Abstract
Background: Remdesivir is a broad-spectrum antiviral that has been approved as promising medicine worldwide for the fatal pandemic COVID-19 disease. There is a debate over its efficacy, with different studies taking into account a variety of factors. Therefore, we conducted this study to evaluate the primary composite outcome of mortality rate, need for mechanical ventilation (MV), and escalation of care among Remdesivir (RDV) and non-Remdesivir (NoRDV) groups. Methods: Patients with moderate and severe PCR-confirmed COVID-19 infection were observed retrospectively, before and after including RDV in the treatment protocol during the period from August 2020 to February 2021. Result: From the 509 hospitalized patients, 35% received Remdesivir, with 64% being severe patients. The median age in both groups was 59 years old, and there was no significant difference between the two groups regarding gender, baseline characteristics, and comorbidities. In contrast, the median hospital length of stay in the RDV group was lower (8 days) than the NoRDV (9 days), p = 0.004. The composite outcome was 17.7% in the RDV group and 22.2% in the NoRDV group, but the difference was statistically insignificant (p-value 0.289). Adjusted logistic regression demonstrated a non-significant lower association of the composite outcome with RDV use (OR 0.623, 95CI% 0.37–1.02), and a significant reduction occurred in patients <60 years old (OR 0.39, 95%CI 0.17–0.83). However, survival analysis for mortality, MV, and transfer to a higher level revealed insignificant differences in the median time between groups. Subgroup analyses showed that RDV utilization had a non-significant effect on the risk of all three outcomes across different groups. Conclusion: Despite controlling all patient characteristics, treatment with RDV did not improve patient outcomes over other antivirals and standard care. There is an urgent need for further studies to investigate and evaluate new therapeutic approaches or combinations.
1. Introduction
Coronavirus disease 2019 (COVID-19) is an ongoing global fatal pandemic that emerged first in Wuhan, China, in December 2019 and has since spread worldwide. On 30 January 2020, the world health organization declared it a “public health emergency of international concern”. The disease causes severe manifestations ranging from multiple organ failure to death. There have been over 520 million confirmed COVID-19 cases worldwide since 18 May 2022, with 6 million deaths reported to the WHO [1]. With no evidence of effective treatment, drastic non-therapeutic measures were implemented worldwide to control the spread of the infection, including cordon sanitaire, travel restrictions, and partial or even complete lockdown [2]. Two years later, COVID-19 had disastrous consequences for the healthcare system. Despite the continuous dedicated clinical research and the number of vaccine doses exceeding 11 billion globally, a significant number of severe cases ended with death.
Throughout this unprecedented public health emergency, the pandemic has prompted research centers worldwide to investigate the efficacy of every available drug or to develop novel treatments [3]. The severe acute respiratory syndrome coronavirus (SARS-CoV-2) pertains to the Beta coronavirus family that comprises SARS-CoV and Middle East respiratory syndrome CoV (MERS-CoV). Consequently, every drug that demonstrated effectiveness in patients with MERS or SARS, in addition to broad-spectrum antivirals, was investigated in COVID-19 [4].
Remdesivir (RDV) is a broad-spectrum antiviral developed in 2009, initially to treat hepatitis C (HCV) and respiratory syncytial virus (RSV) but showed no marked effect. In 2015, it was repurposed and tested for Ebola virus disease [5]. It is a prodrug administered intravenously and activated intracellularly into the active form, a ribonucleotide analog that inhibits the viral DNA-dependent RNA polymerase activity, decreasing the production of viral RNA and hindering its replication [6].
During the COVID-19 pandemic, in March 2020, the United States president announced the availability of RDV for “compassionate use”. In addition, because there is no definitive cure, RDV has been approved or authorized for emergency use in approximately 50 countries to treat COVID-19 [7]. In May, the Food and Drug Administration (FDA) authorized the emergency use of RDV in COVID-19 patients requiring hospitalization [8]. Five months later, the drug was approved as “the first antiviral for the treatment of COVID-19” after three randomized controlled trials that demonstrated its effectiveness in improving recovery and reducing the average hospital stay among mild to severe COVID-19 [8,9,10,11]. RDV is a drug that is administered via intravenous infusion for five to ten days and costs around EUR 2000.
