Abstract
Vaccine breakthrough COVID-19 clusters with high attack rates are very rare. They paralyze affected section/s of the institution; thus, awareness of them is important. This is an analysis of a vaccine breakthrough COVID-19 cluster with an 88% attack rate involving 35 security guards (SGs) of the Teaching Hospital-Peradeniya, Sri Lanka. The identification of the particular combination of factors that resulted in this outbreak’s 88% attack rate was our main objective, because this knowledge is useful to prevent similar clusters of COVID-19 and other similar infections. We traced and documented contact details, risky behaviors, and medical history of all SGs. Contacts of all COVID-19 cases were tested for COVID-19. We created an epi-curve and identified the index case (IC). The epi-curve pattern indicates a propagated source outbreak. No SG was seriously immunocompromised. There was no breach in the local cold chain. The following combination of factors synergistically created this outbreak: communal meals at cramped spaces, unfamiliarity with vaccine breakthrough cases, disregard of the risk of infection from fully vaccinated coworkers, hesitancy to report COVID-19-like symptoms early on, symptomatic treatment of COVID-19-like patients without testing for COVID-19, permission to return home, and the Alpha variant of the SARS-CoV-2 virus.
1. Introduction
Two or three doses of the COVID-19 vaccine do not induce sterilizing immunity in many vaccinated people [1,2,3,4]. Vaccine breakthrough COVID-19 clusters with low attack rates are relatively common, but they usually do not paralyze the affected workplaces. Such clusters with high attack rates are very rare, but they paralyze the affected segments of the institution by taking most of the workers out of work. An awareness of the combination and sequence of events that have led to such COVID-19 vaccine breakthrough outbreaks is useful for preventing similar future outbreaks of COVID-19 and similar infections.
This is an outbreak report regarding a 6 July to 1 August 2021 COVID-19 vaccine breakthrough cluster among security guards (SGs) of the Teaching (General) Hospital-Peradeniya, Sri Lanka, with an 88% (35/40) attack rate. This outbreak was mentioned in the national media and in Sri Lanka’s Parliament. A total of 40 out of 42 SGs of our hospital were vaccinated with two doses of 0.5 ml of the ChAdOx1 nCoV-19 (Covishield) vaccine administered 13 weeks apart. The batch numbers were 4120z025 (first dose) and 4120z028 (second dose). The second dose was given during the week starting from 29 April 2021. Two security guards who joined the team thereafter were unvaccinated. Both had symptomatic COVID-19. Therefore, the overall attack rate for the whole SG cluster was also 88% (37/42).
Motivation behind This Study
We intended to determine the causes that led to this outbreak, to analyze the outbreak’s high attack rate, and to propose measures to prevent similar future outbreaks.
The remainder of this paper has following components. In Section 2, we describe the details of this event from the onset and the way in which we have designed this study. Section 3 describes our findings, including the index case, risky exposure of SGs, the epi-curve of the outbreak, pre-existing medical problems of the SGs, the symptoms they experienced, and the identification of the variant of the virus. In Section 4, we discuss our findings in light of the existing literature, illustrate the factors that led to this outbreak, describe the study’s limitations, and propose preventive measures.
2. Materials and Methods
2.1. Setting
The SGs are based in a small building near the main entrance to the hospital where they rest and their administrative work is completed. We found that the events that led to this outbreak happened here.
2.2. Design
Ours is a retrospective descriptive study. People who tested positive for COVID-19 by RT-PCR with or without symptoms and by RAT with symptoms and a history of exposure 14 days after the second dose of a COVID-19 vaccine are considered to be vaccine breakthrough cases here [5]. We studied all 42 SGs at our hospital. Our study design covered the following aspects: tracing of high-risk contacts of all infected SGs 14 days previous (from the date of positive sample taken) adhering to criteria stipulated by the Ministry of Health, Sri Lanka [6]; tracing high-risk contacts of other SGs; determination of COVID-19 infection status of all high-risk contacts of the COVID-19 positive SGs within 10–15 days; creation of an epi-curve considering the date of onset of symptoms of COVID-19 cases; collection of details of pre-existing medical conditions; symptoms of COVID-19 cases using the relevant protocol of the Centers for Disease Control (CDC) of the United States and that in ref. [7]; scrutinization of the local cold chain; determination of reason/s that led to this outbreak; and potential preventive methods.
2.3. Details of the Event
On 10 July 2021, one symptomatic SG tested positive for COVID-19 by a RAT. Later, we identified her as the index case (IC) of this cluster. Within two hours, six other SGs came forward explaining that they had also developed similar symptoms and were exposed to the IC as well. Four of them tested positive for COVID-19 by RAT. Out of two SGs testing negative, one tested positive for COVID-19 by RT-PCR a day later with a cycle threshold (Ct) value of 14.94. The other SG tested negative by RT-PCR and by a repeated test 15 days later. After the preliminary contact tracing, all other (35) SGs were asked to undergo RT-PCR tests. Of these, 29 tested positive with Ct values of 15.37–38.62. Out of six SGs testing negative, two SGs later tested positive by RAT when they developed several symptoms. The other four SGs tested negative by repeated RT-PCRs performed 10–15 days after the first test.
