Annual Excess Crude Mortality in Europe during the COVID-19 Pandemic: A Longitudinal Joinpoint Regression Analysis of Historical Trends from 2000 to 2021
Abstract
1. Introduction
1.1. Background
1.2. Research Objectives
2. Materials and Methods
2.1. Population
2.2. Data Collection
2.3. Study Design
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Principal Findings
4.2. Comparison with Other Literature
4.3. Practical Implications
4.4. Limitations
4.5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 3 November 2022).
- Centers for Disease Control and Prevention. Excess Deaths Associated with COVID-19. Available online: https://www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htm (accessed on 3 November 2022).
- Shang, W.; Wang, Y.; Yuan, J.; Guo, Z.; Liu, J.; Liu, M. Global Excess Mortality during COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 3 November 2022).
- Singh, M.K.; Mobeen, A.; Chandra, A.; Joshi, S.; Ramachandran, S. A meta-analysis of comorbidities in COVID-19: Which diseases increase the susceptibility of SARS-CoV-2 infection? Comput. Biol. Med. 2021, 130, 104219. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.H.; Tipih, T.; Makoah, N.A.; Vermeulen, J.-G.; Goedhals, D.; Sempa, J.B.; Burt, F.J.; Taylor, A.; Mahalingam, S. Comorbidities in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. mBio 2021, 12, e03647-20. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Ray, P.P. A systematic review and meta-analysis on correlation of weather with COVID-19. Sci. Rep. 2021, 11, 10746. [Google Scholar] [CrossRef]
- De Frel, D.L.; Atsma, D.E.; Pijl, H.; Seidell, J.C.; Leenen, P.J.M.; Dik, W.A.; Van Rossum, E.F.C. The Impact of Obesity and Lifestyle on the Immune System and Susceptibility to Infections Such as COVID-19. Front. Nutr. 2020, 7, 597600. [Google Scholar] [CrossRef]
- Zang, S.-T.; Luan, J.; Li, L.; Yu, H.-X.; Wu, Q.-J.; Chang, Q.; Zhao, Y.-H. Ambient air pollution and COVID-19 risk: Evidence from 35 observational studies. Environ. Res. 2022, 204 Pt B, 112065. [Google Scholar] [CrossRef]
- Rovetta, A. Common Statistical and Methodological Errors in Scientific Investigations: A Simple Guide to Avoid Invalid Conclusions and Unfounded Decisions. OSF Preprints 2022. [Google Scholar] [CrossRef]
- Rovetta, A.; Bhagavathula, A.S. The Impact of COVID-19 on Mortality in Italy: Retrospective Analysis of Epidemiological Trends. JMIR Public Health Surveill. 2022, 8, e36022. [Google Scholar] [CrossRef]
- Giattino, C.; Ritchie, H.; Roser, M.; Ortiz-Ospina, E.; Hasell, J. Excess Mortality during the Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/excess-mortality-covid (accessed on 10 November 2022).
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536, Erratum in Lancet 2022, 399, 1468. [Google Scholar] [CrossRef]
- Greenland, S. Valid P-Values Behave Exactly as They Should: Some Misleading Criticisms of P-Values and Their Resolution With S-Values. Am. Stat. 2019, 73, 106–114. [Google Scholar] [CrossRef]
- Greenland, S. Analysis goals, error-cost sensitivity, and analysis hacking: Essential considerations in hypothesis testing and multiple comparisons. Paediatr. Perinat. Epidemiol. 2021, 35, 8–23. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Joinpoint Trend Analysis Software, v.4.9.1.0. Bethesda, United States. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 20 April 2022).
- Gillis, D.; Edwards, B.P. The utility of joinpoint regression for estimating population parameters given changes in population structure. Heliyon 2019, 5, e02515. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. Death Rate, Crude (per 1000 People). Available online: https://data.worldbank.org/indicator/SP.DYN.CDRT.IN (accessed on 20 October 2022).
- Eurostat. Population Change—Demographic Balance and Crude Rates at National Level. Available online: https://ec.europa.eu/eurostat/databrowser/view/DEMO_GIND__custom_2090018/bookmark/table (accessed on 20 October 2022).