In May 2020, the Egyptian COVID-19 treatment protocol introduced RDV to be used in severe and critically ill cases [12]. Nevertheless, after the updated protocol in November, the drug’s use was expanded to include severe and moderate cases in high-risk populations [13].
Surprisingly, on 15 October 2020, the WHO Solidarity Trial (open-label global trial) published its interim results. The panel analyzed data from this trial, as well as three other randomized controlled trials. Data from over 7000 patients across the four trials were considered. The evidence indicated that there was no significant effect on mortality, need for mechanical ventilation, time to clinical improvement, and other patient-important outcomes [14,15]. However, the data from this trial have not undergone the rigorous review required to allow for constructive scientific discussion.
In April 2022, the Food and Drug Administration (FDA) still authorized the use of RDV as one of the options for COVID-19 hospitalized patients who require supplemental oxygen or oxygen through a high-flow device [8].
The guideline development group recognized that more research is needed, especially to provide higher certainty of the evidence for specific groups of patients. They supported continued enrollment in a trial evaluating RDV. New evidence supporting or refuting the need for RDV is imperative to guide Egyptian regulators’ decision making [16]. Hence, our study aims to assess the impact of introducing RDV in treatment protocol on patient outcomes.
Specific aim: The study aimed to compare the mortality rate, the hospital length of stay, and the need for MV before and after the introduction of RDV in moderate to severe COVID-19 patients. We hypothesized that introducing RDV will improve the following outcomes: reduce mortality rate, escalation to a higher level of care, the need for MV, and lower ICU and ward length of stay in moderate COVID-19 patients.
2. Methodology
Study design: We proposed a retrospective observational comparative follow-up study comparing the mortality rates, and need for MV, hospital length of stay in moderate to severe COVID-19 patients before and after including RDV in the COVID-19 treatment protocol.
Study setting: The Maamora Chest Hospital was converted to a COVID-19 isolation hospital during the pandemic, with 84 beds in isolation wards and 42 critical care beds.
Study sample: All patients fulfilling inclusion criteria from the 1 August 2020 until the end of February 2021 made up the study sample.
Study population: All patients admitted to general wards or intermediate care with a confirmed diagnosis of moderate and severe COVID-19, from 1 August until 31 October (NoRDV cohort), from November until February, with confirmed cases receiving RDV were included (RDV cohort). A confirmed COVID-19 case was defined as a patient having a positive result of the polymerase chain reaction (PCR) test for SARS-CoV-2 with a deep nasopharyngeal swab analyzed by the centralized molecular biology laboratory of the Alexandria Fever Hospital [12,13].
Inclusion criteria: Patients who were 18 years or older meeting the definition of moderate to severe COVID-19 were included. Patients with moderate COVID-19 had pulmonary infiltrates and SpO2%, whereas severe COVID-19 patients had respiratory rates > 30 breaths/min or lung infiltrates > 50 and SpO2 < 92%, and PaO2/FiO2 < 300 [12,13].
Exclusion criteria: Patients who were admitted to critical care, transferred from other hospitals, hospitalized for <24 h, or expired within 24 h of admission, in addition to patients after November 1st who did not receive RDV due to the presence of contraindications were excluded.
Data collection: Data were collected retrospectively from the hospital’s medical records at the pre-specified period and then recorded in the electronic data collection form.
Primary outcome definition: The primary endpoint is a composite outcome measure of escalation to the ICU from a general ward, progression to MV due to respiratory failure, or in-hospital mortality; if any of these outcomes were present, we considered it met our composite endpoint
Other outcomes: Mortality rate is defined as the number of deaths divided by the sum of patient days calculated monthly. The MV rate is the number of admitted patients who deteriorated and required MV per total patient days. In addition, the average monthly length of stay (LOS) is calculated by summing all individual lengths of stay. LOS was assessed for patients who were discharged alive.
3. Statistical Analysis
Since quantitative variables were abnormally distributed, baseline characteristics of the RDV and NoRDV groups were presented as frequency and percent for categorical variables, median, and range for quantitative variables. Aside from the Z test for independent rates, Chi-squared, Fischer exact test, or Mann–Whitney were used to compare the two groups.