We traced the contacts of the IC. Thereafter, for tracing risky contacts, we interviewed all SGs over the telephone over a period of a week and repeated the same procedure to verify the information provided. We cross checked with hospital vaccination records and verified that 40 SGs received two doses of vaccination.
3. Results
3.1. Index Case
Four days before testing positive for COVID-19, the index case (IC) developed symptoms. She was the first person of this cohort to develop symptoms. On day 3 of her illness, the IC went to the outpatient department (OPD) and was prescribed paracetamol, chlorpheniramine, and co-amoxiclav. With this treatment, her symptoms improved partially, and she continued to work. She commuted to work from a boarding house. There was another couple at this boarding house working at two workplaces. They tested positive for COVID-19 on day 3 and day 4 of the IC’s illness. The IC did not report her symptoms or exposure until we later asked her to. She and the other SGs were used to having tea and chatting with each other (without masks) in small rooms, often with all the windows closed. We did not detect any risky exposure on her part other than the exposure to her co-SGs and co-boarders within the 14 days before she developed symptoms.
3.2. Identified Risky Exposures
All SGs had generally complied with COVID-19 prevention guidelines while at their duty stations even after the completion of COVID-19 vaccination. However, they were slack regarding the risk of contracting infection from co-workers. All said that they believed the main risk to them was from COVID-19 patients coming into the hospital. In addition to having tea, they took communal meals in a small room with the windows often closed, sometimes chatting. During night shifts, male SGs sometimes sat close to each other in these rooms without masks, sometimes chatting. All of them were exposed to other SGs on every working day over the 14 days before they were tested. However, they could not accurately recall the names of all co-SGs to whom they were exposed, as several of them mingled with each other in a small space during tea and mealtimes. Two SGs took meals at the public canteen with others (e.g., visitors to the hospital) at the same table within seven days before being diagnosed. We did not find any high-risk contact of the IC or SGs with other hospital workers or patients. All SGs had no history of other high-risk exposure at public places or while using public transport within the 14 days of their first test. All except two SGs living alone had high-risk exposure to other people in their residences.
Two SGs of this cluster lived alone, the IC and one other lived in boarding houses with two other boarders in each place, and all the other SGs lived with their families. All (two) co-boarders of the IC tested positive for COVID-19 within the four days after the IC developed symptoms. Co-boarders of the other SG later tested negative. One or more family members tested positive for COVID-19 in the families of the eight SGs who lived with their families several days after their diagnosis. This includes one family member of an unvaccinated SG. No family member of any SG tested positive 14 days before his/her diagnosis or had COVID-19-like symptoms. Interestingly, the husband of one SG out of five tested negative by an initial RT-PCR test, then developed symptoms and tested positive for COVID-19 at his workplace one day after she tested negative. Nevertheless, she and their children tested negative by subsequent tests as well. This indicates that the infection of the husband was not related to this cluster. Considering all of this information, we identified the IC as the primary case of this cluster [8]. None of the other SGs who developed symptoms reported their COVID-19-like symptoms until the IC was diagnosed.
Among the fully vaccinated, 96% (26/27) of the men and 69% (9/13) of the women contracted COVID-19. None of the SGs was pregnant. The median age of the 35 vaccine breakthrough cases was 48 years (range 28–60 years). Of these, 14 were >50 years old. There were 34 ethnic Sinhalese and one ethnic Tamil. The median age of the five SGs testing negative with the first and repeated RT-PCR tests was 46 years (range 34–52 years). A total of 91% (32/35) of the breakthrough cases were symptomatic. Though some medical experts do not consider asymptomatic infections to be cases, here, we do consider them to be COVID-19 cases [9].
3.3. Epi-Curve
We have created an epi-curve (Figure 1) illustrating the day of onset of the symptoms of those who tested positive for COVID-19 and were symptomatic. This includes the two unvaccinated SGs as well. There were two COVID-19-positive asymptomatic SGs. One SG of this cluster tested positive by RT-PCR on July 16th with a Ct value of 33.56 but remained asymptomatic at a COVID-19 intermediate care center (ICC). He developed symptoms on 6 August. As symptoms progressed, he sought treatment, and on 10 August, he again underwent a RT-PCR test. The test was positive with a Ct value 14.40. We believe he had a reinfection (probably by Delta VOC = variants of concern) unrelated to this cluster. There are more details about this case in the Supplementary File 2.
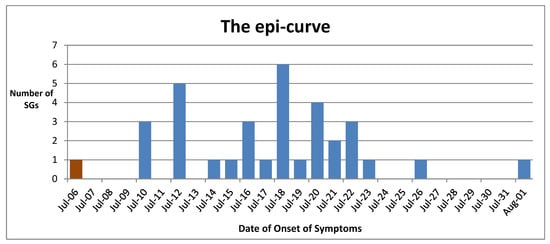
Figure 1.
The epi-curve. This depicts the dates of onset of the first symptoms of 34 symptomatic vaccine breakthrough cases and two unvaccinated cases. The index case is shown in maroon color. X-axis: date, Y-axis: the number of SGs who developed their first symptoms. The dates of onset of symptoms of two unvaccinated cases were 16 and 20 July.
