Assessing the Vaccine Efficacy in Health Care Providers for Combating the COVID-19 Infection: Results from Tertiary Cancer Care Centre
Abstract
:1. Introduction
2. Methodology
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Statistical Analysis
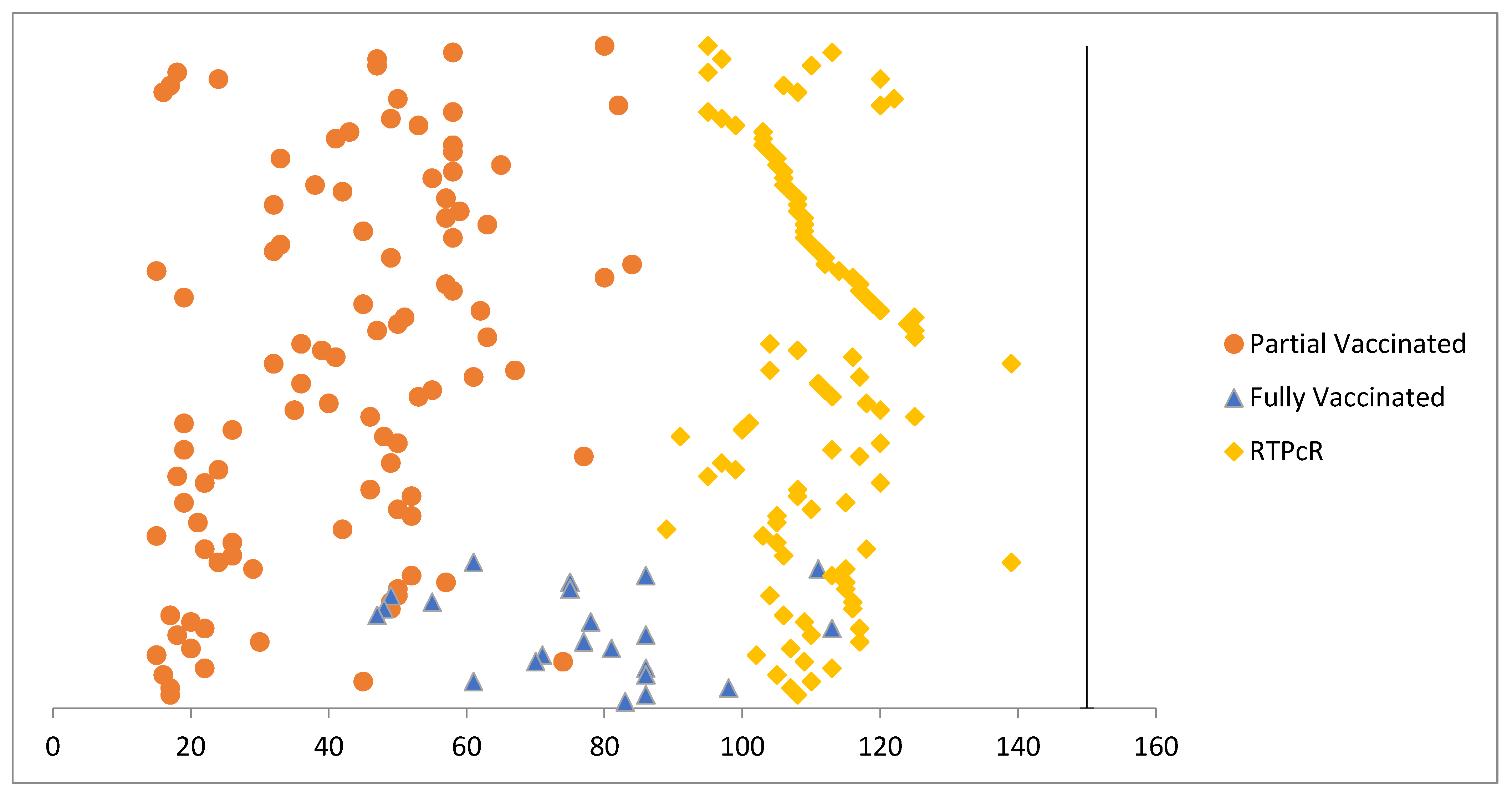
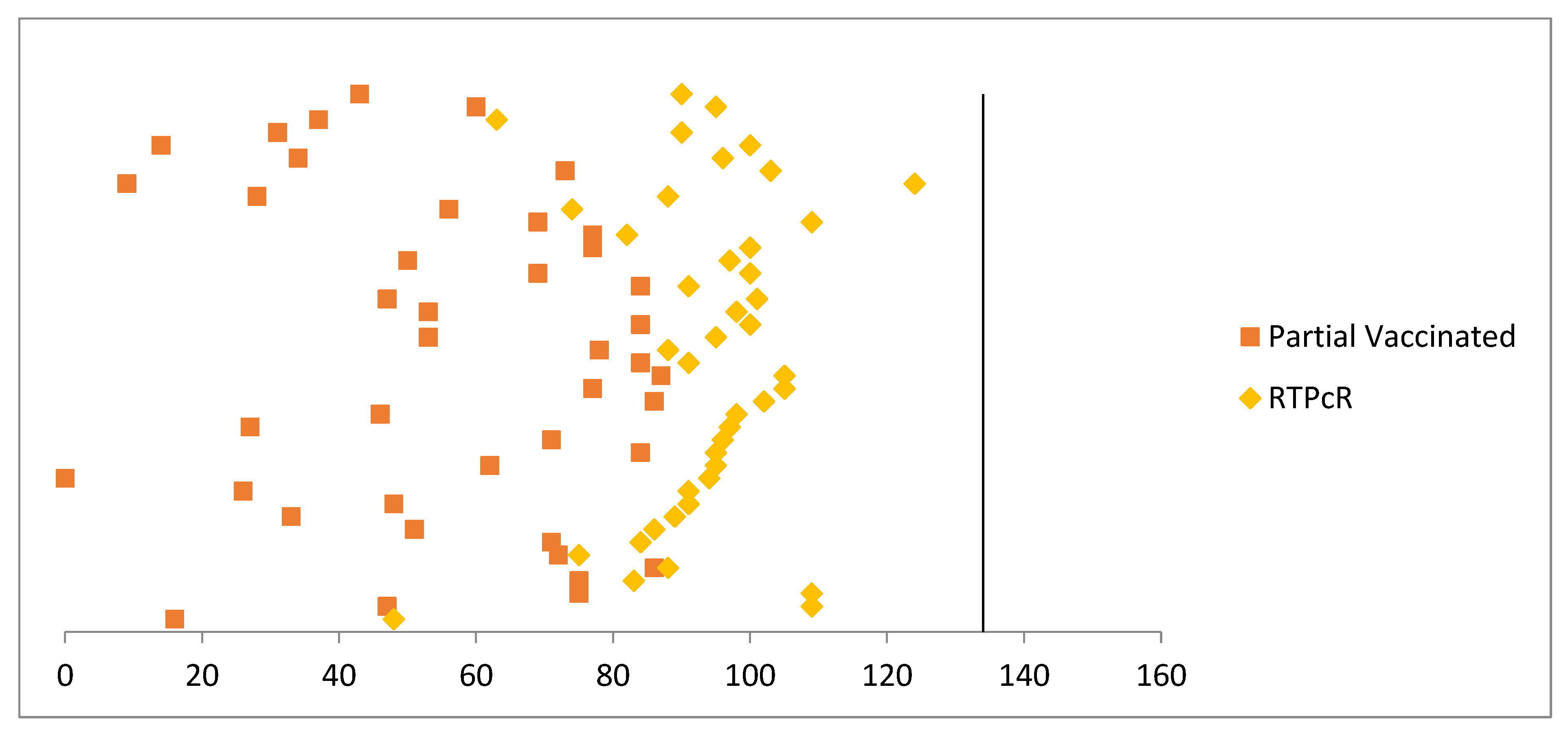
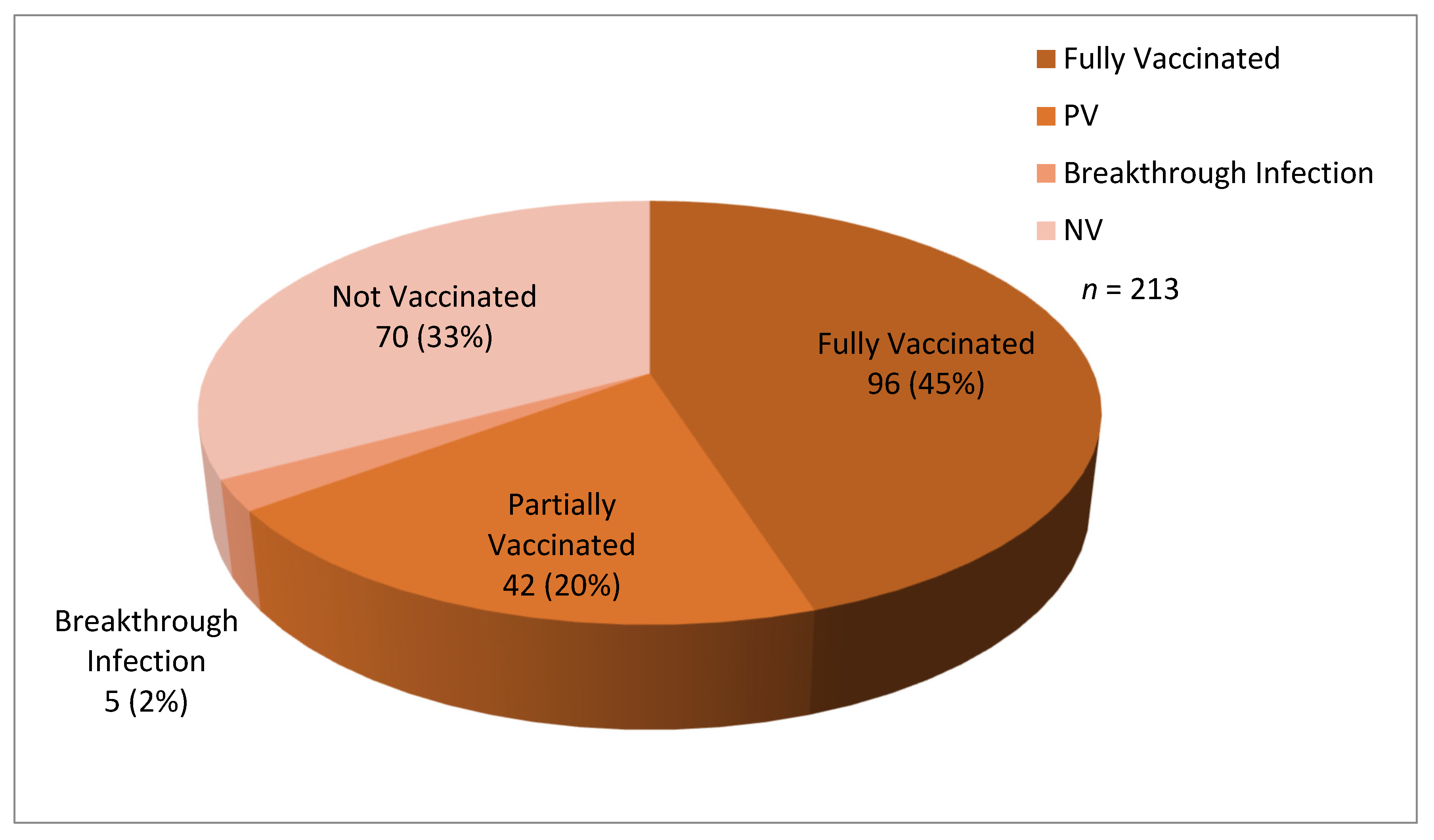
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, M.M.; Groenewold, M.R.; Lessem, S.E.; Xu, K.; Ussery, E.N.; Wiegand, R.E.; Qin, X.; Do, T.; Thomas, D.; Tsai, S.; et al. Update: Characteristics of health care personnel with COVID-19—United States, February 12–July 16, 2020 MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Adaptive Phase IB-II Randomized Clinical Trial of Preventive Vaccine Consisting of Autologous Dendritic Cells Loaded with Antigens from Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), with or without GM-CSF, in Subjects Negative for COVID-19 Infection and Anti-SARS-CoV-2 Antibodies. ClinicalTrials.gov Identifier (NCT Number): NCT04386252. Available online: https://clinicaltrials.gov/ct2/show/NCT04386252 (accessed on 11 December 2020).