- Eurostat. Healthcare Expenditure Statistics. Kirchberg, Luxembourg City, Luxembourg. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Healthcare_expenditure_statistics (accessed on 20 October 2022).
- Eurostat. Physicians, by Speciality. 2020. Kirchberg, Luxembourg City, Luxembourg. Available online: https://ec.europa.eu/eurostat/statistics-explained/images/3/3d/Physicians%2C_by_speciality%2C_2020_Health20.png (accessed on 20 October 2022).
- Eurostat. Healthcare Resource Statistics—Beds. Kirchberg, Luxembourg City, Luxembourg. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Healthcare_resource_statistics_-_beds (accessed on 20 October 2022).
- Institute for Health Metrics and Evaluation. University of Washington, Seattle, United States. COVID-19 Projections. Available online: https://covid19.healthdata.org/ (accessed on 20 October 2022).
- Statistics Kingdom. Multiple Linear Regression Calculator. Melbourne, Australia. Available online: https://www.statskingdom.com/410multi_linear_regression.html (accessed on 20 October 2022).
- Statistics Kingdom. Correlation Confidence Interval Calculator. Melbourne, Australia. Available online: https://www.statskingdom.com/correlation-confidence-interval-calculator.html (accessed on 20 October 2022).
- World Health Organization. Global Excess Deaths Associated with COVID-19 (Modelled Estimates). Available online: https://www.who.int/data/sets/global-excess-deaths-associated-with-covid-19-modelled-estimates (accessed on 14 December 2022).
- Mendiola-Pastrana, I.R.; López-Ortiz, E.; de la Loza-Zamora, J.G.R.; González, J.; Gómez-García, A.; López-Ortiz, G. SARS-CoV-2 Variants and Clinical Outcomes: A Systematic Review. Life 2022, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M.; et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front. Public Health 2022, 10, 873596. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Sheikh, A.; Robertson, C.; Taylor, B. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against Death from the Delta Variant. N. Engl. J. Med. 2021, 385, 2195–2197. [Google Scholar] [CrossRef]
- Gelles, R.L. COVID-19 Vaccine Effectiveness in Children and Adults. U.S. Food and Drug Administration and Centers for Disease Control and Prevention, VRBPAC. 6 April 2022. Available online: https://www.fda.gov/media/157475/download (accessed on 15 November 2022).
- European Medicines Agency and European Centre for Disease Prevention and Control. COVID-19: Joint Statement from ECDC and EMA on the Administration of a Fourth Dose of mRNA Vaccines. 6 April 2022. Available online: https://www.ema.europa.eu/en/documents/public-statement/covid-19-joint-statement-ecdc-ema-administration-fourth-dose-mrna-vaccines_.pdf (accessed on 15 November 2022).
- Lo, C.-H.; Chiu, L.; Qian, A.; Khan, M.Z.; Alhassan, H.A.; Duval, A.J.; Chan, A.T. Association of Primary Care Physicians Per Capita With COVID-19 Vaccination Rates Among US Counties. JAMA Netw. Open 2022, 5, e2147920. [Google Scholar] [CrossRef]
- Armocida, B.; Formenti, B.; Ussai, S.; Palestra, F.; Missoni, E. The Italian health system and the COVID-19 challenge. Lancet Public Health 2020, 5, e253. [Google Scholar] [CrossRef]
- Sandhu, P.; Shah, A.B.; Ahmad, F.B.; Kerr, J.; Demeke, H.B.; Graeden, E.; Marks, S.; Clark, H.; Bombard, J.M.; Bolduc, M.; et al. Emergency Department and Intensive Care Unit Overcrowding and Ventilator Shortages in US Hospitals During the COVID-19 Pandemic, 2020–2021. Public Health Rep. 2022, 137, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Villani, L.; McKee, M.; Cascini, F.; Ricciardi, W.; Boccia, S. Comparison of Deaths Rates for COVID-19 across Europe during the First Wave of the COVID-19 Pandemic. Front. Public Health 2020, 8, 620416. [Google Scholar] [CrossRef] [PubMed]
- Butac.it. Meloni, Restrizioni e Dati. Available online: https://www.butac.it/meloni-restrizioni-dati/ (accessed on 1 November 2022).
