRef] [PubMed]
- Pickering, K.; Galappaththi, E.K.; Ford, J.D.; Singh, C.; Zavaleta-Cortijo, C.; Hyams, K.; Miranda, J.J.; Arotoma-Rojas, I.; Togarepi, C.; Kaur, H.; et al. Indigenous peoples and the COVID-19 pandemic: A systematic scoping review. Environ. Res. Lett. 2023, 18, 033001. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Mata-Sánchez, N.D. Challenges, inequalities and COVID-19: Examples from indigenous Oaxaca, Mexico. Glob. Public Health 2021, 16, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Sergipe. PNAD Periodic. Available online: https://transparencia.aracaju.se.gov.br/prefeitura/wp-content/uploads/periodicos/Periodico_PNAD_Continua_Aju_2021_1.pdf (accessed on 6 September 2024).
- Zhang, J.; Dong, X.; Liu, G.; Gao, Y. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2022, 64, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.N.C.; Abreu, I.N.; Rodrigues, E.P.S.; Freitas, V.O.; Botelho, B.J.S.; Souza, S.L.; Cayres-Vallinoto, I.; Guerreiro, J.F.; Ishak, R.; Vallinoto, A.C.R. Anti-SARS-CoV-2 antibodies among indigenous populations of the Brazilian Amazon: A cross-sectional study. BMJ Open 2022, 12, e054271. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.d.S.; Silva, B.S.d.; Santos, R.S.; Silva, D.M.R.R.; Silva, E.E.D.; Moura, P.H.M.; Souza, J.B.d.; Santana, L.A.d.M.; Fonseca, D.L.M.; Filgueiras, I.S. COVID-19 Vaccination and Serological Profile of a Brazilian University Population. Life 2023, 13, 1925. [Google Scholar] [CrossRef] [PubMed]
- Bedekar, P.; Kearsley, A.J.; Patrone, P.N. Prevalence estimation and optimal classification methods to account for time dependence in antibody levels. J. Theor. Biol. 2023, 559, 111375. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- United Nations. United Nations Declaration on the Rights of Indigenous Peoples. Available online: https://www.acnur.org/fileadmin/Documentos/portugues/BDL/Declaracao_das_Nacoes_Unidas_sobre_os_Direitos_dos_Povos_Indigenas.pdf (accessed on 15 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).