- A Randomized, Double-Blind, Placebo-Bontrolled Phase 3 Study to Assess the Efficacy and Safety of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adults Aged 18 Years and Older. ClinicalTrials.gov Identifier: NCT04505722. Available online: https://clinicaltrials.gov/ct2/show/NCT04505722 (accessed on 11 December 2020).
- A Phase 2a, Randomized, Observer-Blind, Placebo Controlled, Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 SARS-CoV-2 Vaccine in Adults Aged 18 Years and Older. ClinicalTrials.gov Identifier: NCT04405076. Available online: https://clinicaltrials.gov/ct2/show/NCT04405076 (accessed on 11 December 2020).
- A Phase 1/Phase 2, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Trial to Evaluate the Safety, Tolerability and Immunogenicity of V591 (COVID-19 Vaccine) in Healthy Younger and Older Participants. ClinicalTrials.gov Identifier: NCT04498247. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04498247 (accessed on 11 December 2020).
- Phadke, V.K.; Bednarczyk, R.A.; Salmon, D.A.; Omer, S.B. Association between Vaccine Refusal and Vaccine-Preventable Diseases in the United States. JAMA 2016, 315, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Dooling, K.; Marin, M.; Wallace, M.; McClung, N.; Chamberland, M.; Lee, G.M.; Talbot, H.K.; Romero, J.R.; Bell, B.P.; Oliver, S.E. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020 MMWR. Morb. Mortal. Wkly. Rep. 2021, 69, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Explorer. 2021. Available online: https://worldhealthorg.shinyapps.io/covid/ (accessed on 5 June 2021).
- World Health Organization. Interim Recommendations for Use of the ChAdOx1-S [Recombinant] Vaccine against COVID-19 (AstraZeneca COVID-19 Vaccine AZD1222, SII Covishield, SK Bioscience). 2021. Available online: https://apps.who.int/iris/rest/bitstreams/1343289/retrieve (accessed on 16 July 2021).
- Ministry of Health and Family Welfare, Government of India. CoWIN Dashboard. Available online: https://dashboard.cowin.gov.in/ (accessed on 30 June 2021).
- Two Doses of COVID-19 Vaccine Can Help Battle Breakthrough Infections. Available online: https://www.deccanherald.com/national/two-doses-of-covid-19-vaccine-can-help-battle-breakthrough-infections-994195.html (accessed on 5 June 2021).
- Nixon, D.F.; Ndhlovu, L.C. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 385, e7. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl 1.), S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bhadada, S.K.; Misra, A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Breakthrough Infections in Those Vaccinated May Be Higher in India, Finds Study. Available online: https://www.newindianexpress.com/nation/2021/jun/05/breakthrough-infections-in-those-vaccinated-may-be-higher-in-india-finds-study-2312223.html (accessed on 6 June 2021).