3.4. Pre-Existing Medical Conditions
None of the 42 SGs had chronic kidney disease, chronic liver cell disease, autoimmune disease, HIV infection, active cancer, solid organ transplant, hematopoietic stem cell transplant, or were on any systemic immunosuppressive therapy. However, some of them were on treatment for diseases such as hypertension, dyslipidemia, and type 2 diabetes. More details about pre-existing medical conditions are in the Supplementary Files.
3.5. Description of Symptoms
Symptomatic and fully vaccinated SGs had various symptoms for various durations. Figure 2 illustrates a breakdown of these symptoms.
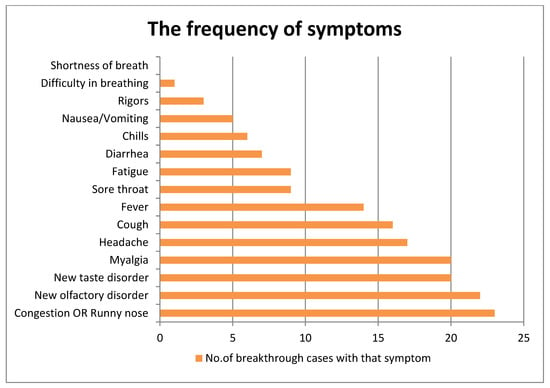
Figure 2.
The frequency of 15 symptoms of breakthrough cases. X-axis: number of breakthrough cases with that symptom, Y-axis: symptom.
Table 1 depicts how the number of breakthrough cases that developed and the number of symptoms that were seen.

Table 1.
Number of breakthrough cases; number of symptoms.
The two unvaccinated SGs had 8 and 10 symptoms. While at intermediate care centers, three SGs (including the two who were unvaccinated) developed severe symptoms and needed to be transferred to a hospital. All SGs recovered. Nonetheless, three SGs including the IC had newly developed shortness of breath and tightness of the chest when climbing a staircase as well as leg ache when inquired after 12 weeks after testing positive (i.e., indicating of long COVID-19), but those symptoms improved.
3.6. Maintenance of the Cold Chain
Vaccines were stored at our hospital for 1–2 days, and storage temperature was monitored. We checked and discovered no evidence of a break in the cold chain at the national, regional, or hospital level.
3.7. Identification of the Variant of the Virus
Four samples of patients whose RT-PCR Ct values were <20 were sent to be identified for a possible variant of concern by variant-spotting kits (not whole-genomic sequencing). All were identified as the Alpha variant of concern of SARS-CoV-2.
3.8. Further Information about SGs
Our Supplementary Files 1 and 2 provide further information on the SGs. Supplementary File 2 also describes some other breakthrough infections occurring at our hospital, including a smaller cluster.
4. Discussion
We concluded that our IC was also the primary case in this COVID-19 vaccine breakthrough cluster [8]. This outbreak illustrates that even basically healthy people who have received the second dose of ChAdOx1 nCoV-19, a widely accepted vaccine, only two months ago (i.e., they were fully vaccinated) could contract COVID-19, become symptomatic, and transmit infection to co-worker congregates and to others living in the same household, resulting in clusters with attack rates as high as 88% under conducive conditions. This underscores the importance of maintaining non-pharmacological interventions even among the fully vaccinated if the COVID-19 incidence in the community is high. We believe that our description of this outbreak should give some useful insights to readers about the potential risk of such outbreaks at their workplaces and the value of early detection of outbreaks to limit the attack rates to a small percentage.
When we observe the epi-curve, the pattern of multiple peaks is compatible with the pattern of a propagated source outbreak. Considering the information mentioned above, we concluded that this is probably a SARS-CoV-2 Alpha variant outbreak. From April to early August 2021, Alpha was the predominant variant circulating in many parts of Sri Lanka, including our hospital catchment area, Kandy [10,11,12].
Figure 3 depicts the proportions of the total number of sequences of the various variants of SARS-CoV-2 over time in Sri Lanka.
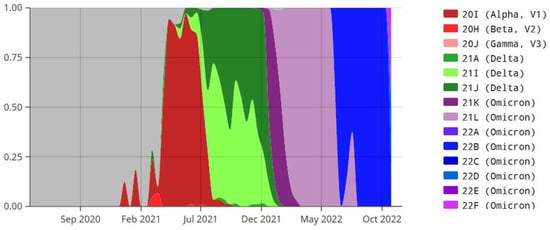
Figure 3.
Proportions of the total number of sequences of the various variants of SARS-CoV-2 over time in Sri Lanka. X-axis: month and year, Y-axis: proportion of various variants of SARS-CoV-2. Panel on the right side of this figure shows the color codes of the various variants of SARS-CoV-2. This figure was adopted from Emma B. Hodcroft. 2021. “CoVariants: SARS-CoV-2 Mutations and Variants of Interest.” https://covariants.org/, accessed on 28 January 2023. Created with CC BY4.0.
As seen in Figure 3, the Alpha VOC was dominant by 6 July 2021 (the date of onset of symptoms of the IC) in Sri Lanka, and there was a transition during July to the Delta VOC becoming dominant by 1 August 2021.
