Emerging SARS-CoV-2 Variants, Inequitable Vaccine Distribution, and Implications for COVID-19 Control in Sub-Saharan Africa
Abstract
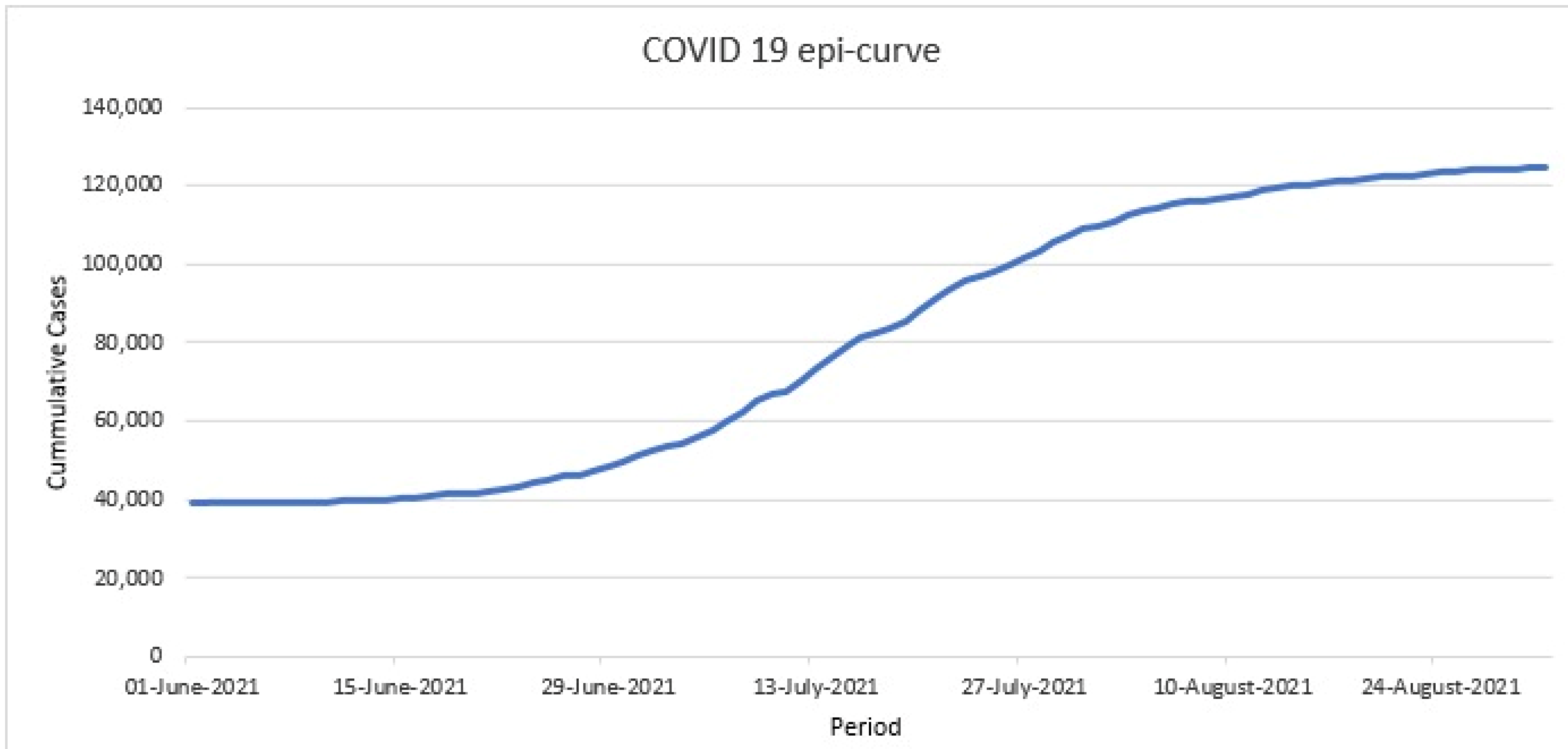
:1. Introduction
2. Drivers of the COVID-19 Pandemic
3. The Emergence of Variants of Concern
4. Current COVID-19 Vaccination Programs
4.1. The Global Rollout and Progress of COVID-19 Vaccination Programs
4.2. Vaccine Hesitancy and Unclear Vaccination Policies
5. Implications of Variants of Concern on the Efficacy of Current Vaccination Programmes on the African Continent
6. The Implications of the Inequitable Distribution of Vaccines on the Emergence of Variants of Concern
7. Enhancing Genomic Sequencing and Vaccine Production Capacity Building in Sub-Saharan Africa Remains Key
8. The Need to Promote Multiple Prevention Strategies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Dzobo, M.; Chitungo, I.; Dzinamarira, T. COVID-19: A Perspective for Lifting Lockdown in Zimbabwe. Pan Afr. Med. J. 2020, 35, 13. [Google Scholar] [CrossRef]
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 8 March 2022).