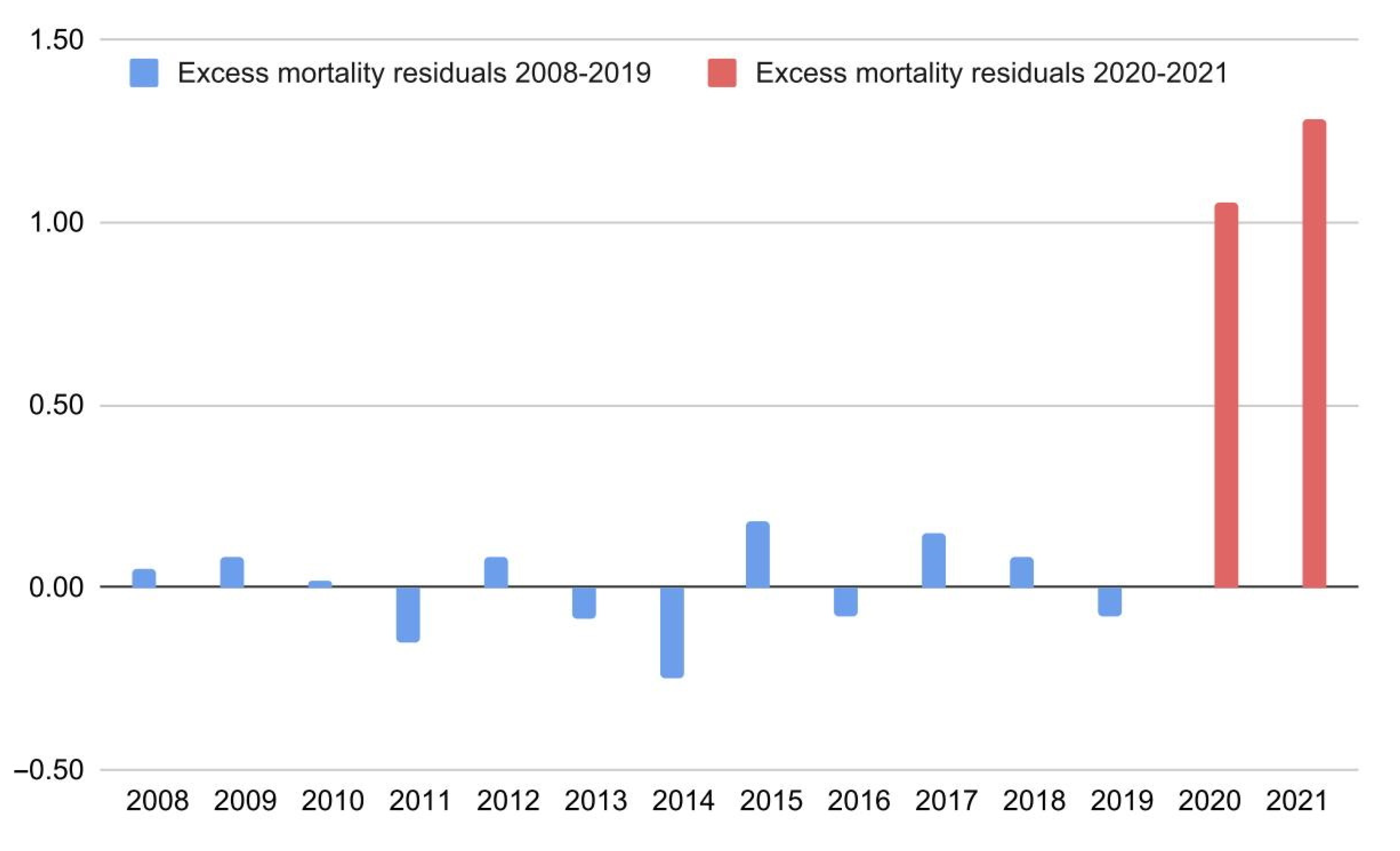
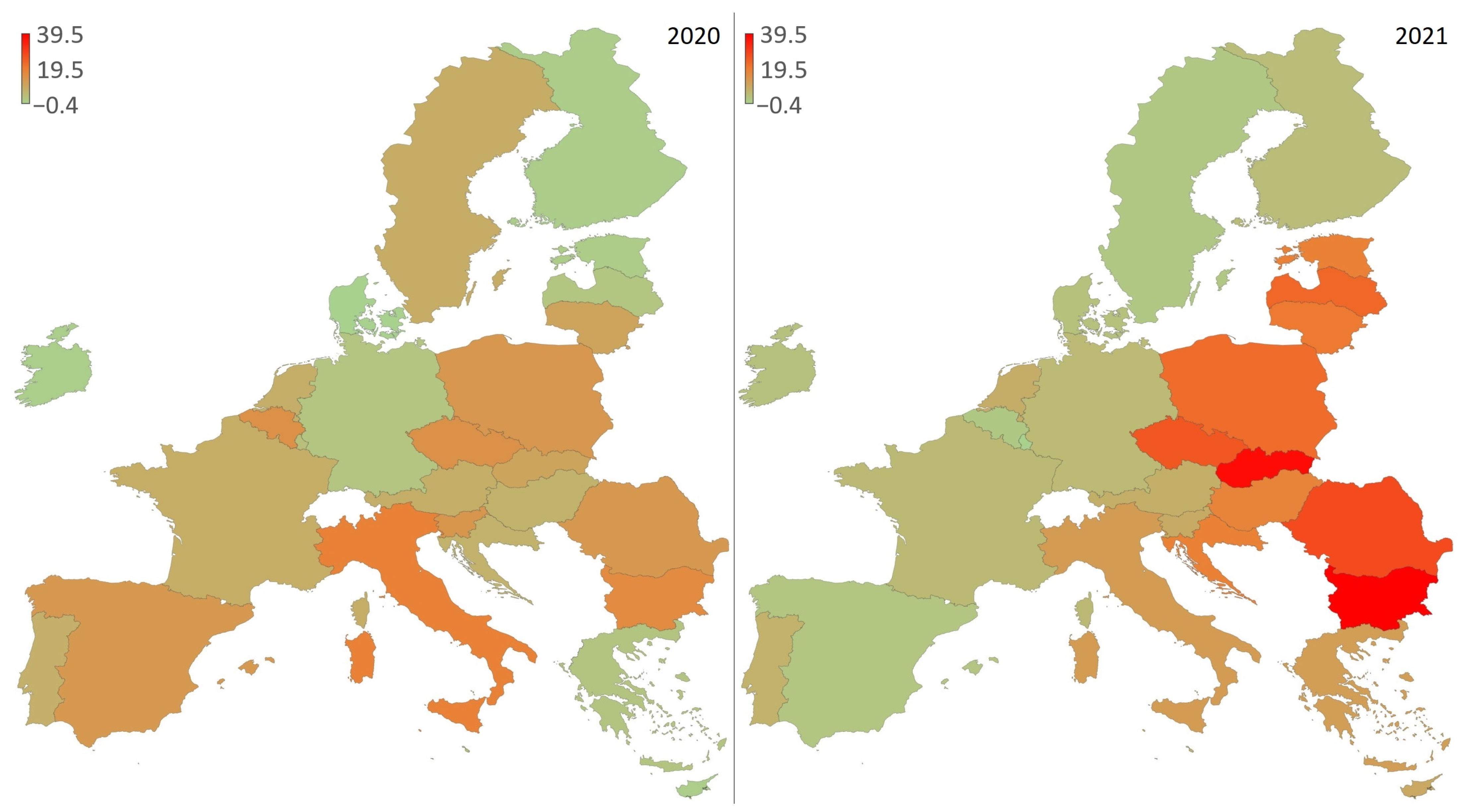
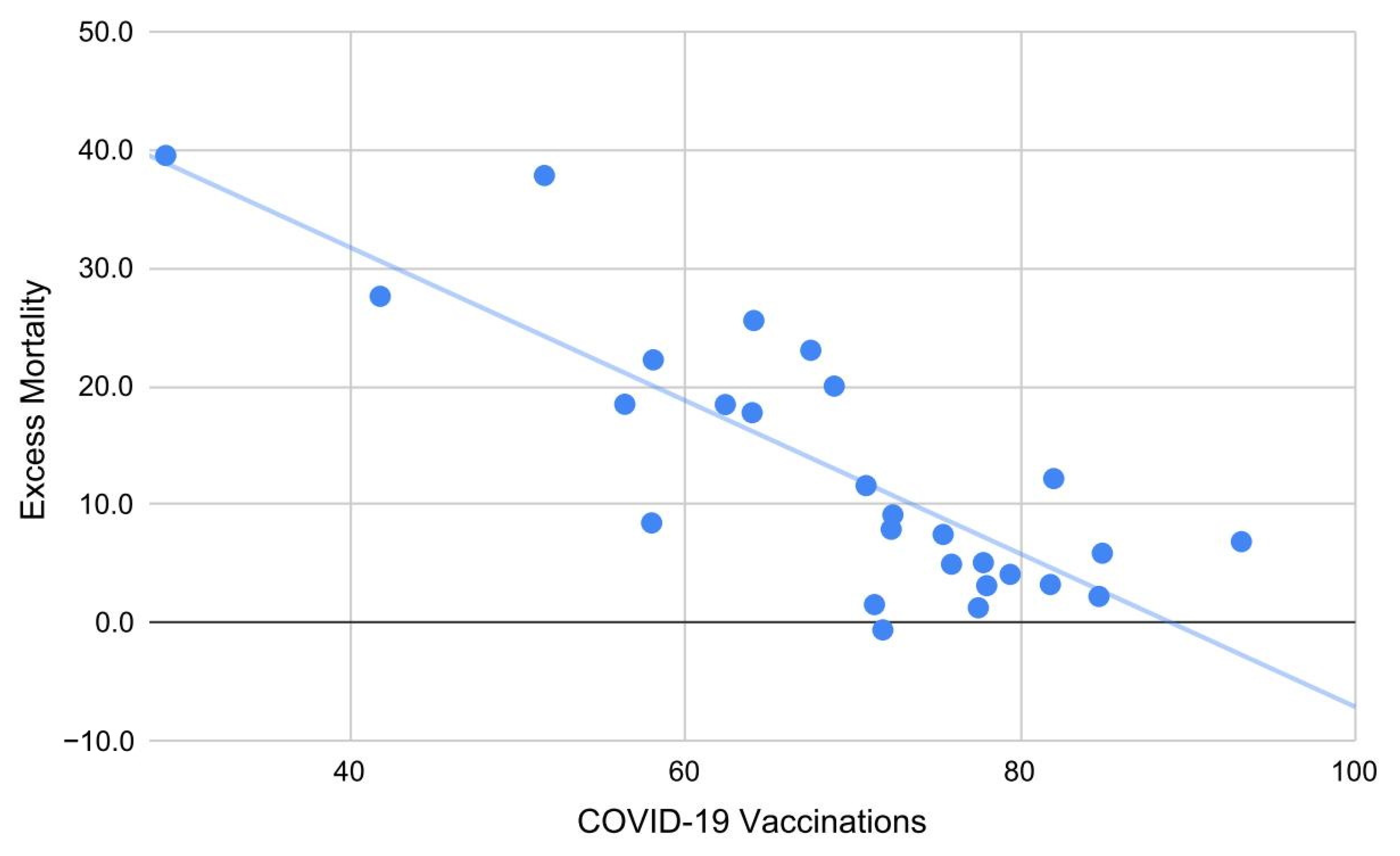
| Country | Predicted 2020 | Observed | Δ% 2020 | Predicted 2021 | Observed | Δ% 2021 |
|---|---|---|---|---|---|---|
| Austria | 9.5 | 10.3 | 7.9 | 9.6 | 10.3 | 7.5 |
| Belgium | 9.6 | 11 | 14.8 | 9.6 | 9.7 | 1.3 |
| Bulgaria | 15.5 | 18 | 16.2 | 15.6 | 21.7 | 39.5 |
| Croatia | 13.2 | 14.1 | 7.0 | 13.3 | 15.8 | 18.5 |
| Cyprus | 7.2 | 7.2 | 0.4 | 7.2 | 7.9 | 9.1 |
| Czech Republic | 10.6 | 12.1 | 14.7 | 10.6 | 13.3 | 25.6 |
| Denmark | 9.4 | 9.4 | −0.4 | 9.5 | 9.8 | 3.2 |
| Estonia | 11.8 | 11.9 | 0.9 | 11.8 | 14 | 18.5 |