Bivariate logistic regression analysis was performed for the composite measure as a dependent and the use of RDV as an explanatory variable. The multivariate analysis was subsequently conducted to adjust for age, gender, disease severity, and comorbidities. Variables were included in the model if the p-value of the univariate analysis was lower than 0.2.
The log-rank test was used to compare the median time to death, MV, and transfer to higher-level separately across groups. The effect of RDV on mortality rate, MV rate, and transfer to higher-level rate was then assessed using multivariable Cox proportional regression analysis while controlling for all other predictors. The level of statistical significance was set at a p-value of 0.05. Subgroup analyses were performed for mortality and MV using the same regression model. Our sample surpassed the minimum required sample size of 213 in both groups, calculated assuming that the proportion of composite outcome in RDV and NoRDV groups equal 35% and 54%, respectively [17], to achieve 80% power at a 5% level of significance was. All statistical analyses and sample size calculations were performed using R software (R version 4.0.5).
4. Results
A total of 509 hospitalized patients were included in the study (PCR-confirmed COVID-19), 175 (35%) of whom were in the RDV group and 334 (65%) in the NoRDV group. The median and minimum ages of both groups were matching (59, 25 years), while the maximum age in the RDV cohort was 91 and for the NoRDV cohort, it was 89 years old. Females represent 55.4% of the NoRDV group and 46.9% of the RDV group; Alexandria residents constituted 88.9% and 89.7% of the RDV and the non-RDV group, respectively, and the comorbidities as shown in (Table 1). A total of 197 patients (40.1% NoRDV, 36% RDV group) were identified as having moderate disease, and 312 (59.9% NoRDV, 64% RDV group) were in the severe disease stratum. There is no statistical difference in the distribution of disease severity between the groups, p = 0.418. The baseline characteristics were well balanced between the groups concerning biochemical laboratory tests (SGOT, SGPT, urea, creatinine, leucocytes cells/mm, lymphocytes %, vital signs (temperature, heart rate, respiratory rate, oxygen saturation, diastolic and systolic blood pressures), as shown in Supplementary Table S3.

Table 1.
Baseline patient characteristics and clinical manifestations.
Each patient in the study received different therapeutic management according to the ongoing updates in COVID protocol adopting to MoHP Egypt Tables S1 and S2.
Our total population was divided into two groups: moderate (n = 197) and severe (n = 312). In the moderate group, 63 (32%) patients received RDV, 78 (39.6%), 6 (3%), and 2 (1%) received Hydroxychloroquine, Ivermectin, Lopinavir/Ritonavir, respectively, while 112 (35.9%) of the severe group received RDV, 74 (23.7%), 25 (8%), and 9 (2.9%) received Hydroxychloroquine, Ivermectin, and Lopinavir/Ritonavir, respectively.
Outcomes: The outcomes of cohorts are presented in Table 2; patients in the RDV group had statistically significant shorter hospital length of stay (median = 8 [range 1–29] days) than patients in the NoRDV group (median = 9 (range 1–65) days) (p = 0.004), and the difference was more evident in severe cases.

Table 2.
Patients’ outcomes.
Death, MV, and transfer to a higher level were the primary composite outcomes in 17.7% of the RDV group and 22% of the non-RDV group. However, the effect was statistically insignificant (p-value 0.289), even after stratification into moderate and severe cases (p = 0.684 and 0.291, respectively). The all-cause mortality rate was slightly higher in the RDV group 17.67 [11.2–24]/1000 patient days than in the NoRDV group, 15.9 [11.8–2]/1000 patient days (Table 2).
The univariable logistic regression analysis to test the effect of RDV use revealed a non-significant lower association with RDV use (OR = 0.75, 95% CI 0.47–1.19) that remained insignificant in the multivariable model (OR 0.623, 95% CI 0.37–1.02) after adjusting for all characteristics with p-value < 0.2 on separate logistic. The patient age and COVID severity are the significant factors for our primary composite outcome (OR 1.597, 2.804), respectively. Other factors such as gender, cardiovascular disease, chronic lung diseases, diabetes, obesity, and chronic kidney disease were not significantly associated with the composite outcome, as shown in Supplementary Table S5.