- Chitungo, I.; Dzobo, M.; Hlongwa, M.; Dzinamarira, T. COVID-19: Unpacking the low number of cases in Africa. Public Health Pract. 2020, 1, 100038. [Google Scholar] [CrossRef]
- Rutayisire, E.; Nkundimana, G.; Mitonga, H.K.; Boye, A.; Nikwigize, S. What works and what does not work in response to COVID-19 prevention and control in Africa. Int. J. Infect. Dis. 2020, 97, 267–269. [Google Scholar] [CrossRef]
- Chitungo, I.; Mhango, M.; Mbunge, E.; Dzobo, M.; Dzinamarira, T. Digital technologies and COVID-19: Reconsidering lockdown exit strategies for Africa. Pan Afr. Med. J. 2021, 39, 93. [Google Scholar] [CrossRef]
- Dzobo, M.; Hlongwa, M.; Denhere, K.; Kampira, V.; Mugoni, M.; Musuka, G.; Dzinamarira, T. COVID-19 resurgence: Lessons learnt to inform the South African response. Disaster Med. Public Health Prep. 2021, 1–6. [Google Scholar] [CrossRef]
- Chirisa, I.; Mavhima, B.; Nyevera, T.; Chigudu, A.; Makochekanwa, A.; Matai, J.; Masunda, T.; Chandaengerwa, E.K.; Machingura, F.; Moyo, S. The impact and implications of COVID-19: Reflections on the Zimbabwean society. Soc. Sci. Humanit. Open 2021, 4, 100183. [Google Scholar] [CrossRef]
- Murewanhema, G.; Dzinamarira, T.; Herrera, H.; Musuka, G. Decision making conundrum as Zimbabwe experiences a harsh third wave of the COVID-19 pandemic. Disaster Med. Public Health Prep. 2021, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Loembé, M.M.; Nkengasong, J.N. COVID-19 vaccine access in Africa: Global distribution, vaccine platforms, and challenges ahead. Immunity 2021, 54, 1353–1362. [Google Scholar] [CrossRef]
- Nkengasong, J.N.; Ndembi, N.; Tshangela, A.; Raji, T. COVID-19 vaccines: How to ensure Africa has access. Nature 2020, 586, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Nachega, J.B.; Sam-Agudu, N.A.; Masekela, R.; van der Zalm, M.M.; Nsanzimana, S.; Condo, J.; Ntoumi, F.; Rabie, H.; Kruger, M.; Wiysonge, C.S. Addressing challenges to rolling out COVID-19 vaccines in African countries. Lancet Glob. Health 2021, 9, e746–e748. [Google Scholar] [CrossRef]
- Kubota, Y.; Shiono, T.; Kusumoto, B.; Fujinuma, J. Multiple drivers of the COVID-19 spread: The roles of climate, international mobility, and region-specific conditions. PLoS ONE 2020, 15, e0239385. [Google Scholar] [CrossRef] [PubMed]
- Murewanhema, G.; Mutsigiri-Murewanhema, F. Drivers of the third wave of COVID-19 in Zimbabwe and challenges for control: Perspectives and recommendations. Pan Afr. Med. J. 2021, 40, 46. [Google Scholar]
- Murewanhema, G.; Burukai, T.V.; Chiwaka, L.; Maunganidze, F.; Munodawafa, D.; Pote, W.; Mufunda, J. The effect of increased mobility on SARS-CoV-2 transmission: A descriptive study of the trends of COVID-19 in Zimbabwe between December 2020 and January 2021. Pan Afr. Med. J. 2021, 39, 125. [Google Scholar] [CrossRef]