SARS-CoV-2-infected people are usually infectious during the first week of the infection [13]. Had the IC and all SGs who initially developed symptoms notified staff, the infection of many others could have been prevented. Our experience is that some healthcare workers (HCW) of all levels of hierarchy hesitate to report and seek treatment unless or until symptoms aggravate due to various combinations of the following: dislike for being isolated at an intermediate care center or at a hospital away from the family and comfort of the home (the home isolation of mild symptomatic cases started later in Sri Lanka); avoidance of ostracization if testing positive; fear of losing income during a financially difficult time (government hospital workers receive paid leave if they contract COVID-19, but some of them lose income from overtime work and private practice); prior commitments that require mobility; unfamiliarity with the possibility of vaccination breakthroughs; “it won’t happen to me” attitude; preoccupation with other problems or suffering from pandemic fatigue, thus ignoring their own health problems; and aversion to the hassle of testing procedures [10]. Our experience of COVID-19 preventive work among our 1900-odd workforce during the pandemic shows that we have to find novel ways to increase reporting of COVID-19-like symptoms by HCW who are knowledgeable but hesitant to report their symptoms due to the above reasons.
On day 3 of her illness, the IC and later another SG visited the hospital OPD. They were not tested for COVID-19, and a common symptomatic treatment was given. This partially relieved their symptoms, further delaying diagnosis and isolation. Unfamiliarity with the risk of vaccine breakthroughs have resulted in this experience. However, HCW known to us later (2022) became familiar with vaccine breakthrough COVID-19 cases. Similar clusters can occur during the initial wave of future epidemics of similar infections. Thus, awareness of this incident is important. Prescriptions to control symptoms without testing of COVID-19-like patients has continued and became widespread in Sri Lanka after mid-2022, mainly because testing facilities became very scarce and unaffordable due to the economic crisis of 2022. We recommend that first-contact doctors should have a high index of suspicion of COVID-19, even when a fully vaccinated patient presents with COVID-19-like symptoms, until the pandemic ends. In contrast to widely held initial presumptions about local exposure to patients causing this outbreak among SGs, we have demonstrated that SGs contracted the infection from colleagues. Other than this cluster (July 2021), 9 and 11 vaccine breakthrough COVID-19 cases were reported to us in June and July, respectively, among some 1800-plus vaccinated employees of our hospital.
Higher numbers of symptoms and lower Ct values have been associated with the subsequent development of clusters by people with COVID-19 infection [14,15]. The IC ultimately had nine symptoms, and the first eight other SGs who developed symptoms had eight, seven, seven, six, five, four, four, and three symptoms. We believe that the presence of many infectious people (13 had Ct values <30, and 10 had Ct values <25) in this congregate (some were tested a few days after the onset of symptoms) repeated exposures to them, which increased contact time among them, and the median age of 48 years also contributed to generating a cluster with an 88% (very high) attack rate [14,16,17]. According to a systematic review of systematic reviews on the COVID-19, fever associated with shortness of breath is the most important prognostic factor for disease progression [18]. None of our breakthrough cases had shortness of breath, and no one needed HDU or ICU care, despite 91% (32/35) being symptomatic.
4.1. Our Preventive Work among the Hospital Workers
Starting from January 2020, our HCW were educated on COVID-19 preventive measures by a multi-disciplinary team initially visiting each unit of the hospital, then through weekly COVID-19 steering committee meetings and unit-in-charge officers’ meetings. Our staff was regularly updated on the latest instructions for preventive measures sent by the Health Ministry via the aforementioned meetings and internal circulars. Our hospital workers were repeatedly reminded by internal circulars, announcements, and notices/posters to adhere to basic precautions against COVID-19 and to report if/when they developed COVID-19-like symptoms or were exposed to a COVID-19-like person at work or elsewhere. However, the compliance of SGs was suboptimal. During periods with high COVID-19 incidence, the vast majority of our workers adhered to some of our instructions, such as correctly wearing a surgical or KN95 mask. Usually, they were keen to protect themselves from contracting infection from patients, but some were slack regarding protection from vaccinated co-workers. For example, communal dining continued in many units. During 2022, the government gradually relaxed COVID-19 control restrictions. The regulation of mandatory face masks at indoor and outdoor public places was removed in June 2022.
Within a month after this episode, we made three surprise visits to the building in which SGs are based in order to check whether they had complied with our COVID-19 prevention advice. The first lunchtime visit proved that they still continued with old practices despite all the hardships they had undergone in the recent past, but during the last visit, we noticed improvements. This is an example of the importance of regular monitoring of the staff to improve their adherence to basic COVID-19 preventive measures. Nevertheless, we could not sustain regular monitoring.
4.2. Comparison of Our Findings with the Recent Literature Related to Our Study
Recent reviews and meta-analyses illustrate that vaccination reduces serious infection, death, and the risk of long COVID-19 [1,2,3,4,19]. None of the vaccine breakthrough cases among approximately 1800-plus vaccinated workers of our hospital died up to January 2023. This recapitulates the importance of COVID-19 vaccination to prevent serious illness and to save lives. After this episode, the hospital workers of Sri Lanka were offered two booster doses of BNT162b2 (Comirnaty) vaccine. Another systematic review and meta-analysis had different findings. They found that the unvaccinated were more susceptible to COVID-19 infection than those fully vaccinated for all VOCs. However, once infected, there were no statistically significant differences in the risk of hospitalization, invasive mechanical ventilation, or mortality [20].