| Finland | 9.9 | 10 | 0.6 | 10.0 | 10.4 | 4.1 |
| France | 9.2 | 9.9 | 8.0 | 9.2 | 9.7 | 5.1 |
| Germany | 11.6 | 11.9 | 2.5 | 11.7 | 12.3 | 4.9 |
| Greece | 11.9 | 12.2 | 2.6 | 12.1 | 13.5 | 11.6 |
| Hungary | 13.6 | 14.5 | 6.8 | 13.7 | 16.1 | 17.8 |
| Ireland | 6.4 | 6.4 | 0.2 | 6.4 | 6.6 | 3.1 |
| Italy | 10.6 | 12.6 | 18.5 | 10.7 | 12 | 12.2 |
| Latvia | 14.9 | 15.2 | 2.2 | 15.0 | 18.4 | 23.0 |
| Lithuania | 14.1 | 15.6 | 10.4 | 14.2 | 17 | 20.0 |
| Luxembourg | 7.0 | 7.3 | 3.9 | 7.0 | 7 | -0.6 |
| Malta | 7.6 | 7.9 | 4.5 | 7.6 | 8 | 5.9 |
| Netherlands | 9.0 | 9.7 | 7.8 | 9.1 | 9.8 | 7.9 |
| Poland | 11.1 | 12.6 | 13.4 | 11.3 | 13.8 | 22.2 |
| Portugal | 11.2 | 12 | 7.4 | 11.3 | 12.1 | 6.8 |
