Association between SARS-CoV-2 Infection and Neuropsychiatric Manifestations
Abstract
:1. Introduction
2. Materials and Methods
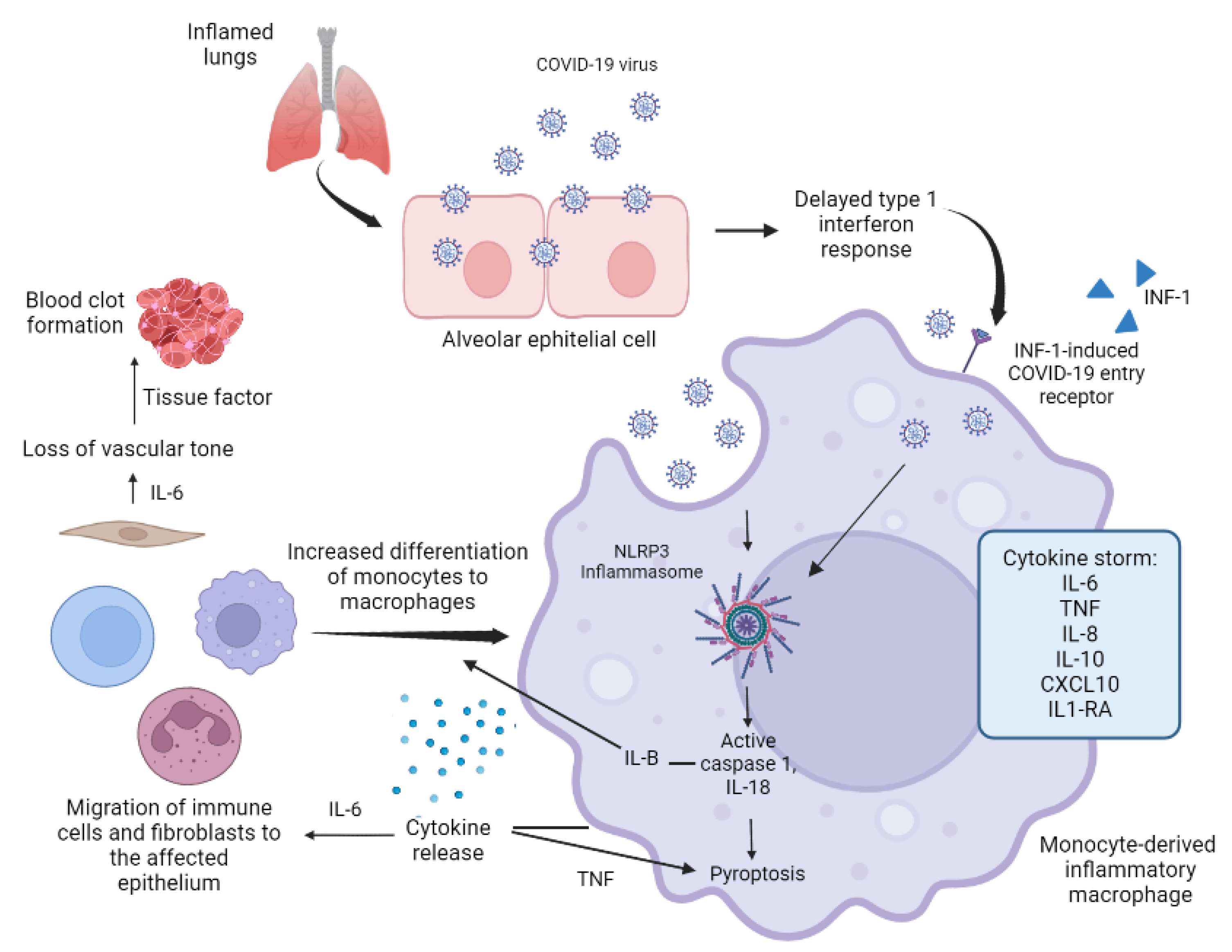
3. COVID-19 and the Cascade of Cytokines
3.1. Pathogenesis of Cytokine Storm
3.2. COVID-19 and the Central Nervous System
4. Neuropsychiatric Manifestations Secondary to COVID-19 Infection
4.1. Sleep Disorders
4.2. Anxiety and Depression
4.3. Acute Psychotic Disorder and Manic Disorder
4.4. Delirium and Confusional State
4.5. Anosmia and Ageusia
4.6. Post-Traumatic Stress Disorder
4.7. Encephalitis and Encephalopathies
5. Chronic Neuropsychiatric Conditions
6. Psychopharmaceuticals as Possible Adjuvant Therapy against SARS-CoV-2
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 13 July 2022).