In addition, upon stratification according to COVID severity, for moderate patients, the RDV was insignificantly associated (OR 0.73, 95% CI 0.25–1.88), while for severe COVID patients, the RDV showed a significant association with reduced composed outcome (OR 0.42, 95% CI 0.17–0.96). However, none of the individual outcomes was significantly associated with the RDV use (p = 0.939, 0.5117, 0.0886) for mortality, MV, and transfer to a higher level, respectively).
According to Kaplan–Meier curves, the log-rank test revealed no difference in median time to death, time to MV, and time to transfer to a higher level (p = 0.48, 0.89, 0.23, respectively) (Figure 1).
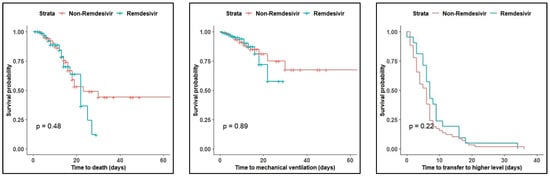
Figure 1.
Kaplan–Meier curves for mortality, mechanical ventilation, and transfer to a higher level.
Moreover, we performed a series of univariate and multivariate Cox proportional hazard regression models for each outcome constituting our composite outcome separately (mortality, mechanical ventilation, or transfer to a higher level) to include the time factor in the analysis. The use of RDV was associated with an insignificant reduction in the MV and escalation of care, and an insignificant increase in mortality. The results are illustrated in Table 3.

Table 3.
Cox hazard regression models for Remdesivir effect on Mortality, MV, and escalation of care.
Subsequent subgroup analyses for the same model to investigate effects in different subgroups also revealed a non-significant effect of RDV utilization on mortality risk and MV (Figure 2 and Figure 3).
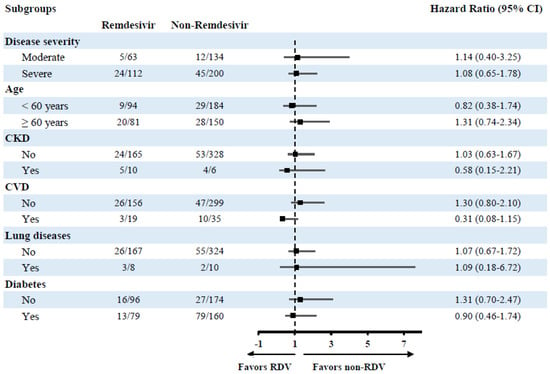
Figure 2.
Subgroup analysis of the risk of mortality in different treatment groups.
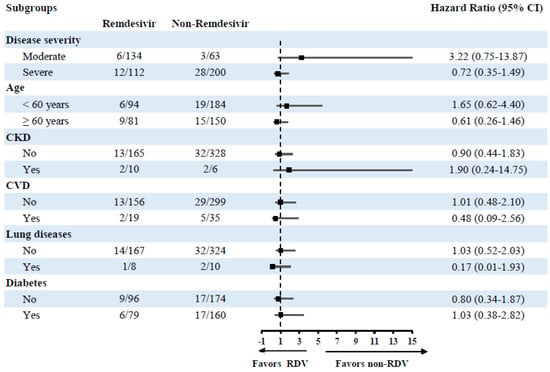
Figure 3.
Subgroup analysis of the risk of mechanical ventilation in different treatment groups.
5. Discussion
The current study is the first in Egypt to assess the effectiveness of RDV compared to other regimens while incorporating all patient characteristics in real situations with limited exclusion criteria.