There are interesting recent reports on vaccine breakthrough COVID-19 among HCW as well. In an Italian hospital during the January 2021–February 2022 period, among 5996 workers who received two vaccine doses, 582 breakthrough infections were observed, and 88% of those were symptomatic. There were no hospitalizations, deaths, or long COVID among the infected [21]. Our findings broadly agree with the findings of this study. Among 64,172 HCW who received two doses of vaccine in 12 European health centers, 797 breakthrough infections were observed up to mid-November 2021 (i.e., a cumulative incidence of 1.2%) [22]. This was before the emergence of the Omicron VOC, and the researchers concluded that vaccination is important for COVID-19 prevention, especially among HCW [22].
A recently published study followed up with 6496 HCW who received a primary vaccine series plus a booster dose in Greece for 22 weeks while the Delta and Omicron VOC were dominant and found 28.4 episodes of breakthrough infections per 100 HCW occurring with a mean gap of 16.2 weeks after the last vaccine dose [23]. In a prospective study conducted in Indonesia, 125 infection-naive and 10 previously infected HCWs who received their primary series of two vaccines were studied. An mRNA-1273 booster increased their anti-SARS-CoV-2 S-RBD antibodies significantly (September–October 2021). However, during the January to March 2022 Omicron wave, about 30% of HCW of both groups became infected but were asymptomatic or had only mild COVID-19 symptoms [24]. In comparison, throughout February 2022, during the peak of the first Omicron wave, 189 breakthrough infections with bothersome symptoms were reported to us from the 1800-plus HCWs of our hospital who had received their primary series vaccination with these SGs. A total of 152 of these 189 received a booster dose of BNT162b2 in early November 2021. Many such breakthroughs were not reported. We believe that there were many mild and asymptomatic cases as well. A systematic review revealed that vaccine effectiveness against infection and symptomatic disease decreased approximately 20–30 percentage points by 6 months [2]. Giving frequent boosters to vulnerable groups such as HCWs is difficult to implement, not sustainable, and the virus will evolve continuously. This indicates that there is a necessity of vaccines ideally providing sterilizing immunity against common VOCs or at least providing longer protection (e.g., the interval between boosters should be at least a year) and higher effectiveness. At present (January 2023), new bivalent COVD-19 vaccines are not available in Sri Lanka. Further, they may not provide the expected enhanced protection against VOC of SARS-CoV-2 [25]
There are no reports on vaccine breakthrough COVID-19 incidence in the general population of Sri Lanka. There is a recent study conducted among some 15.5 million fully vaccinated people in Britain, with a median follow-up time of 149 days. Only <4% of this population reported a vaccine breakthrough infection [26]. A prospective cohort study from Belgium based on data of over eight million fully vaccinated adults showed a breakthrough infection incidence of 11.2 per 100 person years [27].
We did not have access to tests for antibody titers and T cell immunity. A team from the only laboratory in Sri Lanka that could perform those tests has published a relevant paper [28]. Antibodies to the SARS-CoV-2 virus were assessed in two cohorts. One cohort was very similar to our SGs. A total of 336 HCW who received the same ChAdOx1 nCoV-19 vaccine 12 weeks apart were sampled 12 weeks after the second dose. All individuals had SARS-CoV-2-specific total antibodies (IgM, IgG, and IgA), and 94.2% had ACE2-blocking antibodies. Among different age groups, 41.2% to 65.8% had a positive response to the hemagglutination assay to the RBD of the ancestral virus and VOCs, including the Alpha VOC (B.1.1.7 (N501Y)). They had high T cell responses post vaccination. There was no significant difference between the different age groups in the responses to the RBDs of different VOCs in hemagglutination tests [28]. There was no problem in our cold chain. This indicates that our SGs would also have had a good immunity level in July 2021. A total of 69% (22/32) of symptomatic breakthrough cases of our cluster had loss of smell. Estimated global pooled prevalence of loss of smell among COVID-19 patients is 48.47% [29]. A recent meta-analysis demonstrated that COVID-19 patients with loss of smell are less likely to develop severe illness, and those authors describe a possible mechanism as well [30]. Accordingly, early SARS-CoV-2 infection and viral replication occurs in the upper respiratory tract. The cytokines generated by the immune response to this result in the apoptosis of olfactory receptor neurons [30]. No SG was immunocompromised. We can presume that most fully vaccinated SGs elicited a good immune response to the initial infection that caused a loss of smell sensation more frequently among them compared to the general population. Fever is a well-known inflammatory response. Interestingly, all 15 cases who had fever had loss of smell sensation as well. All in all, we believe that repeated exposures to high viral loads would have overwhelmed their immunity, infecting them. Previously, we explained how repeated exposure to high viral loads progressed.
A large British randomized controlled trial has shown that the clinical efficacy of a single dose of the ChAdOx1 nCoV-19 vaccine against symptomatic PCR-positive infection was 70·4% (95% CI 43.6–84.5) for Alpha and 81·5% (67.9–89.4) for non-Alpha lineages [31]. This indicates the fact that the Alpha variant was the responsible pathogen that also likely contributed to this outbreak.