- Halabe, J.; Robledo, Z.; Fajardo, G. Síndrome Post-COVID-10 Certezas E Interrogantes, 1st ed.; Editorial Medica Panamericana: Ciudad de México, Mexico, 2022. [Google Scholar]
- Tripathy, S.; Singh, N.; Singh, A.; Kar, S.K. COVID-19 and psychotic symptoms: The view from psychiatric immunology. Curr. Behav. Neurosci. Rep. 2021, 8, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Zaki, N.F.W.; Qasim, M.; Morsy, N.E.; Dilshad, M.D.M.; Charney, D.S.; Seeman, M.V. Neuropsychiatric consequences of COVID-19 pandemic: A synthetic review from a global perspective. Alpha Psychiatry 2022, 23, 144–154. [Google Scholar] [CrossRef]
- Robles, R.; Rodríguez, E.; Vega-Ramírez, H.; Álvarez-Icaza, D.; Madrigal, E.; Durand, S.; Morales-Chainé, S.; Astudillo, C.; Real-Ramírez, J.; Medina-Mora, M.-E.; et al. Mental health problems among healthcare workers involved with the COVID-19 outbreak. Rev. Bras. Psiquiatr. 2021, 43, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Sheikh, B.; Belzie, L. New-Onset Psychosis Following COVID-19 Infection. Cureus 2021, 13, e17904. [Google Scholar] [CrossRef]
- Bakre, S.; Chugh, K.; Oke, O.; Kablinger, A. COVID-19 Induced Brief Psychotic Disorder: A Case Report and Review of Literature. Case Rep. Psychiatry 2022, 2022, 9405630. [Google Scholar] [CrossRef]
- Hassan, L.; Peek, N.; Lovell, K.; Carvalho, A.F.; Solmi, M.; Stubbs, B.; Firth, J. Disparities in COVID-19 infection, hospitalization and death in people with schizophrenia, bipolar disorder, and major depressive disorder: A cohort study of the UK Biobank. Mol. Psychiatry 2022, 27, 1248–1255. [Google Scholar] [CrossRef]
- Lam, M.H.-B.; Wing, Y.-K.; Yu, M.W.-M.; Leung, C.-M.; Ma, R.C.W.; Kong, A.P.S.; So, W.Y.; Fong, S.Y.-Y.; Lam, S.-P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up: Long-term follow-up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef]
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39. [Google Scholar] [CrossRef]
- Chacko, M.; Job, A.; Caston, F., III; George, P.; Yacoub, A.; Cáceda, R. COVID-19-Induced Psychosis and Suicidal Behavior: Case Report. SN Compr. Clin. Med. 2020, 2, 2391–2395. [Google Scholar] [CrossRef]
- Banerjee, D.; Viswanath, B. Neuropsychiatric manifestations of COVID-19 and possible pathogenic mechanisms: Insights from other coronaviruses. Asian J. Psychiatry 2020, 54, 102350. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-Month neurological and psychiatric outcomes in 236,379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Herdoiza-Arroyo, P.E.; Arriaga, R.J.M.; Valerio, E.B.; Mollá, J.M.; de la Rosa-Gómez, A.; Farfallini, L.; Jiménez, M.J.H.; Santoveña, E.E.E.; Ramírez-Martínez, F.R.; et al. Prevalence of anxiety symptoms and associated clinical and sociodemographic factors in Mexican adults seeking psychological support for grief during the COVID-19 pandemic: A cross-sectional study. Front. Psychiatry 2022, 13, 749236. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, S.J.; Klepacz, L.; Lynch, S.; Tavakkoli, M.; Dornbush, R.; Baharani, R.; Smolin, Y.; Bartell, A. COVID-19 Psychosis: A Potential New Neuropsychiatric Condition Triggered by Novel Coronavirus Infection and the Inflammatory Response? Psychosomatics 2020, 61, 551–555. [Google Scholar] [CrossRef]