In this study, our primary outcome was measured as a composite outcome of death, mechanical ventilation, and transfer to a higher level of care. In hospitalized patients with moderate to severe COVID-19, we found no significant difference in the composite outcome (22.2%) after its inclusion in the MoHP protocol compared to those who received other treatments (17.7%) in hospitalized patients with moderate to severe COVID-19. The association of the composite outcome with RDV was insignificant even after adjusting for all baseline characteristics, which were nearly balanced in the two cohorts. However, upon conducting a multivariate model, we found that age, disease severity, and chronic kidney disease were significant predictors of the composite outcome, but surprisingly, all other comorbidities, including cardiovascular diseases and lung diseases, were not. This finding can be attributed to possible incomplete history in patient records. Moreover, in the strata of patients younger than 60 years old, the odds of patients experiencing the composite outcome are 58% lower in the RDV cohort than in the NoRDV cohort, with no significant effect on older patients. Nevertheless, the effect was solely detected in the transfer to a higher level with no significant reduction in mortality or need for MV. The delays in transfer to a higher level could be interpreted by other factors such as limited critical bed capacity and prioritizing transfer of older patients in higher-risk categories.
Furthermore, there was no difference in overall mortality rate, MV rate, and transfer to a higher level rate, which could be due to a comparison of RDV is conducted against a combination of antivirals and standard care, not standard care alone. The median length of stay reduction was mainly prominent in severe COVID cases.
However, separate adjusted Cox hazard regression models for each endpoint in our composite outcome, RDV revealed a statistically insignificant effect for all three outcomes. The crude, adjusted hazard ratios as well as subgroup analysis for mortality, MV, and transfer to a higher level are consistent with the results of the systematic review and network meta-analysis conducted by Rochwerg et al. on which the WHO updated living guidelines based on the advice against RDV use regardless of the disease severity [3,15]. The pooled data from 7333 patients revealed that RDV had no significant effect on mortality (odds ratio 0.92; 95% CI, 0.80 to1.07), the need for MV (OR 0.88; 95% CI, 0.76–1.03) and the duration of hospitalization (mean difference: −0.5 lower; 95% CI, 3.3 lower–2.3 higher) [3,15]. However, a recent meta-analysis pooled the results of two non-interventional studies estimated a 44% reduction in risk of 28-day mortality [18]. This disparity in the population could be explained by the fact that Pasquini et al. focused on critical patients on MV [19] and Fried et al. reported the risk based on only 44 patients [20]. In addition, a study conducted by Olender SA et al. revealed that by Day 14, RDV was associated with significantly greater recovery and 62% reduced odds of death versus standard of care treatment in severe COVID-19 patients [21]. In our study, the insignificant effect can be attributed to either the difference in the comparison group since we compared against a combination of antivirals and standard care. Another potential explanation is lower power since our sample size was initially calculated based on the primary outcome (composite outcome) rather than secondary outcomes, indicating the need for further studies with a specifically calculated sample size to assess each outcome.
In the previously published studies supporting the use of RDV, Beigel JH et al. concluded that the RDV arm had a lower median time to recovery (10 days; 95% CI, −9–11) compared to the placebo (15 days; 95% CI, 13 to 18) but the effect on mortality was not statistically significant (hazard ratio 0.73; 95% CI, −0.2–1.03) [10]. In an open-label clinical trial, Spinner et al. revealed that 65% and 54% of patients receiving a 5-day course of RDV and patients receiving a 10-day course, respectively, had a two-point clinical improvement at day 14 on a seven-point ordinal scale. This finding indicates that both groups demonstrated a significant clinical improvement from baseline, but the 10-day course had no statistically significant benefit [11]. In 2021, Hussain Alsayed et al. found that early administration of RDV (first 7 days of symptoms onset) could decrease ICU admissions (aHR 0.31; 95% CI, 0.15 to 0.64), MV need (aHR, 0.22; 95% CI, 0.10 to 0.51), and mortality at 28 days (aHR, 0.15; 95% CI, 0.04 to 0.53) than RDV late administration or non RDV group 23 [22] In the fourth study, hospitalized moderate COVID-19 patients receiving a 5-day course of RDV had a statistically significant improvement in clinical status compared to those on standard care (odds ratio = 1.65; 95% CI, 1.09–2.48] after 11 days from treatment initiation. However, the improvement was of vague clinical importance [9]. In our study, a similar improvement percentage was observed in the RDV group (76.6%) and the NoRDV group (76.0%), yet we were unable to assess the effect of RDV on clinical improvement quantitatively since no similar instrument was applied in our local hospital.