| Romania | 13.6 | 15.4 | 13.2 | 13.7 | 17.5 | 27.6 |
| Slovak Republic | 9.8 | 10.8 | 10.3 | 9.8 | 13.5 | 37.8 |
| Slovenia | 10.0 | 11.4 | 13.5 | 10.1 | 11 | 8.4 |
| Spain | 9.2 | 10.4 | 13.0 | 9.3 | 9.5 | 2.2 |
| Sweden | 8.8 | 9.5 | 8.4 | 8.7 | 8.8 | 1.5 |
| EUROPE | 10.5 | 11.6 | 10.0 | 10.6 | 11.9 | 12.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovetta, A. Annual Excess Crude Mortality in Europe during the COVID-19 Pandemic: A Longitudinal Joinpoint Regression Analysis of Historical Trends from 2000 to 2021. COVID 2022, 2, 1778-1786. https://doi.org/10.3390/covid2120128
Rovetta A. Annual Excess Crude Mortality in Europe during the COVID-19 Pandemic: A Longitudinal Joinpoint Regression Analysis of Historical Trends from 2000 to 2021. COVID. 2022; 2(12):1778-1786. https://doi.org/10.3390/covid2120128
Chicago/Turabian StyleRovetta, Alessandro. 2022. "Annual Excess Crude Mortality in Europe during the COVID-19 Pandemic: A Longitudinal Joinpoint Regression Analysis of Historical Trends from 2000 to 2021" COVID 2, no. 12: 1778-1786. https://doi.org/10.3390/covid2120128
APA StyleRovetta, A. (2022). Annual Excess Crude Mortality in Europe during the COVID-19 Pandemic: A Longitudinal Joinpoint Regression Analysis of Historical Trends from 2000 to 2021. COVID, 2(12), 1778-1786. https://doi.org/10.3390/covid2120128