- Ravi, M.; Miller, A.H.; Michopoulos, V. The immunology of stress and the impact of inflammation on the brain and behavior. BJPsych Adv. 2021, 27 (Suppl. S3), 158–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Pearce, L.; Davidson, S.M.; Yellon, D.M. The cytokine storm of COVID-19: A spotlight on prevention and protection. Expert Opin. Ther. Targets 2020, 24, 723–730. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Isung, J.; Aeinehband, S.; Mobarrez, F.; Nordström, P.; Runeson, B.; Asberg, M.; Piehl, F.; Jokinen, J. High interleukin-6 and impulsivity: Determining the role of endophenotypes in attempted suicide. Transl. Psychiatry 2014, 4, e470. [Google Scholar] [CrossRef] [Green Version]
- Vega-Fernández, J.A.; Suclupe-Campos, D.O.; Aguilar-Gamboa, F.R. Neurological damage in SARS-CoV-2 infections. J. Fac. Hum. Med. 2021, 21, 387–398. [Google Scholar] [CrossRef]
- Maes, M.; Junior, W.L.D.T.; Lozovoy, M.A.B.; Mori, M.T.E.; Danelli, T.; de Almeida, E.R.D.; Tejo, A.M.; Tano, Z.N.; Reiche, E.M.V.; Simão, A.N.C. In COVID-19, NLRP3 inflammasome genetic variants are associated with critical disease and these effects are partly mediated by the sickness symptom complex: A nomothetic network approach. Mol. Psychiatry 2022, 27, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- van Vuren, E.J.; Steyn, S.F.; Brink, C.B.; Möller, M.; Viljoen, F.P.; Harvey, B.H. The neuropsychiatric manifestations of COVID-19: Interactions with psychiatric illness and pharmacological treatment. Biomed. Pharmacother. 2021, 135, 111200. [Google Scholar] [CrossRef] [PubMed]
- Steardo, L., Jr.; Steardo, L.; Verkhratsky, A. Psychiatric face of COVID-19. Transl. Psychiatry 2020, 10, 261. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Pallanti, S.; Grassi, E.; Makris, N.; Gasic, G.P.; Hollander, E. Neurocovid-19: A clinical neuroscience-based approach to reduce SARS-CoV-2 related mental health sequelae. J. Psychiatr. Res. 2020, 130, 215. [Google Scholar] [CrossRef]
- Na Zeng, N.; Zhao, Y.-M.; Yan, W.; Li, C.; Lu, Q.-D.; Liu, L.; Ni, S.-Y.; Mei, H.; Yuan, K.; Le Shi, L.; et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: Call for research priority and action. Mol. Psychiatry 2022, 1–11. [Google Scholar] [CrossRef]
- Merikanto, I.; Kortesoja, L.; Benedict, C.; Chung, F.; Cedernaes, J.; Espie, C.A.; Morin, C.M.; Dauvilliers, Y.; Partinen, M.; De Gennaro, L.; et al. Evening-types show highest increase of sleep and mental health problems during the COVID-19 pandemic-multinational study on 19,267 adults. Sleep 2022, 45, zsab216. [Google Scholar] [CrossRef]
- Partinen, M.; Holzinger, B.; Morin, C.M.; Espie, C.; Chung, F.; Penzel, T.; Benedict, C.; Bolstad, C.J.; Cedernaes, J.; Chan, R.N.Y.; et al. Sleep and daytime problems during the COVID-19 pandemic and effects of coronavirus infection, confinement and financial suffering: A multinational survey using a harmonised questionnaire. BMJ Open 2021, 11, e050672. [Google Scholar] [CrossRef]