Based on our findings, the treatment with RDV did not reduce the need for MV nor prevented the progression to severe respiratory distress or even death compared to other regimens.
RDV was the promising medicine for COVID-19 after the “Food and Drug Administration (FDA) issued an Emergency Use Authorization to permit the use of RDV for treatment of COVID-19 patients” based on findings of different studies showing an encouraging effect for the use of RDV in COVID-19 [9,10,11,23]. Later, on 20 November 2020, WHO issued a conditional recommendation against using RDV after disappointing results from the SOLIDARITY trial and the absence of concrete evidence from other studies that RDV improved the survival outcomes. Consequently, WHO excluded RDV from the COVID-19 management list without further updates [3]. Our results coincide with these recommendations. Accordingly, we urge the Egyptian MoHP to widely explore the effect of RDV in other settings and conduct cost-effectiveness analyses to evaluate RDV before the next COVID-19 patient management protocol revision.
6. Limitations
Since this is a one-center study, our findings could only be applied to a population similar to ours. Additionally, the retrospective comparative before and after the design is not the best design to assess effectiveness. However, due to the availability of records before and after the guidelines’ application, the time limitation, the inability of the research team to randomize the treatment, and the absence of a comparable control group in the same period, this design was considered optimal. In addition, due to the retrospective nature of the study, data were collected from a single source—the paper-based patient medical record—so several important variables were missing and could not be ascertained otherwise.
7. Conclusions
Our data revealed that treatment with RDV had no effect on patient outcomes over other antivirals and standard care, including the need for MV, the progression of the disease to severe respiratory distress, or even death. More research is needed to investigate and evaluate new therapeutic approaches: novel antivirals, immuno-modulators, and combination therapy, to improve outcomes in patients with COVID-19 and effectively aid the fight against this intimidating pandemic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid2120126/s1, Table S1: COVID-19 severity, Table S2: difference of May, November 2020 MoHP protocols, Table S3: patients’ characteristics stratified by severity, Table S4: patients’ outcomes stratified by severity, Table S5: Logistic regression, Table S6: all the medications used for hospitalized patients.
Author Contributions
I.A.: conceptualization, methodology, data analysis, a major contributor in writing the manuscript, R.A.: conceptualization, methodology, data management, writing results section, S.S.: material and data preparation, writing the discussion section, D.M.: material and data preparation, writing the discussion section, B.H.: conceptualization, material and data preparation, share in writing introduction and methodology sections, S.A. (Sara Abdulattif): material and data preparation, writing abstract and share in the introduction, S.A. (Shahinda Aly): a literature review, A.F. and D.K.: proofreading and editing of the manuscript, A.A. and E.K.: conceptualization, supervision, medical consultation, review of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Not Applicable.
Institutional Review Board Statement
The study was approved by the Ministry of Health and Population (MoHP) research ethics committee (Com. No/Dec. No: 3-2021/22), and it was carried out in concordance with national guidelines, with waivers of informed consent due to the retrospective nature of the study.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data will be available upon request from the first or corresponding authors.
Conflicts of Interest
All authors declared no conflict of interest.
Abbreviations
| CKD | Chronic kidney disease |
| CVD | Cardiovascular disease |
| COPD | Chronic obstructive pulmonary disorder |
| COVID-19 | Coronavirus associated disease 2019 |
| DAMA | Discharge against medical advice |
| DVT | Deep vein thrombosis, |
| FDA | Food and drug administration |
| HCV | Hepatitis C virus |
| HR | Hazard ratio |
| ICU1 | Intermediate care unit |
| ICU 2 | Intensive care unit |
| ILD | Interstitial lung disease |
| LOS | Length of stay |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| MoHP | Egyptian ministry of health and population |
| MV | Mechanical ventilation |
| NoRDV | Non-Remdesivir |
| OR | Odds ratio |
| PaO2/FiO2 ratio | The ratio of arterial oxygen partial pressure to fractional inspired oxygen |
| PCR | Polymerase chain reaction |
| RDV | Remdesivir |
| SARS-CoV2 | Severe acute respiratory syndrome coronavirus |
| SGPT | Serum glutamic pyruvic transaminase |
| SGOT | Serum glutamic oxaloacetic transaminase |
| SpO2 | Peripheral oxygen saturation |
| TB | Tuberculosis |
References
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Dryhurst, S.; Schneider, C.R.; Kerr, J.; Freeman, A.L.J.; Recchia, G.; van der Bles, A.M.; Spiegelhalter, D.; van der Linden, S. Risk perceptions of COVID-19 around the world. J. Risk Res. 2020, 23, 994–1006. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Therapeutics and COVID-19: Living Guideline, 20 November 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; Aronson, J.K. Remdesivir in COVID-19. BMJ 2020, 369, m1610. [Google Scholar] [CrossRef] [PubMed]
- GILEAD. U.S. Food and Drug Administration Approves Gilead’s Antiviral Veklury® (remdesivir) for Treatment of COVID-19. Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2020/10/us-food-and-drug-administration-approves-gileads-antiviral-veklury-remdesivir-for-treatment-of-covid19 (accessed on 21 June 2022).