There are many reports of COVID-19 clusters and vaccine breakthroughs in the literature. However, we did not find any report of a similarly sized vaccine breakthrough COVID-19 cluster among the fully vaccinated with an identified likely index case, a primary case, and a similar attack rate. There are two similar past reports. A 47-case cluster with a much lower attack rate occurred in spite of augmented preventive measures, leading to partial closure of a major hospital in Singapore [32]. A lesser percentage of those infected had at least one vaccine dose in that cluster. The Delta VOC was responsible for this outbreak. Those authors had more resources; hence, those authors managed to confirm epidemiological findings with viral genomic evidence. Like us, they controlled the spread by case isolation, extensive contact tracing, and quarantine measures. The index case and the primary case of that cluster were two different people, unlike in our cluster. A delay in the identification of the primary case (who was initially treated with antibiotics) contributed to this cluster as well. This group had better infection prevention facilities to prevent transmission while taking meals [32].
A similar 24-case cluster was reported in a gold mine in French Guiana. The attack rate was 60% (15/25) among miners fully vaccinated with the BNT162b2 vaccine. The Gamma VOC was responsible [33]. These mine workers lived in separate rooms but shared face-to-face meals and machine cabins [33].
Communal dining is a part of many cultures and has certain social and health benefits [34]. However, avoiding this practice when the incidence of COVID-19 and similar diseases is high in the locality can help to prevent clusters such as these.
Our Supplementary File 2 provides information about some vaccine breakthrough COVID-19 cases at our hospital (including a small cluster) that occurred after this outbreak. That information supports key messages of our discussion and conclusions.
5. Limitations
We did not have the facilities to perform whole-genome sequencing of the SARS-CoV-2 or for antibody testing, COVID-19 environmental tests of surfaces, or authentication of the SG’s statements by review of closed-circuit camera video footage. This limited us from further investigating and verifying of our findings. However, the World Health Organization’s interim guideline, Contact tracing in the context of COVID-19 -1 February 2021, which was the latest WHO guideline available to us at that time, states that “identifying the source of infection through backward tracing is key to detecting unrecognized chains of transmission and common points of exposure” [35]. Hence, ours is a reasonable but not an ideal method to identify the index case and transmission chain.
6. Conclusions
The key factors that led to this COVID-19 cluster included communal dining in cramped places, unfamiliarity with vaccine breakthrough cases (in July 2021), disregard of the risk of infection from fully vaccinated co-workers, hesitancy to report COVID-19-like symptoms early on, symptomatic treatment administered to COVID-19-like patients without testing for COVID-19 and their release back into society, the Alpha VOC of SARS-CoV-2, congested and poorly ventilated resting areas for workers, and repeated exposure to several infectious patients. These factors acted synergistically, resulting in a very high attack rate. The identification of such factors in readers’ workplaces and controlling them as far as possible will be helpful in preventing similar future outbreaks of COVID-19 and similar infections. Figure 4 graphically summarizes the factors that led to this outbreak.
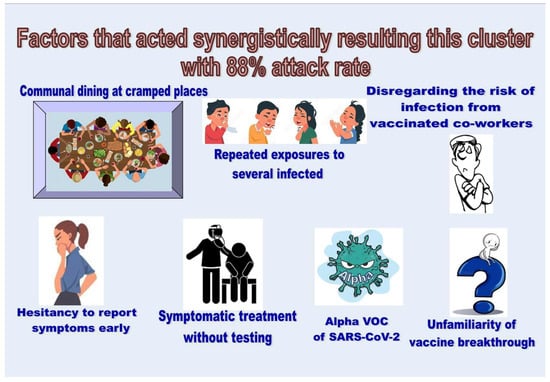
Figure 4.
Factors that acted synergistically resulting in this outbreak with an 88% attack rate.
At present, the local and global tendency is to relax non-pharmacological interventions for COVID-19 containment. A larger percentage of patients at hospitals have a weaker immune status compared to the general population and are thus susceptible to severe infections even from a few infectious HCW. Further studies will be helpful to determine the best and safest time to withdraw basic COVID-19 control measures at hospitals. Communal dining should be discouraged at workplaces with a high risk of COVID-19 transmission until the present pandemic is under control. Many experts are now (2023) aware of the individual factors that have led to this cluster as risk factors that are conducive to COVID-19 transmission. However, knowing that this particular combination of factors acted synergistically and that this sequence of events resulted a vaccine breakthrough COVID-19 cluster with an attack rate as high as 88% (35/40) will be useful in preempting similar clusters of COVID-19 and similar infections (especially respiratory infections with high basic reproduction numbers) in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid3020017/s1.
Author Contributions
Conceptualization, N.D.B.E.; Methodology, N.D.B.E.; Formal Analysis, N.D.B.E.; Investigation, N.D.B.E. and B.A.S.W.; Resources, N.D.B.E.; Data Curation, N.D.B.E. and B.A.S.W.; Writing—Original Draft Preparation, N.D.B.E.; Writing—Review and Editing, B.A.S.W.; Visualization, N.D.B.E.; Project Administration, N.D.B.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Review Committee of the Peradeniya Medical Faculty of Sri Lanka (2021/EC/78) on 4 October 2021.