- Murewanhema, G.; Burukai, T.; Mazingi, D.; Maunganidze, F.; Mufunda, J.; Munodawafa, D.; Pote, W. A descriptive study of the trends of COVID-19 in Zimbabwe from March-June 2020: Policy and strategy implications. Pan Afr. Med. J. 2020, 37, 33. [Google Scholar] [CrossRef]
- Banerjee, A.; Mossman, K.; Grandvaux, N. Molecular determinants of SARS-CoV-2 variants. Trends Microbiol. 2021, 29, 871–873. [Google Scholar] [CrossRef]
- Lou, F.; Li, M.; Pang, Z.; Jiang, L.; Guan, L.; Tian, L.; Hu, J.; Fan, J.; Fan, H. Understanding the Secret of SARS-CoV-2 Variants of Concern/Interest and Immune Escape. Front. Immunol. 2021, 12, 4326. [Google Scholar] [CrossRef]
- WHO. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 19 December 2021).
- Dyson, L.; Hill, E.M.; Moore, S.; Curran-Sebastian, J.; Tildesley, M.J.; Lythgoe, K.A.; House, T.; Pellis, L.; Keeling, M.J. Possible future waves of SARS-CoV-2 infection generated by variants of concern with a range of characteristics. medRxiv 2021, 12, 5730. [Google Scholar] [CrossRef]
- Kouamou, V.; Matarise, R.; Dos Santos, E.; Elose, N.; Manasa, J. SARS-CoV-2 in Zimbabwe: Milestones and challenges faced towards achieving the expected 60% herd immunity. Pan Afr. Med. J. 2021, 39, 255. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Althaus, C.L.; Giovanetti, M.; San, J.E.; Giandhari, J.; Pillay, S.; Naidoo, Y.; Ramphal, U.; Msomi, N. Rapid replacement of the Beta variant by the Delta variant in South Africa. medRxiv 2021. [Google Scholar] [CrossRef]
- Dzobo, M.; Musuka, G.; Mashe, T.; Dzinamarira, T. Inadequate SARS-CoV-2 genetic sequencing capacity in Zimbabwe: A call to urgently address this key gap to control current and future waves. IJID Reg. 2021, 1, 3–4. [Google Scholar] [CrossRef]
- Samanga, R. Covid: Global North’s Power Plays Impede Transparency Amid Pandemic. Available online: https://www.theafricareport.com/153593/covid-global-norths-power-plays-impede-transparency-amid-pandemic/ (accessed on 18 January 2022).
- Zubașcu, F. Do Not Blame South Africa’ for the Omicron Variant. Available online: https://sciencebusiness.net/news/do-not-blame-south-africa-omicron-variant (accessed on 28 January 2022).
- Adamu, Z.; Busari, S. Anger Simmers over Omicron Travel Bans in Southern Africa. Available online: https://edition.cnn.com/2021/12/04/africa/africa-travel-ban-omicron-variant-intl-cmd/index.html (accessed on 18 January 2022).
- MoHCC. COVID-19 Situational Reports. Available online: http://www.mohcc.gov.zw/index.php?option=com_phocadownload&view=category&id=15&Itemid=741 (accessed on 20 December 2021).