- Teran, R.A.; Walblay, K.A.; Shane, E.L.; Xydis, S.; Gretsch, S.; Gagner, A.; Samala, U.; Choi, H.; Zelinski, C.; Black, S.R. SARS-CoV-2 infections among skilled nursing facility residents and staff members—Chicago, Illinois, December 2020–March 2021. Am. J. Transplant. 2021, 21, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; De Marco, A.; Ferreira, I.A.; Meng, B.; Datir, R.P.; Walls, A.C.; Kemp, S.A.; Bassi, J.; Pinto, D.; Silacci-Fregni, C.; et al. Sensitivity of SARS-CoV-2 B. 1.1.7 to mRNA vaccine-elicited antibodies. Nature 2021, 593, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Loes, A.N.; Crawford, K.H.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. Cell Host Microbe 2021, 29, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Weisblum, Y.; Schmidt, F.; Zhang, F.; DaSilva, J.; Poston, D.; Lorenzi, J.C.; Muecksch, F.; Rutkowska, M.; Hoffmann, H.H.; Michailidis, E.; et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020, 9, e61312. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Starr, T.N.; Gilchuk, P.; Zost, S.J.; Binshtein, E.; Loes, A.N.; Hilton, S.K.; Huddleston, J.; Eguia, R.; Crawford, K.H.; et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe 2021, 29, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.N.; Greaney, A.J.; Addetia, A.; Hannon, W.W.; Choudhary, M.C.; Dingens, A.S.; Li, J.Z.; Bloom, J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science 2021, 371, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Denis, K.S.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Olson, S.M.; Self, W.H.; Talbot, H.K.; Lindsell, C.J.; Steingrub, J.S.; Shapiro, N.I.; Ginde, A.A.; Douin, D.J.; Prekker, M.E.; et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021 MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Pollock, B.D.; Shah, N.D.; Farrugia, G.; Virk, A.; Swift, M.; Breeher, L.; Binnicker, M.; Berbari, E.F. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening. Clin. Infect. Dis. 2021, 74, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.D.; Breeher, L.E.; Tande, A.J.; Tommaso, C.P.; Hainy, C.M.; Chu, H.; Murad, M.H.; Berbari, E.F.; Virk, A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin. Infect. Dis. 2021, 73, e1376–e1379. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
| S.No. | Variables | Mean ± S.D/Percentage |
|---|---|---|
| 1. | Age (Years) | 32.34 ± 9.04 |
| 2. | Sex | |
| 625 (62.31%) | |
| 378 (37.68%) | |
| 3. | Vaccination status: | |
| 599 (76.69%) | |
| 182 (23.3%) | |
| 193 (24.71%) | |
| 29 (3.71%) | |
| Total | 1003 |
| Vaccination | Degree of COVID-19 Infection | p-Value | Admission Status | Mean ± S.D | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Critical | Home Isolation | Admitted | ||||
| Not Vaccinated (70/193) | 41 (58.57) | 23 (32.86) | 5 (7.14) | 1(1.43) | 0.207 | 66 (94.29) | 4 (5.71) | 0.967 | |
| Partially Vaccinated (42/182) | 27 (64.29) | 13 (30.95) | 2 (4.76) | 0(0) | 40 (95.24) | 2 (4.76) | 32.50 ± 22.18 | <0.001 | |
| Fully Vaccinated (101/599) | 75 (74.26) | 19 (18.81) | 7 (6.93) | 0(0) | 96 (95.05) | 5 (4.95) | 54.41 ± 26.59 | ||
| Total | 213 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnihotri, S.; Mehta, A.; Sharma, A. Assessing the Vaccine Efficacy in Health Care Providers for Combating the COVID-19 Infection: Results from Tertiary Cancer Care Centre. COVID 2023, 3, 238-245. https://doi.org/10.3390/covid3020018
Agnihotri S, Mehta A, Sharma A. Assessing the Vaccine Efficacy in Health Care Providers for Combating the COVID-19 Infection: Results from Tertiary Cancer Care Centre. COVID. 2023; 3(2):238-245. https://doi.org/10.3390/covid3020018
Chicago/Turabian StyleAgnihotri, Shalini, Anurag Mehta, and Anurag Sharma. 2023. "Assessing the Vaccine Efficacy in Health Care Providers for Combating the COVID-19 Infection: Results from Tertiary Cancer Care Centre" COVID 3, no. 2: 238-245. https://doi.org/10.3390/covid3020018
APA StyleAgnihotri, S., Mehta, A., & Sharma, A. (2023). Assessing the Vaccine Efficacy in Health Care Providers for Combating the COVID-19 Infection: Results from Tertiary Cancer Care Centre. COVID, 3(2), 238-245. https://doi.org/10.3390/covid3020018