Informed Consent Statement
This study was approved by the Ethics Review Committee of the Peradeniya medical faculty of Sri Lanka (2021/EC/78). Written informed consent from all those relevant was obtained individually for publication of their anonymized data.
Data Availability Statement
All data relevant to the study are in the manuscript and the two supplementary files.
Acknowledgments
The authors thank all who cooperated in investigating this cluster and in testing, as well as those who helped us to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O'Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.R.; Kobayashi, T.; Suzuki, H.; Alsuhaibani, M.; Schweizer, M.L.; Diekema, D.J.; Tofaneto, B.M.; Bariani, L.M.; Auler, M.d.A.; Salinas, J.L.; et al. The long-term effectiveness of coronavirus disease 2019 (COVID-19) vaccines: A systematic literature review and meta-analysis. Antimicrob. Steward. Health Epidemiol. 2022, 2, e22. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Chen, X.; Zheng, C.; Liu, H.; Wang, G.; Zhang, B.; Li, Z.; Zhang, W. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: A literature review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Niyas, V.K.; Arjun, R. Breakthrough COVID-19 infections among health care workers after two doses of ChAdOx1 nCoV-19 vaccine. QJM Int. J. Med. 2021, 114, 757–758. [Google Scholar] [CrossRef]
- The Secretary-Ministry of Health of Sri Lanka. Screening and Management of Healthcare Workers Following Exposure to a Confirmed/Suspected Case of COVID-19 (Updated-20th October 2020); Ministry of Health-Sri Lanka: Colombo, Sri Lanka, 2020; No: EPID/400/2019nCov dated 02/11/2020. [Google Scholar]
- Centers for Disease Control of the USA. Public Health Investigations of COVID-19 Vaccine Breakthrough Cases—Case Investigation Protocol. 2021. Available online: https://www.cdc.gov/vaccines/covid-19/downloads/COVID-vaccine-breakthrough-case-investigations-protocol.pdf (accessed on 25 November 2022).
- Giesecke, J. Primary and index cases. Lancet 2014, 384, 2024. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(14)62331-X/fulltext (accessed on 26 January 2023). [CrossRef]
- Gao, W.; Lv, J.; Pang, Y.; Li, L.-M. Role of asymptomatic and pre-symptomatic infections in COVID-19 pandemic. BMJ 2021, 375, n2342. [Google Scholar] [CrossRef]
- Ehelepola, N.D.B.; Wijewardana, B.A.S. An episode of transmission of COVID-19 from a vaccinated healthcare worker to co-workers. Infect. Dis. 2021, 54, 297–302. [Google Scholar] [CrossRef]
- Jeewandara, C.; Jayathilaka, D.; Ranasinghe, D.; Hsu, N.S.; Ariyaratne, D.; Jayadas, T.T.; Arachchige, D.M.P.; Lindsey, B.B.; Gomes, L.; Parker, M.D.; et al. Genomic and Epidemiological Analysis of SARS-CoV-2 Viruses in Sri Lanka. Front. Microbiol. 2021, 12, 722838. [Google Scholar] [CrossRef]
- Allergy, Immunology and Cell Biology Unit of the Department of Immunology and Molecular Medicine of the University of Sri Jayewardenepura, Sri Lanka. USJ Researchers Found Four Mutant Covid Delta Variants with Two New Sri Lankan Mutations (A701S, R24C). University of Sri Jayewardenepura. 30 August 2021. Available online: https://www.sjp.ac.lk/news/usj-researchers-found-four-mutant-covid-delta-variants-with-two-new-sri-lankan-mutations-a701s-r24c/ (accessed on 26 January 2023).
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Alishaq, M.; Nafady-Hego, H.; Jeremijenko, A.; Al Ajmi, J.A.; Elgendy, M.; Vinoy, S.; Fareh, S.B.; Plaatjies, J.V.; Nooh, M.; Alanzi, N.; et al. Risk factors for breakthrough SARS-CoV-2 infection in vaccinated healthcare workers. PLoS ONE 2021, 16, e0258820. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.E.; Frankel, D.N.; Pawlak, M.T.; Casey, T.M.; Cybulski, R.J.; Enriquez, E.; Okulicz, J.F.; Yun, H.C. Risk Factors Associated With COVID-19 Transmission Among US Air Force Trainees in a Congregate Setting. JAMA Netw. Open 2021, 4, e210202. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-Y.; Jian, S.-W.; Liu, D.-P.; Ng, T.-C.; Huang, W.-T.; Lin, H.-H.; The Taiwan COVID-19 Outbreak Investigation Team. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern. Med. 2020, 180, 1156–1163. [Google Scholar] [CrossRef]