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef]
- Chard, A.N.; Datta, S.D.; Tallis, G.; Burns, C.C.; Wassilak, S.G.; Vertefeuille, J.F.; Zaffran, M. Progress toward polio eradication—Worldwide, January 2018–March 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 784. [Google Scholar] [CrossRef]
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.-C. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Vasan, S.; Kim, J.H.; Ake, J.A. Current approaches to HIV vaccine development: A narrative review. J. Int. AIDS Soc. 2021, 24, e25793. [Google Scholar] [CrossRef] [PubMed]
- Kreier, F. ‘Unprecedented achievement’: Who received the first billion COVID vaccinations? Nature 2021, 29. [Google Scholar] [CrossRef]
- Williams, V.; Edem, B.; Calnan, M.; Otwombe, K.; Okeahalam, C. Considerations for establishing successful coronavirus disease vaccination programs in Africa. Emerg. Infect. Dis. 2021, 27, 2009. [Google Scholar] [CrossRef]
- Mundagowa, P.T.; Tozivepi, S.N.; Chiyaka, E.T.; Mukora-Mutseyekwa, F.; Makurumidze, R. Assessment of COVID-19 vaccine hesitancy among Zimbabweans: A rapid national survey. medRxiv 2021. [Google Scholar] [CrossRef]
- Dzinamarira, T.; Nachipo, B.; Phiri, B.; Musuka, G. COVID-19 vaccine roll-out in South Africa and Zimbabwe: Urgent need to address community preparedness, fears and hesitancy. Vaccines 2021, 9, 250. [Google Scholar] [CrossRef]
- Mutombo, P.N.; Fallah, M.P.; Munodawafa, D.; Kabel, A.; Houeto, D.; Goronga, T.; Mweemba, O.; Balance, G.; Onya, H.; Kamba, R.S. COVID-19 vaccine hesitancy in Africa: A call to action. Lancet Glob. Health 2021, 10, e320–e321. [Google Scholar] [CrossRef]
- Cooper, S.; van Rooyen, H.; Wiysonge, C.S. COVID-19 vaccine hesitancy in South Africa: How can we maximize uptake of COVID-19 vaccines? Expert Rev. Vaccines 2021, 20, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Frugoli, A.G.; Prado, R.S.; Silva, T.; Matozinhos, F.P.; Trapé, C.A.; Lachtim, S.A.F. Vaccine fake news: An analysis under the World Health Organization’s 3Cs model. Rev. Esc. Enferm. USP 2021, 55, e03736. [Google Scholar] [CrossRef]
- Mukherjee, P. South Africa Delays COVID Vaccine Deliveries as Inoculations Slow. Available online: https://www.reuters.com/world/africa/exclusive-south-africa-delays-covid-vaccine-deliveries-inoculations-slow-2021-11-24/ (accessed on 27 November 2021).
- Murewanhema, G.; Mukwenha, S.; Dzinamarira, T.; Mukandavire, Z.; Cuadros, D.; Madziva, R.; Chingombe, I.; Mapingure, M.; Herrera, H.; Musuka, G. Optimising COVID-19 Vaccination Policy to Mitigate SARS-CoV-2 Transmission within Schools in Zimbabwe. Vaccines 2021, 9, 1481. [Google Scholar] [CrossRef]
- Murewanhema, G.; Dzinamarira, T.; Herrera, H.; Musuka, G. COVID-19 vaccination for pregnant women in Zimbabwe: A public health challenge that needs an urgent discourse. Public Health Pract. 2021, 2, 100200. [Google Scholar] [CrossRef]
- Murewanhema, G. Vaccination hesitancy among women of reproductive age in resource-challenged settings: A cause for public health concern. Pan Afr. Med. J. 2021, 38, 336. [Google Scholar] [CrossRef]
- Mello, M.M.; Silverman, R.D.; Omer, S.B. Ensuring uptake of vaccines against SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1296–1299. [Google Scholar] [CrossRef]
- Bai, W.; Gu, Y.; Liu, H.; Zhou, L. Epidemiology Features and Effectiveness of Vaccination and Non-Pharmaceutical Interventions of Delta and Lambda SARS-CoV-2 Variants. China CDC Wkly. 2021, 3, 977. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B. 1.1. 529) Variant. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef]
- Murewanhema, G.; Dzinamarira, T.; Madziva, R.; Herrera, H.; Musuka, G. SARS-CoV-2 vaccine-related adverse events in Zimbabwe: The need to strengthen pharmacovigilance in resource-limited settings. Pharmacoepidemiol. Drug Saf. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bloomberg. J&J, Sputnik, and Sinopharm Vaccines Found to be Largely Ineffective in Fighting Omicron: Study. Available online: https://fortune.com/2021/12/17/jj-sputnik-sinopharm-covid-vaccines-ineffective-omicron-study/ (accessed on 20 December 2021).