- Mônico-Neto, M.; Dos Santos, R.V.T.; Moreira Antunes, H.K. The world war against the COVID-19 outbreak: Don’t forget to sleep! J. Clin. Sleep Med. 2020, 16, 1215. [Google Scholar] [CrossRef]
- Jahrami, H.; BaHammam, A.S.; Bragazzi, N.L.; Saif, Z.; Faris, M.; Vitiello, M.V. Sleep problems during the COVID-19 pandemic by population: A systematic review and meta-analysis. J. Clin. Sleep Med. 2021, 17, 299–313. [Google Scholar] [CrossRef]
- Lai, J.; Ma, S.; Wang, Y.; Cai, Z.; Hu, J.; Wei, N.; Wu, J.; Du, H.; Chen, T.; Li, R.; et al. Factors associated with mental health outcomes among health care workers exposed to Coronavirus disease 2019. JAMA Netw. Open 2020, 3, e203976. [Google Scholar] [CrossRef]
- Merikanto, I.; Dauvilliers, Y.; Chung, F.; Holzinger, B.; De Gennaro, L.; Wing, Y.K.; Korman, M.; Partinen, M. Disturbances in sleep, circadian rhythms and daytime functioning in relation to coronavirus infection and Long-COVID—A multinational ICOSS study. J. Sleep Res. 2022, 31, e13542. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.; Gorwood, P. The consequences of the COVID-19 pandemic on mental health and implications for clinical practice. Eur. Psychiatry 2020, 63, e32. [Google Scholar] [CrossRef]
- Racine, N.; McArthur, B.A.; Cooke, J.E.; Eirich, R.; Zhu, J.; Madigan, S. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: A meta-analysis: A meta-analysis. JAMA Pediatr. 2021, 175, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.G.M.; Hagag, R.S. The possible immunoregulatory and anti-inflammatory effects of selective serotonin reuptake inhibitors in coronavirus disease patients. Med. Hypotheses 2020, 144, 110140. [Google Scholar] [CrossRef]
- Gillett G, Jordan I, Severe psychiatric disturbance and attempted suicide in a patient with COVID-19 and no psychiatric history. BMJ Case Rep. 2020, 13, e239191. [CrossRef]
- Lim, S.T.; Janaway, B.; Costello, H.; Trip, A.; Price, G. Persistent psychotic symptoms following COVID-19 infection. BJPsych Open 2020, 6, e105. [Google Scholar] [CrossRef]
- Sasayama, D.; Hattori, K.; Wakabayashi, C.; Teraishi, T.; Hori, H.; Ota, M.; Yoshida, S.; Arima, K.; Higuchi, T.; Amano, N.; et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J. Psychiatr. Res. 2013, 47, 401–406. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: Results from an observational study. Mol. Psychiatry 2021, 26, 5199–5212. [Google Scholar] [CrossRef]
- Barcella, C.A.; Polcwiartek, C.; Mohr, G.H.; Hodges, G.; Søndergaard, K.; Bang, C.N.; Andersen, M.P.; Fosbøl, E.; Køber, L.; Schou, M.; et al. Severe mental illness is associated with increased mortality and severe course of COVID-19. Acta Psychiatr. Scand. 2021, 144, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Y.; Alabdulla, M.; Latoo, J.; Kumar, R.; Albrahim, S.; Wadoo, O.; Haddad, P.M. Mania and hypomania associated with COVID-19: A series of 15 cases seen by the consultation-liaison psychiatry service in Qatar. Qatar. Med. J. 2021, 2021, 65. [Google Scholar] [CrossRef]