- Rosser, J.I.; Tayyar, R.; Giardina, R.; Kolonoski, P.; Kenski, D.; Shen, P.; Steinmetz, L.M.; Hung, L.-Y.; Xiao, W.; Bains, K.; et al. Case-control study evaluating risk factors for SARS-CoV-2 outbreak amongst healthcare personnel at a tertiary care center. Am. J. Infect. Control. 2021, 49, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Hatmi, Z.N. A Systematic Review of Systematic Reviews on the COVID-19 Pandemic. SN Compr. Clin. Med. 2021, 3, 419–436. [Google Scholar] [CrossRef]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Impact of COVID-19 vaccination on long COVID: A systematic review and meta-analysis. medRxiv 2022. [Google Scholar] [CrossRef]
- Lee, C.J.; Woo, W.; Kim, A.Y.; Yon, D.K.; Lee, S.W.; Koyanagi, A.; Kim, M.S.; Tizaoui, K.; Dragioti, E.; Radua, J.; et al. Clinical manifestations of COVID-19 breakthrough infections: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 4234–4245. [Google Scholar] [CrossRef]
- De Maria, L.; Sponselli, S.; Caputi, A.; Stefanizzi, P.; Pipoli, A.; Giannelli, G.; Delvecchio, G.; Tafuri, S.; Inchingolo, F.; Migliore, G.; et al. SARS-CoV-2 Breakthrough Infections in Health Care Workers: An Italian Retrospective Cohort Study on Characteristics, Clinical Course and Outcomes. J. Clin. Med. 2023, 12, 628. [Google Scholar] [CrossRef]
- Porru, S.; Monaco, M.G.L.; Spiteri, G.; Carta, A.; Pezzani, M.D.; Lippi, G.; Gibellini, D.; Tacconelli, E.; Vecchia, I.D.; Sala, E.; et al. SARS-CoV-2 Breakthrough Infections: Incidence and Risk Factors in a Large European Multicentric Cohort of Health Workers. Vaccines 2022, 10, 1193. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Gamaletsou, M.N.; Giannouchos, T.V.; Koukou, D.-M.; Karapanou, A.; Sourri, F.; Syrimi, N.; Lemonakis, N.; Peskelidou, E.; Papanastasiou, K.; et al. Timing of last COVID-19 vaccine dose and SARS-CoV-2 breakthrough infections in fully (boosted) vaccinated healthcare personnel. J. Hosp. Infect. 2022, 132, 46–51. [Google Scholar] [CrossRef]
- Santi, T.; Kamarga, L.; De Samakto, B.; Jo, J. SARS-CoV-2 Breakthrough Infection after mRNA-1273 Booster among CoronaVac-Vaccinated Healthcare Workers. Infect. Chemother. 2022, 54, 774–780. [Google Scholar] [CrossRef]
- Offit, P.A. Bivalent COVID-19 Vaccines–A Cautionary Tale. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef]
- The Open SAFELY Collaborative; Green, A.; Curtis, H.; Hulme, W.; Williamson, E.; McDonald, H.; Bhaskaran, K.; Rentsch, C.; Schultze, A.; MacKenna, B.; et al. Describing the population experiencing COVID-19 vaccine breakthrough following second vaccination in England: A cohort study from OpenSAFELY. BMC Med. 2022, 20, 1–14. [Google Scholar] [CrossRef]
- Stouten, V.; Hubin, P.; Haarhuis, F.; van Loenhout, J.A.F.; Billuart, M.; Brondeel, R.; Braeye, T.; Van Oyen, H.; Wyndham-Thomas, C.; Catteau, L. Incidence and Risk Factors of COVID-19 Vaccine Breakthrough Infections: A Prospective Cohort Study in Belgium. Viruses 2022, 14, 802. [Google Scholar] [CrossRef]
- Jeewandara, C.; Aberathna, I.S.; Gomes, L.; Pushpakumara, P.D.; Danasekara, S.; Guruge, D.; Ranasinghe, T.; Gunasekera, B.; Kamaladasa, A.; Kuruppu, H.; et al. Kinetics of immune responses to the AZD1222/Covishield vaccine with varying dose intervals in Sri Lankan individuals. Immun. Inflamm. Dis. 2022, 10, e592. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, T.S.; Fasunla, A.J.; Orimadegun, A.E. Systematic Review and Meta-analysis of Smell and Taste Disorders in COVID-19. OTO Open 2020, 4, 2473974X20957975. [Google Scholar] [CrossRef]
- Purja, S.; Shin, H.; Lee, J.-Y.; Kim, E. Is loss of smell an early predictor of COVID-19 severity: A systematic review and meta-analysis. Arch. Pharmacal Res. 2021, 44, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Lim, W.-Y.; Tan, G.; Htun, H.; Phua, H.; Kyaw, W.; Guo, H.; Cui, L.; Mak, T.; Poh, B.; Wong, J.; et al. First nosocomial cluster of COVID-19 due to the Delta variant in a major acute care hospital in Singapore: Investigations and outbreak response. J. Hosp. Infect. 2022, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Vignier, N.; Bérot, V.; Bonnave, N.; Peugny, S.; Ballet, M.; Jacoud, E.; Michaud, C.; Gaillet, M.; Djossou, F.; Blanchet, D.; et al. Breakthrough Infections of SARS-CoV-2 Gamma Variant in Fully Vaccinated Gold Miners, French Guiana, 2021. Emerg. Infect. Dis. 2021, 27, 2673–2676. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. Breaking Bread: The Functions of Social Eating. Adapt. Hum. Behav. Physiol. 2017, 3, 198–211. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Contact Tracing in the Context of COVID-19—Interim Guidance. 1 February 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/339128/WHO-2019-nCoV-Contact_Tracing-2021.1-eng.pdf?sequence=24&isAllowed=y (accessed on 28 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).