- Bloomberg. Sinopharm, J&J, Sputnik Vaccines are Weaker against Omicron in New Study. Available online: https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/sinopharm-jj-sputnik-vaccines-are-weaker-against-omicron-in-new-study/articleshow/88342469.cms (accessed on 20 December 2021).
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.G.; Sutherland, A.D.; Suliman, T.; Shaw, M.; Preiser, W. Breakthrough Infections with SARS-CoV-2 Omicron Variant Despite Booster Dose of MRNA Vaccine. 2021. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3981711 (accessed on 23 December 2021).
- Cele, S.; Jackson, L.; Khan, K.; Khoury, D.S.; Moyo-Gwete, T.; Tegally, H.; Scheepers, C.; Amoako, D.; Karim, F.; Bernstein, M. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. MedRXiv 2021. [Google Scholar] [CrossRef]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. COVID super-immunity: One of the pandemic’s great puzzles. Nature 2021, 598, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and waning of natural and hybrid COVID-19 immunity. MedRxiv 2021. [Google Scholar] [CrossRef]
- Dzinamarira, T.; Mukwenha, S.; Mukandavire, Z.; Cuadros, D.F.; Murewanhema, G.; Madziva, R.; Musuka, G. Insights from Zimbabwe’s SARS-CoV-2 genomic surveillance. Lancet Glob. Health 2021, 9, e1624–e1625. [Google Scholar] [CrossRef]
- Dzinamarira, T.; Murewanhema, G.; Musuka, G. Different SARS-CoV-2 variants, same prevention strategies. Public Health Pract. 2022, 3, 100223. [Google Scholar] [CrossRef]
- Lone, S.A.; Ahmad, A. COVID-19 pandemic—An African perspective. Emerg. Microbes Infect. 2020, 9, 1300–1308. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murewanhema, G.; Dzinamarira, T.; Chingombe, I.; Mapingure, M.P.; Mukwenha, S.; Chitungo, I.; Herrera, H.; Madziva, R.; Ngwenya, S.; Musuka, G. Emerging SARS-CoV-2 Variants, Inequitable Vaccine Distribution, and Implications for COVID-19 Control in Sub-Saharan Africa. COVID 2022, 2, 341-349. https://doi.org/10.3390/covid2030023
Murewanhema G, Dzinamarira T, Chingombe I, Mapingure MP, Mukwenha S, Chitungo I, Herrera H, Madziva R, Ngwenya S, Musuka G. Emerging SARS-CoV-2 Variants, Inequitable Vaccine Distribution, and Implications for COVID-19 Control in Sub-Saharan Africa. COVID. 2022; 2(3):341-349. https://doi.org/10.3390/covid2030023
Chicago/Turabian StyleMurewanhema, Grant, Tafadzwa Dzinamarira, Innocent Chingombe, Munyaradzi Paul Mapingure, Solomon Mukwenha, Itai Chitungo, Helena Herrera, Roda Madziva, Solwayo Ngwenya, and Godfrey Musuka. 2022. "Emerging SARS-CoV-2 Variants, Inequitable Vaccine Distribution, and Implications for COVID-19 Control in Sub-Saharan Africa" COVID 2, no. 3: 341-349. https://doi.org/10.3390/covid2030023
APA StyleMurewanhema, G., Dzinamarira, T., Chingombe, I., Mapingure, M. P., Mukwenha, S., Chitungo, I., Herrera, H., Madziva, R., Ngwenya, S., & Musuka, G. (2022). Emerging SARS-CoV-2 Variants, Inequitable Vaccine Distribution, and Implications for COVID-19 Control in Sub-Saharan Africa. COVID, 2(3), 341-349. https://doi.org/10.3390/covid2030023