- Wilson, J.E.; Mart, M.F.; Cunningham, C.; Shehabi, Y.; Girard, T.D.; MacLullich, A.M.J.; Slooter, A.J.C.; Ely, E.W. Delirium. Nat. Rev. Dis. Primers 2020, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.; Sockalingam, S.; Bonato, S.; Rajaratnam, T.; Ravindran, M.; Gosse, P.; Sheehan, K.A. A rapid review of the pathoetiology, presentation, and management of delirium in adults with COVID-19. J. Psychosom. Res. 2020, 141, 110350. [Google Scholar] [CrossRef]
- Veiga Fernández, F.; Cruz Jentoft, A.J. Delirium: Etiology and pathophysiology. Rev. Española Geriatr. Gerontol. 2008, 43 (Suppl. S3), 4–12. [Google Scholar]
- Hamm, B.S.; Rosenthal, L.J. Psychiatric Aspects of Chloroquine and Hydroxychloroquine Treatment in the Wake of Coronavirus Disease-2019: Psychopharmacological Interactions and Neuropsychiatric Sequelae. Psychosomatics 2020, 61, 597–606. [Google Scholar] [CrossRef]
- Coolen, T.; Lolli, V.; Sadeghi, N.; Rovai, A.; Trotta, N.; Taccone, F.S.; Creteur, J.; Henrard, S.; Goffard, J.-C.; De Witte, O.; et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology 2020, 95, e2016–e2027. [Google Scholar] [CrossRef]
- Goral, A.; Feder-Bubis, P.; Lahad, M.; Aharonson-Daniel, L. “In the middle, between anxiety victims and PTSD, there are people that have some kind of a disorder that has no name yet” insights about the traumatic stress consequences of exposure to ongoing threat. Trauma Care 2022, 2, 185–196. [Google Scholar] [CrossRef]
- Yang, J.J.; Jiang, W. Immune biomarkers alterations in post-traumatic stress disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 268, 39–46. [Google Scholar] [CrossRef]
- Shalev, A.; Liberzon, I.; Marmar, C. Post-Traumatic Stress Disorder. N. Engl. J. Med. 2017, 376, 2459–2469. [Google Scholar] [CrossRef]
- American Psychiatric Association. Guia De Consulta De Los Criterios Diagnosticos Del DSM-5 (R): Spanish Edition of the Desk Reference to the Diagnostic Criteria From DSM-5 (R); American Psychiatric Association Publishing: Arlington, TX, USA, 2014. [Google Scholar]
- Eisma, M.C.; Tamminga, A. Grief before and during the COVID-19 pandemic: Multiple group comparisons. J. Pain Symptom Manag. 2020, 60, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.P.; Macdonald, K.I.; Ruiz, I.; Raugh, I.M.; Bartolomeo, L.A.; James, S.H. The impact of the COVID-19 pandemic on negative symptoms in individuals at clinical high-risk for psychosis and outpatients with chronic schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.P. COVID-19 and mental health: A review of the existing literature. Asian J. Psychiatry 2020, 52, 102066. [Google Scholar] [CrossRef] [PubMed]
- Badenoch, J.B.; Rengasamy, E.R.; Watson, C.; Jansen, K.; Chakraborty, S.; Sundaram, R.D.; Hafeez, D.; Burchill, E.; Saini, A.; Thomas, L.; et al. Persistent neuropsychiatric symptoms after COVID-19: A systematic review and meta-analysis. Brain Commun. 2021, 4, fcab297. [Google Scholar] [CrossRef]
- Diaz, J.V.; Soriano, J.B. A Delphi consensus to advance on a Clinical Case Definition for Post COVID-19 condition: A WHO protocol. Res. Sq. 2021, 22, e102–e107. [Google Scholar]
- Lee, S.H.; Shin, H.-S.; Park, H.Y.; Kim, J.L.; Lee, J.J.; Lee, H.; Won, S.-D.; Han, W. Depression as a Mediator of Chronic Fatigue and Post-Traumatic Stress Symptoms in Middle East Respiratory Syndrome Survivors. Psychiatry Investig. 2019, 16, 59–64. [Google Scholar] [CrossRef]