- Rubin, D.; Chan-Tack, K.; Farley, J.; Sherwat, A. FDA Approval of Remdesivir—A Step in the Right Direction. N. Engl. J. Med. 2020, 383, 2598–2600. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. J. Am. Med. Assoc. 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Ministry of Health and Population (MoHP). Management Protocol for COVID-19 Patients Version 1.4/30 May 2020. Available online: http://www.mohp.gov.eg/JobsDetails.aspx?job_id=3061 (accessed on 21 June 2022).
- Ministry of Health and Population (MoHP). Management Protocol for COVID-19 Patients Version 1.4/November 2020. Available online: http://www.mohp.gov.eg/JobsDetails.aspx?job_id=3061 (accessed on 21 June 2022).
- Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; García, C.H.; Kieny, M.-P.; Malekzadeh, R.; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Lamontagne, F.; Agoritsas, T.; MacDonald, H.; Leo, Y.S.; DIaz, J.; Agarwal, A.; Zeng, L.; Cecconi, L.; Chanda, D.; Gotte, M.; et al. A living WHO guideline on drugs for COVID-19. BMJ 2020, 370, m3379. [Google Scholar]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf (accessed on 21 June 2022).
- Fadel, R.; Morrison, A.R.; Vahia, A.; Smith, Z.R.; Chaudhry, Z.; Bhargava, P.; Miller, J.; Kenney, R.M.; Alangaden, G.; Ramesh, M.S.; et al. Early Short-Course Corticosteroids in Hospitalized Patients with COVID-19. Clin. Infect. Dis. 2020, 71, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizadeh, A.; Khiali, S.; Sarbakhsh, P.; Entezari-Maleki, T. Remdesivir for treatment of COVID-19: An updated systematic review and meta-analysis. Eur. J. Pharmacol. 2021, 897, 173926. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, Z.; Montalti, R.; Temperoni, C.; Canovari, B.; Mancini, M.; Tempesta, M.; Pimpini, D.; Zallocco, N.; Barchiesi, F. Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU. J. Antimicrob. Chemother. 2020, 75, 3359–3365. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.W.; Crawford, J.M.; Mospan, A.R.; Watkins, S.E.; Munoz, B.; Zink, R.C.; Elliott, S.; Burleson, K.; Landis, C.; Reddy, K.R.; et al. Patient Characteristics and Outcomes of 11,721 Patients with Coronavirus Disease 2019 (COVID-19) Hospitalized Across the United States. Clin. Infect. Dis. 2021, 72, E558–E565. [Google Scholar] [CrossRef] [PubMed]
- Olender, S.A.; Perez, K.K.; Go, A.S.; Balani, B.; Price-Haywood, E.G.; Shah, N.S.; Wang, S.; Walunas, T.L.; Swaminathan, S.; Slim, J.; et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care. Clin. Infect. Dis. 2021, 73, E4166–E4174. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alsayed, H.; Saheb Sharif-Askari, F.; Saheb Sharif-Askari, N.; Hussain, S.A.A.; Hamid, Q.; Halwani, R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS ONE 2021, 16, e0258643. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0258643 (accessed on 21 June 2022).
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).