- Roessler, M.; Tesch, F.; Batram, M.; Jacob, J.; Loser, F.; Weidinger, O.; Wende, D.; Vivirito, A.; Toepfner, N.; Seifert, M.; et al. Post COVID-19 in children, adolescents, and adults: Results of a matched cohort study including more than 150,000 individuals with COVID-19. BioRxiv 2021, 10, 81. [Google Scholar]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Mohebbi, N.; Talebi, A.; Moghadamnia, M.; Nazari Taloki, Z.; Shakiba, A. Drug Interactions of psychiatric and COVID-19 medications. Basic Clin. Neurosci. 2020, 11, 185–200. [Google Scholar] [CrossRef]
- Goyal, K.; Chauhan, P.; Chhikara, K.; Gupta, P.; Singh, M.P. Fear of COVID 2019: First suicidal case in India! Asian J. Psychiatr. 2020, 49, 101989. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Cougoule, C.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; Becker, K.A.; Reiersen, A.M.; Lenze, E.J.; Seftel, D.; et al. Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: Current evidence and potential mechanisms. Mol. Psychiatry 2021, 26, 7098–7099. [Google Scholar] [CrossRef] [PubMed]
- Diez-Quevedo, C.; Iglesias-González, M.; Giralt-López, M.; Rangil, T.; Sanagustin, D.; Moreira, M.; López-Ramentol, M.; Ibáñez-Caparrós, A.; Lorán, M.E.; Bustos-Cardona, T.; et al. Mental disorders, psychopharmacological treatments, and mortality in 2150 COVID-19 Spanish inpatients. Acta Psychiatr. Scand. 2021, 143, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Brimson, S.; Thitilertdecha, P.; Tencomnao, T. Drugs that offer the potential to reduce hospitalization and mortality from SARS-CoV-2 infection: The possible role of the sigma-1 receptor and autophagy. Expert Opin. Ther. Targets 2021, 25, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Zimniak, M.; Kirschner, L.; Hilpert, H.; Geiger, N.; Danov, O.; Oberwinkler, H.; Steinke, M.; Sewald, K.; Seibel, J.; Bodem, J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci. Rep. 2021, 11, 5890. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidrio, A.L.; Nicolini, H.; Zarate, C.T.; Castro, T.G.; Rojop, I.J.; Magaña, J.M.; López, N.M.; Mendoza, A.D.G. Association between SARS-CoV-2 Infection and Neuropsychiatric Manifestations. COVID 2022, 2, 1270-1286. https://doi.org/10.3390/covid2090094
Vidrio AL, Nicolini H, Zarate CT, Castro TG, Rojop IJ, Magaña JM, López NM, Mendoza ADG. Association between SARS-CoV-2 Infection and Neuropsychiatric Manifestations. COVID. 2022; 2(9):1270-1286. https://doi.org/10.3390/covid2090094
Chicago/Turabian StyleVidrio, Aranza Llorente, Humberto Nicolini, Carlos Tovilla Zarate, Thelma Gonzales Castro, Isela Juárez Rojop, Jaime Martínez Magaña, Nicolás Martínez López, and Alma Delia Genis Mendoza. 2022. "Association between SARS-CoV-2 Infection and Neuropsychiatric Manifestations" COVID 2, no. 9: 1270-1286. https://doi.org/10.3390/covid2090094
APA StyleVidrio, A. L., Nicolini, H., Zarate, C. T., Castro, T. G., Rojop, I. J., Magaña, J. M., López, N. M., & Mendoza, A. D. G. (2022). Association between SARS-CoV-2 Infection and Neuropsychiatric Manifestations. COVID, 2(9), 1270-1286. https://doi.org/10.3390/covid2090094











